Abstract
Our recent report on a parallel decrease in the body weights and serum IGF-I levels of weaver mice suggests that IGF-I’s endocrine function may be impaired in neurodegenerative diseases. To further understand the overall effects of IGF-I deficiency on the postnatal growth, we measured bone mineral density (BMD), bone mineral content (BMC), lean body mass (LBM) and fat mass in male and female weaver mice and wild-type littermates on D21 (prepuberty), D45 (puberty), and D60 (postpuberty) using Dual-Energy X-ray Absorptionmetry (DEXA). In both male and female weaver mice, we found that the levels of circulating IGF-I paralleled those of BMD, BMC, and LBM, but not the fat mass. Male weaver mice have normal fat mass at all three ages studied, whereas female weaver mice showed a trend to increase their fat mass as they mature. To determine whether circulating IGF-I is a determinant of body composition, we crossbred IGF-I transgenic mice with homozygous weaver mice, which resulted in a significant increase in circulating IGF-I levels in both male and female weaver mice and normalization of their BMD, BMC and body weights. In summary, our results demonstrated that normal circulating IGF-I levels are important in maintaining BMD, BMC, and body composition in neurodegenerative diseases, such as hereditary cerebellar ataxia.
Keywords: BMD, body composition, IGF-I, weaver mice
Introduction
The growth hormone (GH) and IGF-I axis plays essential roles in overall pre- and postnatal body growth primarily through stimulating bone growth and improving body composition. In humans, circulating levels of both GH and IGF-I increase during puberty in parallel with increases in bone mineral density (BMD) (Libanati et al., 1999; Moreira-Andres et al., 1995; Richman et al., 2001). Children with GH deficiency (Boot et al., 1997) or IGF-I gene deletion (Woods et al., 1997) exhibit increased total fat and reduced BMD which can be reversed following recombinant human IGF-I (rhIGF-I) therapy (Woods et al., 2000). IGF-I treatment also increases longitudinal growth, body weight, and body fat in children with GH insensitivity syndromes (Ranke et al., 1999), suggesting that IGF-I mediates GH’s effects on growth. An essential role for IGF-I in bone growth and body composition is further reinforced by studies using mouse models of gene deletion and mutation. A decrease in BMD was seen in Igf1 heterozygous knockout mice (He et al., 2006; Mohan and Baylink, 2005) and mice lacking a functional IGF-I gene or IGF-I receptor (IGF-IR) gene (Bikle et al., 2001; Mohan et al., 2003; Zhang et al., 2002). Conversely, mice expressing GH or IGF-I transgene had an increase in BMD (Saban et al., 1996; Zhao et al., 2000). The above evidence demonstrates that IGF-I promotes bone growth with its positive effects on BMD.
Dual Energy X-ray Absortiometry, or DEXA scanning, is currently the most widely used method to measure bone mineral density in humans and animals. First introduced in 1987 (Genant HK, 1987), DEXA determines body composition according to a three-compartment model: BW = BF + BMC + FFM (where BW is body weight, BF is body fat, BMC is bone mineral content and FFM is fat-free mass). GH deficiency (GHD) or GH receptor deficiency (GHRD) in mice and humans is accompanied by increased fat mass, decreased LBM and body weight, as well as decreased circulating IGF-I levels (Bachrach et al., 1998; Boguszewski et al., 2005; Donahue and Beamer, 1993). This poor body composition can be improved by IGF-I treatment, as demonstrated by reduced body fat as well as increased LBM and body weight (Mauras et al., 2000a; Mauras et al., 2000b; Thompson et al., 1995; Woods et al., 2000). To date, although the role of the GH/IGF-I axis in postnatal growth is established, whether IGF-I influences somatic growth in neurodegenerative diseases, as well as in other chronic conditions, has not been evaluated.
The weaver mutant mouse is a commonly used model for studying hereditary cerebellar ataxia due to the degeneration of cerebellum granule neurons (Goldowitz, 1989; Smeyne and Goldowitz, 1989) and is characterized by ataxia, hyperactivity, and tremor (Caviness and Rakic, 1978). Previously, we demonstrated a decrease in IGF-I’s biological activities in the weaver cerebellum during neurodegeneration (Zhong et al., 2002). In the early postnatal period, we also found a parallel decrease in circulating IGF-I levels and body growth retardation in weaver mice (Yao et al., 2005). Because of IGF-I’s role in bone growth and body composition, we hypothesize that these parameters are also affected in weaver mice. To test this hypothesis, we used DEXA to compare BMD and body composition in homozygous and heterozygous weaver mice and their wild-type control littermates at postnatal ages that encompass time periods reaching maturity. To further evaluate the contribution of circulating IGF-I to bone growth and body composition, we cross-bred IGF-I transgenic mice with homozygous weaver mice and re-examined the same parameters after verifying transgene expression. Results of our investigation support the role of IGF-I in bone growth and body composition in mice with neurodegenerative disease.
Materials and Methods
Animal and Tissue Preparation
The care and maintenance of the mice were in strict accordance with the NIH Guide for the Care and Use of Laboratory Animals. Weaver mice were obtained from a colony maintained at the Indiana University Medical Center. This colony was established from mice heterozygous for the weaver gene purchased from the Jackson Laboratory (Bar Harbor, ME). Mutant and wild type mice were maintained on a hybrid B6CBA-AW-J/A background. Homozygous weaver mutants (w/w) were obtained by crossing pairs of heterozygous or homozygous females with heterozygous males. In the IGF-I transgenic study, female w/w mice were crossbred with male IGF-I transgenic mice from a C57B6 genetic background, kindly provided by Dr. A.J. D’Ercole. The generation of IGF-I transgenic mice has been described previously (Ye et al., 1996). These transgenic mice carry a 6.9-kb fusion gene with a 5.7-kb fragment of the 5′ mouse IGF-II genomic regulatory region driving the expression of a human IGF-I cDNA (Dai et al., 1992). IGF-I transgenic mice were bred as heterozygotes, since homozygous transgenic mice are not viable. Herein, we use I/+ to indicate that the transgene is present and use I/− to indicate its absence. Female w/w mice were mated with male I/+ mice to obtain I/+,w/+ mice. Then male I/+,w/+ mice were cross-bred with female w/w mice to derive I/+,w/w and I/−,w/w mice. Mice were provided with food and water ad libitum and placed on a 12:12 hr light/dark cycle. Mice were housed two per cage with the same genotype. Wet-feed was consistently provided to weaver mice to help in their feeding.
Genotyping
Genomic DNA was isolated from tails and the genotypes of both weaver and IGF-I transgenic mice were determined by polymerse chain reaction (PCR) with primers as previously reported (Ye et al., 1996). A point mutation of the K+ ion channel (Girk2) (Lesage et al., 1994) is associated with the weaver mutation (Patil et al., 1995; Slesinger et al., 1996). For AgeI restriction enzyme digestion, the DNA was amplified in a 25 μl reaction volume using primers AgeI-1 (5′-GCT TTT TTA TTC TCC ATA GAG ACA GAA ACC ACC ACC-3′) and AgeI -2 (5′-AAC ACG GAC TGG ATT AAG AAG-3′). The AgeI primer has a C at the penultimate base of the 3′ end instead of a T, which would normally be present in genomic DNA. In the final amplification product, this results in the loss of an AgeI site if the weaver mutation is present. The cycling parameters are: denaturation at 95°C for 2 min., followed by 35 amplification cycles (30 sec. at 95°C, 30 sec. at 66°C, 1 min. at 72°C) and a final extension at 72°C for 5 min. After amplification, 10 μl of PCR products were digested with AgeI in a 20 μlume and evaluated by electrophoresis in an 8% polycarylamide (TBE) gel. The resulting restriction fragments were determined for mutant and normal alleles.
To determine the IGF-I transgene, a primer sequence hIGF-I (359 bp) was used (forward primer: 5′-gga ccg gag acg ctc tgc gg-3′ and reverse primer: 5′-ctg cgg tgg cat gtc act ct-3′). A 40 μl PCR reaction mixture contained 4 μl 10 ×synthesis buffer, 2 μl primer, 1 μl 10mM dNTP, 0.2 μl Taq enzyme (Invitrogene), 6 μl sample, and 26.8 μl ddH2O. The denaturing temperature (Tm) was set at 94°C, annealing Tm at 59°C and extending Tm at 72°C (35 cycle). DNA products were quantified after polyacrylamide gel electrophoresis.
RT-PCR
Total RNAs (cerebellum, liver, heart, kidney, spleen, and muscle) were prepared by an RNAzol method according to manufacturer’s instructions. A 20 μl RT reaction system contained 1 μl of sample (1 μg of total RNA), 1μl polydT, 9μl DEPC water, 4μl 5× synthesis buffer, 1μl 10mM dNTP, 2μl 0.1M DTT, 1μl reverse transcriptase SuperscriptII (Invitrogene) and 1μl RNase inhibitor. Primer sequences were as follows:
hIGF-I (359 bp)
Forward primer: 5′-gga ccg gag acg ctc tgc gg-3′
Reverse primer: 5′-ctg cgg tgg cat gtc act ct-3′
Histone 3.3 (220 bp)
Forward primer: 5′-gca aga gtg cgc cct cta ctg-3′
Reverse primer: 5′-ggc ctc act tgc ctc ctg caa-3′
The reaction was carried out at 42°C for 50 min; then, 70°C for 10 min to denature reverse transcriptase. A 25 μl PCR reaction mixture contained 1 unit DNA polymerase Tfl (Invitrogene), 2.5μl 10 × synthesis buffer, 2.0μl 25mM MgSO4, 0.5μl dNTP, 1.0 μl primer respectively, 2.5 μl RT product and DEPC water. The denaturing temperature was set at 94°C, annealing Tm at 59°C and extending Tm at 72°C (28 cycles). DNA products were quantified after polyacrylamide gel electrophoresis.
Radioimmunoassay (RIA)
All reagents and basic procedures for the mouse IGF-I RIA were from ALPCO Diagnostics, Inc. (Windham, NH). A formic acid-acetone extraction procedure was used to separate IGFs from IGFBPs (Bowsher et al., 1991). Briefly, 10 μl serum was added into 1 ml diluted buffer to make a 1:100 dilution. Next, 100 μl diluted samples were then pipetted into the assay tubes. 100 μl anti-hIGF-I first antibody containing rabbit IgG and rec hIGF-II and 100 μl tracer 125I-IGF-I were added. Tubes were incubated at 4°C for 2 days. Then 500 μl cold anti-rabbit second antibody and precipitation reagent mixture was added. Tubes were mixed with a vortex mixer and incubated at 4°C for 1 hr. After adding 1 ml ice-cold distilled water, tubes were centrifuged at 3,000 g for 30 min at 4°C. Then the supernatant was aspirated and the radioactivity of precipitates was measured in a gamma counter.
Dual-Energy X-ray Absorptionmetry (DEXA)
Male and female weaver mice of three genotypes (+/+, w/+, and w/w) at D21, D45, D60 (n=4–6) and I/+, w/w and I/−, w/w mice at D45 (n=4) were used for the experiments. Mice were anesthetized with Ketamine/Xylazine (Ketamine 9 mg/ml mixed with Xylazine 1 mg/ml) cocktail at a dosage of 100 mg/kg body weight). Each mouse was scanned and DEXA was measured using a Lunar PIXImus densitometer (Lunar Corp, Madison, WI) and a specialized software (software version PIXImus II 2.0.) was used to calculate total bone mineral density (BMD mg/cm2), total bone mineral content (BMC mg), bone-free lean body mass (LBM g), and fat mass (FM, g). The fat percentage (%) was calculated by dividing FM by the sum of fat mass and LBM. All data presented for body composition exclude the head (i.e., sub-cranial body composition)
Statistical Analysis
All values are presented as mean ± SEM. The statistical significance among three genotypes was determined by the Analysis of Variance (ANOVA). The difference between the two groups was assessed by student’s unpaired t test. Two mean values are considered statistically different, if a p value is less than 0.05.
Results
BMD and BMC in weaver mice
We previously reported a parallel reduction in circulating IGF-I levels and body weights in weaver mice(Yao et al., 2005). To further evaluate the effects of IGF-I deficiency on overall body growth we first compared total BMD and BMC in weaver mice of three genotypes (w/w, w/+, +/+). As shown in Table 1, the BMD and BMC of female weaver mice (w/w) were reduced to 91% and 60% of their wild-type controls at D21, 88% and 71% at D45, and 92.6% and 76% at D60, respectively. In D21 male weaver mice, BMD and BMC were reduced to 80% and 57% of their wild-type controls respectively; but both parameters showed a trend to increase from D45 to D60 and approached normal levels after the mice entered puberty. Interestingly, the gene dose dependent fluctuation of both BMD and BMC in weaver mice was parallel to their circulating IGF-I levels (Yao et al., 2005).
Table 1. Total BMD and BMC in weaver mice.
Total BMD and BMC of +/+, w/+ and w/w mice of both genders at postnatal days 21, 45 and 60 were measured by PIXImus instrument. Each value represents means ± SEM. (n= 4–6).
| Female
|
Male
|
||||||
|---|---|---|---|---|---|---|---|
| +/+ | w/+ | w/w | +/+ | w/+ | w/w | ||
| D21 | BMD (mg/cm2) | 29.1±0.1 | 28.5±1.1 | 26.5±0.1a | 30.0±0.4 | 27.2±0.3 | 24±0.4a |
| BMC (mg) | 140±1.8 | 110±5.2 | 84±4.0b | 145±5.0 | 120±3.5 | 82±4.5b | |
| D45 | BMD (mg/cm2) | 39.8±0.5 | 41.8±0.3 | 34.9±0.8a | 42.8±1.2 | 45.5±0.6 | 39.8±0.5 |
| BMC (mg) | 254.5±4.2 | 267±4.0 | 181±6.3a | 278±13.6 | 335±5.0 | 246±7.3 | |
| D60 | BMD (mg/cm2) | 43.1±0.3 | 47.4±0.7 | 39.9±0.6a | 47.7±1.0 | 48.7±0.8 | 48.2±1.2 |
| BMC (mg) | 295±4.4 | 326.1±4.9 | 223±7.3a | 338.3±7.3 | 396.2±6.3a | 331.4±8.8 | |
p<0.05;
p<0.001 compared to age-matched +/+ mice, as assessed by one-way ANOVA.
LBM, body fat and fat percentage of weaver mice
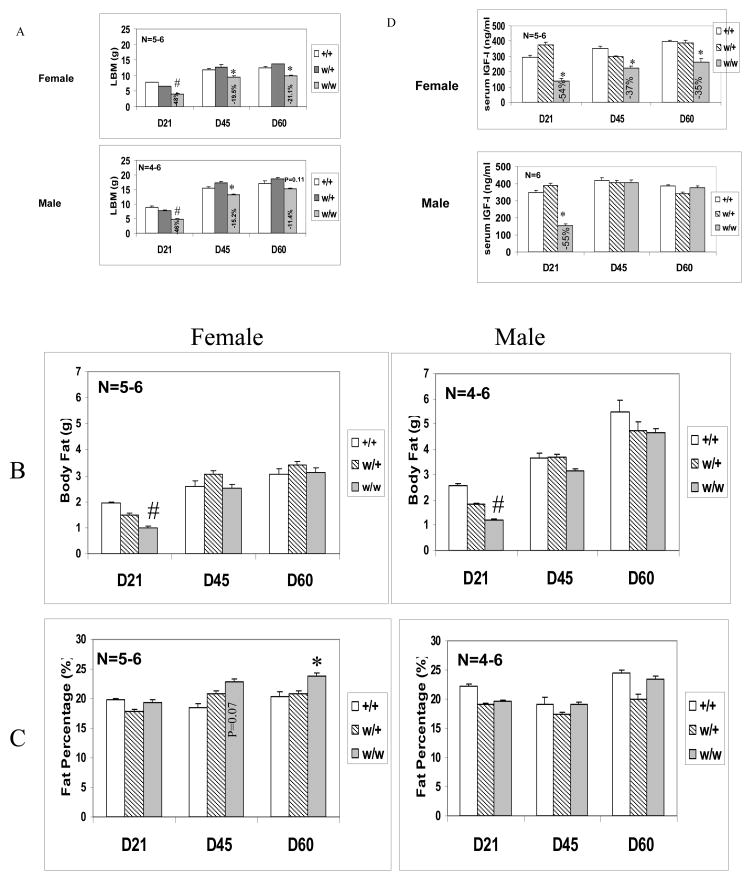
To further evaluate the effects of IGF-I deficiency on body composition, we compared bone-free lean body mass (LBM), fat mass (FM), and fat percentage in male and female weaver mice of three genotypes. LBM accounts for a significant portion of body weight. As shown in figure 1A, the LBM of female weaver mice was reduced to 52%, 80%, and 79% at D21, D45, and D60 of their wild-type mice respectively. In male weaver mice, LBM was reduced to 64% and 85% of their wild-type controls at D21 and D45, respectively. At D60, the LBM of male weaver mice was 89% of their wild-type controls and the difference was not significant. Interestingly, decreases of LBM in weaver mice were parallel to their circulating IGF-I levels. As previously published (Figure 1 D), the circulating IGF-I of female weaver mice was reduced to 46%, 63%, and 65% at D21, D45, and D60 of their wild type mice respectively. In male weaver mice, circulating IGF-I was reduced to 45% of their wild-type controls at D21. However, after male mice entered puberty, their circulating IGF-I levels increased to equal the IGF-I levels of their wild-type littermates. In addition, decreases of LBM at D21 were approximately double the decreases at D45 and D60 in both males and females. This trend was also observed in the reduction of total body fat in weaver mice. At D21, total body fat of female and male weaver mice was 52% and 46% of their wild-type controls respectively. After entering puberty, body fat was comparable to that of wild-type controls in female, but not male, weaver mice (Fig. 1B). The fat percentage was derived by dividing FM by the sum of FM and LBM. Male (all ages) and D21 female weaver mice have fat percentages similar to their wild-type littermates. After entering puberty, there was an increase in fat percentage in female weaver mice, which reached significant levels at D60 (Fig. 1C). These data demonstrated a gender difference in body fat accumulation in weaver mice and, to a lesser extent, in wild-type mice.
Figure 1. Body Composition and circulating IGF-I levels of Weaver Mice.
LBM (A), body fat (B), fat percentage (C), and circulating IGF-I levels (D) of +/+, w/+ and w/w mice of both genders at postnatal date 21, 45 and 60 were measured by DEXA method using a PIXImus instrument and RIA kit, respectively. Each value represents means ± SEM (n= 4–6). #: p<0.001; *: p<0.05 compared to age-matched +/+ mice, as assessed by One-way ANOVA.
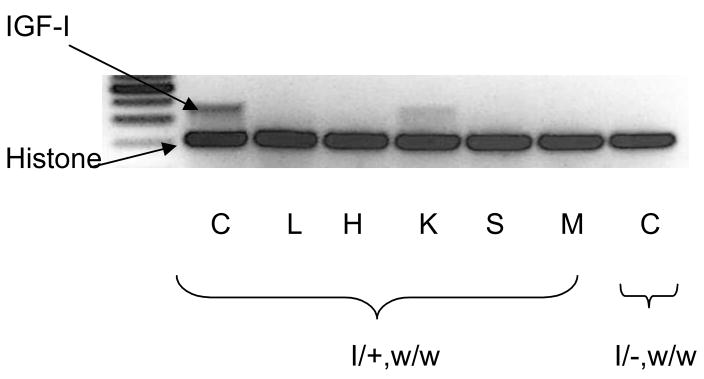
Expression of IGF-I transgene in weaver mice
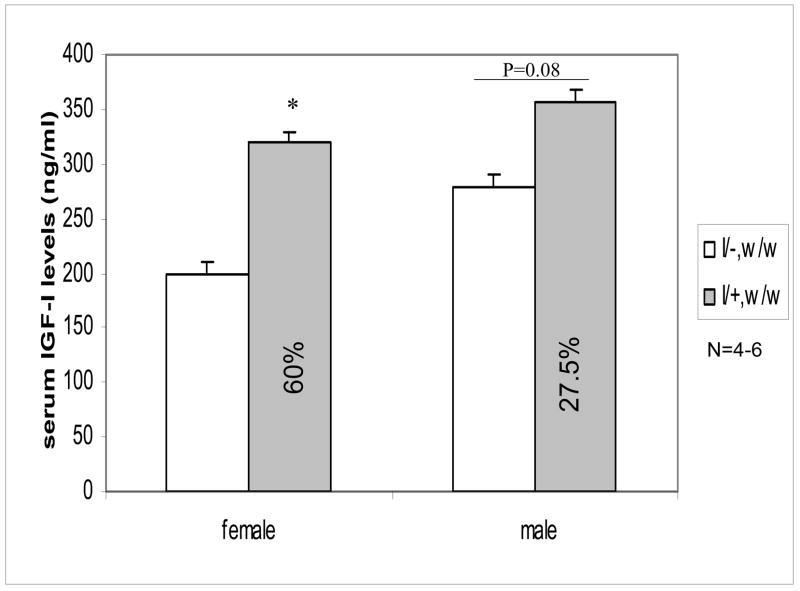
To determine whether IGF-I deficiency is associated with decreased BMD and body composition, we crossbred IGF-I transgenic mice with homozygous weaver mice. To verify the expression of IGF-I transgene, we used RT-PCR to measure transgene expression in cerebellum (C), liver (L), heart (H), kidney (K), spleen (S), and muscle (M) in homozygous weaver mice with (I/+, w/w) or without the transgene (I/−, w/w). IGF-I transgene was predominantly expressed in brain. The transgene also was expressed in the kidney to a much less extent, but not apparently in any other tissue examined (Figure 2). We also found that circulating IGF-I levels in adult IGF-I transgenic mice (460.3 + 29.8 ng/ml; n=3) (2 months) were higher than those in their wild-type littermates (365.3 + 10.9 ng/ml; n=3), though their body weights were at similar levels as those in wild-type mice. Expression of IGF-I transgene significantly increased circulating IGF-I levels at D45 in both male and female homozygous weaver mice (Figure 3), reaching approximately 160 % (females) and 127.5 % (males) of homozygous weaver littermates without the transgene.
Figure 2. Levels of IGF-I Transgene Expression.
The levels of IGF-I transgene mRNA were measure in cerebellum (C), liver (L), heart (H), kidney (K), spleen (S), and muscle (M) of homozygous weaver mice using semi-quantitative RT-PCR and corrected for Histone expression. IGF-I transgene is predominately expressed in brain, but not in other tissues.
Figure 3. Circulating IGF-I Levels in Cross-bred mice.
Serum IGF-I levels were measured in male and female weaver homozygous mice with (I/+, w/w) or without (I/−, w/w) IGF-I transgene at postnatal day 45 using an IGF-I RIA kit. Data are expressed as means ± SEM (n=4–6). *: p<0.05 compared to mean IGF-I levels of their corresponding I/−, w/w mice, as assessed by student t test.
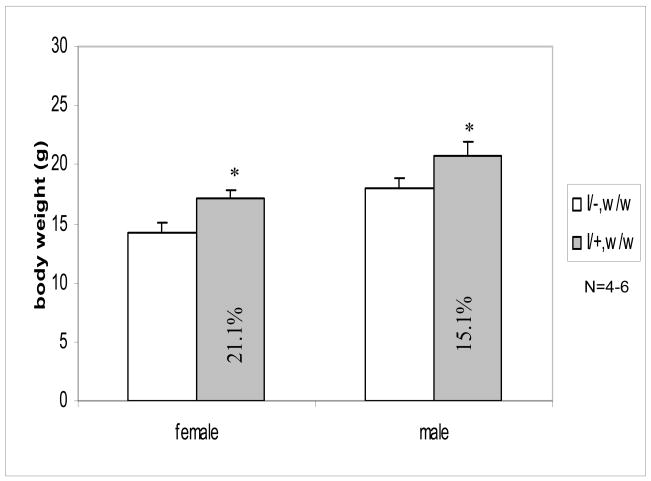
Expression of IGF-I transgene increased body weights of weaver mice
We previously found decreases in the body weights of weaver mice, which paralleled the decreases in IGF-I circulating levels (Yao et al., 2005). Expression of the IGF-I transgene significantly increased body weights of both female and male weaver mice at D45. As shown in Figure 4, body weights of female and male homozygous weaver mice increased to 121.1% and 115.1% when IGF-I transgene was present. These increases normalized the body weights of homozygous weaver mice with those of their wild-type control littermates.
Figure 4. Body Weights of Cross-bred Mice.
Body weights was measured in male and female weaver homozygous mice with (I/+, w/w) or without (I/−, w/w) IGF-I transgene mice at postnatal day 45. Data are expressed as means ± SEM (n=4–6). *: p<0.05 compared to mean body weight of their corresponding I/−, w/w mice, as assessed by student t test.
Expression of IGF-I transgene improved BMD and BMC in weaver mice
To further analyze the effects of IGF-I transgene on bone growth, we analyzed BMD and BMC in homozygous weaver mice with or without IGF-I transgene. As shown in Table 2, the expression of IGF-I transgene increased the total BMD to 110.7% (females) and 117.7% (males) of their homozygous weaver littermates at D45. In the same postnatal period, the increases in total BMC were more significant - to 125.4% (females) and 137.8% (males) of their homozygous weaver littermates.
Table 2. Total BMD and BMC in cross-bred mice.
Total BMD and BMC of homozygous weaver mice in the presence or absence of IGF-I transgene at postnatal date 45. Each value represents means ± SEM. (n= 4).
| IGF-I transgene | female | male | |
|---|---|---|---|
| BMD (mg/cm2) | − | 36.4±0.4 | 42.4±0.8 |
| + | 40.3±0.1a | 49.9±1.3a | |
| BMC (mg) | − | 224±4.1 | 286±12.7 |
| + | 281±7.3b | 394±4.5b |
p<0.05;
p<0.001 compared to age-matched +/+ mice, as assessed by student t test. −: without IGF-I transgene; +: with IGF-I transgene.
Expression of IGF-I transgene improved the body composition of weaver mice
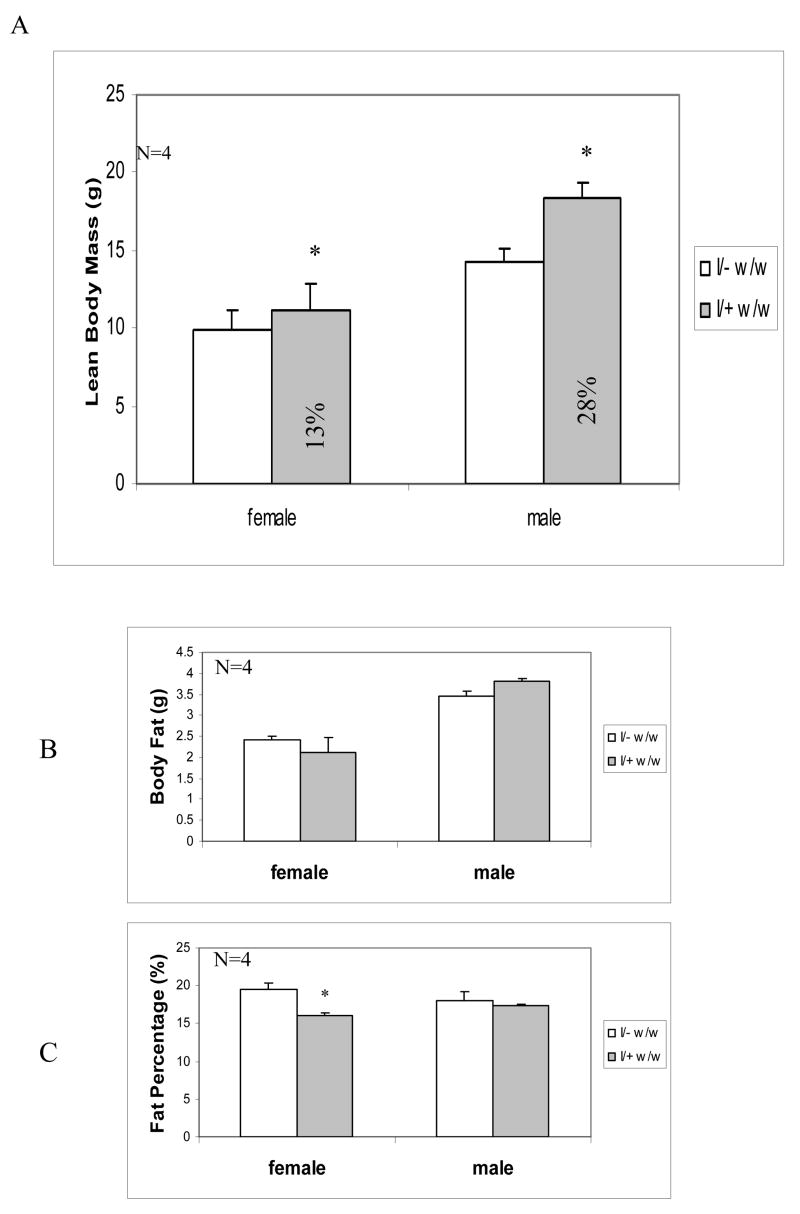
To determine the effects of IGF-I transgene expression on homozygous weaver mice, we compared LBM, total fat mass, and fat percentage in the presence or absence of IGF-I transgene. As shown in Figure 5A, expression of IGF-I transgene increased LBM of D45 weaver mice to 113% (females) and 128% (males) of their homozygous weaver littermates. Similar to their body weights, the body fat of D45 male weaver mice was also higher than their homozygous weaver littermates, though the increases did not reach significant levels (Figure 5B). Interestingly, the increase in body fat didn’t occur in D45 female weaver mice. On the other hand, the fat percentages in male weaver mice were maintained at the same levels as those in control wild-type littermates. However, the expression of IGF-I transgene resulted in a decrease in the fat percentage in D45 homozygous weaver females, compared to their homozygous female weaver littermates without the transgene (Fig. 5C).
Figure 5. Body Composition of Crossbred Mice.
LBM (A), body fat (B), and fat percentage (C) were measured in male and female weaver homozygous mice with (I/+, w/w) or without (I/−, w/w) IGF-I transgene mice at postnatal day 45 by DXEA using a PIXImus instrument. Data are expressed as means ± SEM (n= 4). *: p<0.05 compared to mean levels of homozygous weaver littermates (I/−, w/w), as assessed by student t test.
Discussion
The weaver mutant mouse presents a unique mouse model to study the functional role of the GH/IGF-I axis in postnatal development following neurodegeneration. Our previous report of a parallel decrease in their body weights and circulating IGF-I levels (Yao et al., 2005) supports the hypothesis that IGF-I deficiency contributes to their growth retardation. Our new findings provide additional supporting evidence that IGF-I deficiency also has a significant impact on bone growth and body composition in weaver mice. This conclusion is based on coordinated levels of circulating IGF-I, BMD, and body composition of weaver mice. In addition, homozygous weaver mice carrying an IGF-I transgene have normal body weights, BMD and body composition. These data provide important information concerning potential beneficial therapeutic uses of IGF-I in neurodegenerative diseases, especially those associated with IGF-I deficiency.
The decreases in circulating IGF-I levels are associated with decreases in BMD in several mouse models. For example, BMD was decreased in heterozygous IGF-I gene deletion mice (Mohan and Baylink, 2005), in congenic mice with reduced (~20%) circulating IGF-I (C3H.B6-6T[6T]) but normal GH levels(Bouxsein et al., 2002; Rosen et al., 2005; Rosen et al., 2004; Rosen et al., 1997), and in liver-specific IGF-I knockout mice (LID) (Sjogren et al., 2002; Yakar et al., 2002). The latter mice had normal body weights and their phenotypes became more severe when they crossed with acid labile subunit (ALS) knockout mice (LID+ALS double knockout mice), which resulted in a further reduction in circulating IGF-I levels (Yakar et al., 2002). The decrease of BMD and BMC in weaver mice also paralleled the decrease in circulating IGF-I levels, suggesting that IGF-I deficiency has the same negative impact on bone growth following neurodegeneration. On the other hand, expression of IGF-I transgene in homozygous weaver mice led to 25% (females) and 19% (males) increases in circulating IGF-I levels as well as increases in BMD and BMC. This evidence strongly supports a positive role of IGF-I in neurodegenerative diseases.
The increase in circulating IGF-I levels in this strain of IGF-I transgenic mice has not been reported before. When these transgenic mice were first characterized, transgene IGF-I mRNA was only identified in the brain, but no elevation in serum IGF-I levels was detected (Dai et al., 1992). The increased circulating IGF-I levels in our colony were likely due to changes in transgene expression occurring during their extensive breeding for over 10 years. It is also possible that kidney transgene expression is the source of the elevated circulating IGF-I. Alternately, improved brain function resulting from increased brain IGF-I transgene expression could have improved the health of weaver mice, and in turn stimulated the expression of the endogenous IGF-I gene. The improved bone growth may also contribute to their improved muscle strength and reduced ataxic behavior, as we previously reported (Zhong et al., 2005). Further work will be needed to answer these questions.
IGF-I initiates its growth promoting function on bone via both endocrine and autocrine/paracrine actions (Rosen et al., 1997). Our data suggest that IGF-I regulate BMD primarily through its endocrine action – or via circulating IGF-I levels. At postnatal day 5, homozygous weaver mice have substantially lower body weights than their wild type control littermates and have no ataxic phenotype. At this age, we did not detect any difference in IGF-I mRNA levels in the femora of homozygous weaver mice and their control littermates (data not shown), suggesting that local IGF-I probably did not contribute to the BMD decrease in weaver mice. However, whether bone IGF-I expression plays a role in BMD at older ages is unknown.
Although we found a correlation between circulating IGF-I levels and body growth, BMD, and BMC in w/w mice, the primary causes of IGF-I deficiency in weaver mice carrying a gene mutation in the Girk2 potassium channel (Slesinger et al., 1996), are not clear. Regardless, malnutrition due to lower food intake is unlikely the cause of low IGF-I circulating levels, because homozygous weaver mice consumed the same amount of food as their wild-type littermates (Yao et al., 2007). On the other hand, decreased circulating IGF-I in weaver mice likely results from their lower circulating GH levels (50% lower) (Yao et al., 2007). Since GH also has positive effects on bone mass and BMD (Johansson et al., 1992), it is likely that the decrease in bone growth results from a combined deficiency of the GH/IGF-I axis.
The deficiency of GH/IGF-I not only contributes to poor bone growth, but also to the deteriorated body composition of weaver mice, shown as decreases in LBM and increases in fat mass, especially in females. IGF-I gene deletion in an adult patient resulted in decreased LBM and increased fat mass, both of which were improved following IGF-I treatment (Woods et al., 2000). IGF-I treatment also improved body composition of GHD or GHRD patients (Mauras et al., 2000a; Mauras et al., 2000b), suggesting that GH exerts its function on body composition by interacting with IGF-I. Along this line, expression of IGF-I transgene in homozygous weaver mice normalized the body composition in weaver mice as shown by increased LBM and decreased fat mass. This result lends strong support for a positive effect of IGF-I on body composition following neurodegeneration. On the other hand, GH is also known to have a significant impact on body composition independent of IGF-I. It induces rapid loss of fat mass by stimulating lipolysis and antagonizing the lipogenic actions of insulin (Jorgensen et al., 1994; Laron and Klinger, 1993; Snyder et al., 1988; Svensson et al., 2003; Vahl et al., 1997; Veldhuis et al., 2005). Therefore, decreases in GH levels and/or GH activities likely attenuate its lipolytic action and lead to fat accumulation, as we see in homozygous weaver mice. Since both IGF-I (Yao et al., 2005) and GH hormone (Yao et al., 2007) are decreased in homozygous weaver mice, their poor body composition is most likely the result of the combined deficiency of the GH/IGF-I axis.
We also found a gender bias in bone growth and fat accumulation during the postnatal growth of weaver mice. While male weaver mice have lower fat mass but normal fat percentages; both fat masses and fat percentages of female weaver mice increased at D45 and D60. Expression of IGF-I transgene resulted in gender-dependent fat accumulation in weaver homozygous mice. In female weaver mice, the increase of circulating IGF-I was associated with reduced fat mass and fat percentage, but the opposite was seen in male weaver mice in the presence of IGF-I transgene. There is also a trend of catch-up for BMD, BMC, and LBM in male, but not female, weaver mice after they enter puberty. During puberty, accelerated body growth results from a complex interplay of metabolic hormones, growth factors, and nutrient uptake. Normally, gender specific interactions between gonadal steroid and GH lead to sexual dimorphic skeletal growth and fat accumulation, i.e., estrogen attenuates and/or androgen augments the action of GH (Leung et al., 2004). Besides regulating pituitary GH secretion, estrogen inhibits GH action on hepatocytes (Leung et al., 2003) and induces partial resistance of adipocytes to GH (Ghigo et al., 1999; Moller et al., 1992; Vahl et al., 1997). On the other hand, androgen directly stimulates osteoblast proliferation (Compston, 2001; Moller et al., 1992; Vahl et al., 1997) and promotes skeletal protein synthesis (Bhasin et al., 1997; Grinspoon et al., 1996; Katznelson et al., 1996; Snyder et al., 1999a; Snyder et al., 1999b). Androgen also stimulates bone growth indirectly by increasing IGF-I expression as shown in a conditionally immortalized human fetal osteoblastic cell line, hFOB/AR-6 (Gori et al., 1999) and an isolated organ culture (Maor et al., 1999). Both female and male weaver mice have a normal surge of sex hormones in early puberty. By D60, no genotype-dependent difference was observed in serum and pituitary FSH/LH levels in female weaver mice or in serum and testicular testosterone levels in male weaver mice (Schwartz et al., 1998). Since the gender bias occurs after the onset of puberty (D45), the surges of circulating estrogen and androgen are likely responsible for the differences in BMD and body composition as weaver mice reach sexual maturity.
In conclusion, this is the first study to characterize the effects of the GH/IGF-I axis on postnatal growth, especially bone growth and body composition, following neurodegeneration. We found that a deficiency of the GH/IGF-I axis is responsible for poor bone growth and body composition in weaver mice. The effects are more severe during infancy but become gender dependent after the onset of the puberty. Moreover, the poor bone growth and body composition of homozygous weaver mice can be corrected when an IGF-I transgene is expressed. Results of this investigation expand our knowledge of the functional role of the GH/IGF-I axis in postnatal growth following neurodegeneration and support the therapeutic use of IGF-I in neurodegenerative diseases associated with IGF-I deficiency.
Acknowledgments
This study was supported by the NIH, R01 NS40314, the Riley Children Foundation, and the Lilly Endowment. We are also thankful for the support from the Minor in Aging Fellowship from the School of Medicine at IUPUI and the Grants-in-Aid of Research (GIAR) grant from Sigma Xi.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bachrach LK, et al. Bone mineral, histomorphometry, and body composition in adults with growth hormone receptor deficiency. J Bone Miner Res. 1998;13:415–21. doi: 10.1359/jbmr.1998.13.3.415. [DOI] [PubMed] [Google Scholar]
- Bhasin S, et al. Testosterone replacement increases fat-free mass and muscle size in hypogonadal men. J Clin Endocrinol Metab. 1997;82:407–13. doi: 10.1210/jcem.82.2.3733. [DOI] [PubMed] [Google Scholar]
- Bikle D, et al. The skeletal structure of insulin-like growth factor I-deficient mice. J Bone Miner Res. 2001;16:2320–9. doi: 10.1359/jbmr.2001.16.12.2320. [DOI] [PubMed] [Google Scholar]
- Boguszewski CL, et al. One year of GH replacement therapy with a fixed low-dose regimen improves body composition, bone mineral density and lipid profile of GH-deficient adults. Eur J Endocrinol. 2005;152:67–75. doi: 10.1530/eje.1.01817. [DOI] [PubMed] [Google Scholar]
- Boot AM, et al. Changes in bone mineral density, body composition, and lipid metabolism during growth hormone (GH) treatment in children with GH deficiency. J Clin Endocrinol Metab. 1997;82:2423–8. doi: 10.1210/jcem.82.8.4149. [DOI] [PubMed] [Google Scholar]
- Bouxsein ML, et al. Generation of a new congenic mouse strain to test the relationships among serum insulin-like growth factor I, bone mineral density, and skeletal morphology in vivo. J Bone Miner Res. 2002;17:570–9. doi: 10.1359/jbmr.2002.17.4.570. [DOI] [PubMed] [Google Scholar]
- Bowsher RR, et al. Measurement of insulin-like growth factor-II in physiological fluids and tissues. I. An improved extraction procedure and radioimmunoassay for human and rat fluids. Endocrinology. 1991;128:805–14. doi: 10.1210/endo-128-2-805. [DOI] [PubMed] [Google Scholar]
- Caviness VS, Jr, Rakic P. Mechanisms of cortical development: a view from mutations in mice. Annu Rev Neurosci. 1978;1:297–326. doi: 10.1146/annurev.ne.01.030178.001501. [DOI] [PubMed] [Google Scholar]
- Compston JE. Sex steroids and bone. Physiol Rev. 2001;81:419–447. doi: 10.1152/physrev.2001.81.1.419. [DOI] [PubMed] [Google Scholar]
- Dai Z, et al. Creation of an autocrine model of insulin-like growth factor-I action in transfected FRTL-5 cells. Endocrinology. 1992;130:3175–83. doi: 10.1210/endo.130.6.1375893. [DOI] [PubMed] [Google Scholar]
- Donahue LR, Beamer WG. Growth hormone deficiency in ‘little’ mice results in aberrant body composition, reduced insulin-like growth factor-I and insulin-like growth factor-binding protein-3 (IGFBP-3), but does not affect IGFBP-2, -1 or -4. J Endocrinol. 1993;136:91–104. doi: 10.1677/joe.0.1360091. [DOI] [PubMed] [Google Scholar]
- Genant HKBJ, Steiger P, et al. Osteoporosis Update 1987. Berkeley, California: University of California Press; 1987. Quantitative computed tomography in the assessment of osteoporosis; pp. 49–71. [Google Scholar]
- Ghigo E, et al. Dose-response study of GH effects on circulating IGF-I and IGFBP-3 levels in healthy young men and women. Am J Physiol. 1999;276:E1009–13. doi: 10.1152/ajpendo.1999.276.6.E1009. [DOI] [PubMed] [Google Scholar]
- Goldowitz D. The weaver granuloprival phenotype is due to intrinsic action of the mutant locus in granule cells: evidence from homozygous weaver chimeras. Neuron. 1989;2:1565–75. doi: 10.1016/0896-6273(89)90045-7. [DOI] [PubMed] [Google Scholar]
- Gori F, et al. Effects of androgens on the insulin-like growth factor system in an androgen-responsive human osteoblastic cell line. Endocrinology. 1999;140:5579–86. doi: 10.1210/endo.140.12.7213. [DOI] [PubMed] [Google Scholar]
- Grinspoon S, et al. Loss of lean body and muscle mass correlates with androgen levels in hypogonadal men with acquired immunodeficiency syndrome and wasting. J Clin Endocrinol Metab. 1996;81:4051–8. doi: 10.1210/jcem.81.11.8923860. [DOI] [PubMed] [Google Scholar]
- He J, et al. Postnatal growth and bone mass in mice with IGF-I haploinsufficiency. Bone. 2006;38:826–35. doi: 10.1016/j.bone.2005.11.021. [DOI] [PubMed] [Google Scholar]
- Johansson AG, et al. The bone mineral density in acquired growth hormone deficiency correlates with circulating levels of insulin-like growth factor I. J Intern Med. 1992;232:447–52. doi: 10.1111/j.1365-2796.1992.tb00613.x. [DOI] [PubMed] [Google Scholar]
- Jorgensen JO, et al. Three years of growth hormone treatment in growth hormone-deficient adults: near normalization of body composition and physical performance. Eur J Endocrinol. 1994;130:224–8. doi: 10.1530/eje.0.1300224. [DOI] [PubMed] [Google Scholar]
- Katznelson L, et al. Increase in bone density and lean body mass during testosterone administration in men with acquired hypogonadism. J Clin Endocrinol Metab. 1996;81:4358–65. doi: 10.1210/jcem.81.12.8954042. [DOI] [PubMed] [Google Scholar]
- Laron Z, Klinger B. Body fat in Laron syndrome patients: effect of insulin-like growth factor I treatment. Horm Res. 1993;40:16–22. doi: 10.1159/000183762. [DOI] [PubMed] [Google Scholar]
- Lesage F, et al. Cloning provides evidence for a family of inward rectifier and G-protein coupled K+ channels in the brain. FEBS Lett. 1994;353:37–42. doi: 10.1016/0014-5793(94)01007-2. [DOI] [PubMed] [Google Scholar]
- Leung KC, et al. Estrogen inhibits GH signaling by suppressing GH-induced JAK2 phosphorylation, an effect mediated by SOCS-2. Proc Natl Acad Sci U S A. 2003;100:1016–21. doi: 10.1073/pnas.0337600100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung KC, et al. Estrogen regulation of growth hormone action. Endocr Rev. 2004;25:693–721. doi: 10.1210/er.2003-0035. [DOI] [PubMed] [Google Scholar]
- Libanati C, et al. Studies on the potential mediators of skeletal changes occurring during puberty in girls. J Clin Endocrinol Metab. 1999;84:2807–14. doi: 10.1210/jcem.84.8.5905. [DOI] [PubMed] [Google Scholar]
- Maor G, et al. Testosterone stimulates insulin-like growth factor-I and insulin-like growth factor-I-receptor gene expression in the mandibular condyle--a model of endochondral ossification. Endocrinology. 1999;140:1901–10. doi: 10.1210/endo.140.4.6618. [DOI] [PubMed] [Google Scholar]
- Mauras N, et al. Recombinant human insulin-like growth factor I has significant anabolic effects in adults with growth hormone receptor deficiency: studies on protein, glucose, and lipid metabolism. J Clin Endocrinol Metab. 2000a;85:3036–42. doi: 10.1210/jcem.85.9.6772. [DOI] [PubMed] [Google Scholar]
- Mauras N, et al. Insulin-like growth factor I and growth hormone (GH) treatment in GH-deficient humans: differential effects on protein, glucose, lipid, and calcium metabolism. J Clin Endocrinol Metab. 2000b;85:1686–94. doi: 10.1210/jcem.85.4.6541. [DOI] [PubMed] [Google Scholar]
- Mohan S, Baylink DJ. Impaired skeletal growth in mice with haploinsufficiency of IGF-I: genetic evidence that differences in IGF-I expression could contribute to peak bone mineral density differences. J Endocrinol. 2005;185:415–20. doi: 10.1677/joe.1.06141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan S, et al. Insulin-like growth factor regulates peak bone mineral density in mice by both growth hormone-dependent and -independent mechanisms. Endocrinology. 2003;144:929–36. doi: 10.1210/en.2002-220948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moller N, et al. Dose-response studies on the metabolic effects of a growth hormone pulse in humans. Metabolism. 1992;41:172–5. doi: 10.1016/0026-0495(92)90147-3. [DOI] [PubMed] [Google Scholar]
- Moreira-Andres MN, et al. Correlations between bone mineral density, insulin-like growth factor I and auxological variables. Eur J Endocrinol. 1995;132:573–9. doi: 10.1530/eje.0.1320573. [DOI] [PubMed] [Google Scholar]
- Patil N, et al. A potassium channel mutation in weaver mice implicates membrane excitability in granule cell differentiation. Nat Genet. 1995;11:126–9. doi: 10.1038/ng1095-126. [DOI] [PubMed] [Google Scholar]
- Ranke MB, et al. Experience with insulin-like growth factor I (IGF-I) treatment of growth hormone insensitivity syndrome (GHIS) J Pediatr Endocrinol Metab. 1999;12(Suppl 1):259–66. [PubMed] [Google Scholar]
- Richman C, et al. Postnatal and pubertal skeletal changes contribute predominantly to the differences in peak bone density between C3H/HeJ and C57BL/6J mice. J Bone Miner Res. 2001;16:386–97. doi: 10.1359/jbmr.2001.16.2.386. [DOI] [PubMed] [Google Scholar]
- Rosen CJ, et al. Allelic differences in a quantitative trait locus affecting insulin-like growth factor-I impact skeletal acquisition and body composition. Pediatr Nephrol. 2005;20:255–60. doi: 10.1007/s00467-004-1612-z. [DOI] [PubMed] [Google Scholar]
- Rosen CJ, et al. Congenic mice with low serum IGF-I have increased body fat, reduced bone mineral density, and an altered osteoblast differentiation program. Bone. 2004;35:1046–58. doi: 10.1016/j.bone.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Rosen CJ, et al. Circulating and skeletal insulin-like growth factor-I (IGF-I) concentrations in two inbred strains of mice with different bone mineral densities. Bone. 1997;21:217–23. doi: 10.1016/s8756-3282(97)00143-9. [DOI] [PubMed] [Google Scholar]
- Saban J, et al. Erythroid-specific expression of human growth hormone affects bone morphology in transgenic mice. Bone. 1996;18:47–52. doi: 10.1016/8756-3282(95)00419-x. [DOI] [PubMed] [Google Scholar]
- Schwartz NB, et al. Hypothalamic-pituitary-gonadal axis in the mutant weaver mouse. Neuroendocrinology. 1998;68:374–85. doi: 10.1159/000054387. [DOI] [PubMed] [Google Scholar]
- Sjogren K, et al. Effects of liver-derived insulin-like growth factor I on bone metabolism in mice. J Bone Miner Res. 2002;17:1977–87. doi: 10.1359/jbmr.2002.17.11.1977. [DOI] [PubMed] [Google Scholar]
- Slesinger PA, et al. Functional effects of the mouse weaver mutation on G protein-gated inwardly rectifying K+ channels. Neuron. 1996;16:321–31. doi: 10.1016/s0896-6273(00)80050-1. [DOI] [PubMed] [Google Scholar]
- Smeyne RJ, Goldowitz D. Development and death of external granular layer cells in the weaver mouse cerebellum: a quantitative study. J Neurosci. 1989;9:1608–20. doi: 10.1523/JNEUROSCI.09-05-01608.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder DK, et al. Treatment of obese, diet-restricted subjects with growth hormone for 11 weeks: effects on anabolism, lipolysis, and body composition. J Clin Endocrinol Metab. 1988;67:54–61. doi: 10.1210/jcem-67-1-54. [DOI] [PubMed] [Google Scholar]
- Snyder PJ, et al. Effect of testosterone treatment on bone mineral density in men over 65 years of age. J Clin Endocrinol Metab. 1999a;84:1966–72. doi: 10.1210/jcem.84.6.5741. [DOI] [PubMed] [Google Scholar]
- Snyder PJ, et al. Effect of testosterone treatment on body composition and muscle strength in men over 65 years of age. J Clin Endocrinol Metab. 1999b;84:2647–53. doi: 10.1210/jcem.84.8.5885. [DOI] [PubMed] [Google Scholar]
- Svensson J, et al. Effects of GH and insulin-like growth factor-I on body composition. J Endocrinol Invest. 2003;26:823–31. doi: 10.1007/BF03345231. [DOI] [PubMed] [Google Scholar]
- Thompson JL, et al. The effects of recombinant human insulin-like growth factor-I and growth hormone on body composition in elderly women. J Clin Endocrinol Metab. 1995;80:1845–52. doi: 10.1210/jcem.80.6.7539817. [DOI] [PubMed] [Google Scholar]
- Vahl N, et al. Metabolic effects and pharmacokinetics of a growth hormone pulse in healthy adults: relation to age, sex, and body composition. J Clin Endocrinol Metab. 1997;82:3612–8. doi: 10.1210/jcem.82.11.4388. [DOI] [PubMed] [Google Scholar]
- Veldhuis JD, et al. Endocrine control of body composition in infancy, childhood, and puberty. Endocr Rev. 2005;26:114–46. doi: 10.1210/er.2003-0038. [DOI] [PubMed] [Google Scholar]
- Woods KA, et al. Insulin-like growth factor I gene deletion causing intrauterine growth retardation and severe short stature. Acta Paediatr Suppl. 1997;423:39–45. doi: 10.1111/j.1651-2227.1997.tb18367.x. [DOI] [PubMed] [Google Scholar]
- Woods KA, et al. Effects of insulin-like growth factor I (IGF-I) therapy on body composition and insulin resistance in IGF-I gene deletion. J Clin Endocrinol Metab. 2000;85:1407–11. doi: 10.1210/jcem.85.4.6495. [DOI] [PubMed] [Google Scholar]
- Yakar S, et al. Circulating levels of IGF-1 directly regulate bone growth and density. J Clin Invest. 2002;110:771–81. doi: 10.1172/JCI15463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao W, et al. Role of the GH/IGF-I axis in the growth retardation of weaver mice. Endocrine. 2007;32:227–34. doi: 10.1007/s12020-007-9003-4. [DOI] [PubMed] [Google Scholar]
- Yao W, et al. Igf-I and postnatal growth of weaver mutant mice. Endocrine. 2005;26:117–25. doi: 10.1385/ENDO:26:2:117. [DOI] [PubMed] [Google Scholar]
- Ye P, et al. In vivo actions of insulin-like growth factor-I (IGF-I) on cerebellum development in transgenic mice: evidence that IGF-I increases proliferation of granule cell progenitors. Brain Res Dev Brain Res. 1996;95:44–54. doi: 10.1016/0165-3806(96)00492-0. [DOI] [PubMed] [Google Scholar]
- Zhang M, et al. Osteoblast-specific knockout of the insulin-like growth factor (IGF) receptor gene reveals an essential role of IGF signaling in bone matrix mineralization. J Biol Chem. 2002;277:44005–12. doi: 10.1074/jbc.M208265200. [DOI] [PubMed] [Google Scholar]
- Zhao G, et al. Targeted overexpression of insulin-like growth factor I to osteoblasts of transgenic mice: increased trabecular bone volume without increased osteoblast proliferation. Endocrinology. 2000;141:2674–82. doi: 10.1210/endo.141.7.7585. [DOI] [PubMed] [Google Scholar]
- Zhong J, et al. Inhibition of insulin-like growth factor I activity contributes to the premature apoptosis of cerebellar granule neuron in weaver mutant mice: in vitro analysis. J Neurosci Res. 2002;70:36–45. doi: 10.1002/jnr.10360. [DOI] [PubMed] [Google Scholar]
- Zhong J, et al. Insulin-like growth factor-I protects granule neurons from apoptosis and improves ataxia in weaver mice. J Neurosci Res. 2005;80:481–90. doi: 10.1002/jnr.20490. [DOI] [PubMed] [Google Scholar]