Abstract
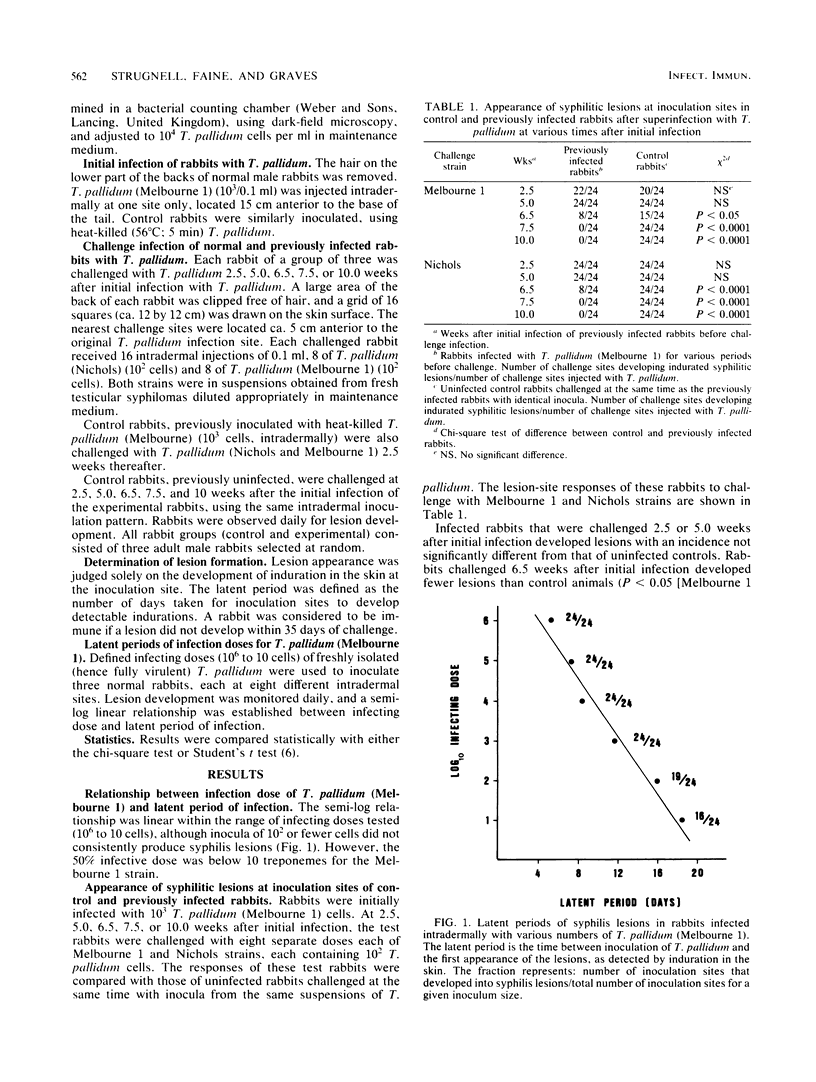
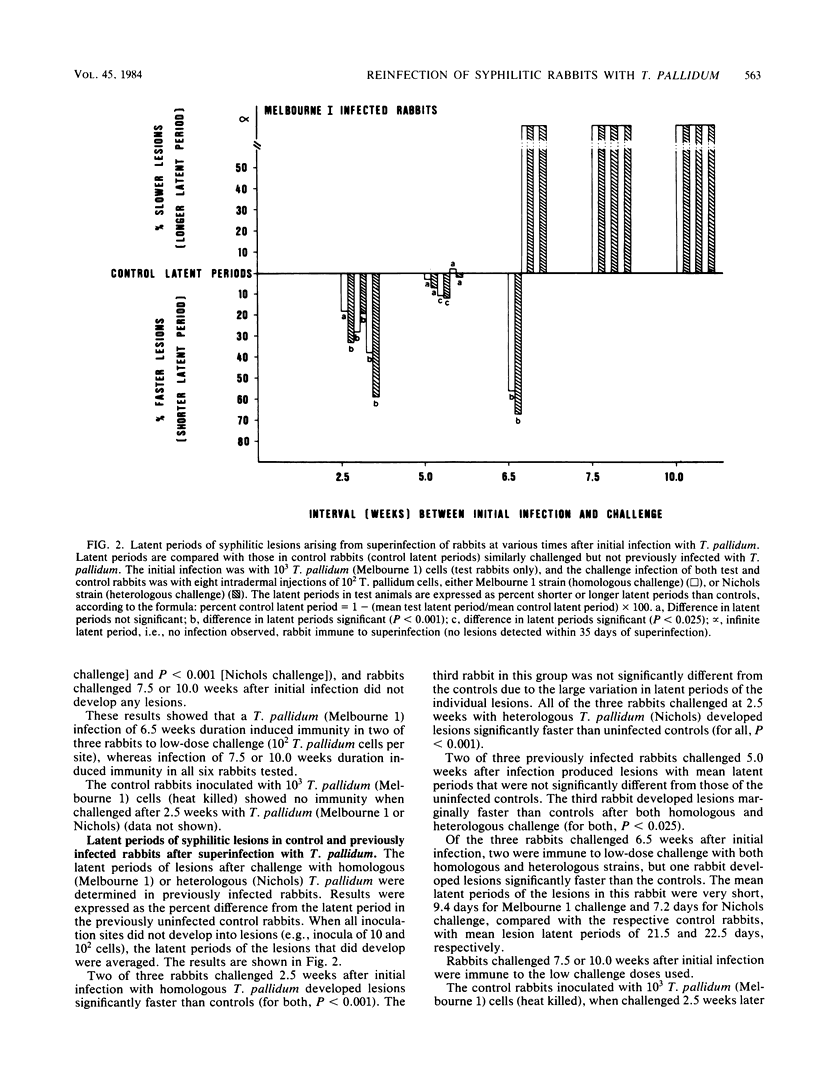
Rabbits infected intradermally with 10(3) Treponema pallidum (Melbourne 1) cells were examined for their susceptibility to reinfection with 10(2) T. pallidum cells (homologous or heterologous strains) at various intervals after the initial infection. At 2.5 weeks after infection, the rabbits were extremely sensitive to reinfection and developed syphilitic lesions significantly faster (i.e., shorter latent periods) than control rabbits that had not received the initial infection. This phenomenon may represent a state of immunosuppression or hypersensitivity in the infected rabbits. Whatever its etiology (at present unknown), it was a transient state since at 5 weeks after infection the rabbits were no longer different from control rabbits in their susceptibility to reinfection. They showed neither immunity (i.e., longer latent periods) nor immunosuppression or hypersensitivity (shorter latent periods) upon reinfection. At 6.5 weeks after infection, two of the three experimental rabbits were fully immune (no lesions upon reinfection), whereas the other rabbit exhibited immunosuppression or hypersensitivity upon reinfection. At 7.5 and 10 weeks after infection, all of the experimental rabbits were immune to reinfection. We conclude that syphilitic rabbits show a biphasic response to reinfection, consisting of an early phase of enhanced sensitivity to T. pallidum and a later phase of immunity to T. pallidum.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker-Zander S. A., Sell S., Lukehart S. A. Serum regulation of in vitro lymphocyte responses in early experimental syphilis. Infect Immun. 1982 Aug;37(2):568–578. doi: 10.1128/iai.37.2.568-578.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker-Zander S., Sell S. A histopathologic and immunologic study of the course of syphilis in the experimentally infected rabbit. Demonstration of long-lasting cellular immunity. Am J Pathol. 1980 Nov;101(2):387–414. [PMC free article] [PubMed] [Google Scholar]
- Baughn R. E., Musher D. M. Aberrant secondary antibody responses to sheep erythrocytes in rabbits with experimental syphilis. Infect Immun. 1979 Jul;25(1):133–138. doi: 10.1128/iai.25.1.133-138.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baughn R. E., Musher D. M. Altered immune responsiveness associated with experimental syphilis in the rabbit: elevated IgM and depressed IgG responses to sheep erythrocytes. J Immunol. 1978 May;120(5):1691–1695. [PubMed] [Google Scholar]
- Bey R. F., Johnson R. C., Fitzgerald T. J. Suppression of lymphocyte response to concanavalin A by mucopolysaccharide material from Treponema pallidum-infected rabbits. Infect Immun. 1979 Oct;26(1):64–69. doi: 10.1128/iai.26.1.64-69.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald T. J. Accelerated lesion development in experimental syphilis. Infect Immun. 1981 Nov;34(2):478–482. doi: 10.1128/iai.34.2.478-482.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald T. J., Johnson R. C. Influence of testicular fluid infected with Treponema pallidum on intradermal lesions. Br J Vener Dis. 1980 Jun;56(3):125–128. doi: 10.1136/sti.56.3.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald T. J. Pathogenesis and immunology of Treponema pallidum. Annu Rev Microbiol. 1981;35:29–54. doi: 10.1146/annurev.mi.35.100181.000333. [DOI] [PubMed] [Google Scholar]
- Graves S. R., Sandok P. L., Jenkin H. M., Johnson R. C. Retention of motility and virulence of Treponema pallidum (Nichols strain) in vitro. Infect Immun. 1975 Nov;12(5):1116–1120. doi: 10.1128/iai.12.5.1116-1120.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves S., Alden J. Limited protection of rabbits against infection with Treponema pallidum by immune rabbit sera. Br J Vener Dis. 1979 Dec;55(6):399–403. doi: 10.1136/sti.55.6.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves S. Sequential changes in susceptibility to Treponema pallidum of rabbits previously infected with Treponema paraluis-cuniculi. Br J Vener Dis. 1981 Feb;57(1):11–14. doi: 10.1136/sti.57.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukehart S. A., Baker-Zander S. A., Lloyd R. M., Sell S. Characterization of lymphocyte responsiveness in early experimental syphilis. II. Nature of cellular infiltration and Treponema pallidum distribution in testicular lesions. J Immunol. 1980 Jan;124(1):461–467. [PubMed] [Google Scholar]
- Lukehart S. A., Baker-Zander S. A., Sell S. Characterization of lymphocyte responsiveness in early experimental syphilis. I. In vitro response to mitogens and Treponema pallidum antigens. J Immunol. 1980 Jan;124(1):454–460. [PubMed] [Google Scholar]
- Maret S. M., Baseman J. B., Folds J. D. Cell-mediated immunity in Treponema pallidum infected rabbits: in vitro response of splenic and lymph node lymphocytes to mitogens and specific antigens. Clin Exp Immunol. 1980 Jan;39(1):38–43. [PMC free article] [PubMed] [Google Scholar]
- Pavia C. S., Baseman J. B., Folds J. D. Selective response of lymphocytes from Treponema pallidum-infected rabbits to mitogens and Treponema reiteri. Infect Immun. 1977 Feb;15(2):417–422. doi: 10.1128/iai.15.2.417-422.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavia C. S., Folds J. D., Baseman J. B. Depression of lymphocyte response to concanavalin A in rabbits infected with Treponema pallidum (Nichols strain). Infect Immun. 1976 Jul;14(1):320–322. doi: 10.1128/iai.14.1.320-322.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavia C. S., Folds J. D., Baseman J. B. Selective in vitro response of thymus-derived lymphocytes from Treponema pallidum-infected rabbits. Infect Immun. 1977 Dec;18(3):603–611. doi: 10.1128/iai.18.3.603-611.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sell S., Baker-Zander S. A., Lloyd R. M. T-cell hyperplasia of lymphoid tissues of rabbits infected with Treponema pallidum: evidence for a vigorous immune response. Sex Transm Dis. 1980 Apr-Jun;7(2):74–84. doi: 10.1097/00007435-198004000-00009. [DOI] [PubMed] [Google Scholar]
- Sell S., Gamboa D., Baker-Zander S. A., Lukehart S. A., Miller J. N. Host response to Treponema pallidum in intradermally-infected rabbits: evidence for persistence of infection at local and distant sites. J Invest Dermatol. 1980 Dec;75(6):470–475. doi: 10.1111/1523-1747.ep12524230. [DOI] [PubMed] [Google Scholar]
- Steiner B., McLean I., Graves S. Redox potential and survival of virulent Treponema pallidum under microaerophilic conditions. Br J Vener Dis. 1981 Oct;57(5):295–301. doi: 10.1136/sti.57.5.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware J. L., Folds J. D., Baseman J. B. Serum of rabbits infected with Treponema pallidum (Nichols) inhibits in vitro transformation of normal rabbit lymphocytes. Cell Immunol. 1979 Feb;42(2):363–372. doi: 10.1016/0008-8749(79)90201-6. [DOI] [PubMed] [Google Scholar]
- Wicher V., Wicher K. In vitro cell response of Treponema pallidum-infected rabbits. I. Lymphocyte transformation. Clin Exp Immunol. 1977 Sep;29(3):480–486. [PMC free article] [PubMed] [Google Scholar]
- Wicher V., Wicher K. In vitro cell response of Treponema pallidum-infected rabbits. II. Inhibition of lymphocyte response to phytohaemagglutinin by serum of T. pallidum-infected rabbits. Clin Exp Immunol. 1977 Sep;29(3):487–495. [PMC free article] [PubMed] [Google Scholar]
- Wong G. H., Steiner B. M., Graves S. R. Effects of anaerobic and microaerophilic conditions of extraction and incubation on the survival of Treponema pallidum in vitro. Br J Vener Dis. 1982 Jun;58(3):139–142. doi: 10.1136/sti.58.3.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Maarsseveen A. C., Bomhof G., Scheper R. J. Demonstration of accelerated and increased migration inhibition factor release in vivo in a PPD retest reaction. Int Arch Allergy Appl Immunol. 1982;67(1):1–6. doi: 10.1159/000232979. [DOI] [PubMed] [Google Scholar]



