Abstract
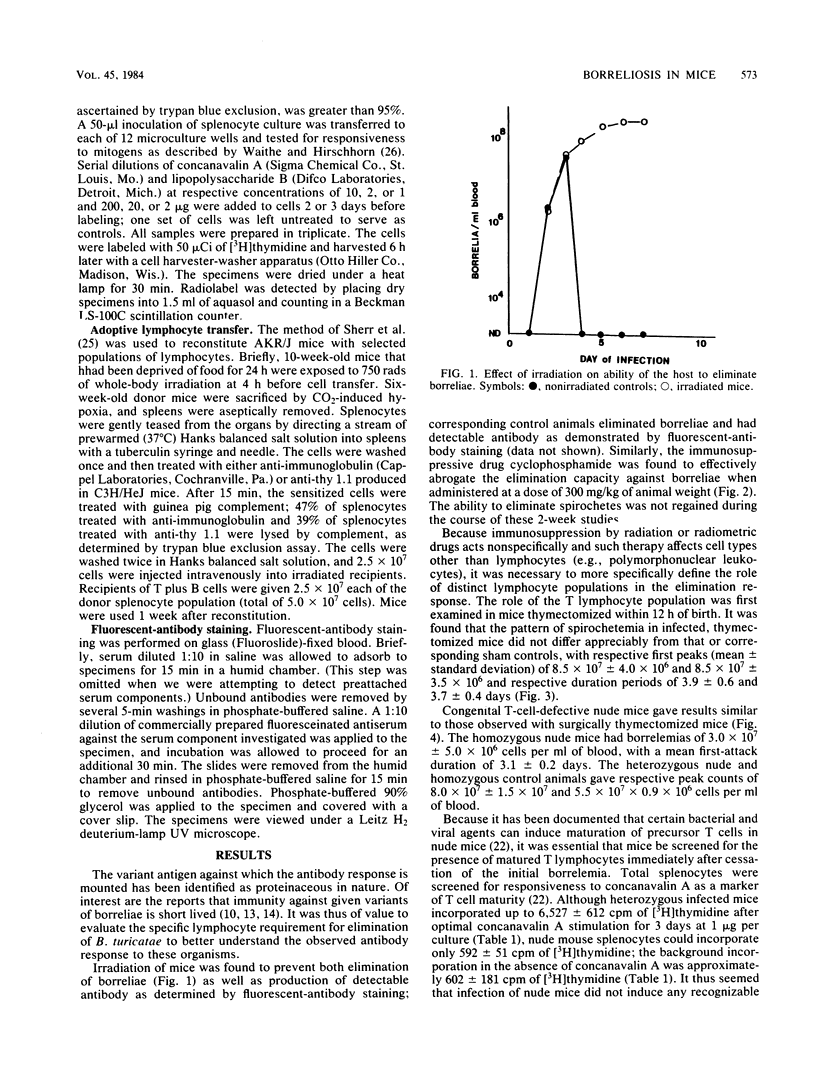
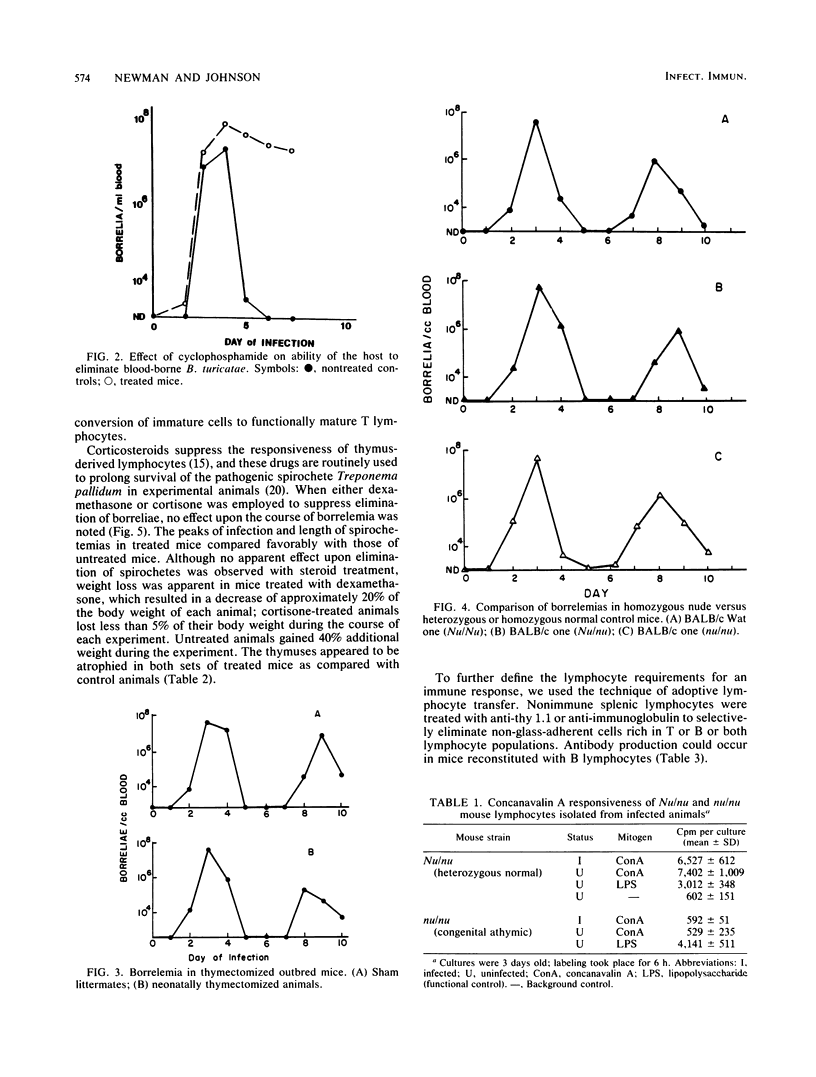
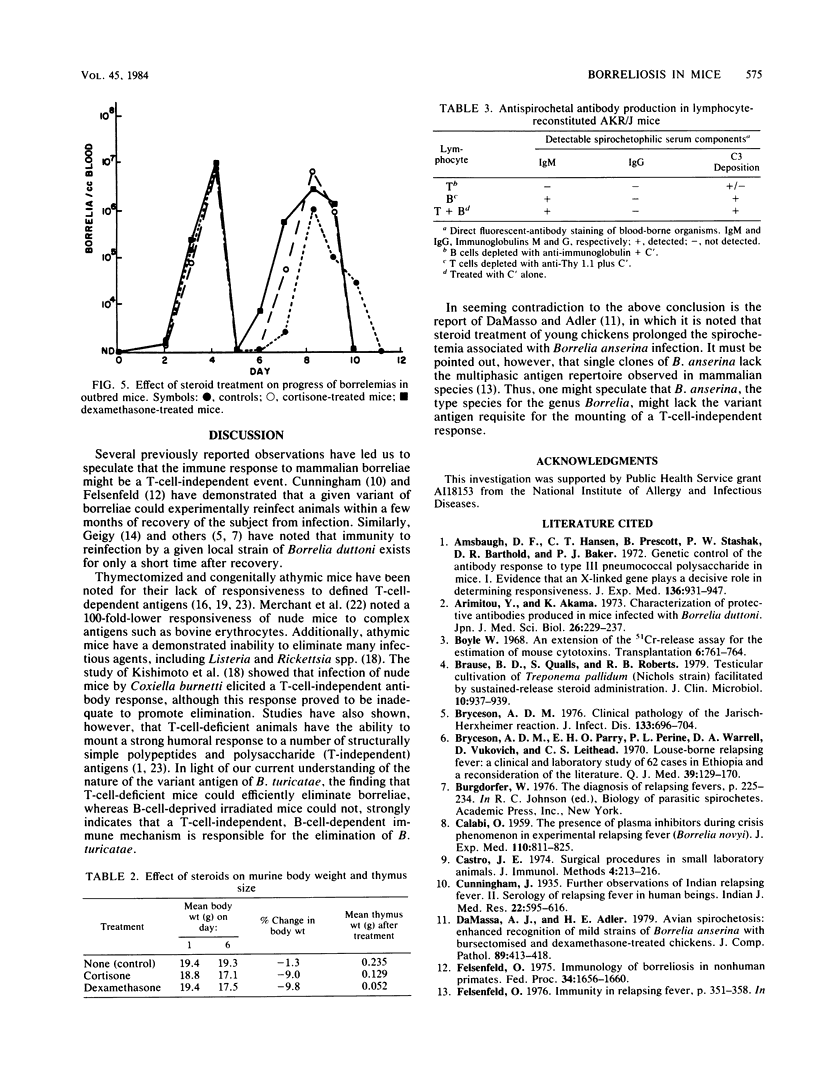
Mice deficient or deprived of thymus-derived lymphocytes eliminated blood-borne Borrelia turicatae with efficiency comparable to that observed in normal littermates. When challenged with 10(5) borreliae, nude mice had mean (+/- standard deviation) primary spirochetemias lasting 3.1 +/- 0.2 days and mean (+/- standard deviation) peak bacterial counts of 3.0 X 10(7) +/- 0.5 X 10(7) cells per ml of blood; in comparison, heterozygous littermates and normal mice had respective primary spirochetemias lasting 3.4 +/- 0.6 and 3.2 +/- 0.2 days and respective peak counts of 8.0 X 10(7) +/- 1.5 X 10(7) and 5.5 X 10(7) +/- 0.9 X 10(7) bacterial cells per ml of blood. No increased responsiveness to concanavalin A was observed in infected nude mice, indicating the sustained lack of maturate T cells in these animals. Thymectomized and steroid-treated mice were also found to eliminate circulating borreliae with efficiency comparable to that observed in control animals. Irradiation of mice abrogated responsiveness to borreliae, but reconstitution with T-cell-depleted splenocytes restored antibody production. It is proposed that elimination of B. turicatae is mediated by a T-cell-independent immune response mechanism.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amsbaugh D. F., Hansen C. T., Prescott B., Stashak P. W., Barthold D. R., Baker P. J. Genetic control of the antibody response to type 3 pneumococcal polysaccharide in mice. I. Evidence that an X-linked gene plays a decisive role in determining responsiveness. J Exp Med. 1972 Oct 1;136(4):931–949. doi: 10.1084/jem.136.4.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arimitsu Y., Akama K. Characterization of protective antibodies produced in mice infected with Borrelia duttonii. Jpn J Med Sci Biol. 1973 Dec;26(5):229–237. doi: 10.7883/yoken1952.26.229. [DOI] [PubMed] [Google Scholar]
- Boyle W. An extension of the 51Cr-release assay for the estimation of mouse cytotoxins. Transplantation. 1968 Sep;6(6):761–764. doi: 10.1097/00007890-196809000-00002. [DOI] [PubMed] [Google Scholar]
- Brause B. D., Qualls S., Roberts R. B. Testicular cultivation of Treponema pallidum (Nichols strains) facilitated by sustained-release steroid administration. J Clin Microbiol. 1979 Dec;10(6):937–939. doi: 10.1128/jcm.10.6.937-939.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryceson A. D. Clinical pathology of the Jarisch-Herxheimer reaction. J Infect Dis. 1976 Jun;133(6):696–704. doi: 10.1093/infdis/133.6.696. [DOI] [PubMed] [Google Scholar]
- Bryceson A. D., Parry E. H., Perine P. L., Warrell D. A., Vukotich D., Leithead C. S. Louse-borne relapsing fever. Q J Med. 1970 Jan;39(153):129–170. [PubMed] [Google Scholar]
- CALABI O. The presence of plasma inhibitors during the crisis phenomenon in experimental relapsing fever (Borrelia novyi). J Exp Med. 1959 Nov 1;110:811–825. doi: 10.1084/jem.110.5.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro J. E. Surgical procedures in small laboratory animals. J Immunol Methods. 1974 Mar;4(2):213–216. doi: 10.1016/0022-1759(74)90064-7. [DOI] [PubMed] [Google Scholar]
- DaMassa A. J., Adler H. E. Avian spirochaetosis: enhanced recognition of mild strains of Borrelia anserina with bursectomized- and dexamethasone-treated chickens. J Comp Pathol. 1979 Jul;89(3):413–420. doi: 10.1016/0021-9975(79)90032-x. [DOI] [PubMed] [Google Scholar]
- Felsenfeld O., Wolf R. H. Immunology of borreliosis in nonhuman primates. Fed Proc. 1975 Jul;34(8):1656–1660. [PubMed] [Google Scholar]
- Kishimoto R. A., Rozmiarek H., Larson E. W. Experimental Q fever infection in congenitally athymic nude mice. Infect Immun. 1978 Oct;22(1):69–71. doi: 10.1128/iai.22.1.69-71.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuchars E., Morgan A., Davies A. J., Wallis V. J. Thymus grafts in thymectomized and normal mice. Nature. 1967 May 20;214(5090):801–802. doi: 10.1038/214801a0. [DOI] [PubMed] [Google Scholar]
- Lukehart S. A., Baker-Zander S. A., Lloyd R. M., Sell S. Effect of cortisone administration on host-parasite relationships in early experimental syphilis. J Immunol. 1981 Oct;127(4):1361–1368. [PubMed] [Google Scholar]
- Mayer M. M., Miller J. A., Shin H. S. A specific method for purification of the second component of guinea pig complement and a chemical evaluation of the one-hit theory. J Immunol. 1970 Aug;105(2):327–341. [PubMed] [Google Scholar]
- Miller J. F., Mitchell G. F., Weiss N. S. Cellular basis of the immunological defects in thymectomized mice. Nature. 1967 Jun 3;214(5092):992–997. doi: 10.1038/214992a0. [DOI] [PubMed] [Google Scholar]
- Newman K., Jr, Johnson R. C. In vivo evidence that an intact lytic complement pathway is not essential for successful removal of circulating Borrelia turicatae from mouse blood. Infect Immun. 1981 Jan;31(1):465–469. doi: 10.1128/iai.31.1.465-469.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherr D., Szewczuk M. R., Siskind G. W. Ontogeny of B lymphocyte function. IV. Kinetics of maturation of B lymphocytes from fetal and neonatal mice when transferred into adult irradiated hosts. J Immunol. 1977 Nov;119(5):1674–1679. [PubMed] [Google Scholar]
- Wright D. J. In vivo and in vitro effect of cyclophosphamide on Borrelia duttoni. Acta Trop. 1979 Jun;36(2):117–122. [PubMed] [Google Scholar]


