Abstract
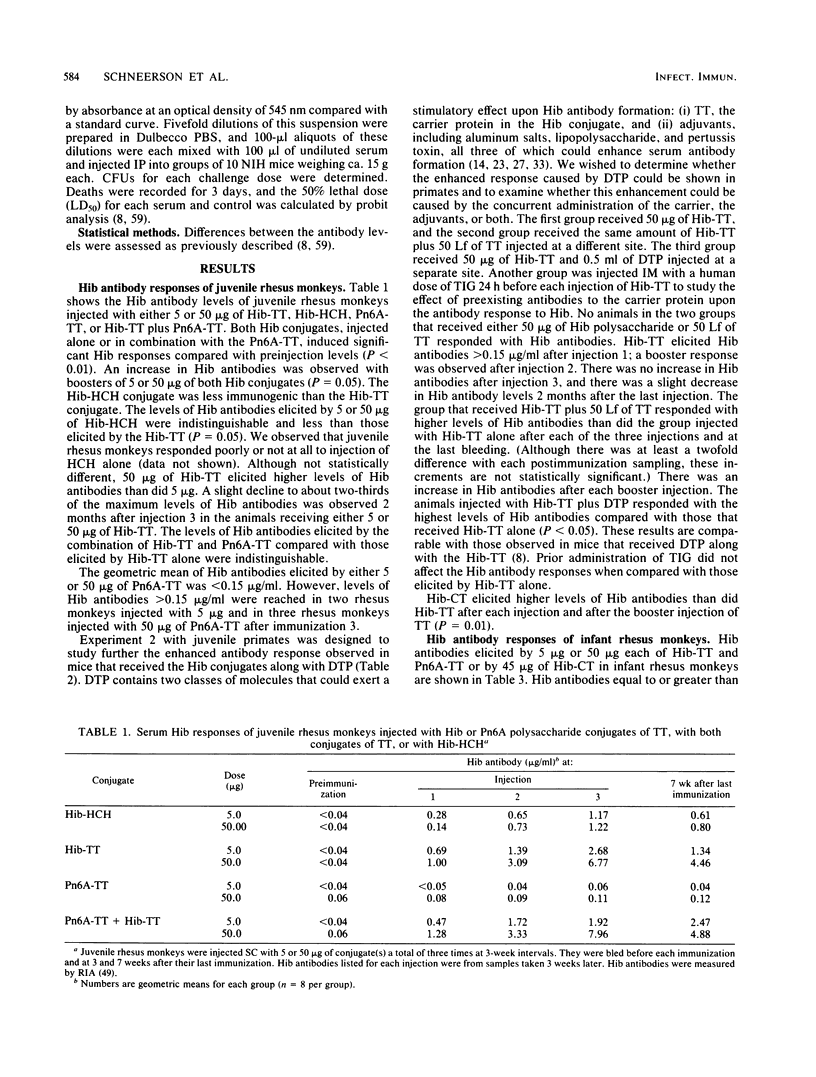
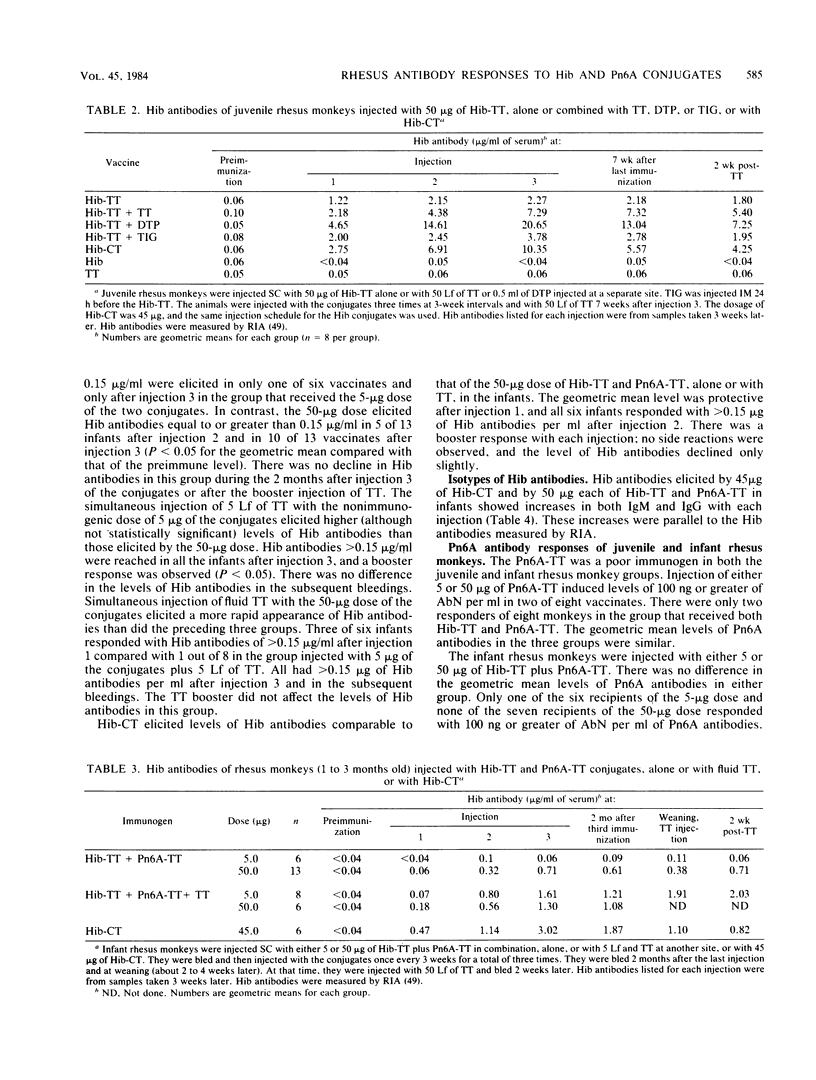
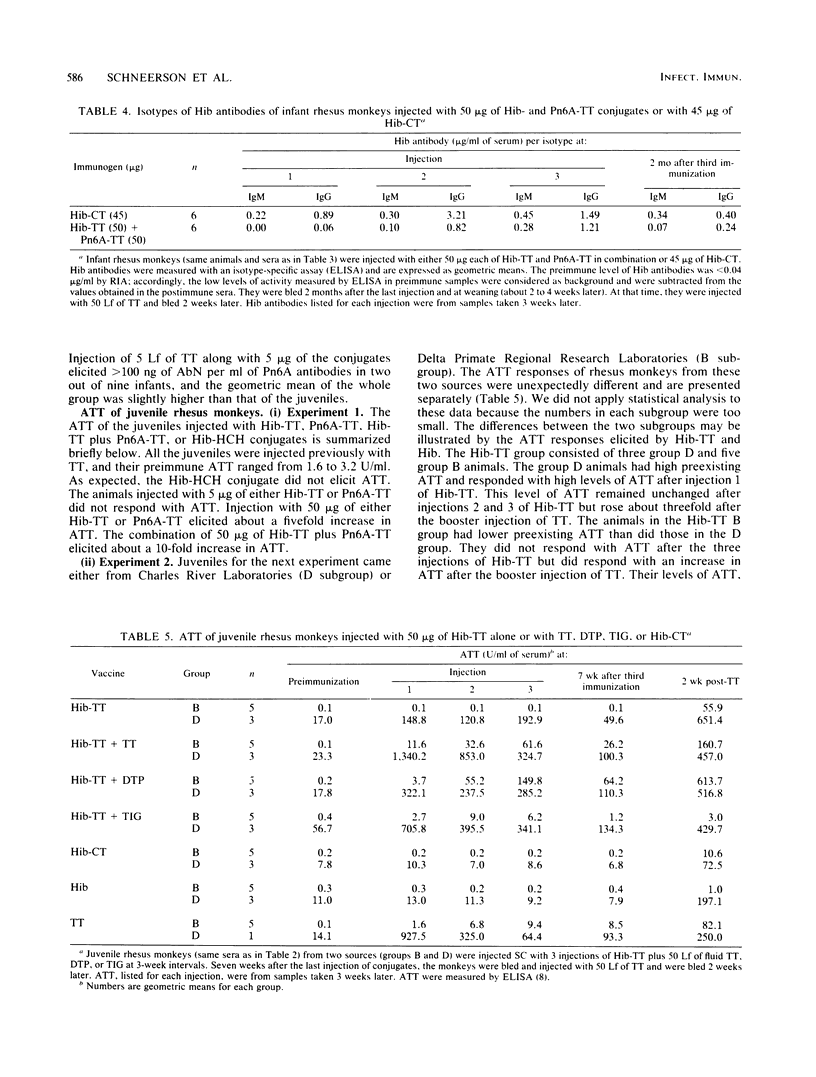
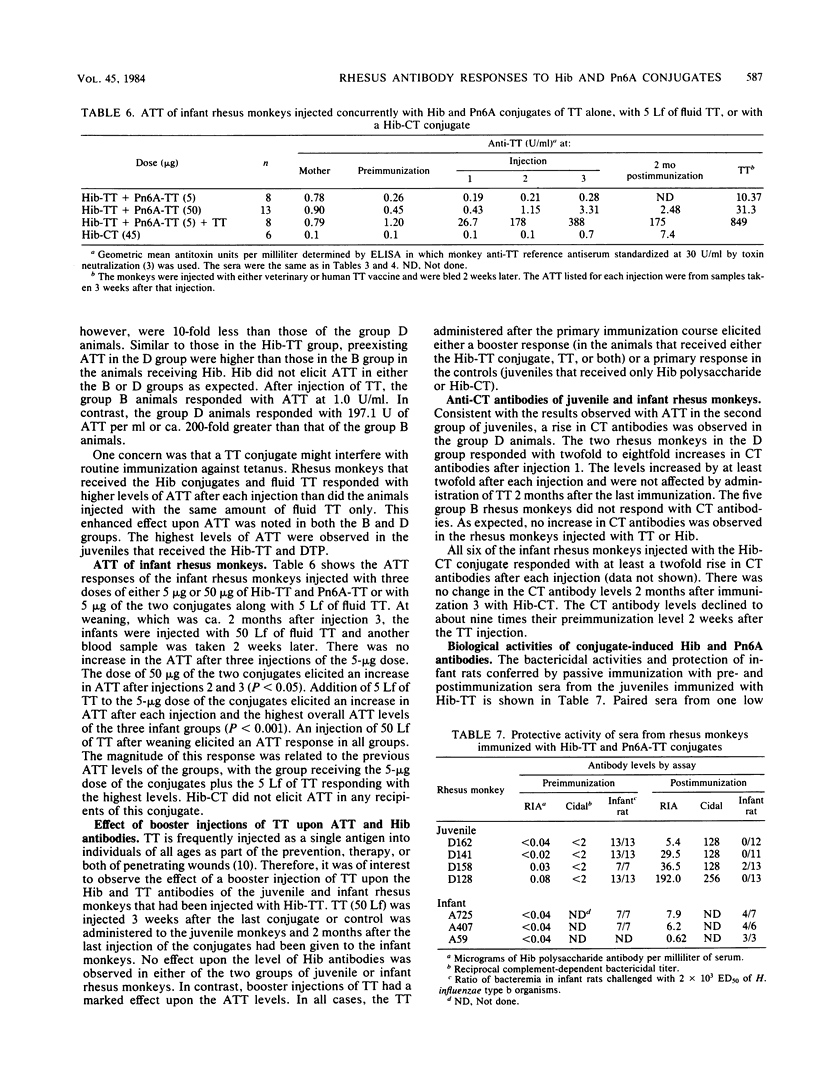
Juvenile and infant rhesus monkeys were injected subcutaneously with saline solutions of Haemophilus influenzae type b (Hib) and pneumococcus type 6A (Pn6A) capsular polysaccharides conjugated to either tetanus toxoid (TT), horseshoe crab hemocyanin, or cholera toxin (CT), and the antibody responses of the monkeys to both bacterial components were measured. All three Hib conjugates were immunogenic and elicited booster responses; their comparative immunogenicity was Hib-CT greater than Hib-TT greater than Hib-horseshoe crab hemocyanin. Hib alone did not elicit antibodies in the juveniles. Juveniles responded earlier and with higher levels of antibodies than did infants. TT, as well as diphtheria-tetanus toxoids-pertussis vaccine adsorbed injected concurrently at a separate site, increased both Hib and TT antibody responses in juveniles (P less than 0.05). Concurrent injection of 5 Lf of fluid TT with a nonimmunogenic 5-micrograms dose in infants elicited levels of Hib antibodies comparable to those elicited by 50 micrograms of Hib-TT. Hib antibodies elicited by the conjugates remained at protective levels in both juveniles and infants 2 months after the last injection, were bactericidal, and conferred passive immunity against bacteremia in infant rats. Passive immunization of juveniles with tetanus immune globulin before each injection of Hib-TT did not suppress Hib antibodies. Hib-TT and Hib-CT elicited increases of Hib antibodies of the immunoglobulin M and G isotypes in the infants. The Pn6A-TT conjugate was considerably less immunogenic than the Hib-TT conjugate; only a few of the juveniles or infants responded with protective levels of Pn6A antibodies. Pn6A antibodies from responders conferred protection in mice against intraperitoneal challenge with Pn6A organisms. TT antibodies were elicited in both juvenile and infant animals after one injection of 50 micrograms of Hib-TT and in the infants injected with 5 micrograms of Hib-TT plus 5 Lf of TT; 5 micrograms of Hib-TT and Pn6A-TT in combination alone did not elicit TT antibodies. Hib-CT elicited CT antibodies in both juveniles and infants.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson P. Antibody responses to Haemophilus influenzae type b and diphtheria toxin induced by conjugates of oligosaccharides of the type b capsule with the nontoxic protein CRM197. Infect Immun. 1983 Jan;39(1):233–238. doi: 10.1128/iai.39.1.233-238.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery O. T., Goebel W. F. CHEMO-IMMUNOLOGICAL STUDIES ON CONJUGATED CARBOHYDRATE-PROTEINS : II. IMMUNOLOGICAL SPECIFICITY OF SYNTHETIC SUGAR-PROTEIN ANTIGENS. J Exp Med. 1929 Sep 30;50(4):533–550. doi: 10.1084/jem.50.4.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barile M. F., Hardegree M. C., Pittman M. Immunization against neonatal tetanus in New Guinea. 3. The toxin-neutralization test and the response of guinea-pigs to the toxoids as used in the immunization schedules in New Guinea. Bull World Health Organ. 1970;43(3):453–459. [PMC free article] [PubMed] [Google Scholar]
- Bayer E. A., Wilchek M. The use of the avidin-biotin complex as a tool in molecular biology. Methods Biochem Anal. 1980;26:1–45. doi: 10.1002/9780470110461.ch1. [DOI] [PubMed] [Google Scholar]
- Beuvery E. C., van Rossum F., Nagel J. Comparison of the induction of immunoglobulin M and G antibodies in mice with purified pneumococcal type 3 and meningococcal group C polysaccharides and their protein conjugates. Infect Immun. 1982 Jul;37(1):15–22. doi: 10.1128/iai.37.1.15-22.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisari F. V., Northrup R. S., Chen L. C. The modulating effect of cholera enterotoxin on the immune response. J Immunol. 1974 Sep;113(3):729–739. [PubMed] [Google Scholar]
- Chu C., Schneerson R., Robbins J. B., Rastogi S. C. Further studies on the immunogenicity of Haemophilus influenzae type b and pneumococcal type 6A polysaccharide-protein conjugates. Infect Immun. 1983 Apr;40(1):245–256. doi: 10.1128/iai.40.1.245-256.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements J. D., Finkelstein R. A. Isolation and characterization of homogeneous heat-labile enterotoxins with high specific activity from Escherichia coli cultures. Infect Immun. 1979 Jun;24(3):760–769. doi: 10.1128/iai.24.3.760-769.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engvall E., Perlmann P. Enzyme-linked immunosorbent assay, Elisa. 3. Quantitation of specific antibodies by enzyme-labeled anti-immunoglobulin in antigen-coated tubes. J Immunol. 1972 Jul;109(1):129–135. [PubMed] [Google Scholar]
- Fairchild R. L., Braley-Mullen H. Characterization of the murine immune response to type 6 pneumococcal polysaccharide. Infect Immun. 1983 Feb;39(2):615–622. doi: 10.1128/iai.39.2.615-622.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finger H., Emmerling P., Schmidt H. Accelerated and prolongated multiplication of antibody-forming spleen cells by Bordetella pertussis in mice immunized with sheep red blood cells. Experientia. 1967 Jul 15;23(7):591–592. doi: 10.1007/BF02137991. [DOI] [PubMed] [Google Scholar]
- Finkelstein R. A., Atthasampunna P. Immunity against experimental cholera. Proc Soc Exp Biol Med. 1967 Jun;125(2):465–469. doi: 10.3181/00379727-125-32121. [DOI] [PubMed] [Google Scholar]
- Finkelstein R. A., Vasil M. L., Holmes R. K. Studies on toxinogenesis in Vibrio cholerae. I. Isolation of mutants with altered toxinogenicity. J Infect Dis. 1974 Feb;129(2):117–123. doi: 10.1093/infdis/129.2.117. [DOI] [PubMed] [Google Scholar]
- Fujita K., Finkelstein R. A. Antitoxic immunity in experimental cholera: comparison of immunity induced perorally and parenterally in mice. J Infect Dis. 1972 Jun;125(6):647–655. doi: 10.1093/infdis/125.6.647. [DOI] [PubMed] [Google Scholar]
- Gold R., Lepow M. L. Present status of polysaccharide vaccines in the prevention of meningococcal disease. Adv Pediatr. 1976;23:71–93. [PubMed] [Google Scholar]
- Gotschlich E. C., Goldschneider I., Artenstein M. S. Human immunity to the meningococcus. IV. Immunogenicity of group A and group C meningococcal polysaccharides in human volunteers. J Exp Med. 1969 Jun 1;129(6):1367–1384. doi: 10.1084/jem.129.6.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henrickson R. V., Maul D. H., Osborn K. G., Sever J. L., Madden D. L., Ellingsworth L. R., Anderson J. H., Lowenstine L. J., Gardner M. B. Epidemic of acquired immunodeficiency in rhesus monkeys. Lancet. 1983 Feb 19;1(8321):388–390. doi: 10.1016/s0140-6736(83)91503-9. [DOI] [PubMed] [Google Scholar]
- Hill J. C. From the National Institute of Allergy and Infectious Diseases. Summary of a workshop on Haemophilus influenzae type B vaccines. J Infect Dis. 1983 Jul;148(1):167–175. doi: 10.1093/infdis/148.1.167. [DOI] [PubMed] [Google Scholar]
- Hirashima M., Yodoi J., Ishizaka K. Formation of IgE-binding factors by rat T lymphocytes. II. Mechanisms of selective formation of IgE-potentiating factors by treatment with Bordetella pertussis vaccine. J Immunol. 1981 Nov;127(5):1804–1810. [PubMed] [Google Scholar]
- Hong K., Kinoshita T., Kitajima H., Inoue K. Inhibitory effect of K-76 monocarboxylic acid, an anticomplementary agent, on the C3b inactivator system. J Immunol. 1981 Jul;127(1):104–108. [PubMed] [Google Scholar]
- Jennings H. J., Lugowski C., Ashton F. E. Conjugation of meningococcal lipopolysaccharide R-type oligosaccharides to tetanus toxoid as route to a potential vaccine against group B Neisseria meningitidis. Infect Immun. 1984 Jan;43(1):407–412. doi: 10.1128/iai.43.1.407-412.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaijser B., Larsson P., Olling S., Schneerson R. Protection against acute, ascending pyelonephritis caused by Escherichia coli in rats, using isolated capsular antigen conjugated to bovine serum albumin. Infect Immun. 1983 Jan;39(1):142–146. doi: 10.1128/iai.39.1.142-146.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karch H., Nixdorff K. Modulation of the IgG subclass responses to lipopolysaccharide by bacterial membrane components: differential adjuvant effects produced by primary and secondary stimulation. J Immunol. 1983 Jul;131(1):6–8. [PubMed] [Google Scholar]
- Kong A. S., Morse S. I. The effect of Bordetella pertussis on the antibody response in mice to type III pneumococcal polysaccharide. J Immunol. 1976 Apr;116(4):989–993. [PubMed] [Google Scholar]
- Lane H. C., Masur H., Edgar L. C., Whalen G., Rook A. H., Fauci A. S. Abnormalities of B-cell activation and immunoregulation in patients with the acquired immunodeficiency syndrome. N Engl J Med. 1983 Aug 25;309(8):453–458. doi: 10.1056/NEJM198308253090803. [DOI] [PubMed] [Google Scholar]
- Melville-Smith M. E., Seagroatt V. A., Watkins J. T. A comparison of enzyme-linked immunosorbent assay (ELISA) with the toxin neutralization test in mice as a method for the estimation of tetanus antitoxin in human sera. J Biol Stand. 1983 Apr;11(2):137–144. doi: 10.1016/s0092-1157(83)80038-9. [DOI] [PubMed] [Google Scholar]
- Mitchell G. F., Humphrey J. H., Wiliamson A. R. Inhibition of secondary anti-hapten responses with the hapten conjugated to type 3 pneumococcal polysaccharide. Eur J Immunol. 1972 Oct;2(5):460–467. doi: 10.1002/eji.1830020516. [DOI] [PubMed] [Google Scholar]
- OSBORN J. J., DANCIS J., JULIA J. F. Studies of the immunology of the newborn infant. II. Interference with active immunization by passive transplacental circulating antibody. Pediatrics. 1952 Sep;10(3):328–334. [PubMed] [Google Scholar]
- Parke J. C., Jr, Schneerson R., Robbins J. B. The attack rate, age incidence, racial distribution, and case fatality rate of Hemophilus influenzae type b meningitis in Mecklenbury County, North Carolina. J Pediatr. 1972 Oct;81(4):765–769. doi: 10.1016/s0022-3476(72)80099-4. [DOI] [PubMed] [Google Scholar]
- Peterson J. W. Protection against experimental cholera by oral or parenteral immunization. Infect Immun. 1979 Nov;26(2):594–598. doi: 10.1128/iai.26.2.594-598.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce N. F., Kaniecki E. A., Northrup R. S. Protection against experimental cholera by antitoxin. J Infect Dis. 1972 Dec;126(6):606–616. doi: 10.1093/infdis/126.6.606. [DOI] [PubMed] [Google Scholar]
- Robbins J. B., Austrian R., Lee C. J., Rastogi S. C., Schiffman G., Henrichsen J., Mäkelä P. H., Broome C. V., Facklam R. R., Tiesjema R. H. Considerations for formulating the second-generation pneumococcal capsular polysaccharide vaccine with emphasis on the cross-reactive types within groups. J Infect Dis. 1983 Dec;148(6):1136–1159. doi: 10.1093/infdis/148.6.1136. [DOI] [PubMed] [Google Scholar]
- Robbins J. B., Parke J. C., Jr, Schneerson R., Whisnant J. K. Quantitative measurement of "natural" and immunization-induced Haemophilus influenzae type b capsular polysaccharide antibodies. Pediatr Res. 1973 Mar;7(3):103–110. doi: 10.1203/00006450-197303000-00001. [DOI] [PubMed] [Google Scholar]
- Robbins J. B. Vaccines for the prevention of encapsulated bacterial diseases: current status, problems and prospects for the future. Immunochemistry. 1978 Nov;15(10-11):839–854. doi: 10.1016/0161-5890(78)90117-7. [DOI] [PubMed] [Google Scholar]
- Schiffman G., Douglas R. M., Bonner M. J., Robbins M., Austrian R. A radioimmunoassay for immunologic phenomena in pneumococcal disease and for the antibody response to pneumococcal vaccines. I. Method for the radioimmunoassay of anticapsular antibodies and comparison with other techniques. J Immunol Methods. 1980;33(2):133–144. doi: 10.1016/s0022-1759(80)80004-4. [DOI] [PubMed] [Google Scholar]
- Schneerson R., Barrera O., Sutton A., Robbins J. B. Preparation, characterization, and immunogenicity of Haemophilus influenzae type b polysaccharide-protein conjugates. J Exp Med. 1980 Aug 1;152(2):361–376. doi: 10.1084/jem.152.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneerson R., Bradshaw M., Whisnant J. K., Myerowitz R. L., Parke J. C., Jr, Robbins J. B. An Escherichia coli antigen cross-reactive with the capsular polysaccharide of Haemophilus influenzae type b: occurrence among known serotypes, and immunochemical and biologic properties of E. coli antisera toward H. influenzae type b. J Immunol. 1972 Jun;108(6):1551–1562. [PubMed] [Google Scholar]
- Schneerson R., Robbins J. B., Barrera O., Sutton A., Habig W. B., Hardegree M. C., Chaimovich J. Haemophilus influenzae type B polysaccharide-protein conjugates: model for a new generation of capsular polysaccharide vaccines. Prog Clin Biol Res. 1980;47:77–94. [PubMed] [Google Scholar]
- Schneerson R., Rodrigues L. P., Parke J. C., Jr, Robbins J. B. Immunity to disease caused by Hemophilus influenzae type b. II. Specificity and some biologic characteristics of "natural," infection-acquired, and immunization-induced antibodies to the capsular polysaccharide of Hemophilus influenzae type b. J Immunol. 1971 Oct;107(4):1081–1089. [PubMed] [Google Scholar]
- Seid R. C., Jr, Sadoff J. C. Preparation and characterization of detoxified lipopolysaccharide-protein conjugates. J Biol Chem. 1981 Jul 25;256(14):7305–7310. [PubMed] [Google Scholar]
- Smith A. L., Smith D. H., Averill D. R., Jr, Marino J., Moxon E. R. Production of Haemophilus influenzae b meningitis in infant rats by intraperitoneal inoculation. Infect Immun. 1973 Aug;8(2):278–290. doi: 10.1128/iai.8.2.278-290.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. H., Peter G., Ingram D. L., Harding A. L., Anderson P. Responses of children immunized with the capsular polysaccharide of Hemophilus influenzae, type b. Pediatrics. 1973 Nov;52(5):637–644. [PubMed] [Google Scholar]
- Snippe H., van Houte A. J., van Dam J. E., De Reuver M. J., Jansze M., Willers J. M. Immunogenic properties in mice of hexasaccharide from the capsular polysaccharide of Streptococcus pneumoniae type 3. Infect Immun. 1983 Jun;40(3):856–861. doi: 10.1128/iai.40.3.856-861.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutliff W. D., Finland M. ANTIPNEUMOCOCCIC IMMUNITY REACTIONS IN INDIVIDUALS OF DIFFERENT AGES. J Exp Med. 1932 May 31;55(6):837–852. doi: 10.1084/jem.55.6.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton A., Schneerson R., Kendall-Morris S., Robbins J. B. Differential complement resistance mediates virulence of Haemophilus influenzae type b. Infect Immun. 1982 Jan;35(1):95–104. doi: 10.1128/iai.35.1.95-104.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svenson S. B., Nurminen M., Lindberg A. A. Artificial Salmonella vaccines: O-antigenic oligosaccharide-protein conjugates induce protection against infection with Salmonella typhimurium. Infect Immun. 1979 Sep;25(3):863–872. doi: 10.1128/iai.25.3.863-872.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas J. W., Bucy R. P., Kapp J. A. T cell-independent responses to an Ir gene-controlled antigen. I. Characteristics of the immune response to insulin complexed to Brucella abortus. J Immunol. 1982 Jul;129(1):6–10. [PubMed] [Google Scholar]
- Wallick S., Claflin J. L., Briles D. E. Resistance to Streptococcus pneumoniae is induced by a phosphocholine-protein conjugate. J Immunol. 1983 Jun;130(6):2871–2875. [PubMed] [Google Scholar]
- Yamamoto K., Takeda Y., Miwatani T., Craig J. P. Evidence that a non-O1 Vibrio cholerae produces enterotoxin that is similar but not identical to cholera enterotoxin. Infect Immun. 1983 Sep;41(3):896–901. doi: 10.1128/iai.41.3.896-901.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yount W. J., Dorner M. M., Kunkel H. G., Kabat E. A. Studies on human antibodies. VI. Selective variations in subgroup composition and genetic markers. J Exp Med. 1968 Mar 1;127(3):633–646. doi: 10.1084/jem.127.3.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zon G., Szu S. C., Egan W., Robbins J. D., Robbins J. B. Hydrolytic stability of pneumococcal group 6 (type 6A and 6B) capsular polysaccharides. Infect Immun. 1982 Jul;37(1):89–103. doi: 10.1128/iai.37.1.89-103.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]



