Abstract
Exosomes are nanovesicles that are released from cells as a mechanism of cell-free intercellular communication. Only a limited number of proteins have been identified from the plasma exosome proteome. Here, we developed a multi-step fractionation scheme incorporating gel exclusion chromatography, rate zonal centrifugation through continuous sucrose gradients, and high-speed centrifugation to purify exosomes from human plasma. Exosome-associated proteins were separated by SDS-PAGE and 66 proteins were identified by LC-MS/MS, which included both cellular and extracellular proteins. Furthermore, we identified and characterized peroxisome proliferator-activated receptor-γ (PPARγ), a nuclear receptor that regulates adipocyte differentiation and proliferation, as well as immune and inflammatory cell functions, as a novel component of plasma-derived exosomes. Given the important role of exosomes as intercellular messengers, the discovery of PPARγ as a component of human plasma exosomes identifies a potential new pathway for the paracrine transfer of nuclear receptors.
Introduction
Exosomes are nanovesicles that are released from cells as a mechanism for the intercellular transfer of membrane and cytoplasmic molecules[1, 2]. Exosome biogenesis involves the inward budding of endosomes into multivesicular bodies to form intraluminal vesicles. Subsequent fusion of multivesicular bodies with the plasma membrane releases intraluminal vesicles to the extracellular space as exosomes. Limited information, however, exists about human plasma exosomes. Exosome-like vesicles isolated by differential ultracentrifugation of human plasma express MHC class I, MHC class II, integrin alpha 2b (CD41, GPIIb), as well as tetraspanin molecules, such as CD9, CD63, and CD81[3]. We have previously shown that TNFR1 exosome-like vesicles circulate in human plasma and co-segregate with, but are distinct from the LDL fraction of human plasma[4]. Here, we developed a multi-step fractionation scheme incorporating gel exclusion chromatography, rate zonal centrifugation through continuous sucrose gradients, and high-speed centrifugation to purify exosomes that co-segregate with the VLDL (very low density lipoprotein) and IDL (intermediate density lipoprotein) fractions of human plasma. LC-MS/MS was utilized to identify 66 proteins that were associated with plasma-derived exosomes. Furthermore, we identified and characterized peroxisome proliferator-activated receptor-γ (PPARγ), an important nuclear receptor that regulates adipocyte differentiation and proliferation, as well as immune and inflammatory cell functions, as a novel component of exosomes that circulate in human plasma.
Methods
Isolation of VLDL and IDL Fractions from Human Plasma
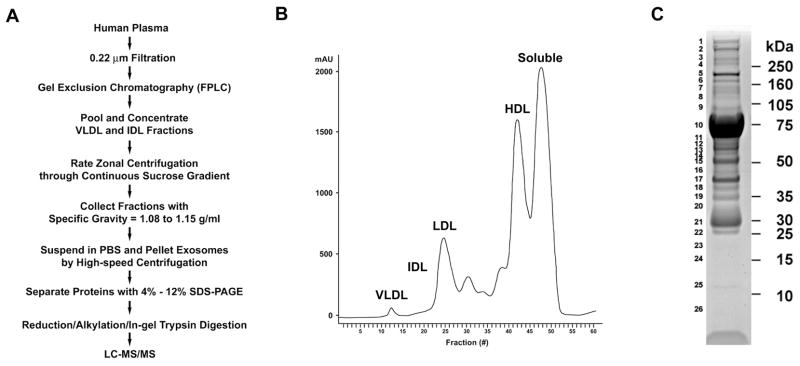
Informed consent was obtained, as per protocol 96-H-0100, which was approved by the National Heart, Lung, and Blood Institute Institutional Review Board. Two plasma samples were passed through a 0.22 μm filter to remove large structures and debris (Figure 1A). To reduce sample complexity and facilitate the identification of plasma exosomes, samples were separated into lipoprotein fractions by fast protein liquid chromatography (FPLC) using two Superose 6 HR 10/30 gel exclusion chromatography columns connected in series (GE Biosciences)[4]. Fractions corresponding to the VLDL and IDL fractions were pooled and concentrated using a 3,000-Da cut-off Microcon centrifugal filter (Millipore). To separate higher density exosomes from lower density lipoprotein particles, concentrated VLDL and IDL fractions (1.27 mg) were overlaid on a continuous sucrose gradient (0.2–2.5 M in 20 mM Tris, pH 8.0) and centrifuged at 175,000 g for 16 h. Fractions (0.5 ml) were collected from the bottom and proteins were quantified. Fractions 5 through 10, which corresponded to specific gravities of 1.08–1.15 g/ml were pooled, diluted in PBS, and exosomes were isolated by high-speed centrifugation at 175,000 × g for 2 h. Proteins present in the exosome pellet were separated by 1D SDS-PAGE. Fractions 1 through 4, which corresponded to specific gravities of 1.16–1.2 g/ml, did not contain proteins and were excluded from the subsequent analysis. Fractions 11 through 16, which corresponded to low-density vesicles with specific gravities of 1.015–1.07 g/ml, were also omitted to exclude lipoprotein particles.
Figure 1. Isolation of Exosomes Co-segregating with the IDL and VLDL Fractions of Human Plasma for Proteomic Analysis.
A. Flowchart for the isolation of exosomes that co-segregate with the IDL and VLDL fractions of human plasma. B. Chromatogram of fractions of human plasma separated by gel exclusion chromatography using FPLC. C. Image of Coomassie blue-stained 4%-12% 1D-SDS PAGE gel of exosomes isolated from human plasma. Molecular mass markers are shown on the right and gel slice numbers are shown on the left.
1D SDS-PAGE and In-Gel Trypsin Digestion
Exosome-associated proteins (100 μg) were separated by SDS-PAGE using 4% - 12% Bis-Tris Nupage gels and visualized with SimplyBlue Coomassie G-250 SafeStain (Invitrogen). Serial gel slices were excised, diced into smaller fragments, destained with 50% acetonitrile in 25 mM NH4HCO3, and dried. Samples were reduced with 10 mM dithiothreitol in 25 mM NH4HCO3 at 56° C for 1 hour and alkylated with 55 mM iodoacetamide for 45 minutes at room temperature. In-gel trypsin digestion was performed using 12.5 ng/μL of sequencing grade modified porcine trypsin (Promega) diluted in 25 mM NH4HCO3 at 37°C overnight. Peptides were extracted with 0.5% formic acid and 50% acetonitrile. Following evaporation of acetonitrile, peptides were purified using a ZipTipC18 column (Millipore, Billerica, MA). The volume of each eluted sample was reduced in a Speedvac to 5 μl to evaporate acetonitrile and adjusted to 20 μl with 0.1% formic acid prior to LC-MS/MS analysis.
Nanospray LC/MS/MS Analysis and Database Search
LC-MS/MS analyses were carried out using a ThermoFinnigan LTQ linear ion trap mass spectrometer, as previously described[5]. Raw data files were searched against the NCBI human Refseq protein sequence database using BioWorks 3.2 software (based on the SEQUEST algorithm). The identified peptide sequences were qualified and filtered using the following threshold: 1) the cross-correlation scores (Xcorr) were greater than 1.5, 2.0, and 2.5 for charge state +1, +2, and +3 peptide ions, respectively, 2) the uniqueness scores of matches ΔCn) were higher than 0.08, and 3) the ranks of the preliminary scores (Rsp) were 10 or less. Using these criteria, the false positive rates of peptide identification, estimated from reverse database searches, were less than 5%. Only proteins identified by at least two peptides were considered significant. The mass spectra of those identified by 2 or 3 peptides were further manually inspected to ensure MS/MS spectra quality.
Immunoblotting of Lipoprotein Fractions
Lipoprotein fractions were separated by gel exclusion chromatography, concentrated using a 3,000-Da cut-off Microcon centrifugal filter, and 20 μg of protein was separated by SDS-PAGE[4]. For analysis of individual tubes from the VLDL and IDL fractions, gel exclusion chromatography samples were precipitated using 10% trichloroacetic acid (TCA) prior to separation by SDS-PAGE.
Rate zonal centrifugation through continuous sucrose gradients was performed to characterize the density of exosomes co-segregating with the IDL fraction of human plasma. Eight fractions of human IDL that had been collected by gel exclusion chromatography were pooled and concentrated using a 3,000-Da cut-off Microcon centrifugal filter. Proteins were overlaid on a continuous sucrose gradient (0.2 M to 2 M in 20 mM Tris, ph 8.0) and centrifuged at 175,000 × g for 16 h. Fractions (0.5 ml) were collected from the bottom of the gradient and proteins were precipitated with 10% TCA. Densitometry was measured using a Palm Abbe Digital Refractometer (Misco).
Immunoblotting was performed as previously described and probed with antibodies against PPARγ (H-100), apolipoprotein B-100, apolipoprotein E, and fibronectin (Santa Cruz Biotechnology)[4].
Immunoelectron Microscopy
Fixation and labeling of exosomes on specimen grids was performed using a modification of previously described methods[6]. Exosomes from the IDL fraction were adsorbed to nickel mesh grids that had been coated with a formvar film and subjected to glow-discharge to increase hydrophilicity. The adsorbed suspensions were fixed for 30 min at 4° C in 2% paraformaldehyde, 0.2% glutaraldehyde in 0.1M sodium phosphate buffer, pH 7.3. The following sequential washes and incubations for immunolabeling were performed at room temperature: 4 washes with PBS; permeabilization and blocking with 0.075% saponin in PBS, 10% normal goat serum, and 1% BSA for 30 min; rabbit anti-PPARγ at 2 μg/ml in PBS with 1% BSA (PBS-BSA) for 1 h; 4 washes with PBS-BSA for 30 min; 1.4 nm gold-conjugated Fab′ goat anti-rabbit IgG (Nanoprobes) at 0.8 μg/ml in PBS-BSA for 1 h; 4 washes with PBS for 30 min; 3 washes with PBS; and re-fixation with 2% glutaraldehyde in 0.1M sodium phosphate buffer, pH 7.3 for 10 min. After washing with water, the immunogold-labeled samples were silver-enhanced for 1 to 1.5 min using the HQ silver enhancement kit (Nanoprobes) and washed again water, followed by positive staining with uranyl acetate. Samples were stabilized with a carbon coating prior to imaging with a JEM 1200EX electron microscope (JEOL USA) equipped with an AMT XR-60 digital camera (Advanced Microscopy Techniques Corp).
Results
Fractionation and Identification of Exosome-associated Proteins that Co-segregate with the VLDL and IDL Fractions of Human Plasma
Proteins present in exosomes isolated from the VLDL and IDL fractions of human plasma (Figure 1B) were separated by 1D SDS-PAGE (Figure 1C) and 26 gel slices were excised, destained, reduced, alkylated, and subjected to in-gel trypsin digestion. LC-MS/MS analysis identified 66 proteins (Table 1), which were characterized as cellular or extracellular (secreted). The 28 cell-associated proteins were classified further as regulators of vesicular trafficking and protein sorting (n = 4), cytoskeletal-associated or motor proteins (n = 5), plasma membrane-associated (n = 7), cytosolic proteins (n = 7), or proteins associated with the nucleus (n = 4) or mitochondria (n = 1). Similarly, the 38 extracellular (secreted) proteins were classified as components of complement pathways (n = 9), hemostasis and thrombosis (n = 6), extracellular matrix (n = 6), lectins (n = 9), or plasma proteins (n = 8). In addition, 8 lipoprotein-related proteins were identified, which may reflect protein components of lipoprotein particles, rather than exosomes, since exosomes were isolated from VLDL and IDL fractions of human plasma.
Table 1.
Proteins Identified as Components of Plasma-derived Exosomes.
| GI Number | Mass (Daltons) | # of Peptides Identified | Gel Slice Number | Exosome-associated Protein | |
|---|---|---|---|---|---|
| I. CELLULAR PROTEINS | |||||
| a. Vesicular Trafficking and Protein Sorting | |||||
| cell cycle progression 1 isoform 1 | gi|75677585 | 87,210 | 2 | 20 | |
| clathrin heavy chain 1 | gi|4758012 | 191,484 | 9 | 5,6 | Adipocytes, B cells, DC, IEC |
| clathrin, heavy polypeptide-like 1 | gi|9257202 | 186,873 | 2 | 5 | |
| Sec8 protein isoform a | gi|82546830 | 110,367 | 2 | 10 | |
| b. Cytoskeletal-related or Motor Proteins | |||||
| actin-like protein | gi|62990121 | 41,885 | 2 | 16 | |
| axonemal dynein heavy chain 7 | gi|151301127 | 461,030 | 2 | 4 | |
| myosin, heavy polypeptide 9, non-muscle | gi|12667788 | 226,402 | 8 | 4 | DC, Mesothelioma cells, Pleural Effusion, Urine |
| ninein isoform 2 | gi|148536869 | 248,105 | 2 | 10 | |
| titin isoform novex-1 | gi|110349713 | 3,006,712 | 2 | 4 | |
| c. Plasma Membrane Proteins | |||||
| ATP-binding cassette, sub-family A, member 7 isoform | gi|150417984 | 234,220 | 2 | 10 | |
| Fc fragment of IgG binding protein | gi|4503681 | 571,890 | 45 | 7,8,9,11,12, 13,14,15,16,17,18,22 | Urine |
| hyperpolarization activated cyclic nucleotide-gated potassium channel 3 | gi|38327037 | 86,032 | 2 | 20 | |
| integrin alpha 2b preprotein | gi|4504745 | 110,005 | 9 | 7,8,22 | Plasma |
| NMDA receptor 1 isoform NR1–3 precursor | gi|11038637 | 103,434 | 2 | 20 | |
| polymeric immunoglobulin receptor | gi|31377806 | 83,153 | 7 | 9 | Urine |
| scavenger receptor cysteine-rich type 1protein M160 precursor | gi|50659091 | 154,468 | 2 | 16 | |
| d. Nuclear Proteins | |||||
| DEAD (Asp-Glu-Ala-Asp) box polypeptide 21 | gi|50659095 | 87,214 | 2 | 10 | |
| delangin isoform B | gi|47578107 | 304,214 | 2 | 3 | |
| mutS homolog 6 | gi|4504191 | 152,655 | 2 | 10 | |
| spectrin repeat containing, nuclear envelope 1 isoform 2 | gi|23097308 | 1,005,181 | 2 | 10 | |
| e. Mitochondrial Proteins | |||||
| arginyl-tRNA synthetase | gi|15149476 | 75,248 | 2 | 17 | |
| f. Cytoplasmic Proteins | |||||
| alpha 1 globin | gi|4504347 | 15,126 | 4 | 23 | DC, Reticulocytes, Urine |
| beta globin | gi|4504349 | 15,867 | 3 | 23 | |
| delta globin | gi|4504351 | 15,924 | 2 | 23 | |
| haptoglobin | gi|4826762 | 45,074 | 5 | 15,16 | |
| haptoglobin-related protein | gi|45580723 | 38,899 | 16 | 15,16,17,18, 19,22,23,24,25 | |
| hornerin | gi|57864582 | 282,260 | 3 | 1 | Urine |
| peroxiredoxin 2 isoform b | gi|33188452 | 15,858 | 2 | 22 | Breast Milk, Urine |
| II. EXTRACELLULAR PROTEINS | |||||
| a. Complement Pathways | |||||
| clusterin isoform 1 | gi|42716297 | 57,702 | 11 | 16,17,18 | Breast Milk, Urine |
| complement component 1 inhibitor precursor | gi|73858568 | 52,844 | 5 | 8,9 | |
| complement component 1, q subcomponent, B chain precursor | gi|87298828 | 26,591 | 3 | 18,19 | Pleural Fluid |
| complement component 1, r subcomponent | gi|66347875 | 80,069 | 24 | 9,11,12,13,18 | |
| complement component 3 precursor | gi|4557385 | 187,018 | 2 | 11 | Urine |
| complement component 4 binding protein, | 3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21, | ||||
| alpha chain precursor | gi|4502503 | 61,671 | 32 | 23 | |
| complement component 4 binding protein, beta chain isoform 1 precursor | gi|4502505 | 26,264 | 5 | 15,16,17 | |
| complement component 4A preproprotein | gi|67190748 | 190,535 | 3 | 7,8,9 | Pleural Fluid |
| complement component 4B preproprotein | gi|50345296 | 190,535 | 17 | 4,5,6,7,8,9,10,11,12,13,15,18 | |
| b. Extracellular Matrix Proteins | |||||
| fibronectin 1 isoform 3 preproprotein | gi|16933542 | 256,752 | 58 | 3,4,5,6,7,17 | Mesothelioma Cells, Reticulocyte, Urine |
| laminin, gamma 1 precursor | gi|145309326 | 174,283 | 2 | 4 | |
| proteoglycan 4 | gi|67190163 | 150,947 | 19 | 1,2,3,15,16, 18,21,23,24,25 | |
| reelin isoform a | gi|27436938 | 388,259 | 2 | 3 | |
| thrombospondin 1 precursor | gi|40317626 | 127,496 | 13 | 6,7,8,9 | Urine |
| vitronectin precursor | gi|18201911 | 52,278 | 2 | 12 | Urine |
| c. Lectins | |||||
| collectin sub-family member 10 | gi|5453619 | 30,602 | 3 | 17 | |
| ficolin 1 precursor | gi|8051584 | 32,217 | 3 | 18 | |
| ficolin 2 isoform a precursor | gi|61744445 | 31,413 | 3 | 17 | |
| ficolin 3 isoform 2 precursor | gi|27754778 | 29,358 | 7 | 17,18,21 | |
| galectin 3 binding protein | gi|5031863 | 63,277 | 19 | 7,8,9,10,11,12,13,20 | Urine |
| mannan-binding lectin serine protease 1 isoform 1 precursor | gi|21264357 | 49,052 | 6 | 11,12 | |
| mannan-binding lectin serine protease 1 isoform 2 precursor | gi|21264359 | 48,607 | 2 | 15 | |
| mannan-binding lectin serine protease 2 isoform 1 precursor | gi|21264363 | 47,662 | 3 | 22 | Urine |
| soluble mannose-binding lectin precursor | gi|4557739 | 24,021 | 5 | 18,19 | |
| d. Thrombosis and Hemostasis | |||||
| fibrinogen, alpha chain isoform alpha preproprotein | gi|11761629 | 67,661 | 16 | 1,3,4,6,7,8, 10,11,12,13,14,15,16,17, 18,19,20,21,22 | Urine |
| fibrinogen, beta chain preproprotein | gi|70906435 | 52,315 | 26 | 12,13,14,15,16 | Pleural Fluid |
| fibrinogen, gamma chain isoform gamma-A precursor | gi|70906437 | 46,468 | 17 | 6,7,8,9,14,15,16,17 | |
| multimerin 1 | gi|45269141 | 137,980 | 2 | 6 | |
| protein S (alpha) | gi|4506117 | 70,646 | 20 | 7,8,9,10,11,12,13,15,17,18 | |
| von Willebrand factor preprotein | gi|4507907 | 225,727 | 25 | 3,4,5,6,7 | |
| e. Plasma Proteins | |||||
| albumin precursor | gi|4502027 | 66,473 | 24 | 3,6,7,8,9,10,11,12 | Adipocytes, DC, Pleural Fluid, Urine |
| alpha-2-macroglobulin precursor | gi|66932947 | 160,812 | 60 | 1,2,3,4,5,6,7, 8,9,10,11,12,13,15 | Adipocytes, DC |
| angiotensinogen preproprotein | gi|4557287 | 46,761 | 11 | 11,12,13 | Mast Cells |
| CD5 molecule-like | gi|5174411 | 37,957 | 18 | 15,16,17,20 | |
| immunoglobulin J chain | gi|21489959 | 17,967 | 5 | 21,22 | |
| paraoxonase 1 | gi|19923106 | 39,600 | 3 | 15 | |
| serine (or cysteine) proteinase inhibitor, clade A, member 1 | gi|50363219 | 46,606 | 5 | 13 | |
| transthyretin | gi|4507725 | 13,761 | 2 | 23 | |
| III. LIPOPROTEINS | |||||
| apolipoprotein A-II preproprotein | gi|4502149 | 9,304 | 2 | 25 | |
| apolipoprotein B precursor | gi|4502153 | 512,787 | 186 | 1,2,3,4,5,6,7,8,9,11,12,14,15 | |
| apolipoprotein D precursor | gi|4502163 | 19,303 | 2 | 19 | |
| apolipoprotein E precursor | gi|4557325 | 34,237 | 17 | 17,18,19,20,21,22,23,24,25 | Urine |
| apolipoprotein L1 isoform b precursor | gi|21735616 | 41,129 | 11 | 15,16,17,18,19,25,26 | Breast Milk |
| lipoprotein Lp(a) precursor | gi|5031885 | 224,414 | 18 | 1,2,3,4,5,6,7 | |
| low density lipoprotein-related protein 1 | gi|126012562 | 504,575 | 9 | 1,2,3,4 | |
| low density lipoprotein-related protein 1B precursor | gi|93102379 | 515,370 | 2 | 2 |
Columns denote functional classification, GI number, protein mass (Daltons), number of peptides identified, and whether the protein had previously been reported as a component of exosomes released by adipocytes, B cells, dendritic cells (DC), intestinal epithelial cells (IEC), mast cells, mesothelioma cells, reticulocytes, breast milk, plasma, pleural effusions, or urine (see supplemental material for references).
References are provided for publications reporting proteins that have previously been identified within exosomes released by adipocytes[1], B cells[2], dendritic cells (DC) [3, 4], intestinal epithelial cells (IEC) [5], mast cells[6], mesothelioma cells[7], reticulocytes[8–11], breast milk[12], plasma[13], pleural effusions[14], or urine[15].
N. Aoki, S. Jin-no, Y. Nakagawa, N. Asai, E. Arakawa, N. Tamura, T. Tamura, and T. Matsuda, Identification and characterization of microvesicles secreted by 3T3-L1 adipocytes: redox- and hormone-dependent induction of milk fat globule-epidermal growth factor 8-associated microvesicles, Endocrinology 148 (2007) 3850–3862.
R. Wubbolts, R. S. Leckie, P. T. Veenhuizen, G. Schwarzmann, W. Mobius, J. Hoernschemeyer, J. W. Slot, H. J. Geuze, and W. Stoorvogel, Proteomic and biochemical analyses of human B cell-derived exosomes. Potential implications for their function and multivesicular body formation, J Biol Chem 278 (2003) 10963–10972.
E. Segura, C. Nicco, B. Lombard, P. Veron, G. Raposo, F. Batteux, S. Amigorena, and C. Thery, ICAM-1 on exosomes from mature dendritic cells is critical for efficient naive T-cell priming, Blood 106 (2005) 216–223.
C. Thery, M. Boussac, P. Veron, P. Ricciardi-Castagnoli, G. Raposo, J. Garin, and S. Amigorena, Proteomic analysis of dendritic cell-derived exosomes: a secreted subcellular compartment distinct from apoptotic vesicles, J Immunol 166 (2001) 7309–7318.
G. van Niel, G. Raposo, C. Candalh, M. Boussac, R. Hershberg, N. Cerf-Bensussan, and M. Heyman, Intestinal epithelial cells secrete exosome-like vesicles, Gastroenterology 121 (2001) 337–349.
K. Al-Nedawi, J. Szemraj, and C. S. Cierniewski, Mast cell-derived exosomes activate endothelial cells to secrete plasminogen activator inhibitor type 1, Arterioscler Thromb Vasc Biol 25 (2005) 1744–1749.
J. P. Hegmans, M. P. Bard, A. Hemmes, T. M. Luider, M. J. Kleijmeer, J. B. Prins, L. Zitvogel, S. A. Burgers, H. C. Hoogsteden, and B. N. Lambrecht, Proteomic analysis of exosomes secreted by human mesothelioma cells, Am J Pathol 164 (2004) 1807–1815.
R. M. Johnstone, M. Adam, J. R. Hammond, L. Orr, and C. Turbide, Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes), J Biol Chem 262 (1987) 9412–9420.
R. M. Johnstone, A. Mathew, A. B. Mason, and K. Teng, Exosome formation during maturation of mammalian and avian reticulocytes: evidence that exosome release is a major route for externalization of obsolete membrane proteins, J Cell Physiol 147 (1991) 27–36.
S. Rieu, C. Geminard, H. Rabesandratana, J. Sainte-Marie, and M. Vidal, Exosomes released during reticulocyte maturation bind to fibronectin via integrin alpha4beta1, Eur J Biochem 267 (2000) 583–590.
L. Blanc, C. Barres, P. Bette-Bobillo, and M. Vidal, Reticulocyte-secreted exosomes bind natural IgM antibodies: involvement of a ROS-activatable endosomal phospholipase iPLA2, Blood 110 (2007) 3407–3416.
C. Admyre, S. M. Johansson, K. R. Qazi, J. J. Filen, R. Lahesmaa, M. Norman, E. P. Neve, A. Scheynius, and S. Gabrielsson, Exosomes with immune modulatory features are present in human breast milk, J Immunol 179 (2007) 1969–1978.
M. P. Caby, D. Lankar, C. Vincendeau-Scherrer, G. Raposo, and C. Bonnerot, Exosomal-like vesicles are present in human blood plasma, Int Immunol 17 (2005) 879–887.
M. P. Bard, J. P. Hegmans, A. Hemmes, T. M. Luider, R. Willemsen, L. A. Severijnen, J. P. van Meerbeeck, S. A. Burgers, H. C. Hoogsteden, and B. N. Lambrecht, Proteomic analysis of exosomes isolated from human malignant pleural effusions, Am J Respir Cell Mol Biol 31 (2004) 114–121.
T. Pisitkun, R. F. Shen, and M. A. Knepper, Identification and proteomic profiling of exosomes in human urine, Proc Natl Acad Sci U S A 101 (2004) 13368–13373.
PPARγ Co-segregates with, but is Distinct from the IDL Fraction of Human Plasma
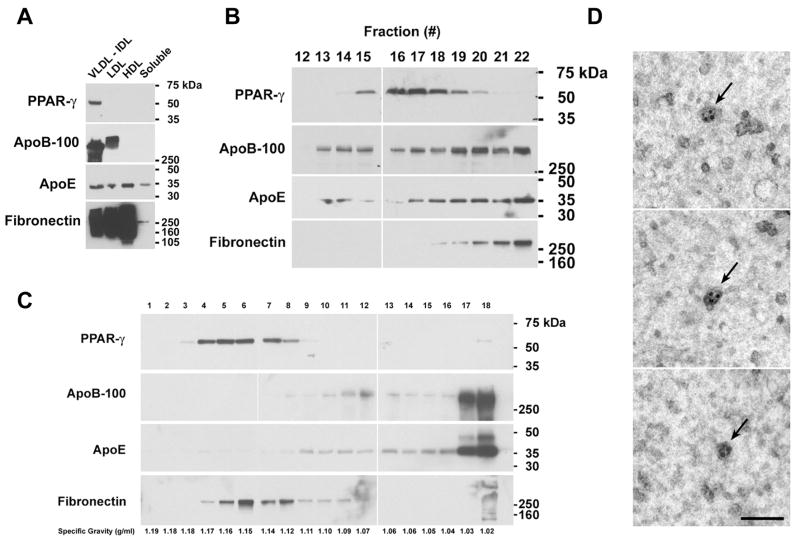
A single peptide (TENSWSNKAK) from gel slice number 15 was identified as PPARγ, coactivator 1α (PPARGC1A), a transcriptional coactivator that binds the nuclear receptor, PPARγ. Because of the important role of PPARγ in adipocyte differentiation and diabetes, experiments were performed to characterize PPARGC1A and PPARγ as exosome-associated proteins. Western blots of lipoprotein fractions that had been separated by gel exclusion chromatography revealed that PPARγ was present in the pooled VLDL/IDL fraction, but not in LDL (low density lipoprotein), HDL (high density lipoprotein), or soluble protein fractions (Figure 2A). Western blots were stripped and reprobed with antibodies that react with ApoB-100 and Apo-E to confirm the collection of plasma lipoprotein fractions. ApoB-100 was detected in the VLDL, IDL, and LDL fractions, whereas ApoE was present in all lipoprotein fractions. Similarly, fibronectin was detected in all lipoprotein fractions, with only a small amount co-fractionating with soluble proteins. PPARGC1A, however, could not detected by Western blotting.
Figure 2. Characterization of PPARγ Exosomes that Co-segregate with the IDL Fraction of Human Plasma.
A. Samples (20 μg) of FPLC fractions were immunoblotted and reacted with antibodies against PPARγ. Membranes were stripped and re-probed with antibodies against apolipoprotein B-100, apolipoprotein E, and fibronectin. This blot is representative of two independent experiments that demonstrated the same result. B. Proteins from individual tubes that corresponded to the VLDL and IDL fractions of human plasma were precipitated with 10% TCA, immunoblotted, and reacted with antibodies against PPARγ Membranes were stripped and re-probed with antibodies against apolipoprotein B-100, apolipoprotein E, and fibronectin. Individual tube numbers are shown on top. This blot is representative of five independent experiments that demonstrated the same result. C. A sample containing 435 μg of IDL proteins from human plasma was subjected to rate zonal centrifugation through a continuous sucrose gradient followed by immunoblotting. Lane numbers correspond to the fractions collected. Specific gravity of individual fractions are shown below. This blot is representative of two independent experiments that demonstrated the same result. D. Characterization by immunoelectron microscopy of PPARγ exosomes that co-segregate with the IDL fraction of human plasma. Exosomes from the IDL fraction of human plasma were concentrated and visualized by immunogold electron microscopy using antibodies that react with PPARγ. Three separate fields are shown. The arrows indicate PPARγ exosomes each labeled with 2–4 silver-enhanced gold particles (5–12 nm). The bar denotes 100 nm.
Additional Western blots were performed to define whether PPARγ co-segregated with the VLDL or IDL fractions of human plasma (Figure 2B). Proteins present in tubes 12 through 22 were precipitated with 10% TCA and Western blots were performed. Peak PPARγ expression was detected in tubes 15 – 20, which corresponded to the IDL fraction, but not in tubes 12 or 13, which corresponded to the VLDL fraction. Peak expression of ApoB-100, ApoE and fibronectin did not co-segregate with PPARγ, but appeared to migrate with the LDL shoulder. This showed that PPARγ exosomes co-segregate with IDL particles on the basis of size.
To confirm that the PPARγ exosomes were not a component of IDL lipoprotein vesicles, the IDL fraction was subjected to rate zonal centrifugation through continuous sucrose gradients to fractionate vesicles by density. As shown in Figure 2C, ApoB-100 and ApoE sedimented to peak density of 1.02–1.03 gm/ml, which is consistent with IDL particles. In contrast, PPARγ sedimented to a peak density of 1.14–1.17 gm/ml. This shows that PPAR-γ is not a component of a lipoprotein particle, such as IDL, but instead fractionated at a density consistent with an exosome. Similarly, fibronectin sedimented to a peak density of 1.12–1.16 gm/ml, which is also consistent with an exosome. PPARγ exosomes present in the IDL fraction of human plasma were then visualized by immunoelectron microscopy (Figure 2D). A sub-population of exosomes, containing one or more silver grains that represented specific immunoreactivity for PPARγ appeared as irregularly shaped vesicles with a diameter of 36–50 nm. Control samples, which were incubated without the primary antibody, did not reveal immunolabeling of exosomes. Taken together, these data demonstrate that PPARγ containing exosomes, which co-segregate with IDL on the basis of size, circulate in human plasma.
Discussion
Exosomes are small, secreted membrane vesicles that mediate cell-free intercellular communication via the transfer of membrane and cytosolic proteins. We previously utilized gel exclusion chromatography to purify TNFR1 exosome-like vesicles from the LDL fraction of human plasma[4]. The advantage of this approach is that it allows the separation of distinct populations of vesicles on the basis of size. This reduces sample complexity, which is important for protein identification as human plasma is a complex biological fluid that may contain greater than 1 million different protein species reflecting alternative splicing, post-translational modification, proteolytic processing, and clonal immunoglobulin populations[7].
Here, we report on the proteomic analysis and characterization of exosomes that circulate in human plasma. We developed a purification scheme that utilized gel exclusion chromatography, rate zonal centrifugation through continuous sucrose gradients, and high-speed sedimentation to purify exosomes from human blood. Plasma that had been filtered through a 0.22 μm filter to remove large structures was fractionated by gel filtration chromatography to isolate VLDL and IDL fractions, which typically range from approximately 20–80 nm. We hypothesized that plasma exosomes, which typically are 30–100 nm in diameter, would co-segregate with VLDL and IDL particles. VLDL and IDL fractions were next subjected to rate zonal centrifugation through continuous sucrose gradients to separate higher density exosomes from lower density VLDL and IDL particles. Plasma exosomes were pelleted by high-speed centrifugation and fractionated by 1D SDS-PAGE. Proteins present within individual gel slices were digested with trypsin and identified by LC-MS/MS.
This multi-step purification scheme identified 66 proteins associated with human plasma exosomes, including a wide range of cellular proteins, both cytosolic and membrane-associated, as well as extracellular or secreted proteins (Table 1). Only one protein (integrin alpha 2b) had previously been shown to be expressed by plasma-derived exosomes[3], while many proteins had not previously been found to be associated with exosomes. Several of the cell-associated proteins identified were related to vesicular trafficking or protein sorting, which is consistent with the formation of exosomes via endosomal sorting pathways that target proteins to multivesicular bodies[2]. For example, clathrin, which has previously been identified as an exosome-associated protein, can associate with Hrs, an adaptor protein that binds ubiquitinated membrane proteins and participates in protein sorting into multivesicular bodies[8].
The LC-MS/MS analysis of plasma exosomes also identified a single peptide that corresponded to PPARGC1A, a transcriptional activator that binds PPARγ. PPARγ is a member of the nuclear receptor superfamily that regulates normal adipocyte differentiation and proliferation, as well as the uptake and storage of fatty acids[9]. PPARγ is also expressed by vascular endothelium, macrophages, and immune cells which allows it to regulate inflammation, immunity, endothelial function, bone morphogenesis, atherosclerosis and cancer. Because of the important role of PPARγ in the regulation of gene transcription in multiple cell types that modulate lipid and glucose homeostasis, as well as inflammatory and immune responses, we elected to characterize further the expression of PPARGC1A and PPARγ in plasma exosomes. PPARγ, but not PPARGC1A, was confirmed as an exosome-associated protein. Western blots demonstrated PPARγ expression within vesicles that co-segregated with the IDL, but not the VLDL fraction of human plasma on the basis of size and sedimented to a peak specific gravity of 1.15–1.16 g/ml on continuous sucrose gradients, which is consistent with an exosome. In contrast, IDL particles containing apolipoprotein B-100 and apolipoprotein E sedimented to a peak specific gravity of 1.02–1.03 g/ml, which showed that exosomes expressing PPARγ are distinct from IDL particles. These data suggest that plasma-derived exosomes represent a new mechanism by which PPARγ might be transferred between cells in a paracrine fashion to modulate gene expression.
In conclusion, we have developed a multi-step fractionation scheme to obtain plasma exosomes for LC-MS/MS analysis, which resulted in the identification of 66 proteins in the proteome of human plasma exosomes. Both secreted and cell-associated proteins were identified, including many involved in vesicular trafficking pathways. Furthermore, we used biochemical analyses and immunoelectron microscopy to identify PPARγ as a novel component of plasma-derived exosomes. Given the role of exosomes as intercellular messengers, this identifies a potential new pathway for paracrine signaling by nuclear receptors.
Acknowledgments
This work was funded by the Division of Intramural Research, NHLBI, NIH. We are grateful to Patricia S. Connelly of the NHLBI Electron Microscopy Core Facility for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Thery C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol. 2002;2:569–579. doi: 10.1038/nri855. [DOI] [PubMed] [Google Scholar]
- 2.van Niel G, Porto-Carreiro I, Simoes S, Raposo G. Exosomes: a common pathway for a specialized function. J Biochem (Tokyo) 2006;140:13–21. doi: 10.1093/jb/mvj128. [DOI] [PubMed] [Google Scholar]
- 3.Caby MP, Lankar D, Vincendeau-Scherrer C, Raposo G, Bonnerot C. Exosomal-like vesicles are present in human blood plasma. Int Immunol. 2005;17:879–887. doi: 10.1093/intimm/dxh267. [DOI] [PubMed] [Google Scholar]
- 4.Zhang J, Hawari FI, Shamburek RD, Adamik B, Kaler M, Islam A, Liao DW, Rouhani FN, Ingham M, Levine SJ. Circulating TNFR1 exosome-like vesicles partition with the LDL fraction of human plasma. Biochem Biophys Res Commun. 2008;366:579–584. doi: 10.1016/j.bbrc.2007.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang G, Wu WW, Zeng W, Chou CL, Shen RF. Label-free protein quantification using LC-coupled ion trap or FT mass spectrometry: Reproducibility, linearity, and application with complex proteomes. J Proteome Res. 2006;5:1214–1223. doi: 10.1021/pr050406g. [DOI] [PubMed] [Google Scholar]
- 6.Thery C, Amigorena S, Raposo G, Clayton A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol. 2006;Chapter 3 doi: 10.1002/0471143030.cb0322s30. Unit 3 22. [DOI] [PubMed] [Google Scholar]
- 7.Anderson L. Candidate-based proteomics in the search for biomarkers of cardiovascular disease. J Physiol. 2005;563:23–60. doi: 10.1113/jphysiol.2004.080473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raiborg C, Bache KG, Gillooly DJ, Madshus IH, Stang E, Stenmark H. Hrs sorts ubiquitinated proteins into clathrin-coated microdomains of early endosomes. Nat Cell Biol. 2002;4:394–398. doi: 10.1038/ncb791. [DOI] [PubMed] [Google Scholar]
- 9.Heikkinen S, Auwerx J, Argmann CA. PPARgamma in human and mouse physiology. Biochim Biophys Acta. 2007;1771:999–1013. doi: 10.1016/j.bbalip.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]