Abstract
Objective
Because self-rated health (SRH) is strongly associated with health outcomes, it is important to identify factors that individuals take into account when they assess their health. We examined the role of valued life activities (VLAs), the wide range of activities deemed to be important to individuals, in SRH assessments.
Study Design and Setting
Data were from 3 cohort studies of individuals with different chronic conditions – rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), and chronic obstructive pulmonary disease (COPD). Each cohort’s data were collected through structured telephone interviews. Logistic regression analyses identified factors associated with ratings of fair/poor SRH. All analyses included sociodemographic characteristics, general and disease-specific health-related factors, and general measures of physical functioning.
Results
Substantial portions of each group rated their health as fair/poor (RA 37%, SLE 47%, COPD 40%). In each group, VLA disability was strongly associated with fair/poor health (RA: OR=4.44 [1.86,10.62]; SLE: OR=3.60 [2.10,6.16]; COPD: OR=2.76 [1.30,5.85], even after accounting for covariates.
Conclusion
VLA disability appears to play a substantial role in individual perceptions of health, over and above other measures of health status, disease symptoms, and general physical functioning.
Keywords: self-rated health, disability, valued life activities, rheumatoid arthritis, systemic lupus erythematosus, chronic obstructive pulmonary disease
What is new?
Disability in valued life activities (VLAs) appears to play a substantial role in individual perceptions of health, over and above other measures of health status, disease symptoms, and general physical functioning.
Physical functioning measures traditionally used to estimate health status may not be the most important gauges of functioning associated with individuals’ assessments of their health.
VLA disability may signal poor health in a more concrete fashion, by reflecting difficulty performing life roles and in leisure and free-time activities, than difficulty in isolated physical functions such as stooping, reaching, or climbing stairs.
Assisting individuals in finding satisfying replacement activities for those that are affected by health may allow them to maintain a more positive sense of health and well-being.
There has been a strong, consistent relationship between self-rated health (SRH) and morbidity, even after controlling for demographic, medical, and psychosocial factors that affect health [1-12] . Further, SRH is a better predictor of mortality than clinical data, medical records, or physician assessments of physical health [6,9-11,13].
Because SRH is so strongly and reliably linked to health outcomes, it is important to identify factors that individuals take into account, either explicitly or implicitly, when they make assessments of their health. Functioning, measured in various ways, has consistently been associated with SRH [14-20]. However, most measures of functioning in these studies have focused on physical impairments or disability in basic, low-level activities, in particular, activities of daily living (ADLs). Few, if any, studies have examined a broader range of activities that include higher level functioning. Valued life activities (VLAs) comprise the wide range of life activities deemed to be important by the individual, ranging from self-care to leisure activities [21,22]. In several chronic diseases, VLA disability has been closely linked with dissatisfaction with functioning [23,24] and psychological distress [25-28]. Thus, there is a good rationale to believe that VLAs also play a role in SRH. In this paper, we seek to extend the research on SRH by assessing the relationship between VLA disability and SRH among individuals with three chronic health conditions: rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), and chronic obstructive pulmonary disease (COPD). By studying three clinically disparate but seriously disabling conditions, we wished to identify the commonalities that shed light on VLA disability as a key determinant of SRH and the consistency of the relationship between VLA disability and SRH.
Methods
Samples
Data for these analyses were drawn from three longitudinal studies of adults with chronic health conditions. The principal data source for each is an annual telephone interview that includes questions on demographics, symptoms, medications, comorbidities, functioning (including VLAs), and SRH. Each was approved by the University of California, San Francisco Committee on Human Research.
Rheumatoid arthritis (RA) study
The RA sample consisted of respondents to the 2006 wave of the UCSF RA Panel Study (n=441). The RA Panel was constructed in 1982 from a random sample of rheumatologists practicing in Northern California. Participants were recruited from lists maintained by participating rheumatologists of all persons with RA presenting to their offices over a one-month period, and expressing an interest to participate in the study. The original RA Panel consisted of 822 patients; there were subsequently four additional enrollment periods in 1989-90, 1995, 1999, and 2003, during which 203, 131, 122, and 169 individuals were enrolled, respectively. Yearly retention has averaged 93%; the 7% attrition includes deaths.
Systemic lupus erythematosus (SLE) study
The SLE sample was drawn from the 2006 interview of the UCSF Lupus Outcomes Study (n=795). Participants were recruited from both clinical and community-based sources, including UCSF-affiliated clinics (22%), non-UCSF rheumatology offices (11%), lupus support groups and conferences (26%), and newsletters, websites and other forms of publicity (41%). All SLE diagnoses were confirmed by medical record review. The first interviews were conducted in 2002-2003, and have been conducted annually thereafter. Yearly retention has averaged 93%; the 7% attrition includes deaths.
Chronic obstructive pulmonary disease (COPD) study
Subjects were drawn from a sample that originated as a large, population-based study of US adults aged 55 to 75 with obstructive lung disease recruited through random digit dial telephone interviews in 2001. During recruitment, subjects were asked if they had ever been diagnosed with chronic bronchitis, emphysema, or COPD (chronic obstructive lung disease) by a medical doctor; if so, they were included in the COPD cohort. Annual retention among the original sample has averaged approximately 80% through 2006, over five telephone interview waves. In addition, in 2006, another 300 individuals with COPD were recruited from northern California using the same method, for a total sample size of 585 in 2006.
Variables
Valued life activity (VLA) disability
Development of the VLA disability scale for RA has been previously described in detail [22]. Briefly, the VLA scale has its roots in a study in which a measure of 75 life activities, later grouped into 13 domains, was developed to determine the impact of arthritis [29,30]. Respondents have been asked over multiple waves to identify activities in addition to those queried that have been affected by their condition. Revisions have been made to the VLA scale based on those accumulated responses as well as analysis of previous versions of the scale [22].
Versions used for the SLE and COPD cohorts, although not identical, were adapted from the RA scale. In each case, items were omitted if responses in previous waves indicated very infrequent performance or added if responses to open-ended questions suggested the need. Usually additions or deletions were related to the age of the cohort or specific aspects of the disease. The VLAs assessed for each cohort are shown in the Appendix. For RA, SLE, and COPD, 33, 33, and 28 activities, respectively, were queried.
Assessment of disability with the VLA scale represents advancement over previous instruments in three ways. First, a wide spectrum of activities is included, ranging from self-care to recreation and social participation. Second, the VLA scale takes personal value into account. Activities that are not applicable to an individual (e.g., “taking care of children” if the individual has no children) or not important to the individual (e.g., “cooking” if only the spouse cooks) are not included in scoring. Finally, unlike most disability indices, the VLA scale asks respondents to attribute performance difficulties to the health condition under study rather than generically. This is important because comorbidities are frequent in these populations.
In telephone interviews, participants rated the difficulty of performing VLAs (0=no difficulty, 1=a little difficulty, 2=a great deal of difficulty, and 3=unable to perform). Activities that participants deemed unimportant to them, or that they did not do for reasons unrelated to their disease, were not rated and were not included in summary scoring. VLA disability scores were calculated as the mean difficulty of all rated items.
Self-rated heath (SRH)
Individuals were asked to rate their health on either a four-point (excellent, good, fair, or poor; RA) or five-point (excellent, very good, good, fair, or poor; SLE and COPD) scale.
Covariates
Analyses examining the relationship between VLA disability and SRH controlled for sociodemographic and health-related characteristics that may contribute to perceptions of health status [5,12,31-34]. Sociodemographic characteristics included age, sex, race (white non-Hispanic vs. all others), education (more than high school vs. high school graduation or less), and marital status (married/with partner vs. other).
Health-related factors
Fatigue was assessed for all groups, although different measures of fatigue were used in each group. In the RA panel, participants rated fatigue over the past 2 weeks as “none,” “mild,” “moderate,” “severe,” or “very severe.” In SLE sample, the 4-item Vitality subscale from the SF-36 was used [35]. These items query the frequency of feelings of energy and tiredness on a 6-point scale, ranging from “all of the time” to “none of the time.” Scores are typically calculated to range from 0 to 100, with higher scores reflecting greater vitality. In the COPD cohort, participants were asked to rate the severity of their fatigue or physical tiredness in the past four weeks, with 0 being “no fatigue” and 10, “extremely fatigued.” All fatigue scores were rescaled to range from 0 to 10, with higher scores reflecting greater fatigue, so that all results would be scaled in the same metric.
Additional variables were included for each disease group to briefly characterize disease severity or activity. For RA, a rating of pain at the time of the interview on a scale of 0 (no pain) to 100 (very severe pain) [36] was added. For SLE, a rating of disease activity was included. Participants were asked to rate the activity of their SLE during the past three months on a scale of 0 (no activity) to 10 (the most activity). This item is taken from the Systemic Lupus Activity Questionnaire, a validated self-report measure of SLE disease activity [37]. For the COPD cohort, current smoking status and the COPD Severity scale [38] were added. The COPD Severity Score is based on responses to questionnaire items from five domains --- respiratory symptoms, prior systemic corticosteroid use, other COPD medication use, previous hospitalization and intubation for respiratory disease, and home oxygen use. Scores can range from 0 to 31, with higher scores representing greater severity.
Physical limitations
Different measures of physical limitations were also used for each group. In the RA panel, the Health Assessment Questionnaire (HAQ) was used [36]. The HAQ was developed specifically to measure functioning among individuals with arthritis. Items query difficulty in 20 specific functions, grouped into 8 categories: arising, dressing and grooming, eating, walking, personal hygiene, reaching, gripping, and other activities. Difficulty is rated from 0 (no difficulty) to 3 (unable). Overall scores are calculated as the mean of the eight category scores, and range from 0 to 3, with higher scores representing worse functioning.
The SLE study used the 10-item Physical Functioning subscale of the SF-36 [35]. Items assess limitations in bathing and dressing; lifting and carrying; bending, kneeling, or stooping; walking; stair climbing; and moderate and vigorous activities. Scores on this subscale are typically scored to range from 0 to 100, with higher scores representing better functioning.
The COPD cohort used the 10-item Physical Limitations scale from the Health and Retirement Survey [39]. Items assess difficulty in stooping, crouching, or kneeling; walking; sitting or standing for long periods; lifting or carrying; and pushing objects. Difficulty is rated from 0 (no difficulty) to 3 (unable); overall scores are calculated as the mean across all items, ranging from 0 to 3, with higher scores representing worse function.
All physical limitations measures were rescaled to range from 0 to 10, with higher scores reflecting worse function.
Analysis
All analyses were stratified by disease group cohort. Means and frequencies were calculated. To examine differences in VLA disability scores among individuals according to SRH, analysis of variance (ANOVA) models with p-value adjustments were estimated.
In logistic regression analyses, to be consistent with past research, individuals who rated their health as fair or poor were compared to those who rated their health as good, very good, or excellent. Three multiple logistic regression analyses were conducted to determine predictors of fair/poor health, first including physical limitations as the only measure of function (Model 1), second including VLA disability scores without physical limitations (Model 2), and finally, including both physical limitations and VLA disability scores in the model to determine their independent contributions to predicting fair/poor SRH (Model 3). In each model, all covariates were included (i.e., sociodemographic and health-related factors).
Sensitivity analyses were conducted to determine whether alternative operationalizations of the SRH measure yielded consistent results. We considered a binary outcome comparing excellent/very good SRH to good/fair/poor SRH and an ordinal outcome that included all original response categories, poor through excellent. Models of these alternative outcomes paralleled those described above.
RESULTS
Subject characteristics
Characteristics of individuals in each cohort are shown in Table 1. In each group, the majority were female, white non-Hispanic, married, and with education greater than high school although there were notable differences among the groups. The SLE group was the youngest and included the greatest proportion of women, reflecting the age and gender distribution of the disease. The RA group also included a large portion of women, again reflecting the gender distribution of the disease. The COPD group was the oldest and included the fewest women, and also was more likely to report comorbid conditions. Fatigue levels were roughly equivalent among the groups, but physical functioning was considerably worse in the SLE group. The disease-specific measures revealed moderate levels of symptoms for each group.
Table 1.
Subject Characteristics
| RA (n = 441) |
SLE (n = 795) |
COPD (n = 418) |
|
|---|---|---|---|
| Sociodemographic factors | |||
| Age, 66+ years; % (n) | 40.7 (180) | 10.1 (80) | 53.3 (223) |
| Female; % (n) | 85.1 (376) | 92.2 (735) | 62.0 (259) |
| White, non-Hispanic; % (n) | 83.7 (370) | 68.1 (543) | 93.1 (389) |
| Education, > high school; % (n) | 69.0 (305) | 86.8 (692) | 67.2 (281) |
| Married or with partner; % (n) | 62.0 (274) | 59.9 (476) | 54.1 (226) |
| Health-related measures | |||
| Comorbidities; % (n) | |||
| 1 | 39.4 (162) | 34.0 (271) | 30.4 (127) |
| 2 or more | 24.0 (106) | 16.6 (132) | 49.8 (208) |
| Fatigue (range 0 – 10; higher = greater fatigue) | 4.9 (2.7) | 5.5 (2.3) | 4.8 (2.5) |
| Physical functioning (range 0 – 10; higher = worse functioning) | 3.6 (2.4) | 6.0 (1.2) | 3.3 (2.4) |
| Disease–specific measures | |||
| Rheumatoid arthritis (RA) | |||
| Pain rating; means (SD) (0=no pain, 100=very severe pain) | 30.0 (27.1) | ||
| Systemic lupus erythematosus (SLE) | |||
| Rating of SLE activity; mean (SD) (0=none, 10=severe) | 4.2 (2.8) | ||
| Chronic obstructive pulmonary disease (COPD) | |||
| Current smoker | 22.5 (94) | ||
| COPD Severity Score; mean (SD) (range 0-31, high=greater severity) | 7.8 (6.3) |
Self-rated health and VLA disability: bivariate analyses
Overall, VLA disability scores ranged from 0.53 (COPD) to 0.78 (SLE) (Table 2). Within each disease sample, there was a consistent trend for increasing VLA disability with worsening SRH (Figure 1), with most differences between health status rating groups statistically significant (Table 2). Substantial portions of each group rated their health as fair or poor: RA, 37%; SLE, 47%; and COPD, 40%.
Table 2.
Bivariate Associations between VLA Disability and Self-rated Health Status
| Total | Excellent | Very good | Good | Fair | Poor | |
|---|---|---|---|---|---|---|
| RA | ||||||
| N | 441 | 32 (7.3%) | --- | 246 (55.8%) | 131 (29.7%) | 32 (7.3%) |
| VLA disability | 0.55 (0.49) | 0.14 (0.18) | --- | 0.39 (0.37) | 0.79 (0.45) | 1.26 (0.58) a |
| SLE | ||||||
| N | 795 | 33 (4.2%) | 131 (16.5%) | 258 (32.4%) | 257 (32.3%) | 116 (14.6%) |
| VLA disability | 0.78 (0.63) | 0.06 (0.11) | 0.26 (0.32) | 0.57 (0.46) | 1.00 (0.56) | 1.51 (0.56) b |
| COPD | ||||||
| N | 585 | 56 (9.6%) | 124 (21.2%) | 173 (29.6%) | 146 (25.0%) | 86 (14.7%) |
| VLA disability | 0.53 (0.62) | 0.18 (0.43) | 0.14 (0.17) | 0.32 (0.36) | 0.72 (0.53) | 1.44 (0.64) c |
Note: F-tests for all ANOVA significant at p <.0001.
In RA sample, VLA difficulty was significantly different (p < .01) among all groups.
In SLE sample, VLA difficulty was not significantly different between excellent and very good groups. All other comparisons were significantly different (p < .01).
In COPD sample, VLA difficulty was not significantly different among “excellent,” “very good,” and “good” groups. All other comparisons were significantly different (p<.01).
Figure 1.
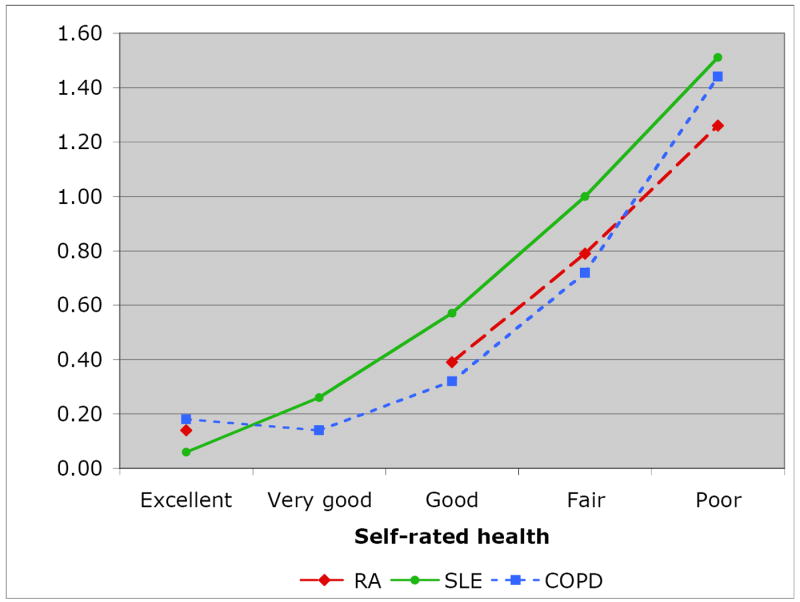
Mean VLA Difficulty Rating for Self-rated Health Groups
Multivariate associations with self-rated health
Sociodemographic factors played little independent role in assessments of fair/poor SRH, with two excetions: in the RA cohort, married/partnered respondents were more likely to report fair/poor SRH ORs from 1.58 to 1.73 in Models 1, 2, and 3 ,Table 3); and in the COPD cohort, and education was inversely related to fair/poor SRH (ORs ranged from 0.55 to 0.65, Table 3). Two or more comorbid conditions were associated with fair/poor SRH for the SLE and COPD groups. In the RA and COPD samples, greater fatigue was significantly associated with fair/poor SRH. Disease-specific measures also made significant contributions to SRH: for RA, greater pain; in SLE, ratings of disease activity; and in COPD, COPD severity score and current smoking were each associated with a higher risk of fair/poor SRH.
Table 3.
Factors Significantly Associated with Fair/Poor Health Status Ratings
| Model 1 Fx lims only |
Model 2 VLA difficulty only |
Model 3 Fx lims & VLA difficulty |
||
|---|---|---|---|---|
| RA | ||||
| Sociodemographic | ||||
| Married/partner | 1.58 (0.96, 2.61)* | 1.72 (1.03, 2.89) | 1.73 (1.03, 2.91) | |
| Health-related | ||||
| Fatigue | 1.29 (1.14, 1.45) | 1.26 (1.12, 1.42) | 1.25 (1.11, 1.42) | |
| Disease-specific | ||||
| Pain rating | 1.01 (1.00, 1.02) | 1.01 (1.00, 1.02) | 1.01 (1.00, 1.02) | |
| Function | ||||
| Functional limitations | 1.41 (1.25, 1.60) | --- | ||
| VLA total | --- | 7.19 (3.82, 13.51) | 5.19 (2.21, 12.21) | |
| AIC | 454.47 | 439.13 | 439.99 | |
| SLE | ||||
| Sociodemographic | ||||
| Married/partner | 1.32 (0.91, 1.90) | 1.34 (0.92, 1.95) | ||
| Female | 0.58 (0.30, 1.14) | .60 (0.31, 1.14) | 0.55 (0.28, 1.07) | |
| Education, > high school | 1.44 (0.84, 2.44) | |||
| Health-related | ||||
| Comorbidities, 2+ | 1.66 (1.01, 2.71) | 1.97 (1.20, 3.24) | 1.76 (1.06, 2.90) | |
| Disease-specific | ||||
| Rating of SLE activity | 1.28 (1.19, 1.38) | 1.20 (1.11, 1.30) | 1.20 (1.10, 1.29) | |
| Function | ||||
| Functional limitations | 2.55 (2.13, 3.06) | --- | 1.65 (1.30, 2.10) | |
| VLA total | --- | 7.88 (5.33, 11.64) | 3.89 (2.35, 6.43) | |
| AIC | 789.68 | 776.26 | 761.65 | |
| COPD | ||||
| Sociodemographic | ||||
| Female | 0.62 (0.34, 1.12) | 0.64 (0.35, 1.16) | ||
| Education, > high school | 0.65 (0.36, 1.17) | 0.55 (0.30, 0.99) | 0.60 (0.33, 1.09) | |
| Health-related | ||||
| Comorbidities, 2+ | 3.60 (1.63, 7.97) | 3.83 (1.74, 8.41) | 3.41 (1.53, 7.60) | |
| Fatigue | 1.53 (1.32, 1.77) | 1.47 (1.26, 1.70) | 1.45 (1.25, 1.69) | |
| Disease-specific | ||||
| Current smoking | 2.05 (1.04, 4.05) | 1.94 (0.97, 3.89) | 1.92 (0.96, 3.85) | |
| COPD severity score | 1.10 (1.04, 1.16) | 1.06 (1.00, 1.13) | 1.06 (1.00, 1.13) | |
| Function | ||||
| Functional limitations | 1.31 (1.13, 1.52) | --- | 1.18 (1.00, 1.40) | |
| VLA total | --- | 3.96 (1.86, 8.42) | 2.71 (1.16, 6.28) | |
| AIC | 357.96 | 355.84 | 354.07 |
- RA: pain rating.
- SLE: rating of lupus activity.
- COPD: COPD Severity Score, current smoking status.
Only associations significant at p≤0.20 are shown.
AIC = Akaieke’s Information Criterion
In Model 1, which included physical functioning but not VLA disability, poorer physical function was consistently associated with fair/poor SRH (RA: OR=1.41 [1.25,1.60]; SLE: OR=2.55 [2.13,3.06]; COPD: OR=1.31 [1.13,1.52]).
In the second stage of the regression analyses (Model 2), VLA disability scores were added to the models without including physical functioning. In each case, greater VLA disability was strongly associated with fair/poor SRH (RA: OR=7.19 [3.82,13.51]; SLE: OR=7.88 [5.33,11.64]; COPD: OR=3.96 [1.86,8.42]). For each condition, the Akaike’s Information Criterion (AIC) was lower for Model 2 than Model 1, indicating better fit of Model 2. However, the difference between Models 1 and 2 was slight for COPD.
Model 3 included both physical functioning and VLA disability. For all three conditions, VLA disability retained its significant association with SRH (RA: OR=5.19 [2.21,12.12]; SLE: OR=3.89 [2.35,6.43]; COPD: OR=2.71 [1.16,6.28]). Differences were noted among the groups in the role of physical functioning and in changes in the fit of the model. For RA, physical functioning was not statistically significant, and the AIC suggested that it should be excluded from the model. For SLE, both physical functioning and VLA disability were statistically significant. Although inclusion of both attenuated the size of the odds ratios, both retained significant independent relationships with SRH, and the AIC suggested that the model including both was the best model. For COPD, both physical functioning and VLA disability made significant independent contributions. There was less attenuation of the odds ratios than in the SLE model, but the AIC again suggested only slight differences in the models.
Sensitivity analyses
In the two sensitivity analyses, the same factors were observed to have significant associations with SRH, with no substantive differences in the interpretation of the results. Therefore, the results of these analyses are not shown.
Discussion
VLA disability was strongly associated with SRH, even after taking sociodemographic, and general and disease-specific health-related factors into account. The association of both VLA disability and physical functioning with SRH is consistent with previous research, which has generally found that SRH is influenced by physical activity and/or functioning. Benyamini and colleagues proposed that SRH was influenced by concrete experiences that were indicators of disease [16]. In that study, when participants were asked what factors were important in rating their health, the most highly rated response was “your ability to do the things you need to do and the things you want to do” – a concept closely aligned with that of valued life activities. Duke also noted that individuals who rated their health as fair or poor were more likely to reduce or abandon valued activities [40].
Differences in the relationship between VLA disability and SRH existed among the disease groups. For RA and SLE, VLA disability was clearly a better predictor of SRH than physical functioning, based on both the odds ratios and the model AICs. While VLA disability was also a strong predictor of SRH in COPD, its role appeared to be weaker. The difference in the role of VLA disability may be due to differences in the samples or may reflect attributes of the diseases themselves. One possible explanation is the gender composition of the groups. Both the RA and SLE groups were predominantly women (85% and 92%, respectively). Previous research suggests that women are attuned to a broader range of factors when rating their health, whereas men tend to focus primarily on serious, life-threatening disease [41]. This supposition is further supported by the impact that comorbidities appeared to have on SRH in the COPD group compared to the RA and SLE groups.
Differences were also noted among the disease groups in the relative importance of physical functioning as compared to VLA disability. For RA, VLA disability was clearly the better predictor of SRH. For SLE, both physical functioning and VLA disability were important, and inclusion of both variables produced the strongest model. It is possible that the importance of both physical functioning and VLA disability for the SLE group can be attributed to the poorer physical functioning and greater VLA disability of this group. For COPD, VLA disability was a stronger predictor, but superiority compared to physical functioning was less clear.
There are potential limitations to this study. Our assessment of VLAs may have been incomplete. However, we have accumulated the lists of VLAs over several years through an iterative process with respondent feedback, supporting their comprehensiveness. Even with the addition of new activities, there is no reason to believe that the overall tenor of these results would change. Factors other than those included here may have affected the association of VLA disability with SRH. The three cohorts may be unrepresentative of individuals with the three conditions in some way; however, each cohort was assembled in a broad-based fashion, not limited to recruitment from tertiary medical centers, and each is among the largest disease-specific cohorts assembled for observational study.
There may be some overlap between physical functioning and VLA disability. The two are related theoretically [42], which is one compelling reason to attempt to tease out their independent effects. Inclusion of both factors did appear to attenuate the coefficient sizes, but we saw significant and independent effects of both variables in the SLE and COPD groups, and examination of differences in the associations among the disease groups may have shed light on the way that each influences SRH.
That VLA disability was more strongly associated with SRH than physical functioning is of particular interest because the physical functioning measures that we used are similar to those traditionally used to estimate health status in clinical research, and which are often used as primary outcomes, intended to measure disability. Our results suggest, however, that in some cases, these functional measures may not be the only, or the most important gauge of functioning associated with individuals’ assessments of their health. VLA disability may signal poor health in a more concrete fashion, by reflecting difficulty performing life roles and in leisure and free-time activities, than difficulty in isolated physical functions such as stooping, reaching, or climbing stairs.
There are potentially important practical implications to these findings. It may not be possible to improve disease symptoms or effects, or functional limitations, among individuals with chronic conditions. It may, however, be possible to assist these individuals in moderating the effect of disease on VLAs, and thus on perceived health and well-being. The relationship between depressed affect and poor SRH is well documented [43, 44], as is the relationship between activity loss and depressed affect [25,26,45-49]. In contrast, replacing lost valued activities appears to be associated with more positive well-being [40,49]. If, indeed, SRH is influenced by valued activity loss, or VLA disability, then perhaps assisting individuals in finding satisfying replacement activities will allow them to maintain a more positive sense of health and well-being. Although the mechanism by which SRH appears to influence mortality and morbidity is still not understood, given the strong and consistent relationship of SRH with mortality and morbidity, such activity replacement may even affect that relationship.
In summary, disability in valued life activities appears to play a substantial role in how individuals perceive their health status, over and above other measures of health status and chronic disease symptoms, as shown in this paper, as well as their psychological status as demonstrated in previous studies [22,25,26]. Self-rated health perceptions are powerful predictors of future health, morbidity, and mortality. Whether it is possible to modify these perceptions by modifying the factors feeding into those perceptions is unknown. More importantly, whether changing health perceptions will change an individual’s illness or health trajectory, or whether these perceptions are solely reactive is also unknown. Nonetheless, patient’s global health ratings are often used as outcome measures in clinical research and trials, so it is important to understand how individuals arrive at those assessments. These results suggest that VLA disability is one important factor that individuals consider when making assessments of their health.
Acknowledgments
Financial support was provided by NIAMS grant AR50015, NIAMS P60 AR053308, the State of California Lupus Fund, and NHLBI HL067438.
We gratefully acknowledge the contribution of the interviewers and data managers for the three studies (Marissa San Pedro, David Burian, Steven King, Rosemary Prem, Janet Stein, Gillian Earnest, Stephanie Rush, and Field Research Corporation). We also appreciate
Appendix
Activities in VLA Scales.
| RA | SLE | COPD |
|---|---|---|
| Take care of basic needs, such as bathing, washing, getting dressed, or taking care of personal hygiene | x | x |
| Walk or get around inside your home | x | x |
| Walk outside, just to get around, in the area around your home or other places you need to go on a regular basis | x | x |
| Get around your community by car or public transportation | x | x |
| Go to appointments such as going to the doctor or dentist or going to have your hair cut/done | x | |
| Sleep | x | x |
| Eating/chewing | ||
| Preparing meals and cooking | x | x |
| Light housework such as dusting or laundry | x | x |
| Heavier housework such as vacuuming, changing sheets, or cleaning floors | x | x |
| Other work around the house such as making minor home repairs or working in the garage fixing things | x | x |
| Shop and do errands | x | x |
| Take care of your children/ grandchildren | x | x |
| Taking care of other family members such as your spouse or parent or other people close to you | x | x |
| Working at a job for pay | x | x |
| Taking care of household business such as paying bills or scheduling repairs | x | x |
| Gardening or working in your yard | x | x |
| Participating in activities with your children/grandchildren | x | |
| Visiting with friends or family members in their homes | x | x |
| Having friends and family members visit you in your home | x | x |
| Participating in leisure activities outside your home, such as playing cards or bingo or going to movies/restaurants | x | x |
| Participating in leisure activities in your home, such as reading, watching television, or listening to music | x | x |
| Going to parties, celebrations, or other social events | x | x |
| Participate in physical recreational activities, such as walking for exercise, playing golf, bicycling, swimming or water aerobics | Participate in moderate physical recreational activities such as dancing, playing golf, or bowling | x |
| Participate in vigorous physical recreational activities such as walking for exercise, jogging, bicycling, swimming, or water aerobics | x | |
| Do things outdoors or in the sun | ||
| Working on hobbies or crafts, or creative activities, such as sewing, woodwork, or painting | x | x |
| Traveling out of town | x | x |
| Participate in religious or spiritual activities | x | x |
| Doing volunteer work | x | x |
| Take care of social communications, such as writing letters, sending emails, or making telephone calls | x | |
| Intimate relations | x | x |
| Having and taking care of a pet | x | |
| Meeting new people | ||
| Going to school or participating in other educational activities, like taking computer classes or adult education | x | |
| Talk either on the phone or in person |
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Idler EL, Angel RJ. Self-rated health and mortality in the NHANES-I Epidemiologic Follow-up Study. Am J Public Health. 1990;80:446–452. doi: 10.2105/ajph.80.4.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Idler EL, Kasl SV. Self-ratings of health: do they also predict change in functional ability? J Gerontol: Soc Sci. 1995;50:S344–S353. doi: 10.1093/geronb/50b.6.s344. [DOI] [PubMed] [Google Scholar]
- 3.Bosworth H, Siegler I, Brummett B, Barefoot J, Williams R, Clapp-Channing N, et al. The association between self-rated health and mortality in a well-characterized sample of coronary artery disease patients. Med Care. 1999;37:1226–1236. doi: 10.1097/00005650-199912000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Schoenfeld D, Malmrose L, Blazer D, Gold D, Seeman T. Self-rated health and mortality in the high-functioning elderly--a closer look at healthy individuals: MacArthur field study of successful aging. J Gerontol: Med Sci. 1994;49:M109–M115. doi: 10.1093/geronj/49.3.m109. [DOI] [PubMed] [Google Scholar]
- 5.Lee Y. The predictive value of self assessed general, physical, and mental health on functional decline and mortality in older adults. J Epidemiol Community Health. 2000;54:123–129. doi: 10.1136/jech.54.2.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bernard S, Kincade J, Konrad T, Arcury T, Rabiner D, Woomert A, et al. Predicting mortality from community surveys of older adults: the importance of self-rated functional ability. J Gerontol: Soc Sci. 1997;52:S155–S163. doi: 10.1093/geronb/52b.3.s155. [DOI] [PubMed] [Google Scholar]
- 7.Greiner PA, Snowdon DA, Greiner LH. The relationship of self-rated function and self-rated health to concurrent functional ability, functional decline, and mortality: findings from the Nun Study. J Gerontol: Soc Sci. 1996;51:S234–41. doi: 10.1093/geronb/51b.5.s234. [DOI] [PubMed] [Google Scholar]
- 8.Wolinsky F, Tierney W. Self-rated health and adverse health outcomes: an exploration and refinement of the trajectory hypothesis. J Gerontol: Soc Sci. 1998;53B:S336–S340. doi: 10.1093/geronb/53b.6.s336. [DOI] [PubMed] [Google Scholar]
- 9.Heistaro S, Jousilahti P, Lahelma E, Vartiainen E, Puska P. Self rated health and mortality: a long term prospective study in eastern Finland. J Epidemiol Community Health. 2001;55:227–232. doi: 10.1136/jech.55.4.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pijls LT, Feskens EJ, Kromhout D. Self-rated health, mortality, and chronic diseases in elderly men. The Zutphen Study, 1985-1990. Am J Epidemiol. 1993;138:840–848. doi: 10.1093/oxfordjournals.aje.a116787. [DOI] [PubMed] [Google Scholar]
- 11.Wilcox VL, Kasl SV, Idler EL. Self-rated health and physical disability in elderly survivors of a major medical event. J Gerontol: Soc Sci. 1996;51:S96–S104. doi: 10.1093/geronb/51b.2.s96. [DOI] [PubMed] [Google Scholar]
- 12.Idler E, Benyamini Y. Self-rated health and mortality: A review of twenty-seven community studies. J Health Soc Behav. 1997;38:21–37. [PubMed] [Google Scholar]
- 13.Lee S, Moody-Ayers S, Landefeld C, Walter L, Lindquist K, Segal M, et al. The relationship between self-rated health and mortality in older black and white Americans. J Am Geriatr Soc. 2007;55:1624–1629. doi: 10.1111/j.1532-5415.2007.01360.x. [DOI] [PubMed] [Google Scholar]
- 14.Guccione A, Anderson J, Anthony J, Meenan R. The correlates of health perceptions in rheumatoid arthritis. J Rheumatol. 1995;22:432–439. [PubMed] [Google Scholar]
- 15.Idler E, Hudson S, Leventhal H. The meanings of self-ratings of health: a qualitative and quantitative approach. Research on Aging. 1999;21:458–476. [Google Scholar]
- 16.Benyamini Y, Leventhal E, Leventhal H. Self-assessments of health: What do people know that predicts their mortality? Research on Aging. 1999;21:477–500. [Google Scholar]
- 17.Mantyselka P, Turunen J, Ahonen R, Kumpusalo E. Chronic pain and poor self-rated health. JAMA. 2003;290:2435–2442. doi: 10.1001/jama.290.18.2435. [DOI] [PubMed] [Google Scholar]
- 18.Smedstad L, Kvien T, Moum T, Vaglum P. Correlates of patients’ global assessment of arthritis impact. A 2-year study of 216 patients with RA. Scand J Rheumatol. 1997;26:259–265. doi: 10.3109/03009749709105313. [DOI] [PubMed] [Google Scholar]
- 19.Perruccio A, Power J, Badley E. Arthritis onset and worsening self-rated health: a longitudinal evaluation of the role of pain and activity limitations. Arthritis Rheum (Arthritis Care Res) 2005;53:571–577. doi: 10.1002/art.21317. [DOI] [PubMed] [Google Scholar]
- 20.Okano G, Miyake H, Mori M. Leisure time physical activity as a determinant of self-perceived health and fitness in middle-aged male employees. J Occup Health. 2003;45:286–292. doi: 10.1539/joh.45.286. [DOI] [PubMed] [Google Scholar]
- 21.Ditto P, Druley J, Moore K, Danks J, Smucker W. Fates worse than death: The role of valued life activities in health-state evaluations. Health Psychol. 1996;15(5):332–343. doi: 10.1037/0278-6133.15.5.332. [DOI] [PubMed] [Google Scholar]
- 22.Katz P, Morris A, Yelin E. Prevalence and predictors of disability in valued life activities among individuals with rheumatoid arthritis. Ann Rheum Dis. 2006;65:763–769. doi: 10.1136/ard.2005.044677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Katz P, Yelin E, Lubeck D, Buatti M, Wanke L. Satisfaction with function: What type of function do rheumatoid arthritis patients value most? Arthritis Rheum. 2001;44:S185. [Google Scholar]
- 24.Neugebauer A, Katz PP, Pasch LA. Effect of valued activity disability, social comparisons, and satisfaction with ability on depressive symptoms in rheumatoid arthritis. Health Psychol. 2003;22:253–262. doi: 10.1037/0278-6133.22.3.253. [DOI] [PubMed] [Google Scholar]
- 25.Katz P, Yelin E. The development of depressive symptoms among women with rheumatoid arthritis. Arthritis Rheum. 1995;38:49–56. doi: 10.1002/art.1780380108. [DOI] [PubMed] [Google Scholar]
- 26.Katz P, Yelin E. Activity loss and the onset of depressive symptoms: Do some activities matter more than others? Arthritis Rheum. 2001;44:1194–1202. doi: 10.1002/1529-0131(200105)44:5<1194::AID-ANR203>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 27.Katz P. Function, disability, and psychological well-being. Adv Psychosom Med. 2004;25:41–62. doi: 10.1159/000079057. [DOI] [PubMed] [Google Scholar]
- 28.Katz P, Yelin E, Eisner M, Chen H, Blanc P. Prevalence and psychological impact of disability in valued life activities in COPD and related airways conditions. Proc Am Thorac Soc. 2005;2:A519. [Google Scholar]
- 29.Schumaker H, editor. Primer on the rheumatic diseases. 10. Atlanta, Georgia: Arthritis Foundation; 1993. [Google Scholar]
- 30.GOLD (Global Initiative for Chronic Obstructive Lung Disease) Global strategies for the diagnosis, management, and prevention of chronic obstructive pulmonary disease 2003 update. 2003 doi: 10.1164/rccm.200703-456SO. [DOI] [PubMed] [Google Scholar]
- 31.Yelin E, Lubeck D, Holman H, Epstein W. The impact of rheumatoid arthritis and osteoarthritis: The activities of patients with rheumatoid arthritis and osteoarthritis compared to controls. J Rheumatol. 1987;14:710–717. [PubMed] [Google Scholar]
- 32.Katz P, Yelin E. Life activities of persons with rheumatoid arthritis with and without depressive symptoms. Arthritis Care Res. 1994;7:69–77. doi: 10.1002/art.1790070205. [DOI] [PubMed] [Google Scholar]
- 33.Baron-Epel O, Kaplan G. General subjective health status or age-related subjective health status: does it make a difference? Soc Sci Med. 2001;53:1373–1381. doi: 10.1016/s0277-9536(00)00426-3. [DOI] [PubMed] [Google Scholar]
- 34.Benyamini Y, Idler E. Community studies reporting association between self-rated health and mortality. Research on Aging. 1999;21:392–401. [Google Scholar]
- 35.Hays J, Schoenfeld D, Blazer D, Gold D. Global self-ratings of health and mortality: hazard in the North Carolina Piedmont. J Clin Epidemiol. 1996;49:969–979. doi: 10.1016/0895-4356(96)00138-2. [DOI] [PubMed] [Google Scholar]
- 36.Idler EL, Russell LB, Davis D. Survival, functional limitations, and self-rated health in the NHANES I Epidemiologic Follow-up Study, 1992. First National Health and Nutrition Examination Survey. Am J Epidemiol. 2000;152:874–883. doi: 10.1093/aje/152.9.874. [DOI] [PubMed] [Google Scholar]
- 37.Ware J, Kosinski M, Keller S. SF-36 Physical and Mental Health Summary Scales: A user’s manual. Boston, MA: The Health Institute, New England Medical Center; 1994. [Google Scholar]
- 38.Fries J, Spitz P, Kraines R, Holman H. Measurement of patient outcome in arthritis. Arthritis Rheum. 1980;23:137–45. doi: 10.1002/art.1780230202. [DOI] [PubMed] [Google Scholar]
- 39.Eisner M, Trupin L, Katz P, Yelin E, Earnest G, Balmes J, et al. Development and validation of a survey-based COPD severity score. Chest. 2005;127:1890–1897. doi: 10.1378/chest.127.6.1890. [DOI] [PubMed] [Google Scholar]
- 40.Health and Retirement Survey website:. http://www.umich.edu/~hrswww/docs/qnaires/online.html
- 41.Duke J, Leventhal H, Brownlee S, Leventhal E. Giving up and replacing activities in response to illness. J Gerontol: Psychol Sci. 2002;57B:P367–P376. doi: 10.1093/geronb/57.4.p367. [DOI] [PubMed] [Google Scholar]
- 42.Benyamini Y, Leventhal E, Leventhal H. Gender differences in processing information for making self-assessments of health. Psychosom Med. 2000;62:354–364. doi: 10.1097/00006842-200005000-00009. [DOI] [PubMed] [Google Scholar]
- 43.Williamson G. Extending the activity restriction model of depressed affect: evidence from a sample of breast cancer patients. Health Psychol. 2000;19:339–347. [PubMed] [Google Scholar]
- 44.Williamson G. Pain, functional disability, and depressed affect. In: Williamson G, Shaffer D, Parmelee P, editors. Physical illness and depression in older adults. New York: Kluwer Academic/Plenum Publishers; 2000. pp. 51–64. [Google Scholar]
- 45.Williamson G, Schulz R. Pain, activity restriction, and symptoms of depression among community-residing elderly adults. J Gerontol: Psychol Sci. 1992;47:P367–P372. doi: 10.1093/geronj/47.6.p367. [DOI] [PubMed] [Google Scholar]
- 46.Williamson G, Schulz R. Activity restriction mediates the association between pain and depressed affect: a study of younger and older adult cancer patients. Psychol Aging. 1995;10:369–378. doi: 10.1037//0882-7974.10.3.369. [DOI] [PubMed] [Google Scholar]
- 47.Benyamini Y, Lomranz J. The relationship of acivity restriction and replacement with depressive symptoms among older adults. Psychology Aging. 2004;19:362–366. doi: 10.1037/0882-7974.19.2.362. [DOI] [PubMed] [Google Scholar]


