Abstract
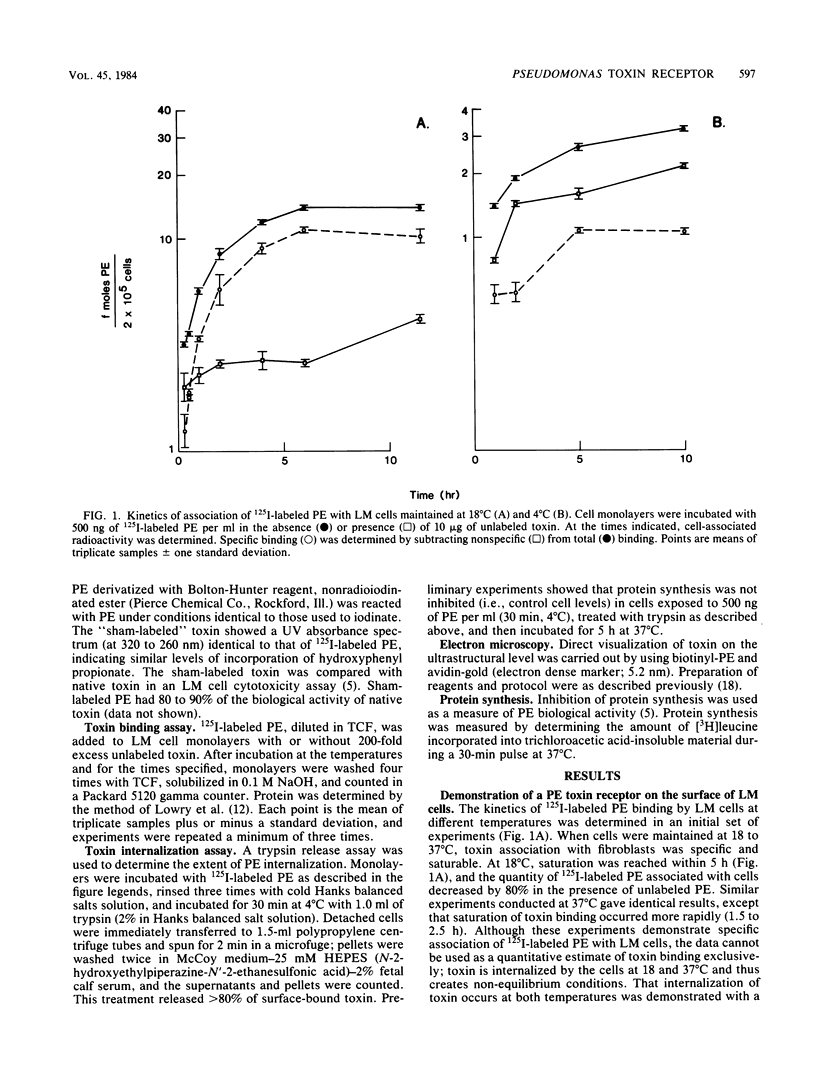
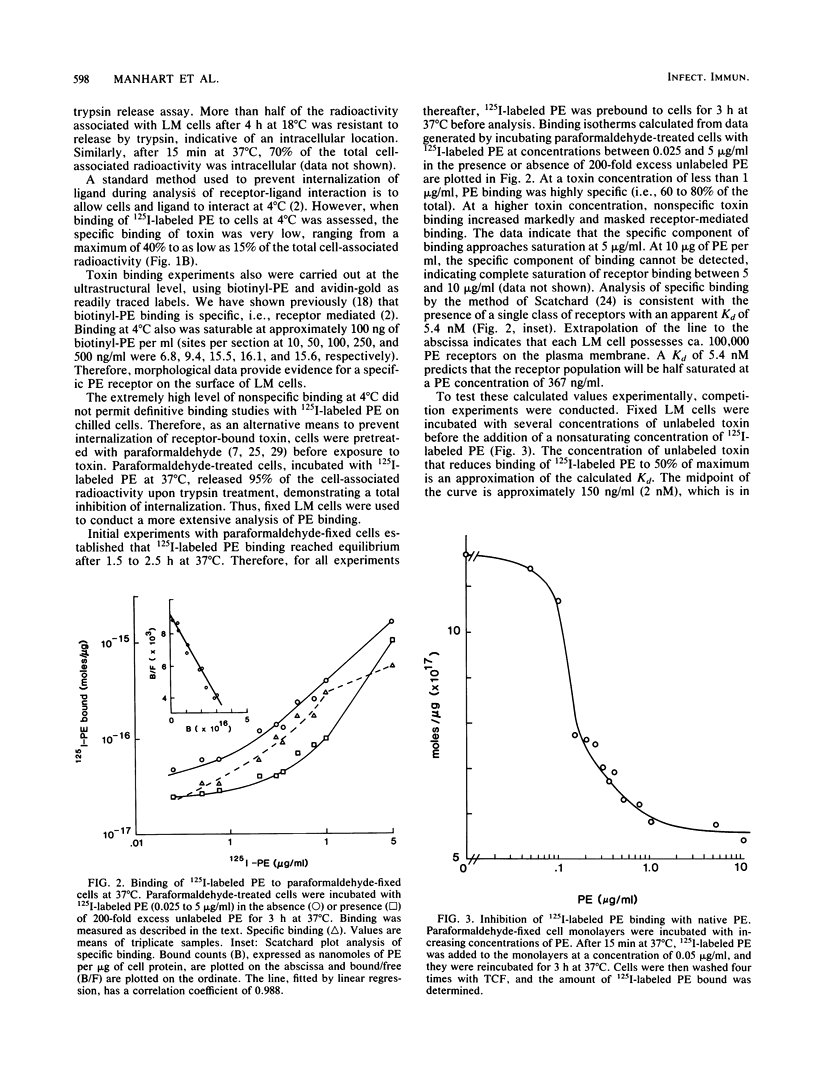
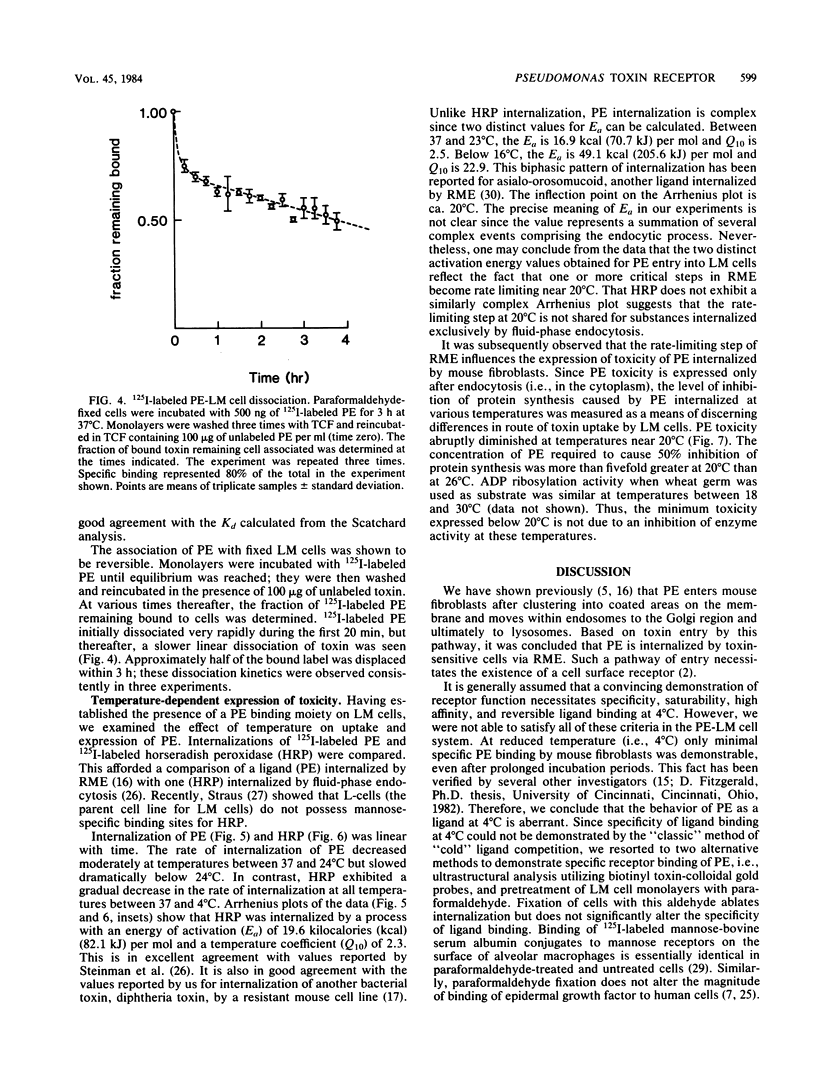
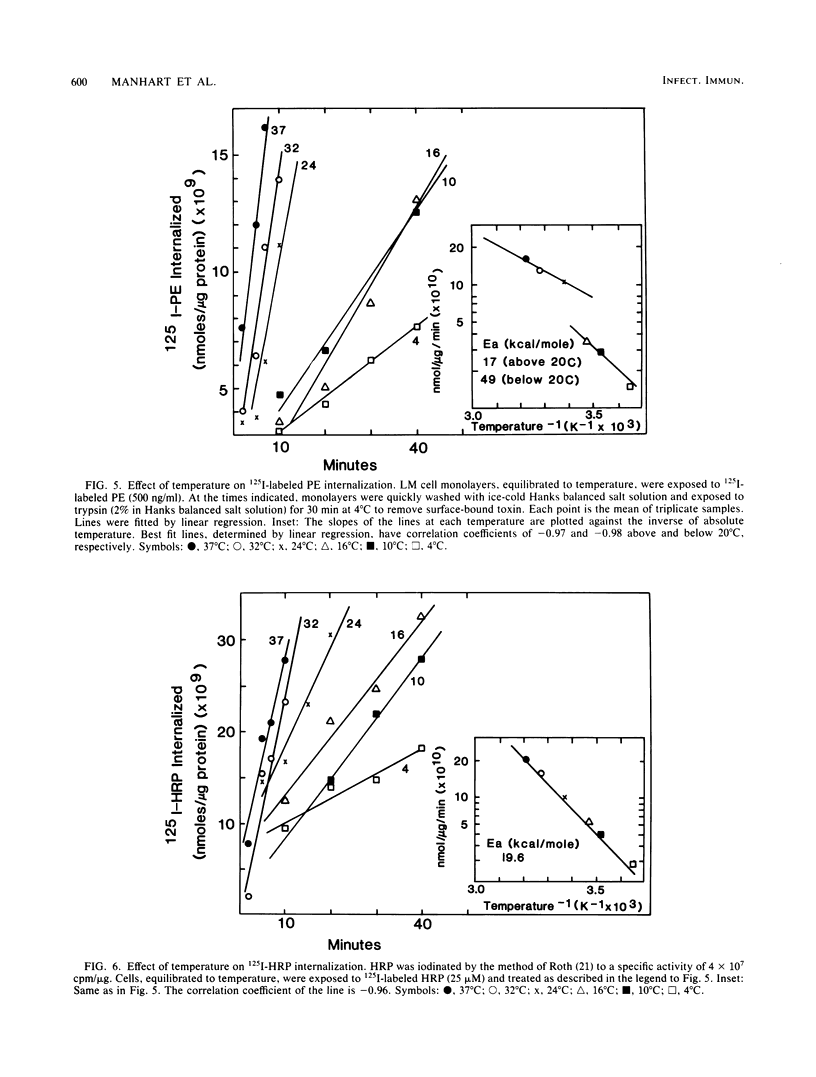
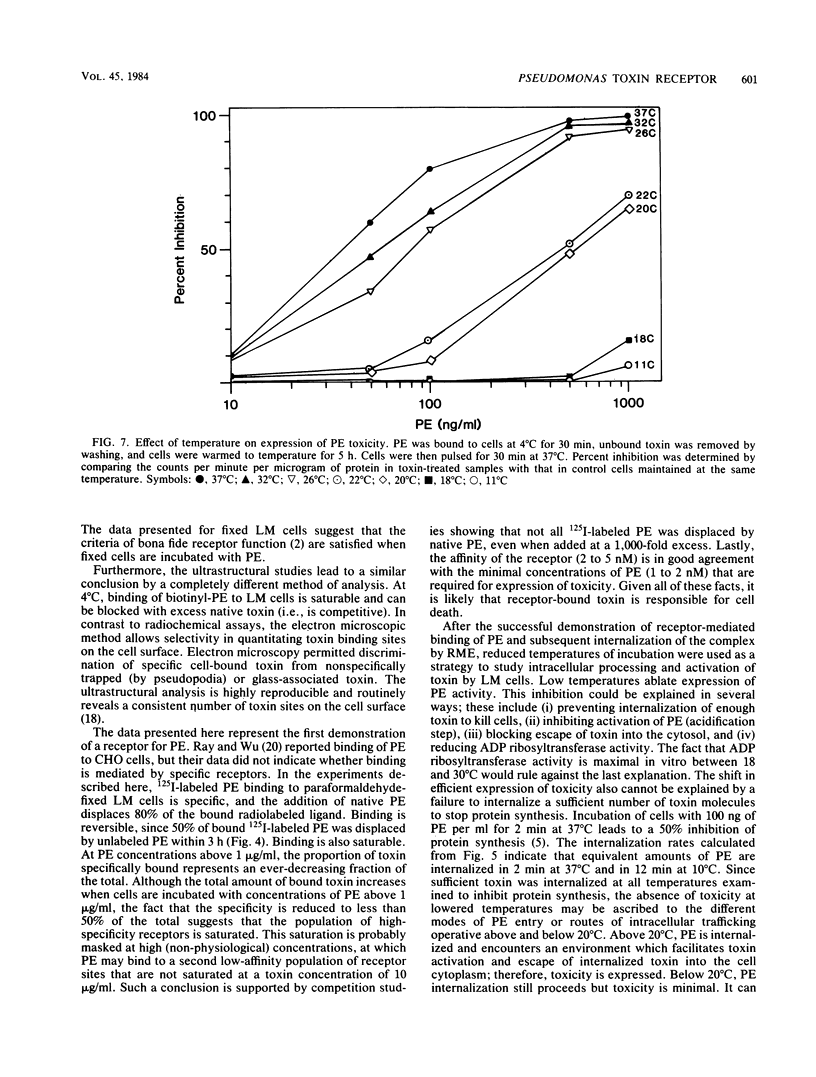
Pseudomonas exotoxin A enters mouse LM fibroblasts by receptor-mediated endocytosis and ultimately causes cell death. Here we present evidence for the existence of a specific receptor for the toxin. Toxin association with LM cells at 18 and 37 degrees C, but not at 4 degrees C, was highly specific. At 37 degrees C, the association increased with time, reaching a steady state by 5 h. Binding to paraformaldehyde-fixed cells at 37 degrees C was saturable (Kd = 5.4 nM), was reversible, and indicated ca. 100,000 binding sites per cell. It is believed that receptor-bound toxin is responsible for cell death. Once the kinetics of toxin entry were described, we examined the effect of reduced temperatures on the intracellular processing of toxin and thus its expression. Toxin-induced inhibition of protein synthesis was minimal at temperatures below 20 degrees C. This was seen even though at 20 degrees C sufficient toxin was internalized to kill cells, and toxin enzyme activity was maximal. Internalization of 125I-labeled toxin, but not of 125I-labeled horseradish peroxidase (marker of fluid-phase endocytosis), became rate limiting at 20 degrees C or below. These data suggest that reduced temperatures block a step in the receptor-mediated endocytic pathway essential for the expression of Pseudomonas toxin activity.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bolton A. E., Hunter W. M. The labelling of proteins to high specific radioactivities by conjugation to a 125I-containing acylating agent. Biochem J. 1973 Jul;133(3):529–539. doi: 10.1042/bj1330529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuatrecasas P. Commentary. Insulin receptors, cell membranes and hormone action. Biochem Pharmacol. 1974 Sep 1;23(17):2353–2361. doi: 10.1016/0006-2952(74)90224-x. [DOI] [PubMed] [Google Scholar]
- Dorland R. B., Middlebrook J. L., Leppla S. H. Receptor-mediated internalization and degradation of diphtheria toxin by monkey kidney cells. J Biol Chem. 1979 Nov 25;254(22):11337–11342. [PubMed] [Google Scholar]
- Dunn W. A., Hubbard A. L., Aronson N. N., Jr Low temperature selectively inhibits fusion between pinocytic vesicles and lysosomes during heterophagy of 125I-asialofetuin by the perfused rat liver. J Biol Chem. 1980 Jun 25;255(12):5971–5978. [PubMed] [Google Scholar]
- FitzGerald D., Morris R. E., Saelinger C. B. Receptor-mediated internalization of Pseudomonas toxin by mouse fibroblasts. Cell. 1980 Oct;21(3):867–873. doi: 10.1016/0092-8674(80)90450-x. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Anderson R. G., Brown M. S. Coated pits, coated vesicles, and receptor-mediated endocytosis. Nature. 1979 Jun 21;279(5715):679–685. doi: 10.1038/279679a0. [DOI] [PubMed] [Google Scholar]
- Haigler H. T., McKanna J. A., Cohen S. Direct visualization of the binding and internalization of a ferritin conjugate of epidermal growth factor in human carcinoma cells A-431. J Cell Biol. 1979 May;81(2):382–395. doi: 10.1083/jcb.81.2.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helenius A., Kartenbeck J., Simons K., Fries E. On the entry of Semliki forest virus into BHK-21 cells. J Cell Biol. 1980 Feb;84(2):404–420. doi: 10.1083/jcb.84.2.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglewski B. H., Kabat D. NAD-dependent inhibition of protein synthesis by Pseudomonas aeruginosa toxin,. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2284–2288. doi: 10.1073/pnas.72.6.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kielian M. C., Keränen S., Käriäinen L., Helenius A. Membrane fusion mutants of Semliki Forest virus. J Cell Biol. 1984 Jan;98(1):139–145. doi: 10.1083/jcb.98.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leppla S. H. Large-scale purification and characterization of the exotoxin of Pseudomonas aeruginosa. Infect Immun. 1976 Oct;14(4):1077–1086. doi: 10.1128/iai.14.4.1077-1086.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh M., Bolzau E., Helenius A. Penetration of Semliki Forest virus from acidic prelysosomal vacuoles. Cell. 1983 Mar;32(3):931–940. doi: 10.1016/0092-8674(83)90078-8. [DOI] [PubMed] [Google Scholar]
- Middlebrook J. L., Dorland R. B., Leppla S. H. Association of diphtheria toxin with Vero cells. Demonstration of a receptor. J Biol Chem. 1978 Oct 25;253(20):7325–7330. [PubMed] [Google Scholar]
- Moehring J. M., Moehring T. J. Strains of CHO-K1 cells resistant to Pseudomonas exotoxin A and cross-resistant to diphtheria toxin and viruses. Infect Immun. 1983 Sep;41(3):998–1009. doi: 10.1128/iai.41.3.998-1009.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris R. E., Manhart M. D., Saelinger C. B. Receptor-mediated entry of Pseudomonas toxin: methylamine blocks clustering step. Infect Immun. 1983 May;40(2):806–811. doi: 10.1128/iai.40.2.806-811.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris R. E., Saelinger C. B. Diphtheria toxin does not enter resistant cells by receptor-mediated endocytosis. Infect Immun. 1983 Nov;42(2):812–817. doi: 10.1128/iai.42.2.812-817.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris R. E., Saelinger C. B. Visualization of intracellular trafficking: use of biotinylated ligands in conjunction with avidin-gold colloids. J Histochem Cytochem. 1984 Jan;32(1):124–128. doi: 10.1177/32.1.6690597. [DOI] [PubMed] [Google Scholar]
- Oka J. A., Weigel P. H. Recycling of the asialoglycoprotein receptor in isolated rat hepatocytes. Dissociation of internalized ligand from receptor occurs in two kinetically and thermally distinguishable compartments. J Biol Chem. 1983 Sep 10;258(17):10253–10262. [PubMed] [Google Scholar]
- Ray B., Wu H. C. Chinese hamster ovary cell mutants defective in the internalization of ricin. Mol Cell Biol. 1982 May;2(5):535–544. doi: 10.1128/mcb.2.5.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth J. Methods for assessing immunologic and biologic properties of iodinated peptide hormones. Methods Enzymol. 1975;37:223–233. doi: 10.1016/s0076-6879(75)37018-3. [DOI] [PubMed] [Google Scholar]
- Salisbury J. L., Condeelis J. S., Satir P. Receptor-mediated endocytosis: machinery and regulation of the clathrin-coated vesicle pathway. Int Rev Exp Pathol. 1983;24:1–62. [PubMed] [Google Scholar]
- Sandvig K., Olsnes S. Entry of the toxic proteins abrin, modeccin, ricin, and diphtheria toxin into cells. I. Requirement for calcium. J Biol Chem. 1982 Jul 10;257(13):7495–7503. [PubMed] [Google Scholar]
- Schreiber A. B., Schlessinger J., Edidin M. Interaction between major histocompatibility complex antigens and epidermal growth factor receptors on human cells. J Cell Biol. 1984 Feb;98(2):725–731. doi: 10.1083/jcb.98.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman R. M., Silver J. M., Cohn Z. A. Pinocytosis in fibroblasts. Quantitative studies in vitro. J Cell Biol. 1974 Dec;63(3):949–969. doi: 10.1083/jcb.63.3.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbot P. J., Vance D. E. Biochemical studies on the entry of sindbis virus into BHK-21 cells and the effect of NH4Cl. Virology. 1982 Apr 30;118(2):451–455. doi: 10.1016/0042-6822(82)90365-8. [DOI] [PubMed] [Google Scholar]
- Tietze C., Schlesinger P., Stahl P. Mannose-specific endocytosis receptor of alveolar macrophages: demonstration of two functionally distinct intracellular pools of receptor and their roles in receptor recycling. J Cell Biol. 1982 Feb;92(2):417–424. doi: 10.1083/jcb.92.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigel P. H., Oka J. A. Temperature dependence of endocytosis mediated by the asialoglycoprotein receptor in isolated rat hepatocytes. Evidence for two potentially rate-limiting steps. J Biol Chem. 1981 Mar 25;256(6):2615–2617. [PubMed] [Google Scholar]
- Wolkoff A. W., Klausner R. D., Ashwell G., Harford J. Intracellular segregation of asialoglycoproteins and their receptor: a prelysosomal event subsequent to dissociation of the ligand-receptor complex. J Cell Biol. 1984 Feb;98(2):375–381. doi: 10.1083/jcb.98.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]


