Abstract
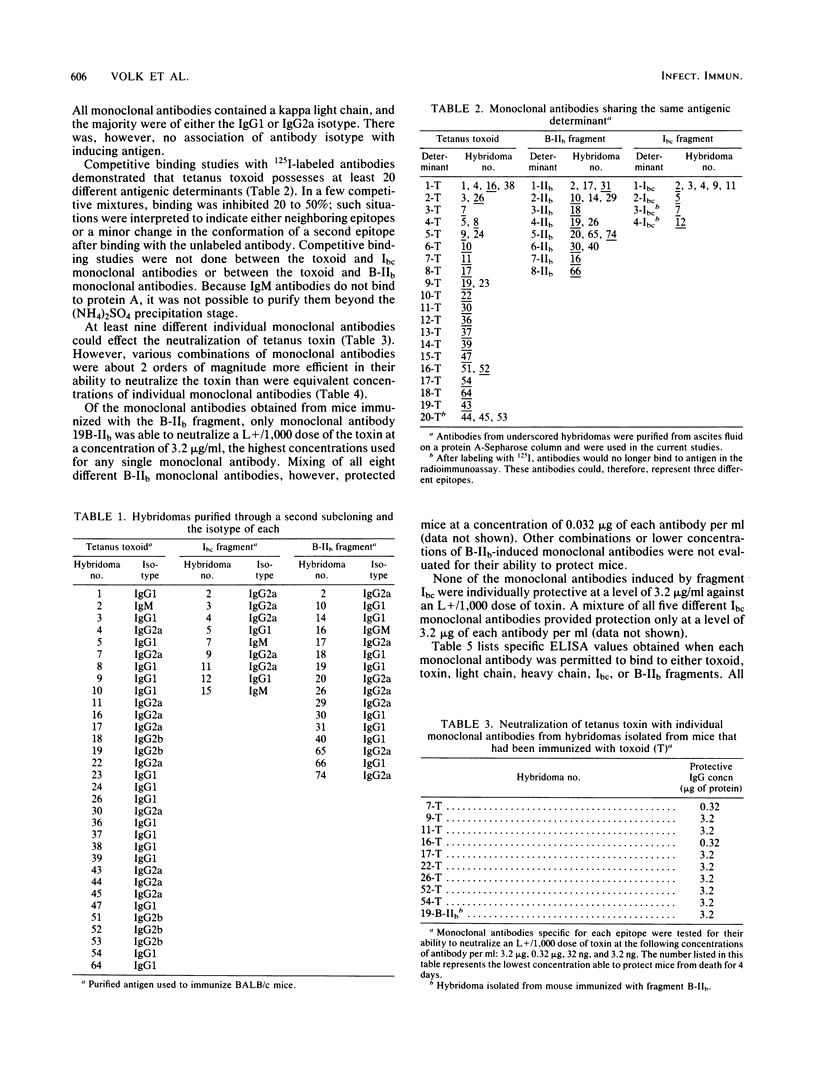
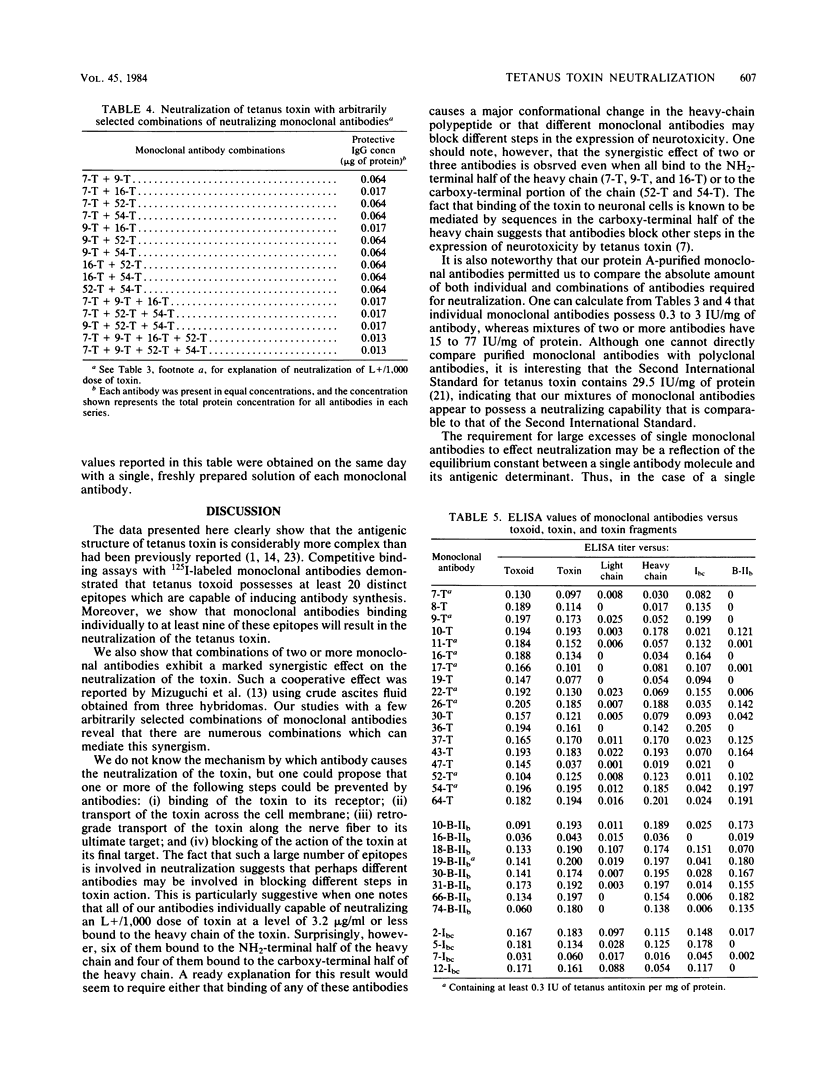
Fifty-seven hybridomas producing antibodies to tetanus toxoid or to the Ibc or B-IIb fragment of the toxin were isolated independently. Competitive inhibition studies demonstrated that monoclonal antibodies from mice immunized with the toxoid bound to at least 20 different epitopes on the toxoid molecule. Similar competitive binding studies revealed eight distinct epitopes on the B-IIb fragment and three to five epitopes on the Ibc fragment of the toxin. Neutralization of toxicity was effected by nine distinct monoclonal antibodies from hybridomas of toxoid-immunized mice and by one monoclonal antibody from B-IIb-immunized mice. Mixtures of two, three, and four different monoclonal antibodies in a variety of combinations exerted a synergistic effect of ca. 200-fold over that observed with individual monoclonal antibodies, indicating that efficient neutralization may involve the simultaneous binding of at least two antibody molecules to different specific regions of the toxin molecule. Only one toxoid-induced monoclonal antibody failed to bind to tetanus toxin. All neutralizing antibodies bound to epitopes on the heavy chain of tetanus toxin. Six of these were directed toward epitopes on the NH2-terminal half, whereas four bound to epitopes on the carboxy-terminal half of the heavy chain. Only one monoclonal antibody bound preferentially to the light chain, but two other monoclonal antibodies appeared to bind to both chains, indicating some homology between these two chains.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahnert-Hilger G., Bizzini B., Goretzki K., Müller H., Völckers C., Habermann E. Monoclonal antibodies against tetanus toxin and toxoid. Med Microbiol Immunol. 1983;172(2):123–135. doi: 10.1007/BF02124513. [DOI] [PubMed] [Google Scholar]
- Bizzini B., Akert K., Glicksman M., Grob P. Preparation of conjugates using two tetanus toxin derived fragments: their binding to gangliosides and isolated synaptic membranes and their immunological properties. Toxicon. 1980;18(5-6):561–572. doi: 10.1016/0041-0101(80)90083-5. [DOI] [PubMed] [Google Scholar]
- Bizzini B., Grob P., Akert K. Papain-derived fragment IIc of tetanus toxin: its binding to isolated synaptic membranes and retrograde axonal transport. Brain Res. 1981 Apr 6;210(1-2):291–299. doi: 10.1016/0006-8993(81)90902-1. [DOI] [PubMed] [Google Scholar]
- Bizzini B., Grob P., Glicksman M. A., Akert K. Use of the B-IIb tetanus toxin derived fragment as a specific neuropharmacological transport agent. Brain Res. 1980 Jul 7;193(1):221–227. doi: 10.1016/0006-8993(80)90959-2. [DOI] [PubMed] [Google Scholar]
- Bizzini B., Stoeckel K., Schwab M. An antigenic polypeptide fragment isolated from tetanus toxin: chemical characterization, binding to gangliosides and retrograde axonal transport in various neuron systems. J Neurochem. 1977 Mar;28(3):529–542. doi: 10.1111/j.1471-4159.1977.tb10423.x. [DOI] [PubMed] [Google Scholar]
- Bizzini B. Tetanus toxin. Microbiol Rev. 1979 Jun;43(2):224–240. doi: 10.1128/mr.43.2.224-240.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ey P. L., Prowse S. J., Jenkin C. R. Isolation of pure IgG1, IgG2a and IgG2b immunoglobulins from mouse serum using protein A-sepharose. Immunochemistry. 1978 Jul;15(7):429–436. doi: 10.1016/0161-5890(78)90070-6. [DOI] [PubMed] [Google Scholar]
- Kenimer J. G., Habig W. H., Hardegree M. C. Monoclonal antibodies as probes of tetanus toxin structure and function. Infect Immun. 1983 Dec;42(3):942–948. doi: 10.1128/iai.42.3.942-948.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda M., Yoneda M. Antigenic substructure of tetanus neurotoxin. Biochem Biophys Res Commun. 1977 Jul 11;77(1):268–274. doi: 10.1016/s0006-291x(77)80192-7. [DOI] [PubMed] [Google Scholar]
- Matsuda M., Yoneda M. Isolation and purification of two antigenically active, "complimentary" polypeptide fragments of tetanus neurotoxin. Infect Immun. 1975 Nov;12(5):1147–1153. doi: 10.1128/iai.12.5.1147-1153.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuguchi J., Yoshida T., Sato Y., Nagaoka F., Kondo S., Matuhasi T. Requirement of at least two distinct monoclonal antibodies for efficient neutralization of tetanus toxin in vivo. Naturwissenschaften. 1982 Dec;69(12):597–598. doi: 10.1007/BF00396359. [DOI] [PubMed] [Google Scholar]
- Nagel J., Cohen H. Studies on tetanus antitoxins. II. Demonstration of at least four antitoxins of different specificity in antitoxic sera. J Immunol. 1973 May;110(5):1388–1395. [PubMed] [Google Scholar]
- Neubauer V., Helting T. B. Structure of tetanus toxin: the arrangement of papain digestion products within the heavy chain-light chain framework of extracellular toxin. Biochim Biophys Acta. 1981 Mar 27;668(1):141–148. doi: 10.1016/0005-2795(81)90157-4. [DOI] [PubMed] [Google Scholar]
- Parham P. Antigenic determinants of the HLA-B7 molecule; Bw6- and B7-specific determinants are spatially separate. Immunogenetics. 1983;18(1):1–16. doi: 10.1007/BF00401351. [DOI] [PubMed] [Google Scholar]
- Raynaud M., Bizzini B., Turpin A. Hétérogénéité de l'anatoxine tétanique. Ann Inst Pasteur (Paris) 1971 Jun;120(6):801–815. [PubMed] [Google Scholar]
- Robinson J. P., Hash J. H. A review of the molecular structure of tetanus toxin. Mol Cell Biochem. 1982 Oct 1;48(1):33–44. doi: 10.1007/BF00214820. [DOI] [PubMed] [Google Scholar]
- Sheppard A. J., Cussell D., Hughes M. Production and characterization of monoclonal antibodies to tetanus toxin. Infect Immun. 1984 Feb;43(2):710–714. doi: 10.1128/iai.43.2.710-714.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaun J., Lyng J. Replacement of the international standard for tetanus antitoxin and the use of the standard in the flocculation test. Bull World Health Organ. 1970;42(4):523–534. [PMC free article] [PubMed] [Google Scholar]
- Wellhöner N. H. Tetanus neurotoxin. Rev Physiol Biochem Pharmacol. 1982;93:1–68. doi: 10.1007/BFb0032668. [DOI] [PubMed] [Google Scholar]



