Abstract
The mechanical properties of triton-permeabilized ventricular preparations isolated from 4, 18 and 24-month-old F344 rats were analyzed to provide information about the molecular mechanisms that lead to age-related increases in diastolic myocardial stiffness in these animals. Passive stiffness (measured in solutions with minimal free Ca2+) did not change with age. This implies that the aging-associated dysfunction is not due to changes in titin or collagen molecules. Ca2+-activated preparations exhibited a characteristic short-range force response: force rose rapidly until the muscle reached its elastic limit and less rapidly thereafter. The elastic limit increased from 0.43 ± 0.01 % l0 (where l0 is the initial muscle length) in preparations from 4-month-old animals to 0.49 ± 0.01 % l0 in preparations from 24-month-old rats (p<0.001, ANOVA). Relative short-range force was defined as the maximum force produced during the short-range response normalized to the prevailing tension. This parameter increased from 0.110 ± 0.002 to 0.142 ± 0.002 over the same age-span (p<0.001, ANOVA). Analytical gel electrophoresis showed that the maximum stiffness of the preparations during the short-range response and the relative short-range force increased (p=0.031 and p=0.005 respectively) with the relative content of slow β myosin heavy chain molecules. Elastic limit values did not correlate with myosin isoform content. Simulations based on these results suggest that attached β myosin heavy chain cross-bridges are stiffer than links formed by α myosin heads. In conclusion, elevated content of stiffer β myosin heavy chain molecules may contribute to aging-associated increases in myocardial stiffness.
Introduction
During diastole, blood flows into the ventricular chambers and the tissue in the ventricular walls is stretched. If the ventricular tissue becomes too stiff, the walls present an excessive resistance to inflowing blood and ventricular filling is impaired. This is termed diastolic dysfunction [1]. Epidemiological studies show that the incidence of the condition in human populations increases markedly with age [2, 3].
Experiments performed with animal models of aging may provide new information about the molecular mechanisms that contribute to aging-associated diastolic dysfunction. Fischer 344 rats (F344 rats) are an interesting model to use for this type of work because Pacher et al. [4] showed (using in vivo pressure-volume measurements) that diastolic myocardial stiffness is elevated in these animals at 24–26 months of age. Diastolic dysfunction has also been confirmed in old F344 rats using echocardiography [5]. F344 rats therefore exhibit aging-associated diastolic dysfunction that may mimic the human condition.
Previous measurements [4] have shown that collagen content and collagen cross-linking are elevated in F344 rats at 23 months of age. This suggests that the increased myocardial stiffness observed in the old animals by Pacher et al. could reflect an increase in the stiffness of extracellular matrix structures [6]. Age-related changes in titin-based stiffness are probably unlikely in this animal model because rats already express the vast majority (>95%) of their titin as the shorter, stiffer N2B isoform [7]. However it is possible that aging-associated changes in other sarcomeric proteins could contribute to myocardial stiffening.
Previous studies performed using canine myocardial preparations [8] showed that the stiffness due to passive structures (primarily titin and collagen filaments) was only 2% of the stiffness measured when the preparations were maximally activated with calcium. The implication of this result is that cross-bridges that remain attached between the myofilaments during filling [9, 10] can make a significant contribution to diastolic myocardial stiffness. This observation is particularly relevant to aging-associated myocardial dysfunction in F344 rats because the relative content of the slowly cycling β myosin heavy chain is greater in myocardium from old animals than it is in the hearts of young adults [11, 12]. If the β myosin heads make a greater contribution to myocardial stiffness than fast-cycling α heads, aging-associated myocardial stiffness may be linked to changes in myosin gene expression.
The present study was designed to test whether the increased ventricular stiffness observed in old F344 rats reflects elevated ‘passive’ stiffness (due to titin, collagen, elastin and intermediate filaments) or elevated ‘active’ stiffness (due to myosin heads bound to actin filaments in relaxing myocardium) [1, 6, 13]. The working hypothesis was that the aging-associated diastolic dysfunction reflects elevated β myosin heavy chain content.
Methods
Preparations
Multicellular cardiac preparations were obtained by mechanically disrupting samples of left ventricular tissue (Polytron Homogenizer, Brinkman Instruments, Westbury, NY) isolated from female 4, 18 and 24-month-old Fischer 344 rats after they had been anesthetized by intraperitoneal injection (50 mg kg −1) of pentobarbital. The preparations were chemically permeabilized using Triton X-100 (30 minutes, 1% v/v) and stored on ice for up to 12 hours before use. All of the rats were purchased from a National Institute on Aging colony maintained by Harlan (Indianapolis, IN). Animal use was approved by the Institutional Animal Care and Use Committee at the University of Kentucky.
Solutions
The relaxing solution used for isolating the myocardial preparations contained (in mmol L−1): 100 KCl, 10 imidazole, 4 ATP, 2 EGTA and 5 MgCl2 and two protease inhibitors (phenylmethylsulfonide 500 μmol L−1 and leupeption 40 μmol L−1). All pCa (= −log10[Ca2+]) solutions contained (in mmol L−1): 20 imidazole, 14.5 creatine phosphate, 7 EGTA, 4 MgATP, 1 free Mg2+, free Ca2+ ranging from 1 nmol L−1 (pCa 9.0) to 32 μmol L−1 (pCa 4.5) and sufficient KCl to adjust the ionic strength to 180 mmol L−1. The precise composition of each pCa solution was determined using Maxchelator software (version 2.50) and NIST stability constants [14].
Mechanical Measurements
Preparations were attached between a force transducer (resonant frequency 600 Hz, model 403, Aurora Scientific, Aurora, Ontario, Canada) and a motor (step time 0.6 ms, model 312B, Aurora) by crimping their ends into metal troughs (shaped from 27 gauge tubing) with overlays of 4-0 nylon monofilament. The technique is illustrated in Fig 1B of Campbell & Moss, 2002[15]. The samples were stretched in pCa 9.0 solution (by manually adjusting the manipulator holding the motor) until sarcomere length (measured by video microscopy [8]) was ~2.26 μm. The mean sarcomere lengths of the preparations from the different age-groups were not statistically different (4-month-old: 2.248 ± 0.009 μm, 18-month-old: 2.257 ± 0.007 μm, 24-month-old: 2.251 ± 0.010 μm, p>0.05, ANOVA). Cross-sectional area (1.62 ± 1.27 × 10−8 m2) was estimated from the video images by assuming that each preparation had a circular profile. The average length of the myocardial sample once the sarcomere length had been set was 574 ± 25 μm. There were no statistically significant differences in the dimensions of the preparations from the different animal age-groups (separate ANOVA tests for cross-sectional area and preparation length, p>0.05). All experiments were performed at 15°C using SLControl software [16].
Each preparation was initially activated in pCa 4.5 solution. Once tension had attained steady-state, the muscle was subjected to 3 ‘saw-tooth’ lengthening/shortening perturbations (magnitude 0.04 l0, velocity ±0.12 l0 s−1, inter-perturbation interval 100 ms, where l0 is the muscle length) and a rapid shortening/re-stretch maneuver (0.2 l0, 20 ms duration) (Fig 1) before being returned to pCa 9.0 solution. Similar trials were subsequently performed for each preparation using solutions with pCa values ranging from 9.0 to 5.0.
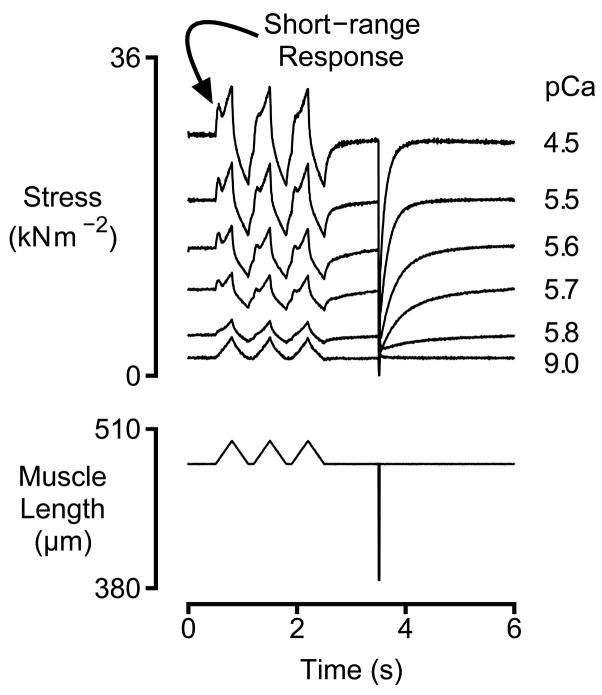
Figure 1.
Experimental Records. Representative force (top panel) and muscle length (bottom panel) records for a left-ventricular preparation immersed in solutions with different free Ca2+ concentrations. Activated preparations are disproportionately stiff for small movements. This behavior is attributed to the muscle’s short-range response.
Myosin heavy chain analysis
The relative content of α and β myosin heavy chain molecules was determined for 30 of the 62 ventricular preparations studied in this work after the relevant mechanical measurements had been completed. Samples were prepared for subsequent electrophoresis using procedures previously described by Tikunov et al. [17] and run on 0.75 mm thick, 10 cm square, 7% acrylamide (50:1 with bis-acrylamide) resolving gels containing 35% glycerol for 20 hours at a temperature of 4°C using a constant current of 3.8 mA. Gels were stained using a commercially-available kit (Silver Stain Plus, Bio-Rad, Hercules, CA) and scanned using a conventional flat-bed device (V500 Photo, Epson, Long Beach, CA).
The optical scans were analyzed using GelBandFitter, a custom-written computer program developed in Matlab (The Mathworks, Nattick, MA) (http://www.gelbandfitter.org). The program used a simplex algorithm to calculate the best least-squares fit of two overlapping asymmetric Gaussian curves (G(x), Eq 1) to the densitometry profile of each selected gel lane (Fig 5A).
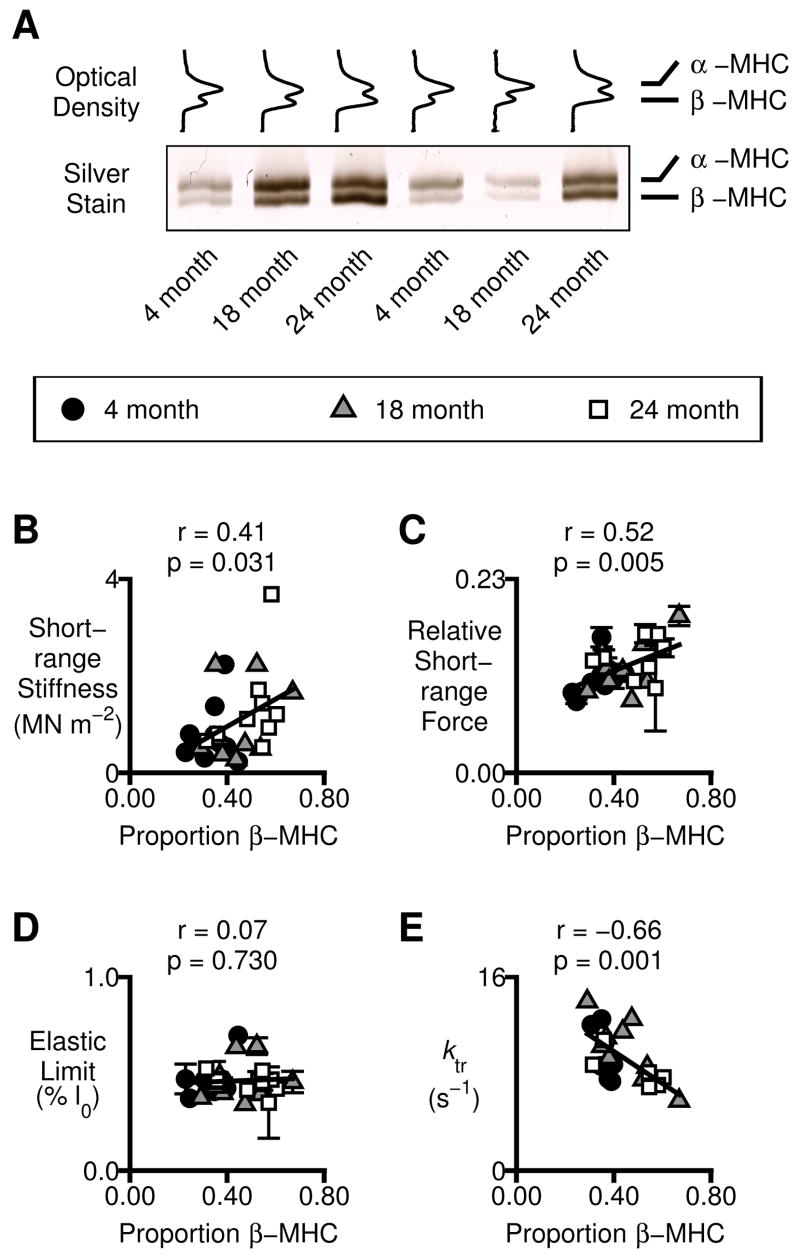
Figure 5.
Myosin heavy chain effects. A) Optical density profiles and a representative gel showing electrophoretic separation of the α and β myosin heavy chain isoforms in samples prepared from the individual preparations used for the mechanical measurements. B–D) Mechanical properties measured in individual preparations plotted as a function of the relative β myosin heavy chain content. B) Short-range stiffness measured in pCa 4.5 solution. C) Relative short-range force (short-range force normalized to Pss, mean ± SEM of all available estimates for the preparation). D) Elastic limit (mean ± SEM of all available estimates). E) ktr measured in pCa 4.5 solution.
| (Eq 1) |
The relative expression of β myosin heavy chain was calculated as Area2/(Area1+Area2) where Area1 and Area2 are the areas under the asymmetric Gaussian curves centered on the upper (α) and lower (β) myosin gel bands respectively, γ is a parameter that defines the width of the Gaussians, λ describes their asymmetry, and x1 and x2 are the positions of the peak values of the two Gaussians. This method is likely to provide a more appropriate estimate of relative protein content than calculations that require separating protein bands into distinct regions of interest when the density profile does not fall to zero between the bands [18, submitted to Electrophoresis, PDF attached as Supplementary Data].
Data Analysis
Mechanical records were analyzed in Matlab using custom-written routines. ktr, the rate of tension recovery following the rapid shortening/re-stretch maneuver, was calculated by fitting a single exponential function to the recovery time-course. Relative tension (Fig 4) was defined as the steady-state isometric force value (Pss, Fig 2C) divided by the corresponding value measured for the preparation in maximally-activating pCa 4.5 solution.
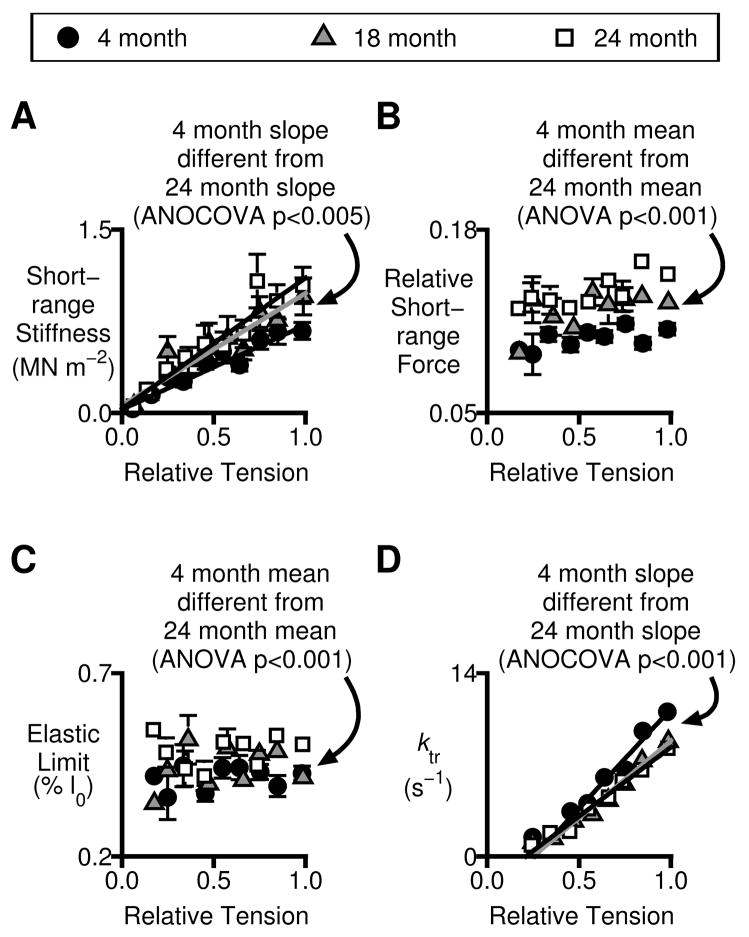
Figure 4.
Short-range mechanical properties and tension recovery kinetics. All plots show mechanical properties plotted as functions of relative isometric tension. Symbols show mean ± SEM values measured in a given pCa solution. Statistical analyses are described in the text. A) Short-range stiffness. B) Relative short-range force (short-range force normalized to Pss). C) Elastic limit. D) ktr.
Figure 2.
Analysis Algorithms. A&B) Representative force and muscle length records for an activated preparation during a lengthening movement. The grey circles demarcate phases of the force response that were modeled by linear regression. A) The additional force produced by straining attached cross-bridges (middle panel) was estimated by subtracting a linear relationship (estimated from the last third of the stretch response) from the measured force record. The short-range force and elastic limit were calculated as the force and length increments respectively when the strain-induced cross-bridge force was maximal. B) Short-range stiffness was estimated from the initial slope of an XY plot of force against muscle length and expressed as a Young’s Modulus. C) Force record for the pCa 4.5 condition shown in Fig 1. Pss was the steady-state force at a given level of Ca2+ activation. ktr was calculated by fitting a single exponential function to the portion of the force record following the large shortening/re-stretch perturbation.
Short-range stiffness, short-range force and elastic limit values were calculated for each stretch response at each level of Ca2+ activation. Stiffness values were derived from the slopes of regression lines fitted to the first 20 ms of XY plots of force against muscle length (Fig 2B) and expressed as Young’s Moduli. Values for the short-range force and the elastic limit were determined using the algorithm illustrated in Fig 2A. The first step in this process was to estimate the additional force produced by straining attached cross-bridges. This was done by subtracting a linear relationship extrapolated from the last third of the measured response from the experimental record. This ‘difference’ signal (Fig 2A, middle panel) was then analyzed to ascertain whether its peak value exceeded a threshold set to 4 times the mean noise level in the experimental force trace. If it did, the short-range force was calculated as the experimentally recorded force value at the time-point corresponding to the maximum ‘difference’ value. The elastic limit was calculated as the imposed length change at the same time-point. If the ‘difference’ signal did not exceed the noise threshold, the muscle was not considered to exhibit a short-range response and short-range force and elastic limit values were not assigned.
The most important advantage of this technique is that it provided an unambiguous way of analyzing short-range force responses that were of a comparable magnitude to the forces produced when the preparations were stretched in pCa 9.0 solution. It was also straightforward to implement automatically and (since it did not require user intervention) was unlikely to be affected by user bias. The approach is different from that described in several of the corresponding author’s previous publications [15, 19] but it is probably more appropriate for preparations (like rat ventricular samples) in which tension continues to rise once the preparation has been stretched beyond the elastic limit.
Mathematical Modeling
Fig 6A shows a simple model that was used to simulate tension responses to imposed ramp stretches. The model was implemented in Matlab and consists of a linear elastic spring (stiffness κp) representing titin molecules and collagen filaments arranged in parallel with a population (N) of 10,000 cycling cross-bridges. The cross-bridges cycled between a single attached state and a single detached state with the attachment and detachment rate constants given by f and g respectively. The attachment rate constant f was the same for every cross-bridge in a given simulation but the detachment rate constant for each cross-bridge varied with muscle length x as
| (Eq 2) |
where g0 and x are constants and x0 is the muscle length at which the cross-bridge attached. Each attached cross-bridge produced a force [Fcb+κcb(x−x0)]. Attachment/detachment events were implemented using stochastic techniques [20] and a time-step of 1 ms. Test calculations showed that the simulated forces responses were not systematically different if the time-step was reduced to 0.5 ms. Pseudo-random numbers were generated using the Mersenne Twister algorithm [21] and a seed generated from the computer clock. κp and x were set to 100 Fcb nm−1 and 0.83 s−1 nm−1 respectively to produce simulated short-range responses that were qualitatively similar to the experimental records obtained in maximally-activating Ca2+ solutions. The initial value of κcb (0.030 Fcb nm−1) was chosen in the same way but, as described in the Results section, one set of simulations was performed with κcb increased by 24% to 0.037 Fcb nm−1.
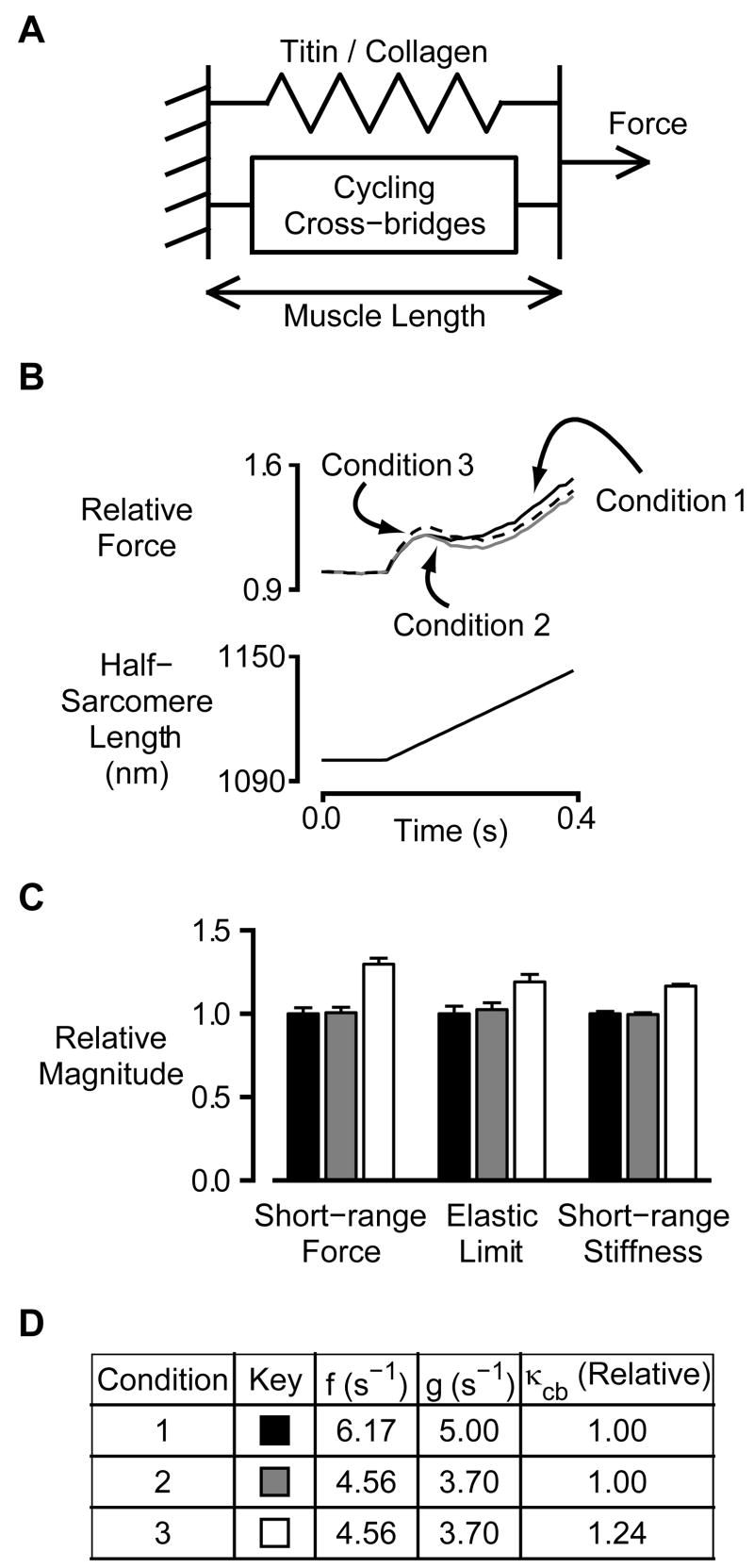
Figure 6.
Simulations. A) Schematic representation of the mathematical model. B) Representative force simulations. C) Mean ± SEM relative values for short-range force, elastic limit and short-range stiffness parameters calculated for the three simulation conditions. D) Summary table.
Statistics
Calculations were performed using the Matlab Statistics Toolbox (Ver 2008a). The potential significance of between-group differences in mean values was assessed using one-way ANOVA tests. ANOCOVA procedures were used to assess experimental effects when the response variable was itself modeled as a linear function of a predictor variable. The method indicates whether regression lines are statistically different. Post-hoc analyzes for both ANOVA and ANOCOVA tests were performed using Tukey’s honestly-significant difference criterion. Results are reported as mean ± SEM.
Results
Fig 1 shows representative force records for a rat multicellular ventricular preparation subjected to three stretch/shortening cycles followed by a rapid shortening/re-stretch perturbation. As previously shown for fast skeletal muscle fibers [19], slow skeletal muscle fibers [15] and canine myocardium [8], activated preparations exhibited short-range mechanical properties; tension rose more quickly during the initial phase of an imposed stretch than thereafter [22–27]. Fig 1 also shows that the magnitude of the short-range response increased with the level of Ca2+ activation. This behavior is consistent with the hypothesis that short-range force responses result from the strain and subsequent cyclic-reattachment of variable numbers of myosin heads [15, 19].
Previous experiments performed using chemically permeabilized canine myocardium showed that the short-range stiffness of the preparation was temporarily reduced by ~50% following the first movement [8] in all Ca2+-activating conditions. The effects observed in the present experiments performed using rat myocardium were not nearly as dramatic. The mean second stretch stiffness in pCa 4.5 solution was 0.984 ± 0.021 of the corresponding first stretch value. This value is not statistically different from unity (t-test, p>0.05) and there were no statistically significant differences in the ratio for the preparations from the different aged animals (4 month-old animals: 0.978 ± 0.027, 18 month-old animals: 0.947 ± 0.045, 24 month-old animals: 1.032 ± 0.040, ANOVA, p>0.05). The magnitude of the short-range force response (measured from the prevailing tension at the beginning of the movement) was not substantially reduced in the second stretch responses either (statistical data not shown). The peak short-range force did however normally occur at a lower absolute level because tension had not completely recovered to the steady-state isometric level in the 100 ms interval between the first and second length changes (Fig 1). The responses to the second and third movements were indistinguishable in agreement with previous measurements made using skeletal and cardiac preparations [8, 15, 19].
These analyzes show that even if preceding movement reduces the short-range mechanical response of rat myocardium the effects of the mechanical perturbation have largely subsided after 100 ms at fixed length. All of the results presented in the remainder of this manuscript pertain to the short-range force response elicited by the first lengthening movement.
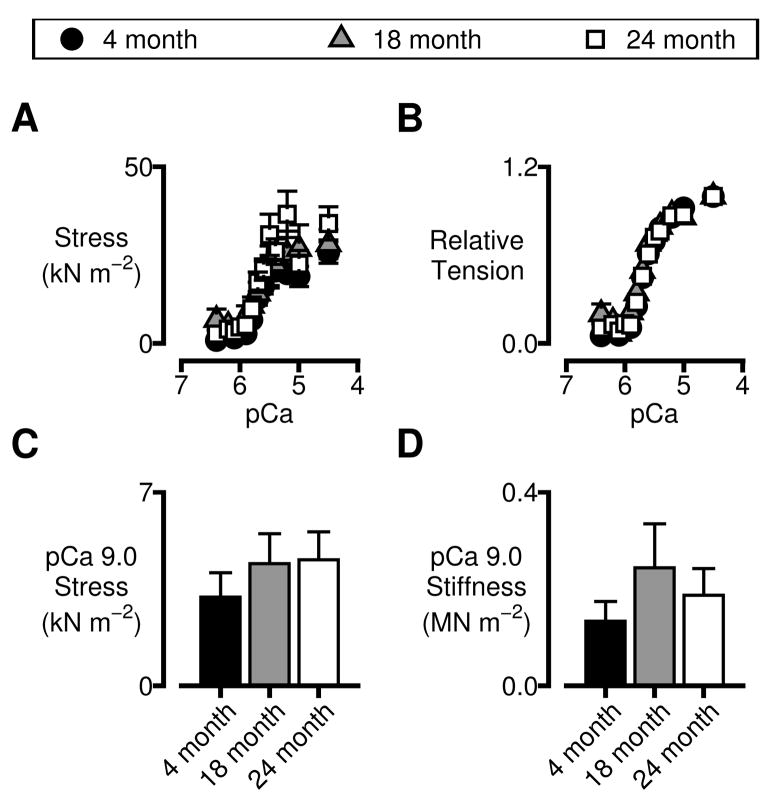
Steady-state isometric force values (Pss, Fig 2C) normalized to each preparation’s cross-sectional area and maximum isometric force respectively are shown in Figs 3A and B. These relationships were modeled using Hill-type curves of the form y = A+B×[Ca2+]n/([Ca2+]n+[Ca2+50]n) where A is indicative of passive (Ca2+-independent) force, B is a constant, n is the Hill coefficient and Ca2+50 is the free calcium concentration at half-maximal force development. Maximum force values and Hill coefficients were not different for the preparations from the different aged animals (p>0.05 for both parameters, ANOVA, data not shown). The pCa50 values for the 18-month-old preparations (5.68 ± 0.01) were lower (p<0.02, ANOVA) than the corresponding data for the 4-month-old (5.64 ± 0.01) and 24-month-old (also 5.64 ± 0.01) animals. This corresponds to a slight leftward-shift of the tension-pCa curve for the preparations from the 18-month-old animals. The shift is an interesting effect but it is relatively small and the physiological significance is unclear. Taken together these data suggest that the force-generating capacity of left ventricular myocardium does not change substantially with age in F344 rats.
Figure 3.
Isometric force values and passive mechanical properties. Symbols and bars show mean ± SEM values. A&B) Force values normalized to preparation cross-sectional area (A) and maximum isometric tension (B) for preparations isolated from the different aged animals. C&D) Mechanical properties measured in pCa 9.0 solution. Isometric tension (C) and stiffness (D) values were not different for the different age-groups (ANOVA, p>0.05 in each case).
Figs 3C and D show measured values of tension and stiffness respectively for preparations immersed in pCa 9.0 solutions. These properties reflect the mechanical behavior of the left-ventricular samples in the absence of large numbers of attached cross-bridges. They did not change significantly with age (ANOVA, p>0.05 for both properties). This implies that the mechanical responses of titin molecules and collagen filaments do not change with age in this animal model.
Other mechanical properties do however change with age. Figs 4A, B and C show measured values of short-range stiffness, relative short-range force (short-range force normalized to Pss) and elastic limit respectively for myocardium isolated from rats of different ages. (The algorithms used to calculate these parameters are shown schematically in Fig 2.) Analysis of Covariance (ANOCOVA) showed that short-range stiffness increased more rapidly (p<0.005) as a function of relative isometric tension in 24-month-old rats than in 4-month-old animals. ANOVA tests showed that relative short-range forces and elastic limits were also greater in 24-month-old than in 4-month-old rats (p<0.001 for both parameters). Neither, of these variables was linearly related to relative isometric tension (ANOCOVA, p>0.05). Finally, ktr values increased less rapidly with relative tension in preparations from the 24-month-old rats than in preparations from the 4-month-old animals (Fig 4D, ANOCOVA, p<0.001). Similar age-dependent slowing of tension recovery has been reported in Fischer 344 x Brown Norway rats [11, 12] (a different animal strain).
The works cited immediately above showed that there was a linear relationship between ktr values measured in maximally-activating Ca2+ solutions and the proportion of myosin heavy chain molecules expressed in the individual experimental preparations as the slow β isoform. Fig 5 shows the results of analyzes designed to ascertain whether a similar relationship exists in preparations from F344 rats and whether any of the short-range mechanical properties also correlate with the relative content of β myosin heavy chain protein.
Fig 5A shows a representative gel and optical density profiles for myocardial preparations isolated from the different-aged animals. The relative expression of the slow β myosin heavy chain isoform increased with age from a mean value of 0.35 ± 0.02 in preparations from 4-month-old animals to a mean value of 0.50 ± 0.04 in preparations from 24-month-old animals (ANOVA, p<0.005). Panels B to D in Fig 5 show the relationships between several different mechanical properties and the relative expression of the slow β myosin heavy chain in the experimental preparations. The short-range stiffness in maximally activating pCa 4.5 solution (Fig 5B) and the relative short-range force (Fig 5C) both increased with increasing β myosin heavy chain expression (p=0.031 and p=0.005 respectively). These are important statistical correlations because they provide the first direct evidence linking myocardial short-range mechanical properties to sarcomeric protein content. The elastic limit did not correlate with myosin heavy chain expression (Fig 5D) while the maximal ktr value (Fig 5E) decreased with the relative proportion of β myosin heavy chain molecules in line with previous results obtained using a different rat strain [11, 12].
Fig 6A shows a schematic representation of a simple mathematical model that was developed to aid in the interpretation of the experimental results. The steady-state isometric force predicted by the model scales with the simulation rate constants as f/(f+g0) while ktr is equivalent to f+g0. The mechanical experiments described in this work do not establish independent values for these two parameters. Basal simulations (Condition 1, Fig 6) were therefore initiated with g0 assigned a plausible value of 5 s−1 and f set to 6.17 s−1 so that the predicted ktr value for the condition equaled the mean value determined for maximally-activated preparations from 4-month-old rats (Fig 4D).
Maximal steady-state force values did not vary with age in the present experiments (Fig 3A). A second series of simulations (Condition 2, Fig 6) was therefore performed using values of f and g0 calculated so that (1) f+g0 equaled 8.26 s−1 (the mean ktr value for maximally-activated preparations from 24-month-old rats) and (2) the ratio f/(f+g0) was the same for Conditions 1 and 2. The resulting rate constants (f = 4.56 s−1, g0 = 3.70 s−1) were uniquely determined by these criteria once a value had been assigned to g0 for Condition 1.
Simulated force records for these two conditions are shown in Fig 6B. Mean ± SEM relative values for short-range force, elastic limit and short-range stiffness calculated from sets of 10 simulated records are shown in Fig 6C. The predicted short-range properties for Conditions 1 and 2 are not statistically different (separate t-tests for each parameter, p>0.05 in each case). This implies that the age-dependent changes in short-range mechanical properties shown in Fig 4 cannot be explained (at least with this model) by simple manipulation of the attachment and detachment rate constants.
A third set of simulations (Condition 3, Fig 6) was then performed using the same values for f and g0 as Condition 2 but a cross-bridge stiffness κcb that was 24% greater. This modification matched the proportional age-dependent increase in pCa 4.5 short-range stiffness observed in the preparations from the 4 and 24-month-old animals. The results of the simulations (Fig 6B and C) show that this modification also increased the predicted values for the short-range force and the elastic limit. The simulated relative increases for these two variables were 29 ± 4 % and 17 ± 1 % respectively. The relative increases in the corresponding experimental values (24 month-old: 4 month-old values) were 34% and 18%.
Discussion
Although advanced age is one of the most important risk factors for diastolic heart failure [2, 3], the molecular mechanisms that contribute to the aging-associated dysfunction are not clear [1]. The increased myocardial stiffness observed in affected patients could reflect increased passive stiffness (due primarily to titin and collagen filaments) or increased active stiffness (due to cross-bridges that remain bound to actin filaments during diastole). The results documented in this work show that, at least in F344 rats, and under the present experimental conditions, passive stiffness does not change with age (Fig 3C and D). This suggests that the aging-associated increase in diastolic ventricular stiffness observed in these animals in vivo [4] is not due to changes in titin, collagen, elastin and/or intermediate filaments.
The working hypothesis for this study was that aging-associated diastolic dysfunction reflects increased relative content of slowly-cycling β myosin heavy chain molecules. The experiments confirmed that the Ca2+-activated preparations from old hearts were stiffer than those isolated from young hearts (Fig 4A) and that the older preparations contained a greater proportion of β myosin heavy chain protein (Fig 5A). The measurements also showed that the stiffness of the maximally-activated preparations increased with the relative β myosin heavy chain content (Fig 5B).
These are important observations but it could be argued that they are not directly relevant to diastolic dysfunction because the cross-bridge component of myocardial stiffness did not become appreciable until the preparations were activated in solutions with pCa values of ~5.8 (Fig 1). The free Ca2+ concentration for this condition is much higher than the level (~pCa 6.7, ~200 nM) normally assumed to prevail during diastole in working hearts [28]. This comparison suggests that the experimental results relating to Ca2+-dependent mechanical properties obtained in this work are only relevant to systolic function. While this is a tenable view it should also be recognized that the tension-pCa relationship in the current experimental preparations may be very different from that found in living tissue. Gao et al. [29] showed that the tension-pCa curve was right-shifted by more than 0.4 log units in rat trabeculae on chemical skinning. The current experiments were also performed at 15°C rather than at physiological temperature.
The current data also suggest that there may be important differences between the diastolic mechanical properties of myocardium from large and small animals. Previous work [8] performed under very similar experimental conditions using permeabilized preparations isolated from canine myocardium showed that the stiffness of the sample immersed in pCa 9.0 solution was 2% of the measured stiffness when the preparation was maximally-activated in pCa 4.5 solution. The corresponding value measured in the current experiments was 18.0 ± 0.5 %. The molecular mechanisms responsible for the large difference in the values for rat and canine myocardium are not clear but variations in titin content could contribute. Dogs express roughly 50% of their myocardial titin as the N2BA isoform while rat myocardial titin is almost exclusively of the shorter (stiffer) N2B type [30].
Another possibility is that while the imposed movements forcibly detached some cross-bridges in the experiments using canine myocardium, myosin heads in rat preparations are attaching and detaching from the thin filaments so rapidly that the relatively slow length changes used in these experiments did not dramatically reduce the number of attached cross-bridges. There are several lines of evidence to support this hypothesis. First, ktr in the canine samples at very low levels of Ca2+ activation was ~0.3 s−1. In the present experiments, the lowest measured value was ~1.4 s−1. This indicates that even at low levels of Ca2+ activation, cross-bridges cycle quicker in rat than in canine myocardium. Second, the relative short-range force in canine myocardium was ~0.25 irrespective of the level of Ca2+ activation. In the present measurements it was 0.125 ± 0.002. This is consistent with the hypothesis that cross-bridges were not stretched as far in the rat myocardium as they were in the experiments performed with canine tissue. Third, the history-dependent changes in the short-range response of canine myocardium were very clear and lasted for several seconds, even at the highest levels of Ca2-activation. In rat myocardium, the short-range response had completely recovered (if it had been perturbed at all) after 100 ms.
It is interesting to note here that (in contrast to the situation in relaxed frog fibers [31]) the short-range force response recovered more quickly than developed tension. This suggests that myosin heads attach quite quickly to thin filaments in rat myocardium but that it takes additional time for the steady-state populations of pre-power-stroke and post-power-stroke cross-bridges to redevelop.
If the present experiments (a) rule out changes in passive mechanical properties and (b) show that cycling cross-bridges not make a substantial contribution to myocardial stiffness at a typical diastolic Ca2+ concentration of 200 nM, why do the older F344 rats exhibit increased diastolic myocardial stiffness and associated cardiovascular dysfunction? Unfortunately, the present study does not provide definitive answers. One possibility is that the sarcomere length changes that occur during ventricular filling are sufficiently quick to stretch even rapidly cycling cross-bridges. If the sarcomere length in the ventricular wall increases by 0.25 μm during diastole and the diastolic period lasts two-thirds of the cardiac cycle, the mean inter-filamentary sliding velocity at a typical rat heart rate of 400 beats per minute (~1.3 l0 s−1) will be ~11 times faster than that used in the present experiments. Another theory is that diastolic function is determined primarily by the earliest part of diastole when the ventricular walls are still relaxing and substantial numbers of cross-bridges may still be attached between the myofilaments.
Although the current experiments do not provide direct evidence to support the original hypothesis that elevated diastolic myocardial stiffness in aged F344 rats reflects increased relative content of β myosin heavy chain they do not show that the hypothesis is definitely wrong. The correlations shown in Figs 5B and 5C also imply that the age-dependent increases in the stiffness of Ca2+-activated myocardium are linked to increased β myosin heavy chain content. This finding could be important for aging-associated systolic dysfunction and is consistent with a simple kinetic argument. β myosin heads have a higher duty cycle than α isoform molecules [32]. This means that a greater proportion of the β than of the α myosin heads will be attached to actin filaments at the beginning of an imposed movement, which in turn implies that β myosin molecules should make a relatively greater contribution to ventricular stiffness.
Although this argument has an appealing simplicity, it does not explain all of the experimental data relating to the elastic limit. As well as having a higher duty ratio, β myosin heads are thought to detach from actin filaments more slowly than α molecules [11]. This means that, all other things being equal, β heads will be stretched further by a given movement before they detach. This would be detected in the present experiments as an increase in the myocardium’s elastic limit. (It is important to realize here that the earlier experiments performed using canine tissue [8] showed that the elastic limit increases with stretch velocity in myocardial samples. This indicates that myosin heads do not detach only once they have been stretched to a critical length in cardiac muscle under these experimental conditions. This is different from the situation observed in maximally-activated skeletal muscle fibers where, at least for stretches above a certain velocity, the elastic limit is independent of the speed of stretching [26, 33].) While it is true that the elastic limit (Fig 4C) and the proportion of myosin heads expressed as the β isoform were both increased in preparations from the 24-month-old animals, the two parameters were not correlated in individual preparations (Fig 5D). This contradicts the straightforward expectation outlined above.
These apparent discrepancies can be overcome if the myosin detachment rate constant, g, is strain-dependent. (This is one of the assumptions incorporated in the mathematical model - Eq. 2). Myosin heads will therefore become increasingly more likely to detach as they are stretched from their initial bound position. This reduces the expected effect of isoform expression on the elastic limit but raises another question. Why is the elastic limit increased in the preparations from the older animals (Fig 4C)?
The observed changes could be a residual effect of the slowed myosin kinetics. In other words, the strain-dependence of the myosin detachment rate may not be sufficient to completely eliminate changes in the elastic limit. Another possibility is that the elastic limit is also influenced by age-related isoform and/or post-translational changes in other myofilament proteins (e.g. C-protein and/or tropomyosin). A third scenario is that the myosin heads produce less force in older preparations and therefore have to be stretched further before they detach at a critical load.
The experiments described in this paper are important because (1) they present the first evidence linking myocardial short-range mechanical properties to sarcomeric protein content (and thus gene expression) and (2) they suggest a potential molecular mechanism that could contribute to diastolic dysfunction in elderly populations. They are however subject to some limitations.
The mechanical measurements would have been better if they had been performed using sarcomere length feedback. Although previous work [8, 16] has shown that this does not change the basic features of myocardial short-range properties, it is possible that the technique would have reduced experimental variability in some of the measured parameters. These experiments would however have been exceedingly technically demanding and were not considered practical because of the limited availability of the aged rats.
Another drawback of the present experiments is that the age-related changes in myosin isoform expression observed in this animal model were, on average, quite modest. The proportion of myosin molecules expressed as the slow β isoform increased from 0.35 ± 0.02 in preparations from the 4 month-old animals to 0.50 ± 0.03 in preparations from the 24-month-old rats. The relative expression of the β isoform in the 18-month-old preparations was more variable, ranging from 0.29 to 0.67 in different preparations. This heterogeneity might explain why the mechanical data for these animals, while generally lying between the mean values measured for the young and old preparations, was somewhat scattered [34]. It might be possible to make stronger conclusions about the relative effects of α and β myosin molecules on myocardial stiffness in future work by using animal models (such as rats treated with propylthiouracil [35, 36]) that exhibit larger and more consistent variations in myosin isoform expression.
Acknowledgments
This work was supported by American Heart Association Scientist Development Grant 0630079N, NIH AG021862, NIH HL 090749 (all to KSC) and the University of Kentucky Research Challenge Trust Fund.
Footnotes
Subject Codes: [14] Contractile Function, [34] Mathematical Modeling, [37] Myocardial biology, [38] Myocardial contractility, [51] Sarcomeric proteins
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kass DA, Bronzwaer JG, Paulus WJ. What mechanisms underlie diastolic dysfunction in heart failure? Circulation Research. 2004 Jun 25;94(12):1533–42. doi: 10.1161/01.RES.0000129254.25507.d6. [DOI] [PubMed] [Google Scholar]
- 2.Kitzman DW. Diastolic heart failure in the elderly. Heart Failure Reviews. 2002 Jan;7(1):17–27. doi: 10.1023/a:1013745705318. [DOI] [PubMed] [Google Scholar]
- 3.Owan TE, Redfield MM. Epidemiology of diastolic heart failure. Progress in Cardiovascular Diseases. 2005 Mar–Apr;47(5):320–32. doi: 10.1016/j.pcad.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 4.Pacher P, Mabley JG, Liaudet L, Evgenov OV, Marton A, Hasko G, et al. Left ventricular pressure-volume relationship in a rat model of advanced aging-associated heart failure. American Journal of Physiology Heart and Circulatory Physiology. 2004 Nov;287(5):H2132–7. doi: 10.1152/ajpheart.00405.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boluyt MO, Converso K, Hwang HS, Mikkor A, Russell MW. Echocardiographic assessment of age-associated changes in systolic and diastolic function of the female F344 rat heart. Journal of Appled Physiology. 2004 Feb;96(2):822–8. doi: 10.1152/japplphysiol.01026.2003. [DOI] [PubMed] [Google Scholar]
- 6.Borlaug BA, Kass DA. Mechanisms of diastolic dysfunction in heart failure. Trends in Cardiovascular Medicine. 2006 Nov;16(8):273–9. doi: 10.1016/j.tcm.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 7.Cazorla O, Freiburg A, Helmes M, Centner T, McNabb M, Wu Y, et al. Differential expression of cardiac titin isoforms and modulation of cellular stiffness. Circulation Research. 2000;86(1):59–67. doi: 10.1161/01.res.86.1.59. [DOI] [PubMed] [Google Scholar]
- 8.Campbell KS, Patel JR, Moss RL. Cycling cross-bridges increase myocardial stiffness at sub-maximal levels of Ca2+ activation. Biophysical Journal. 2003;84:3807–15. doi: 10.1016/S0006-3495(03)75108-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pohlmann L, Kroger I, Vignier N, Schlossarek S, Kramer E, Coirault C, et al. Cardiac Myosin-Binding Protein C Is Required for Complete Relaxation in Intact Myocytes. Circulation Research. 2007 Oct 26;101(9):928–38. doi: 10.1161/CIRCRESAHA.107.158774. [DOI] [PubMed] [Google Scholar]
- 10.Shutt RH, Ferrier GR, Howlett SE. Increases in diastolic [Ca2+] can contribute to positive inotropy in guinea pig ventricular myocytes in the absence of changes in amplitudes of Ca2+ transients. American Journal of Physiology - Heart & Circulatory Physiology. 2006 Oct;291(4):H1623–34. doi: 10.1152/ajpheart.01245.2005. [DOI] [PubMed] [Google Scholar]
- 11.Rundell VL, Manaves V, Martin AF, de Tombe PP. Impact of beta-myosin heavy chain isoform expression on cross-bridge cycling kinetics. American Journal of Physiology - Heart & Circulatory Physiology. 2005 Feb;288(2):H896–903. doi: 10.1152/ajpheart.00407.2004. [DOI] [PubMed] [Google Scholar]
- 12.Fitzsimons DP, Patel JR, Moss RL. Aging-dependent depression in the kinetics of force development in rat skinned myocardium. American Journal of Physiology Heart and Circulatory Physiology. 1999 May;276(5 Pt 2):H1511–9. doi: 10.1152/ajpheart.1999.276.5.H1511. [DOI] [PubMed] [Google Scholar]
- 13.Zile MR, Brutsaert DL. New concepts in diastolic dysfunction and diastolic heart failure: Part II: causal mechanisms and treatment. Circulation. 2002 Mar 26;105(12):1503–8. doi: 10.1161/hc1202.105290. [DOI] [PubMed] [Google Scholar]
- 14.Patton C, Thompson S, Epel D. Some precautions in using chelators to buffer metals in biological solutions. Cell Calcium. 2004 May;35(5):427–31. doi: 10.1016/j.ceca.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 15.Campbell KS, Moss RL. History-dependent mechanical properties of permeabilized rat soleus muscle fibers. Biophysical Journal. 2002;82(2):929–43. doi: 10.1016/S0006-3495(02)75454-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Campbell KS, Moss RL. SLControl: PC-based data acquisition and analysis for muscle mechanics. American Journal of Physiology Heart and Circulatory Physiology. 2003 Aug 7;285(6):H2857–H64. doi: 10.1152/ajpheart.00295.2003. [DOI] [PubMed] [Google Scholar]
- 17.Tikunov BA, Sweeney HL, Rome LC. Quantitative electrophoretic analysis of myosin heavy chains in single muscle fibers. Journal of Appled Physiology. 2001 May;90(5):1927–35. doi: 10.1152/jappl.2001.90.5.1927. [DOI] [PubMed] [Google Scholar]
- 18.Mitov MI, Greaser ML, Campbell KS. GelBandFitter - A computer program for analysis of closely spaced electrophoretic bands. 2008 doi: 10.1002/elps.200800583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Campbell KS, Moss RL. A thixotropic effect in contracting rabbit psoas muscle: prior movement reduces the initial tension response to stretch. Journal of Physiology. 2000;525.2:531–48. doi: 10.1111/j.1469-7793.2000.00531.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Campbell KS. Filament compliance effects can explain tension overshoots during force development. Biophysical Journal. 2006 Dec 1;91(11):4102–9. doi: 10.1529/biophysj.106.087312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matsumoto M, Nishimura T. Mersenne Twister: A 623-dimensionally equidistributed uniform pseudorandom number generator. ACM Transactions on Modeling and Computer Simulation. 1998;8(1):3–30. [Google Scholar]
- 22.Edman KAP. The force bearing capacity of frog muscle fibres during stretch: its relation to sarcomere length and fibre width. Journal of Physiology. 1999;519.2:515–26. doi: 10.1111/j.1469-7793.1999.0515m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Edman KAP, Elzinga G, Noble MIM. Enhancement of mechanical performance by stretch during tetanic contractions of vertebrate skeletal muscle fibers. Journal of Physiology. 1978;281:139–55. doi: 10.1113/jphysiol.1978.sp012413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Flitney FW, Hirst DG. Crossbridge detachment and sarcomere ‘give’ during stretch of active frog’s muscle. Journal of Physiology. 1978;276:449–65. doi: 10.1113/jphysiol.1978.sp012246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Getz EB, Cooke R, Lehman SL. Phase transition in force during ramp stretches of skeletal muscle. Biophysical Journal. 1998;75:2971–83. doi: 10.1016/S0006-3495(98)77738-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lombardi V, Piazzesi G. The contractile response during steady lengthening of stimulated frog muscle fibres. Journal of Physiology. 1990;431:141–71. doi: 10.1113/jphysiol.1990.sp018324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stienen GJ, Versteeg PG, Papp Z, Elzinga G. Mechanical properties of skinned rabbit psoas and soleus muscle fibres during lengthening: effects of phosphate and Ca2+ Journal of Physiology. 1992;451:503–23. doi: 10.1113/jphysiol.1992.sp019176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bers DM. Excitation-contraction coupling and cardiac contractile force. Dordrecht: Kluwer Academic Publishers; 1991. [Google Scholar]
- 29.Gao WD, Backx PH, Azan-Backz M, Marban E. Myofilament Ca2+ sensitivity in intact versus skinned rat ventricular muscle. Circulation Research. 1994;74:408–15. doi: 10.1161/01.res.74.3.408. [DOI] [PubMed] [Google Scholar]
- 30.Wu Y, Cazorla O, Labeit D, Labeit S, Granzier H. Changes in titin and collagen underlie diastolic stiffness diversity of cardiac muscle. Journal of Molecular and Cellular Cardiology. 2000 Dec;32(12):2151–62. doi: 10.1006/jmcc.2000.1281. [DOI] [PubMed] [Google Scholar]
- 31.Campbell KS, Lakie M. A cross-bridge mechanism can explain the thixotropic short-range elastic component of relaxed frog skeletal muscle. Journal of Physiology. 1998;510.3:941–62. doi: 10.1111/j.1469-7793.1998.941bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Palmiter KA, Tyska MJ, Dupuis DE, Alpert NR, Warshaw DM. Kinetic differences at the single molecule level account for the functional diversity of rabbit cardiac myosin isoforms. Journal of Physiology. 1999;519.3:669–78. doi: 10.1111/j.1469-7793.1999.0669n.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roots H, Offer GW, Ranatunga KW. Comparison of the tension responses to ramp shortening and lengthening in intact mammalian muscle fibres: crossbridge and non-crossbridge contributions. J Muscle Res Cell Motil. 2007;28(2–3):123–39. doi: 10.1007/s10974-007-9110-0. [DOI] [PubMed] [Google Scholar]
- 34.Boluyt MO, Devor ST, Opiteck JA, White TP. Regional variation in cardiac myosin isoforms of female F344 rats during aging. Journal of Gerontology: Biological Sciences. 1999 Aug;54(8):B313–7. doi: 10.1093/gerona/54.8.b313. [DOI] [PubMed] [Google Scholar]
- 35.Fitzsimons DP, Patel JR, Moss RL. Role of myosin heavy chain composition in kinetics of force development and relaxation in rat myocardium. Journal of Physiology. 1998;513(1):171–83. doi: 10.1111/j.1469-7793.1998.171by.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu Y, Peng J, Campbell KB, Labeit S, Granzier H. Hypothyroidism leads to increased collagen-based stiffness and re-expression of large cardiac titin isoforms with high compliance. Journal of Molecular and Cellular Cardiology. 2007 Jan;42(1):186–95. doi: 10.1016/j.yjmcc.2006.09.017. [DOI] [PubMed] [Google Scholar]