Abstract
Epithelial-to-mesenchymal transitions (EMT) are important in renal development, fibrosis, and cancer. Loss of function of the tumor suppressor VHL leads to many features of EMT, and it has been hypothesized that the pivotal mediator is down-regulation of the adherens junction (AJ) protein E-cadherin. Here we show that VHL loss-of-function also has striking effects on the expression of the tight junction (TJ) components occludin and claudin 1 in vitro in VHL-defective clear cell renal cell carcinoma (CCRCC) cells and in vivo in VHL-defective sporadic CCRCCs (compared with normal kidney). Occludin is also down-regulated in premalignant foci in kidneys from patients with germline VHL mutations, consistent with a contribution to CCRCC initiation. Reexpression of E-cadherin was sufficient to restore AJ but not TJ assembly, indicating that the TJ defect is independent of E-cadherin down-regulation. Additional experiments show that activation of hypoxia inducible factor (HIF) contributes to both TJ and AJ abnormalities, thus the VHL/HIF pathway contributes to multiple aspects of the EMT phenotype that are not interdependent. Despite the independent nature of the defects, we show that treatment with the histone deacetylase inhibitor sodium butyrate, which suppresses HIF activation, provides a method for reversing EMT in the context of VHL inactivation.
INTRODUCTION
A key characteristic of epithelial surfaces is the formation of specialized intercellular junctions at points of cell–cell contact. It is increasingly appreciated that these junctions have extensive roles beyond their function in cellular cohesion including determination of epithelial permeability and providing inputs modulating proliferation and differentiation. Two important types of intercellular junctions in the kidney epithelium are the tight junction (TJ) and adherens junction (AJ; Tsukita et al., 2001; Conacci-Sorrell et al., 2002; Matter and Balda, 2003). AJs are formed on the basolateral cell membrane and are composed of transmembrane cadherins linked to intracellular catenin proteins (Conacci-Sorrell et al., 2002). AJs are involved in cell–cell adhesion and also regulate β-catenin availability (Nelson and Nusse, 2004). TJs are formed on the apical surface of the lateral membrane and are analagous in structure to AJs, with specific transmembrane proteins (occludin and claudins), which are linked to the actin cytoskeleton via intracellular adaptor proteins, the zona occludens (ZO) family of proteins (Tsukita et al., 2001). In addition to its traditional roles in maintaining apical-basal polarity and controlling paracellular permeability the TJ is also now recognized as a signaling hub. TJ regulation of pathways involving raf kinase, rho GTPase, and the Y-box transcription factor ZONAB control important cellular processes such as cell proliferation, gene transcription, and cellular differentiation (Braga, 2002; Matter and Balda, 2003; Gonzalez-Mariscal et al., 2007; Guillemot et al., 2008).
VHL is a classical tumor suppressor gene which acts as a gatekeeper in renal epithelium (Latif et al., 1993; Kaelin, 2002). It is established that the encoded protein, VHL (von Hippel-Lindau), is required for regulation of hypoxia-inducible factor (HIF) explaining why loss-of-function of VHL is associated with angiogenic signaling and other aspects of the cancer phenotype (Wang and Semenza, 1993; Maxwell et al., 1999). VHL also has effects that are independent of its role in degradation of HIF-α subunits, including regulation of RNA polymerase II, atypical protein kinase C, cellular senescence, matrix assembly, and microtubule stabilization (Ohh et al., 1998; Okuda et al., 2001; Hergovich et al., 2003; Kuznetsova et al., 2003; Young et al., 2008).
As yet, the role of VHL in maintaining normal behavior of renal epithelial cells is not clear and how VHL loss-of-function would initiate the process that leads to malignant transformation is incompletely understood. Recently compelling evidence has emerged for a role of VHL in maintaining expression of the AJ component E-cadherin (Esteban et al., 2006b; Krishnamachary et al., 2006; Evans et al., 2007). Down-regulation of E-cadherin is a hallmark feature of cancer (Hanahan and Weinberg, 2000), and this provides a candidate mechanism linking VHL loss-of-function via HIF activation to tumor initiation. Importantly, it has been suggested that repression of E-cadherin in renal epithelial cells results in a comprehensive epithelial-to-mesenchymal transition (EMT; Krishnamachary et al., 2006; Evans et al., 2007). EMT describes a coordinated set of changes including dissolution of both TJ and AJ cell–cell adhesions and loss of apical-basal polarity accompanied by cytoskeletal rearrangements and increased cell motility (Thiery and Sleeman, 2006). In the kidney, EMT is not only relevant to renal cancer; normal renal development requires a transition from epithelium to mesenchyme, followed by a transition from mesenchyme to epithelium (Dressler, 2002). Furthermore, in progression of human kidney disease it is believed that EMT is important in renal fibrosis (Kalluri and Neilson, 2003; Boutet et al., 2006). More broadly, the effect of HIF activation on E-cadherin implied that oxygen gradients may modulate E-cadherin expression in other epithelia.
Recently it was shown that VHL status also has a marked effect on formation of TJs (Calzada et al., 2006). Here we investigate the mechanism by which this occurs, the potential contribution to early events initiating tumorigenesis and dedifferentiation of renal epithelium and its relation to HIF activation and down-regulation of E-cadherin.
MATERIALS AND METHODS
Cell Culture, Plasmids, Reagents, and Antibodies
Clear cell renal cell carcinoma (CCRCC) cell lines and stable transfectants expressing wild-type VHL were described previously (Esteban et al., 2006b). Cells were cultured in RPMI 1640 (Invitrogen, Carlsbad, CA) with 10% fetal bovine serum, glutamine, and penicillin/streptomycin. Infection with pCMVR retroviral vectors was performed as described (Esteban et al., 2006b); pooled populations were selected with G418 (Invitrogen). Inserts encoding human E-cadherin, human occludin, and human claudin 1 were generated by PCR of cDNAs provided by Dr. Hart (John Vane Science Centre, London, United Kingdom), Dr. Nusrat (Emory University School of Medicine, Atlanta, GA), and Dr. Karl Matter. Bicistronic retroviral plasmids encoding enhanced green fluorescent protein (EGFP) and either empty vector or a constitutively active form of HIF-1α (bearing substitutions in the two regulatory prolines), and infection procedures, were described previously (Esteban et al., 2006a). All plasmids were verified by sequencing.
Sodium butyrate (Sigma, St. Louis, MO) was added to confluent cultures at the indicated concentrations, and analysis was performed up to 3 d later.
Antibodies used were as follows: HIF-1α (Transduction Laboratories. Lexington, KY); HIF-2α and E-cadherin (Cancer Research UK, London); α-tubulin (Sigma); occludin, ZO-1, claudin 1 (Zymed Laboratories, South San Francisco, CA); carbonic anhydrase IX (CAIX; clone M75, provided by Dr. Pastorekova, Institute of Virology, Bratislava, Slovak Republic), and glucose transporter 1 (GLUT1; Alpha Diagnostics, San Antonio, TX).
Immunolabeling
Immunofluorescence microscopy and immunohistochemistry were performed as described (Mandriota et al., 2002; Esteban et al., 2006b). For immunofluorescence studies, cells were cultured on coverslips at confluence for up to 4 d, and fixed with cold methanol. Coverslips were mounted with Vectashield containing DAPI (Vector Laboratories, Burlingame, CA). Multiple fields were analyzed; moreover, the staining was repeated at least twice using coverslips from independent experiments. For immunohistochemical analysis we used kidney samples from five patients with VHL disease and from 10 patients with VHL-defective sporadic CCRCC (widespread HIF activation independent of predicted oxygen gradients—indicative of VHL inactivation—was verified in each tumor; data not shown). Quantitative analyses of junction assembly were performed across nine independent fields. Percentage junction assembly was determined for each cell in a given field, based on the percentage of cell membrane labeled for the TJ protein. The overall score for a single field was the mean junction assembly of all cells present in that field (∼50 cells per field).
Immunoblotting and Quantitative Real-Time Reverse Transcription PCR
Urea-SDS buffer was used to prepare cell lysates (Maxwell et al., 1999); protein concentration was measured using BCA (Pierce, Rockford, IL). ECL or ECL plus (Amersham Pharmacia, Piscataway, NJ) were used for visualization. For quantitative real-time reverse transcription (RT)-PCR analysis, SYBR Green (Abgene, Epsom, United Kingdom) or an On-Demand Taqman assay (for ZO-1 and claudin 1; Applied Biosystems, Foster City, CA), and an Opticon 2 PCR machine (MJ Research, Waltham, MA), were used. Primer sequences for SYBR Green analysis are available upon request. Samples were analyzed in triplicate, and the mean is presented. Expression of β-actin was not influenced by VHL status and was used for normalization. Statistical analysis was performed using Student's t test.
Small Interfering RNA Transfections
Small interfering RNA (siRNA) transfections were performed using Lipofectamine RNAiMAX (Invitrogen), according to the instructions of the manufacturer, at an oligonucleotide concentration of 50 nM. Sequences for siRNA oligos for firefly luciferase, HIF-1α, and HIF-2α, were as described (Esteban et al., 2006b). Lysates were prepared, and coverslips were fixed, up to 5 d after transfection. Similar results were obtained using an independent nonoverlapping set of siRNA oligos for HIF-1α and HIF-2α (data not shown). To silence E-cadherin, reverse siRNA transfections were performed using Lipofectamine RNAiMAX (Invitrogen), according to the manufacturer's instructions. siRNA oligonucleotides were purchased from Ambion (Austin, TX; ECA1, ID s2769; ECA2, ID s2770) and used at a concentration of 20 nM.
RESULTS
VHL Loss-of-Function Down-Regulates the Expression of TJ Constituents in Renal Epithelial Cells In Vitro and In Vivo
To investigate how VHL influences TJs, we first analyzed the effect of reexpressing VHL in VHL-defective CCRCC cell lines on the expression of the TJ components ZO-1, occludin, and claudin 1 (Fanning et al., 1999; Balkovetz, 2006). As previously reported, Western blotting showed that ZO-1 protein levels were not significantly affected by VHL reexpression (Figure 1A). In contrast, RCC4 and RCC10 CCRCC cells expressing VHL contained higher levels of occludin and claudin 1 than in the parental cell lines (Figure 1A). As expected, E-cadherin was also expressed at a higher level in the presence of VHL. Occludin was expressed more strongly in RCC4/VHL than RCC10/VHL, whereas E-cadherin and claudin 1 were expressed at a higher level in RCC10/VHL than RCC4/VHL (Figure 1A).
Figure 1.
VHL loss-of-function down-regulates the expression of occludin and claudin 1 in CCRCC cell lines. (A) Western blot showing that occludin and claudin 1 protein levels are increased when VHL is expressed in RCC4 and RCC10 cells. HIF-1α, HIF-2α, E-cadherin, ZO-1, and α-tubulin (loading control) are also shown. (B) Phase-contrast captures and immunofluorescence labeling for ZO-1, occludin, and claudin 1, in RCC4 and RCC10 cells. Labeling for claudin 1 in RCC4 cells was less intense than in RCC10. Bar, 40 μm. (C) Quantitative real-time RT-PCR analysis of occludin, claudin 1, ZO-1, and GLUT1 mRNA, in RCC4 and RCC10 cells and corresponding stable transfectants expressing VHL. Three independent experiments and the corresponding mean are presented. Differences in occludin, claudin 1, and GLUT1 mRNA expression levels were statistically significant (p < 0.05).
Immunofluorescence labeling for ZO-1 can be used to evaluate TJ integrity in cells cultured in vitro; ZO-1 localization is cytoplasmic in sparse nontransformed epithelial cells, but distributes to TJs in confluent monolayers. It has been previously demonstrated that reexpression of VHL improves epithelial-like morphology and facilitates ZO-1 assembly at TJs in CCRCC cell lines (Calzada et al., 2006). Immunofluorescence analysis revealed localization of ZO-1, occludin, and claudin 1, at points of cell–cell contact between VHL-expressing RCC4 and RCC10 cells. Occludin and claudin 1 were not detected by immunofluorescence in the parental cell lines, and the signal from ZO-1 was weaker and discontinuous (Figure 1B). Quantitative real-time RT-PCR analysis showed that reexpression of VHL increases occludin mRNA and claudin 1 mRNA in both RCC4 and RCC10 cells (Figure 1C); mRNA for ZO-1 was not substantially changed on VHL reexpression. The analysis of GLUT1 mRNA levels (a well-characterized HIF target) confirmed that HIF activation was suppressed by VHL (Figure 1C).
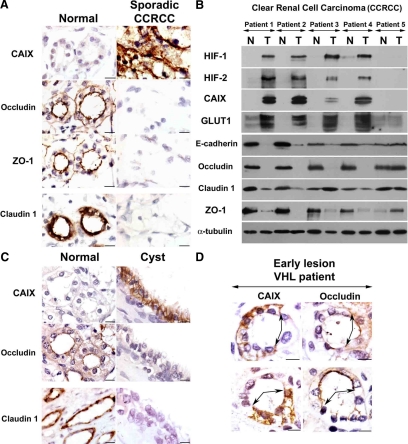
We next performed immunohistochemistry for occludin and claudin 1 on surgical samples from patients with sporadic VHL-defective CCRCC and patients with inherited VHL disease. In unaffected kidney tissue, expression of ZO-1 and occludin was detected in distal tubules and the collecting duct, and at a lower level in the glomerulus and proximal tubule; claudin 1 showed a more restricted distribution, being present in the glomerulus, thin descending limb of the loop of Henle, and the collecting duct. Sporadic CCRCC showed very little labeling for ZO-1, occludin, and claudin 1, compared with the adjacent normal tissue (Figure 2A). Similar results were observed in CCRCC samples from VHL patients (data not shown). As expected, sporadic CCRCC showed intense labeling for carbonic anhydrase IX (CAIX), a marker of HIF activation (Figure 2A). In addition, we performed immunoblotting of extracts of paired normal tissue (N) and tumor (T) from five patients with sporadic CCRCC. CCRCC represents the most frequent form of renal cancer (∼80%), and ∼60–80% have biallelic VHL inactivation. Constitutive activation of HIF-1α and HIF-2α (and the HIF targets GLUT1 and CAIX), consistent with VHL inactivation, was evident in four of the five CCRCC samples analyzed (Figure 2B). High levels of HIF in these four tumors were associated with lower protein levels of occludin and E-cadherin than in the paired normal tissue. Interestingly, ZO-1 protein levels were also reduced in these tumors compared with normal tissue, which may reflect a more profound disturbance of TJs in CCRCC in vivo than in cell lines in vitro. Western blotting for claudin 1 showed variable results between different patients. Levels of occludin, claudin 1, E-cadherin, and ZO-1 were not decreased compared with normal tissue in the single tumor sample (patient 5), which did not show a high level of HIF activation. A limitation to these comparisons between tumors and paired uninvolved renal tissue is that the latter includes all segments of the nephron, together with interstitial cells. This may explain variability in levels of cell–cell adhesion molecules in the normal tissue between the different patients. We also analyzed whether TJ might be altered in premalignant lesions of VHL inactivation, using samples of kidneys of patients with VHL disease. We observed that occludin and claudin 1 expression was low in cystic lesions compared with nearby tubules expressing these proteins (Figure 2C). Furthermore, in the earliest lesions of VHL inactivation (inside otherwise normal tubules), which we identified by CAIX labeling (Mandriota et al., 2002), occludin expression was reduced compared with adjacent cells (Figure 2D). VHL-defective early lesions were not identified within segments of the nephron positive for claudin 1, so we could not examine effects on expression of this protein in the same way.
Figure 2.
TJs are disrupted after VHL inactivation in renal-derived cells in vivo. (A) Serial sections from a representative sporadic VHL-defective CCRCC, which also includes adjacent unaffected tissue. Labeling was performed for ZO-1, occludin, claudin 1, and CAIX. Micrographs show representative fields of normal and tumor tissue from different areas of the same section. (B) Western blot with the indicated antibodies of five sporadic VHL-defective CCRCC tumors and paired unaffected kidney tissue. N, normal tissue; T, tumor. Consistent with VHL inactivation in the majority of sporadic CCRCCs, four of five tumors showed HIF activation (patients 1–4). E-cadherin, occludin, and ZO-1 were down-regulated in all four tumors displaying HIF activation; results with claudin 1 were more variable. (C) Serial sections labeled with the indicated antibodies of kidney tissue containing a cyst from a VHL patient (images are presented as in A). Ten independent cysts were analyzed with similar results. (D) Serial sections containing an early lesion of VHL inactivation (in an otherwise normal tubule) labeled for the HIF target gene CAIX and occludin. Early lesions from five VHL patients were analyzed and showed similar results. Bar, 10 μm.
Thus, VHL loss-of-function in kidney epithelial cells is associated with TJ disruption both in vitro and in vivo, and this is associated with reduced expression of occludin and claudin 1.
Disruption of AJs and TJs in VHL-defective CCRCC Cells Are Not Interdependent
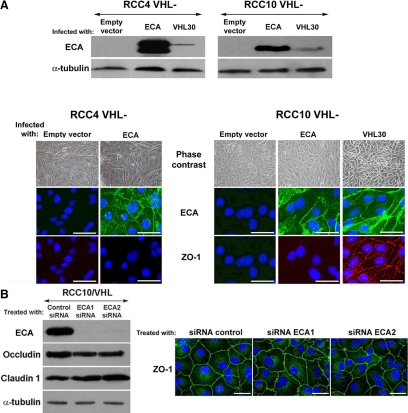
EMT involves a series of coordinated changes resulting in increased motility, loss of cell–cell adhesion, and fibroblastoid morphology (Nieto, 2002; Thiery and Sleeman, 2006). Down-regulated expression of E-cadherin is regarded as a key aspect of EMT in many cancers (Krishnamachary et al., 2006; Russell and Ohh, 2007). To evaluate whether reduced E-cadherin is responsible for alterations in cell shape and TJs in VHL-defective CCRCC cells, we infected RCC4 and RCC10 cells with a retroviral vector encoding human E-cadherin; empty vector was used as a negative control. E-cadherin expression was confirmed by Western analysis (Figure 3) and was substantially higher than in pools of cells reexpressing VHL, especially for RCC4. Double immunofluorescence for E-cadherin (in green) and ZO-1 (in red) showed intense E-cadherin staining at AJs in RCC10 and RCC4 cells overexpressing E-cadherin (Figure 3), but neither cell shape nor ZO-1 assembly was improved. Assembly of the transmembrane TJ proteins occludin or claudin 1 were also unaffected by E-cadherin overexpression (Supplemental Figure S1A). To further investigate the relationship between AJ and TJ assembly in CCRCC cells, we used two different siRNAs to silence E-cadherin in RCC10 cells. Despite markedly reduced E-cadherin levels no effect was observed on ZO-1 assembly in cells treated with either siRNA compared with control treated cells (Figure 3B). Treatment of RCC10/VHL cells with E-cadherin–blocking antibodies (HECD1 and SHE78-7) also had no effect on TJ assembly (data not shown).
Figure 3.
VHL regulates TJ assembly independently of E-cadherin. (A) Western blot for E-cadherin using lysates from VHL negative RCC10 and RCC4 cells overexpressing E-cadherin; VHL 30 and empty vector were used as controls. Phase-contrast captures and immunofluorescence (double with ZO-1) of the same cell lines is also shown. E-cadherin was below the immunofluorescence detection threshold in the pool of RCC4 cells infected with VHL 30. (B) Western blot analysis of E-cadherin, occludin, and claudin 1 in RCC10/VHL cells treated with either a control oligo or two independent E-cadherin siRNA oligos. Representative phase-contrast photomicrographs and immunofluorescence captures of ZO-1 assembly are also shown. Bar, 40 μm. ECA, E-cadherin.
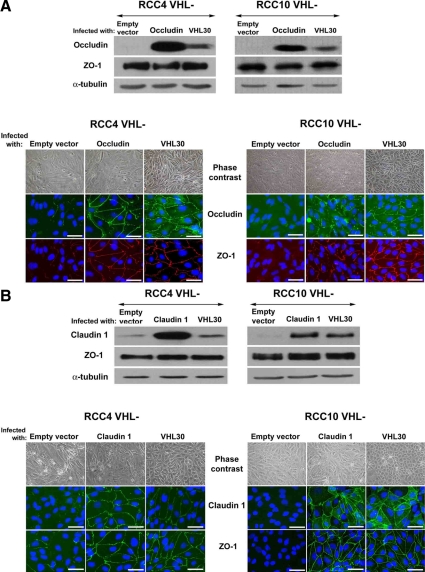
In parallel, RCC4 cells and RCC10 cells were separately infected with retroviruses encoding human occludin or human claudin 1. Corresponding protein levels were also high compared with cells infected with empty vector (Figure 4, A and B), whereas ZO-1 expression was unchanged. Overexpressed occludin and claudin 1 significantly distributed to TJs and increased ZO-1 assembly in VHL-defective RCC4 and RCC10 cells (Figure 4, A and B). Interestingly, this was more evident with claudin 1 and was in neither case associated with significant restoration of epithelial-like cell shape. Overexpression of either occludin or claudin 1 did not restore E-cadherin assembly (Supplemental Figure S1B).
Figure 4.
Reexpression of occludin or claudin 1 restores TJs in VHL negative CCRCC cells. VHL-defective RCC4 and RCC10 cell pools infected with retroviruses encoding (A) occludin, or (B) claudin 1. Empty vector and VHL 30 were used as controls. Immunofluorescence and phase contrast captures, and Western blots with the indicated antibodies are shown. Double labeling for ZO-1 and occludin is presented in A, and single labeling for claudin 1 and ZO-1 is shown in B. Bar, 40 μm.
Therefore, disorganization of AJs and TJs due to VHL inactivation are not interrelated and can be separately improved through overexpression of E-cadherin or TJ transmembrane constituents respectively. Moreover, breakage of either AJs or TJs, at least on its own, does not seem to be responsible for the fibroblastoid morphology observed in these VHL-defective CCRCC cell lines.
Type IIC VHL Mutations Restore Epithelial Cytoarchitecture in VHL-defective RCC4 and RCC10 Cell Lines
The three major clinical manifestations of VHL disease are CCRCC, pheochromocytomas, and hemangioblastomas of the retina and CNS (Kaelin, 2002). VHL disease can be divided into four clinical subtypes depending on the risk of these manifestations: I (high risk of CCRCC and hemangioblastoma and low risk of pheochromocytoma); IIA (low risk of CCRCC and high risk of hemangioblastoma and pheochromocytoma); IIB (high risk of all three manifestations); and IIC (pheochromocytoma only). Functional analysis of genetic mutations associated with these subtypes of VHL disease offers potential insight into which molecular consequences of VHL inactivation underlie development of CCRCC (Clifford et al., 2001; Hoffman et al., 2001; Li et al., 2007). There is a correlation between the risk of CCRCC and the ability of VHL mutants to regulate HIF. Type I and type IIB mutations effectively abolish the ability of VHL to regulate HIF, type IIA mutations have an incomplete effect, and type IIC mutations regulate HIF normally. Rescue of aspects of the VHL-defective phenotype by type IIC VHL mutations in CCRCC cell lines is consistent with effects that are caused by HIF activation, although other consequences of VHL loss of function may also be rescued, for example, altered microtubule stability (Hergovich et al., 2003).
We expressed the type IIC disease–associated VHL mutations encoding V84L and L188V in VHL-defective RCC10 cells. Empty vector and vectors encoding VHL19 and VHL30 were used as positive controls. These VHL isoforms have molecular weights of ∼19 and 30 kDa, with VHL 19 arising from an alternative transcription initiation site at codon 54 (Kaelin, 2002). Both isoforms suppress HIF activation and tumor growth in xenograft assays, but VHL 19 does not rescue fibronectin assembly. Real-time RT-PCR analysis of PHD3/EGLN3 mRNA (a well-characterized HIF target) confirmed suppression of HIF by VHL 19, VHL 30, VHL V84L, and VHL L188V (Figure 5A). In addition, VHL 19, VHL 30, and both type IIC VHL mutations restored epithelial-like cell shape and TJ assembly in RCC10 pools (Figures 5B). Western blotting for occludin and claudin 1 showed substantial up-regulation in RCC10 cells expressing VHL 19, VHL 30, and the two type IIC VHL mutations, compared with empty vector (Figure 5C). We also expressed type IIC VHL mutants and wild-type VHLs in VHL-defective RCC4 cells and observed similar results. Type IIC mutants suppressed mRNA levels of the HIF target gene PHD3 and rescued both epithelial-like cell shape and assembly of occludin and ZO-1, compared with an empty vector. Occludin expression levels were also increased (Figure 5, D–F).
Figure 5.
Type IIC VHL mutations restore epithelial characteristics in VHL-defective RCC10 and RCC4 cells. Quantitative real-time RT-PCR analysis of PHD3 mRNA confirms suppression of HIF in VHL-defective RCC10 (A) and RCC4 (D) pools infected with VHL 19, VHL 30, and VHL V84L and VHL L188V. Three independent experiments and the corresponding mean are presented; differences were statistically significant (p < 0.05). (B) Phase-contrast images of RCC10 cell pools and immunofluorescence for ZO-1, occludin, and claudin 1. Bar, 40 μm. (C) Western blot for occludin, claudin 1, and α-tubulin in RCC10 cell pools. (E) Phase-contrast images of RCC4 cell pools and immunofluorescence for ZO-1 and occludin. Bar, 40 μm. (F) Western blot for occludin and α-tubulin in RCC4 cell pools.
Therefore, two different VHL mutant molecules that are not associated with increased CCRCC risk, and that retain their ability to regulate HIF, are competent to restore epithelial cell shape and TJs in VHL-defective RCC4 and RCC10 CCRCC cell lines.
HIF Participates in the EMT-like Phenotype of VHL-defective CCRCC Cell Lines
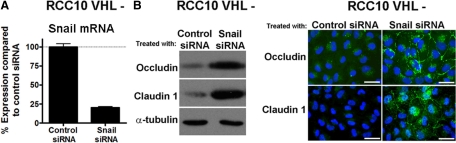
The experiments using VHL type IIC mutations suggest that HIF may be involved in inducing EMT in CCRCC cell lines. To investigate this further, we selectively knocked down HIF-1α or HIF-2α in VHL-defective RCC10 cells, using previously validated siRNA oligos (Esteban et al., 2006b). Specific reduction of the corresponding HIF-α isoform, when compared with control oligos, was verified by Western blotting (Figure 6A). HIF-1α knockdown increased protein levels of claudin 1 and to a lesser extent occludin, whereas ZO-1 levels remained unchanged. Immunofluorescence microscopy demonstrated that silencing HIF-1α increased assembly of the TJ proteins ZO-1 (85 ± 1.12%), occludin (81.5 ± 1.59%), and claudin 1 (74.5 ± 3.80%); in addition, HIF-1α siRNA induced an epithelial-like cell shape (Figure 6A). HIF-2α siRNA also increased occludin and claudin 1 levels, but the effect on TJ assembly and cell shape was less striking and more variable than treatment with HIF-1α siRNA (ZO-1, 42 ± 2.50%; occludin, 48 ± 2.38%; claudin 1, 27.5 ± 3.68%; Figure 6A). To further confirm the role of HIF-1α in the changes of VHL-negative RCC10 cells, we also expressed a constitutively active HIF-1α in VHL-expressing RCC10 cells. This resulted in a substantial alteration in cell shape, reduced occludin expression, and decreased ZO-1/occludin assembly (Figure 6B). Taken together with the above experiments expressing VHL type IIC mutations, our results strongly support a role for HIF in inducing EMT in VHL-defective RCC10 cells. E-box–dependent transcriptional repressors are known to down-regulate both AJ and TJ proteins and provide a potential route for down-regulation of multiple cell adhesion molecules at the mRNA level (Ikenouchi et al., 2003; Kajita et al., 2004). Furthermore several of these repressors have been previously reported to be up-regulated in VHL-defective renal cancer cell lines (Esteban et al., 2006b; Krishnamachary et al., 2006; Evans et al., 2007). Using real-time quantitative RT-PCR, we analyzed the effect of VHL status on a panel of repressors in both the RCC10 and RCC4 cell backgrounds. SNAIL1 was found to be the most highly up-regulated transcriptional repressor (about sixfold) in RCC10 VHL-defective cells compared with cells expressing VHL (Supplemental Table S1). To determine whether SNAIL1 knockdown could rescue the TJ assembly in RCC10 VHL-defective cells, we used siRNA to silence SNAIL1. Treatment resulted in ∼80% knockdown of SNAIL1, and Western blotting analysis showed increased expression of the TJ proteins occludin and claudin 1, compared with a control siRNA (Figure 7). Improved assembly of ZO-1, occludin and claudin 1 was also observed (Figure 7). These results indicate that E-box transcriptional repressors, such as SNAIL1, are likely to mediate, at least in part, the TJ assembly defect observed in RCC10 VHL-defective cells.
Figure 6.
HIF-1α is involved in the EMT-like phenotype of VHL-negative RCC10 cells. (A) Western blot with the indicated antibodies of VHL-defective RCC10 cells treated with control siRNA oligos, and siRNA oligos specific for HIF-1α or HIF-2α. A representative experiment is shown. Phase-contrast images and immunofluorescence labeling for ZO-1, occludin, and claudin 1 from the same siRNA-transfected cells. Immunofluorescence captures comparing different siRNA treatments were taken using similar exposure times. Quantification analyses of junction assembly was also performed across nine independent fields (∼50 cells per field) to permit comparison of the effects of silencing the individual HIF-α subunits. Error bars, SEM. (B) Western blot for HIF-1α and occludin, phase-contrast captures, and immunofluorescences for ZO-1 and occludin of VHL-positive RCC10 cells infected with a constitutively active form of HIF-1α. A representative experiment is shown. Bar, 40 μm.
Figure 7.
SNAIL1 is involved in the EMT-like phenotype of VHL-negative RCC10 cells. (A) Quantitative real-time RT-PCR analysis of SNAIL1 mRNA levels in VHL-defective RCC10 cells treated with either control siRNA oligos or siRNA oligos specific for SNAIL1. Three independent experiments and the corresponding mean are presented. Snail1 knockdown was statistically significant (p < 0.05). (B) Representative Western blot and immunofluorescence captures show the expression level and TJ labeling of occludin and claudin 1 in RCC10 cell treated with SNAIL1 siRNA oligos. Bar, 40 μm.
Sodium Butyrate Improves Epithelial Characteristics in VHL-defective RCC4 and RCC10 Cells
Recent reports have shown that inhibition of histone deacetylases (HDACs) can decrease HIF protein levels and transactivation in VHL-defective cell lines and in VHL competent cells exposed to hypoxia. Therefore we hypothesized that sodium butyrate, a short-chain fatty acid that inhibits HDACs and that is in clinical use (Bi and Jiang, 2006; Bilton et al., 2006; Minucci and Pelicci, 2006), might increase expression of cell junction molecules and promote epithelial-like differentiation in VHL-defective CCRCC cells. Indeed treatment of VHL-defective RCC10 and RCC4 cells significantly restored epithelial-like cell shape and resulted in increased labeling for ZO-1, occludin, and claudin 1 at intercellular junctions (Figure 8, A and B). In parallel, Western blotting showed down-regulation of HIF-1α protein levels (HIF-2α levels were much less affected) and of the target gene GLUT1 (Figure 8, A and B), with concomitant increased expression of E-cadherin, occludin, and claudin 1. Therefore sodium butyrate markedly improves epithelial differentiation and increases expression of cell–cell adhesion molecules in VHL-negative CCRCC cells, while decreasing HIF-1α protein levels.
Figure 8.
Sodium butyrate improves epithelial characteristics in VHL-defective RCC4 and RCC10 cells. VHL-defective RCC4 (A) and RCC10 cells (B) were grown to confluence and treated for 3 d with the indicated doses of sodium butyrate, before phase-contrast imaging and immunofluorescence labeling, or immunoblotting. RCC4 and RCC10 cells stably expressing VHL were included for comparison in the immunoblot analysis. Bars, 40 μm.
DISCUSSION
In this study we have investigated whether the expression of transmembrane TJ constituents is affected by VHL loss-of-function, the role of HIF activation, and whether loss of the AJ protein E-cadherin is the driving force behind a comprehensive EMT.
As expected, we confirmed that TJ assembly is disrupted in VHL-defective CCRCC cells in vitro. Importantly, we show that TJ disruption is coincident with HIF activation in the earliest detectable premalignant lesions in the kidneys of patients with VHL disease. This implies that VHL is necessary for maintenance of normal TJs in human renal epithelium and raises the possibility that TJ disruption could contribute to evolution from normal epithelium to tumor. This is plausible because TJ disruption may facilitate tubular disintegration, enhancing migration, and increasing proliferation. (Mullin, 2004; Matter and Balda, 2007). Furthermore, there is evidence of down-regulation of occludin in malignant transformation in other settings (Wang et al., 2005, 2007; Osanai et al., 2006).
The role of VHL in TJ maintenance reinforces that VHL loss-of-function leads to a dedifferentiated phenotype in renal tubular cells. This may provide insight into the paradox that although VHL is a gatekeeper tumor suppressor on genetic grounds (Kaelin, 2002), VHL status seems to have little effect on proliferation of renal epithelium (Mandriota et al., 2002). One attractive hypothesis is that aspects of dedifferentiation allow renal epithelial cells to become tolerant to subsequent genetic events, which would otherwise result in apoptosis.
Previously it was been suggested that the down-regulation of E-cadherin is sufficient to produce a comprehensive EMT in VHL-defective cells (Krishnamachary et al., 2006; Russell and Ohh, 2007). We tested this directly by reexpressing E-cadherin in VHL-defective cells, although it localized to cell–cell contacts it did not rescue TJ formation, nor did it restore an epithelial-like cell shape. Furthermore we also silenced E-cadherin expression in RCC10 cells and observed no effect on TJ assembly (Figure 3). Taken together this implies that disruption of TJs is not dependent on E-cadherin down-regulation in these cells and is consistent with other reports showing that AJ assembly in some other settings is not a prerequisite for TJ formation (Potempa and Ridley, 1998; Chen et al., 2000; Theard et al., 2007). Notably we found that VHL status had a marked effect on expression of occludin and claudin 1, which involved up-regulation at the mRNA level. Occludin and claudin 1 reexpression were able to significantly rescue ZO-1 organization. This is consistent with the mechanism of TJ disruption involving down-regulation of these key TJ components and provides further evidence that TJ effects are not mediated via effects on E-cadherin expression and the AJ. Interestingly, in our studies claudin 1 reexpression had a more substantial effect than occludin reexpression, which is consistent with a more severe phenotype in claudin-1 than occludin knockout mice (Saitou et al., 2000; Furuse et al., 2002).
Mechanistically we show that HIF activation is necessary and sufficient for loss of TJs in RCC10 cells. In RCC4 cells expression of type IIC mutants restored epithelial cell shape and TJ assembly, suggesting the involvement of HIF. However siRNA for HIF components in RCC4 cells did not show effective rescue of TJ components (data not shown), which may indicate the need for a more absolute and/or prolonged knockdown than was achieved in our experiments. Support for the latter comes from a study showing that infection of VHL-defective RCC4 cells with retroviruses encoding either a dominant negative version of HIF-1α or shRNA for HIF-1α increases transepithelial resistance, indicating improved TJ integrity (Krishnamachary et al., 2006). There is also previous evidence that VHL can exert a HIF independent role in TJ maintenance from studies of 786-O cells (Calzada et al., 2006). Taken together, it is likely that both HIF-dependent and -independent mechanisms contribute to TJ disruption in VHL-defective CCRCC cell lines.
Understanding further how VHL loss-of-function acts through HIF-dependent and -independent mechanisms to alter epithelial-cell differentiation in general, and formation of intercellular junctions in particular, may provide potential routes to interfere with CCRCC tumorigenesis. We and others have shown that the mRNA for a series of transcriptional repressors including SNAIL1, ZEB2, and E47, are up-regulated in VHL-defective CCRCC cell lines (Esteban et al., 2006b; Krishnamachary et al., 2006; Evans et al., 2007; Ikenouchi et al., 2003; Ohkubo and Ozawa, 2004; Martinez-Estrada et al., 2006). Using chromatin immunoprecipitation (ChIP), two recent reports show that HIF can bind to the promoter region of Twist in FaDu (human hypopharyngeal carcinoma) and H1299 (lung cancer) cells (Yang et al., 2008) and to the SNAIL1 promoter in hypoxic SKOV3 (ovarian cancer) cells, although the latter appears to be dependent on Notch activation (Sahlgren et al., 2008). Taken together with our report, these studies suggest that HIF is likely to up-regulate different transcriptional repressors in a number of cell models. Previous studies have suggested that E-box repressors play a role in suppression of E-cadherin in RCC cells. Our data indicates that up-regulation of SNAIL1 may also contribute to the disruption of TJ assembly in RCC10 VHL-defective cells (Figure 7). However, the relationship between HIF, the transcriptional repressors and EMT is likely to be quite complex, with the possibility that different repressors and combinations of repressors can synergize to effect an EMT in a tissue and cell type–specific manner.
Nevertheless, the identification of HIF as being capable of TJ disruption implies that hypoxia could disturb TJ function in epithelia. One system in which TJs have a crucial function and have been studied extensively is the cerebral circulation, where they form seals between adjacent capillary endothelial cells and are crucial for the integrity of the blood brain barrier (Wolburg and Lippoldt, 2002). Intriguingly, hypoxia has been associated with changes in both paracellular permeability and localization of TJ proteins in cerebral microvessel endothelial cells (Mark and Davis, 2002), and it will be interesting to determine if these changes are mediated by HIF activation, which could lead to unwanted effects in the clinical development of HIF activators.
There is considerable interest in decreasing HIF activation in cancer. Recent studies have established that HDAC inhibitors decrease HIF activation in tumor cell lines (Kim et al., 2001, 2007; Fath et al., 2006; Kong et al., 2006; Qian et al., 2006), although the mechanism(s) by which HDAC modulate HIF activity remains controversial (Bilton et al., 2006). Our experiments demonstrate that sodium butyrate, an HDAC inhibitor in clinical use (Davie, 2003), improves epithelial-like cell shape and TJ assembly in VHL-defective RCC4 and RCC10 cells and increases expression of E-cadherin, occludin, and claudin 1. The interplay between HIF-1α and HIF-2α in CCRCC tumorigenesis, and their respective target selectivity, is complex and incompletely understood. The majority of HIF target genes are regulated by both isoforms. A subset of HIF target genes has emerged, however, which show preferential regulation by a particular isoform, though this regulation may be dependent on additional factors, including the cell type (Raval et al., 2005). The rather selective inhibitory effect of sodium butyrate on HIF-1α protein levels compared with HIF-2α is interesting and supports our siRNA experiments indicating that HIF-1α is more important than HIF-2α in mediating EMT in VHL-defective CCRCC cells. A caveat to this interpretation is that HIF-2α transactivation may be suppressed by sodium butyrate despite a preserved level of protein (Fath et al., 2006); consistent with this, GLUT1, which is known to be activated by HIF-2 but not HIF-1 in RCC4 cells (Raval et al., 2005), was potently down-regulated in our experiments. In addition, although we used sodium butyrate primarily to suppress HIF, it is also possible that the effect of sodium butyrate is not solely mediated via repression of HIF. For example HDAC modulate the function of other transcription factors including the repressor SNAIL1 (Peinado et al., 2004), and HDAC inhibitors have been shown to prevent TGF-β–mediated EMT in renal tubular cells in vitro (Yoshikawa et al., 2007).
In summary, VHL inactivation in the kidney epithelium triggers a down-regulation of cell–cell adhesion molecules involved in both AJs and TJs, which results in a dismantling of intercellular junctions and EMT. Further understanding as to how VHL loss-of-function achieves this, and the role of HIF, will be important in understanding the role of VHL in normal renal epithelium. In this regard, we and others have shown that mRNA for a series of transcriptional repressors including SNAIL1, ZEB2, E47, and Twist, are up-regulated via HIF in VHL-defective cells (Esteban et al., 2006b; Krishnamachary et al., 2006; Evans et al., 2007). It is likely that the mechanism linking VHL to different aspects of EMT involves complex interplay between HIF-dependent and -independent recruitment of overlapping combinations of these repressors. From a therapeutic perspective it is attractive that inhibiting HDACs may offer a route to comprehensive reversal of the EMT.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by a Cancer Research UK grant to P.H.M. and M.A.E. and by the EU framework 6 integrated project Euroxy.
Abbreviations used:
- AJ
adherens junction
- CAIX
carbonic anhydrase IX
- CCRCC
clear cell renal cell carcinomas
- ECA
E-cadherin
- EMT
epithelial-to-mesenchymal transition
- GLUT1
glucose transporter 1
- HDAC
histone deacetylase
- HIF
hypoxia-inducible factor
- N
normal tissue
- PHD
prolyl hydroxylase domain
- T
tumor tissue
- TJ
tight junction
- VHL
von Hippel-Lindau
- ZO
zona occludens.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E08-06-0566) on January 14, 2009.
REFERENCES
- Balkovetz D. F. Claudins at the gate: determinants of renal epithelial tight junction paracellular permeability. Am. J. Physiol. Renal Physiol. 2006;290:F572–F579. doi: 10.1152/ajprenal.00135.2005. [DOI] [PubMed] [Google Scholar]
- Bi G., Jiang G. The molecular mechanism of HDAC inhibitors in anticancer effects. Cell Mol. Immunol. 2006;3:285–290. [PubMed] [Google Scholar]
- Bilton R., Trottier E., Pouyssegur J., Brahimi-Horn M. C. ARDent about acetylation and deacetylation in hypoxia signalling. Trends Cell Biol. 2006;16:616–621. doi: 10.1016/j.tcb.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Boutet A., De Frutos C. A., Maxwell P. H., Mayol M. J., Romero J., Nieto M. A. Snail activation disrupts tissue homeostasis and induces fibrosis in the adult kidney. EMBO J. 2006;25:5603–5613. doi: 10.1038/sj.emboj.7601421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braga V. M. Cell-cell adhesion and signalling. Curr. Opin. Cell Biol. 2002;14:546–556. doi: 10.1016/s0955-0674(02)00373-3. [DOI] [PubMed] [Google Scholar]
- Calzada M. J., Esteban M. A., Feijoo-Cuaresma M., Castellanos M. C., Naranjo-Suarez S., Temes E., Mendez F., Yanez-Mo M., Ohh M., Landazuri M. O. von Hippel-Lindau tumor suppressor protein regulates the assembly of intercellular junctions in renal cancer cells through hypoxia-inducible factor-independent mechanisms. Cancer Res. 2006;66:1553–1560. doi: 10.1158/0008-5472.CAN-05-3236. [DOI] [PubMed] [Google Scholar]
- Chen Y., Lu Q., Schneeberger E. E., Goodenough D. A. Restoration of tight junction structure and barrier function by down-regulation of the mitogen-activated protein kinase pathway in ras-transformed Madin-Darby canine kidney cells. Mol. Biol. Cell. 2000;11:849–862. doi: 10.1091/mbc.11.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifford S. C., Cockman M. E., Smallwood A. C., Mole D. R., Woodward E. R., Maxwell P. H., Ratcliffe P. J., Maher E. R. Contrasting effects on HIF-1alpha regulation by disease-causing pVHL mutations correlate with patterns of tumourigenesis in von Hippel-Lindau disease. Hum. Mol. Genet. 2001;10:1029–1038. doi: 10.1093/hmg/10.10.1029. [DOI] [PubMed] [Google Scholar]
- Conacci-Sorrell M., Zhurinsky J., Ben-Ze'ev A. The cadherin-catenin adhesion system in signaling and cancer. J. Clin. Invest. 2002;109:987–991. doi: 10.1172/JCI15429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davie J. R. Inhibition of histone deacetylase activity by butyrate. J. Nutr. 2003;133:2485S–2493S. doi: 10.1093/jn/133.7.2485S. [DOI] [PubMed] [Google Scholar]
- Dressler G. R. Development of the excretory system. In: Rossant R. J., Tam P., editors. Mouse Development. Toronto, ON, Canada: Academic Press; 2002. pp. 395–420. [Google Scholar]
- Esteban M. A., Harten S. K., Tran M. G., Maxwell P. H. Formation of primary cilia in the renal epithelium is regulated by the von Hippel-Lindau tumor suppressor protein. J. Am. Soc. Nephrol. 2006a;17:1801–1806. doi: 10.1681/ASN.2006020181. [DOI] [PubMed] [Google Scholar]
- Esteban M. A., Tran M. G., Harten S. K., Hill P., Castellanos M. C., Chandra A., Raval R., O'Brien T S., Maxwell P. H. Regulation of E-cadherin expression by VHL and hypoxia-inducible factor. Cancer Res. 2006b;66:3567–3575. doi: 10.1158/0008-5472.CAN-05-2670. [DOI] [PubMed] [Google Scholar]
- Evans A. J., et al. VHL promotes E2 box-dependent E-cadherin transcription by HIF-mediated regulation of SIP1 and snail. Mol. Cell. Biol. 2007;27:157–169. doi: 10.1128/MCB.00892-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanning A. S., Mitic L. L., Anderson J. M. Transmembrane proteins in the tight junction barrier. J. Am. Soc. Nephrol. 1999;10:1337–1345. doi: 10.1681/ASN.V1061337. [DOI] [PubMed] [Google Scholar]
- Fath D. M., Kong X., Liang D., Lin Z., Chou A., Jiang Y., Fang J., Caro J., Sang N. Histone deacetylase inhibitors repress the transactivation potential of hypoxia-inducible factors independently of direct acetylation of HIF-alpha. J. Biol. Chem. 2006;281:13612–13619. doi: 10.1074/jbc.M600456200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuse M., Hata M., Furuse K., Yoshida Y., Haratake A., Sugitani Y., Noda T., Kubo A., Tsukita S. Claudin-based tight junctions are crucial for the mammalian epidermal barrier: a lesson from claudin-1-deficient mice. J. Cell Biol. 2002;156:1099–1111. doi: 10.1083/jcb.200110122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Mariscal L., Lechuga S., Garay E. Role of tight junctions in cell proliferation and cancer. Prog. Histochem. Cytochem. 2007;42:1–57. doi: 10.1016/j.proghi.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Guillemot L., Paschoud S., Pulimeno P., Foglia A., Citi S. The cytoplasmic plaque of tight junctions: a scaffolding and signalling center. Biochim. Biophys. Acta. 2008;1778:601–613. doi: 10.1016/j.bbamem.2007.09.032. [DOI] [PubMed] [Google Scholar]
- Hanahan D., Weinberg R. A. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- Hergovich A., Lisztwan J., Barry R., Ballschmieter P., Krek W. Regulation of microtubule stability by the von Hippel-Lindau tumour suppressor protein pVHL. Nat. Cell Biol. 2003;5:64–70. doi: 10.1038/ncb899. [DOI] [PubMed] [Google Scholar]
- Hoffman M. A., Ohh M., Yang H., Klco J. M., Ivan M., Kaelin W. G., Jr von Hippel-Lindau protein mutants linked to type 2C VHL disease preserve the ability to downregulate HIF. Hum. Mol. Genet. 2001;10:1019–1027. doi: 10.1093/hmg/10.10.1019. [DOI] [PubMed] [Google Scholar]
- Ikenouchi J., Matsuda M., Furuse M., Tsukita S. Regulation of tight junctions during the epithelium-mesenchyme transition: direct repression of the gene expression of claudins/occludin by Snail. J. Cell Sci. 2003;116:1959–1967. doi: 10.1242/jcs.00389. [DOI] [PubMed] [Google Scholar]
- Kaelin W. G., Jr Molecular basis of the VHL hereditary cancer syndrome. Nat. Rev. Cancer. 2002;2:673–682. doi: 10.1038/nrc885. [DOI] [PubMed] [Google Scholar]
- Kajita M., McClinic K. N., Wade P. A. Aberrant expression of the transcription factors snail and slug alters the response to genotoxic stress. Mol. Cell. Biol. 2004;24:7559–7566. doi: 10.1128/MCB.24.17.7559-7566.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalluri R., Neilson E. G. Epithelial-mesenchymal transition and its implications for fibrosis. J. Clin. Invest. 2003;112:1776–1784. doi: 10.1172/JCI20530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M. S., et al. Histone deacetylases induce angiogenesis by negative regulation of tumor suppressor genes. Nat. Med. 2001;7:437–443. doi: 10.1038/86507. [DOI] [PubMed] [Google Scholar]
- Kim S. H., Kim K. W., Jeong J. W. Inhibition of hypoxia-induced angiogenesis by sodium butyrate, a histone deacetylase inhibitor, through hypoxia-inducible factor-1alpha suppression. Oncol. Rep. 2007;17:793–797. [PubMed] [Google Scholar]
- Kong X., Lin Z., Liang D., Fath D., Sang N., Caro J. Histone deacetylase inhibitors induce VHL and ubiquitin-independent proteasomal degradation of hypoxia-inducible factor 1alpha. Mol. Cell Biol. 2006;26:2019–2028. doi: 10.1128/MCB.26.6.2019-2028.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamachary B., Zagzag D., Nagasawa H., Rainey K., Okuyama H., Baek J. H., Semenza G. L. Hypoxia-inducible factor-1-dependent repression of E-cadherin in von Hippel-Lindau tumor suppressor-null renal cell carcinoma mediated by TCF3, ZFHX1A, and ZFHX1B. Cancer Res. 2006;66:2725–2731. doi: 10.1158/0008-5472.CAN-05-3719. [DOI] [PubMed] [Google Scholar]
- Kuznetsova A. V., Meller J., Schnell P. O., Nash J. A., Ignacak M. L., Sanchez Y., Conaway J. W., Conaway R. C., Czyzyk-Krzeska M. F. von Hippel-Lindau protein binds hyperphosphorylated large subunit of RNA polymerase II through a proline hydroxylation motif and targets it for ubiquitination. Proc. Natl. Acad. Sci. USA. 2003;100:2706–2711. doi: 10.1073/pnas.0436037100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latif F., et al. Identification of the von Hippel-Lindau disease tumor suppressor gene. Science. 1993;260:1317–1320. doi: 10.1126/science.8493574. [DOI] [PubMed] [Google Scholar]
- Li L., Zhang L., Zhang X., Yan Q., Minamishima Y. A., Olumi A. F., Mao M., Bartz S., Kaelin W. G., Jr. Hif linked to differential kidney cancer risk seen with type 2a and Type 2b Vhl mutations. Mol. Cell. Biol. 2007 doi: 10.1128/MCB.00282-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandriota S. J., et al. HIF activation identifies early lesions in VHL kidneys: evidence for site-specific tumor suppressor function in the nephron. Cancer Cell. 2002;1:459–468. doi: 10.1016/s1535-6108(02)00071-5. [DOI] [PubMed] [Google Scholar]
- Mark K. S., Davis T. P. Cerebral microvascular changes in permeability and tight junctions induced by hypoxia-reoxygenation. Am. J. Physiol. Heart Circ Physiol. 2002;282:H1485–H1494. doi: 10.1152/ajpheart.00645.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Estrada O. M., Culleres A., Soriano F. X., Peinado H., Bolos V., Martinez F. O., Reina M., Cano A., Fabre M., Vilaro S. The transcription factors Slug and Snail act as repressors of Claudin-1 expression in epithelial cells. Biochem. J. 2006;394:449–457. doi: 10.1042/BJ20050591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matter K., Balda M. S. Signalling to and from tight junctions. Nat. Rev. Mol. Cell Biol. 2003;4:225–236. doi: 10.1038/nrm1055. [DOI] [PubMed] [Google Scholar]
- Matter K., Balda M. S. Epithelial tight junctions, gene expression and nucleo-junctional interplay. J. Cell Sci. 2007;120:1505–1511. doi: 10.1242/jcs.005975. [DOI] [PubMed] [Google Scholar]
- Maxwell P. H., Wiesener M. S., Chang G. W., Clifford S. C., Vaux E. C., Cockman M. E., Wykoff C. C., Pugh C. W., Maher E. R., Ratcliffe P. J. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999;399:271–275. doi: 10.1038/20459. [DOI] [PubMed] [Google Scholar]
- Minucci S., Pelicci P. G. Histone deacetylase inhibitors and the promise of epigenetic (and more) treatments for cancer. Nat. Rev. Cancer. 2006;6:38–51. doi: 10.1038/nrc1779. [DOI] [PubMed] [Google Scholar]
- Mullin J. M. Epithelial barriers, compartmentation, and cancer. Sci. STKE. 2004;2004:pe2. doi: 10.1126/stke.2162004pe2. [DOI] [PubMed] [Google Scholar]
- Nelson W. J., Nusse R. Convergence of Wnt, beta-catenin, and cadherin pathways. Science. 2004;303:1483–1487. doi: 10.1126/science.1094291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto M. A. The snail superfamily of zinc-finger transcription factors. Nat. Rev. Mol. Cell Biol. 2002;3:155–166. doi: 10.1038/nrm757. [DOI] [PubMed] [Google Scholar]
- Ohh M., Yauch R. L., Lonergan K. M., Whaley J. M., Stemmer-Rachamimov A. O., Louis D. N., Gavin B. J., Kley N., Kaelin W. G., Jr, Iliopoulos O. The von Hippel-Lindau tumor suppressor protein is required for proper assembly of an extracellular fibronectin matrix. Mol. Cell. 1998;1:959–968. doi: 10.1016/s1097-2765(00)80096-9. [DOI] [PubMed] [Google Scholar]
- Ohkubo T., Ozawa M. The transcription factor Snail downregulates the tight junction components independently of E-cadherin downregulation. J. Cell Sci. 2004;117:1675–1685. doi: 10.1242/jcs.01004. [DOI] [PubMed] [Google Scholar]
- Okuda H., Saitoh K., Hirai S., Iwai K., Takaki Y., Baba M., Minato N., Ohno S., Shuin T. The von Hippel-Lindau tumor suppressor protein mediates ubiquitination of activated atypical protein kinase C. J. Biol. Chem. 2001;276:43611–43617. doi: 10.1074/jbc.M107880200. [DOI] [PubMed] [Google Scholar]
- Osanai M., Murata M., Nishikiori N., Chiba H., Kojima T., Sawada N. Epigenetic silencing of occludin promotes tumorigenic and metastatic properties of cancer cells via modulations of unique sets of apoptosis-associated genes. Cancer Res. 2006;66:9125–9133. doi: 10.1158/0008-5472.CAN-06-1864. [DOI] [PubMed] [Google Scholar]
- Peinado H., Ballestar E., Esteller M., Cano A. Snail mediates E-cadherin repression by the recruitment of the Sin3A/histone deacetylase 1 (HDAC1)/HDAC2 complex. Mol. Cell. Biol. 2004;24:306–319. doi: 10.1128/MCB.24.1.306-319.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potempa S., Ridley A. J. Activation of both MAP kinase and phosphatidylinositide 3-kinase by Ras is required for hepatocyte growth factor/scatter factor-induced adherens junction disassembly. Mol. Biol. Cell. 1998;9:2185–2200. doi: 10.1091/mbc.9.8.2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian D. Z., Kachhap S. K., Collis S. J., Verheul H. M., Carducci M. A., Atadja P., Pili R. Class II histone deacetylases are associated with VHL-independent regulation of hypoxia-inducible factor 1α. Cancer Res. 2006;66:8814–8821. doi: 10.1158/0008-5472.CAN-05-4598. [DOI] [PubMed] [Google Scholar]
- Raval R. R., Lau K. W., Tran M. G., Sowter H. M., Mandriota S. J., Li J. L., Pugh C. W., Maxwell P. H., Harris A. L., Ratcliffe P. J. Contrasting properties of hypoxia-inducible factor 1 (HIF-1) and HIF-2 in von Hippel-Lindau-associated renal cell carcinoma. Mol. Cell. Biol. 2005;25:5675–5686. doi: 10.1128/MCB.25.13.5675-5686.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell R. C., Ohh M. The role of VHL in the regulation of E-cadherin: a new connection in an old pathway. Cell Cycle. 2007;6:56–59. doi: 10.4161/cc.6.1.3668. [DOI] [PubMed] [Google Scholar]
- Sahlgren C., Gustafsson M. V., Jin S., Poellinger L., Lendahl U. Notch signaling mediates hypoxia-induced tumor cell migration and invasion. Proc. Natl. Acad. Sci. USA. 2008;105:6392–6397. doi: 10.1073/pnas.0802047105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitou M., Furuse M., Sasaki H., Schulzke J. D., Fromm M., Takano H., Noda T., Tsukita S. Complex phenotype of mice lacking occludin, a component of tight junction strands. Mol. Biol. Cell. 2000;11:4131–4142. doi: 10.1091/mbc.11.12.4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theard D., Steiner M., Kalicharan D., Hoekstra D., van Ijzendoorn S. C. Cell polarity development and protein trafficking in hepatocytes lacking E-cadherin/beta-catenin-based adherens junctions. Mol. Biol. Cell. 2007;18:2313–2321. doi: 10.1091/mbc.E06-11-1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiery J. P., Sleeman J. P. Complex networks orchestrate epithelial-mesenchymal transitions. Nat. Rev. Mol. Cell Biol. 2006;7:131–142. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- Tsukita S., Furuse M., Itoh M. Multifunctional strands in tight junctions. Nat. Rev. Mol. Cell Biol. 2001;2:285–293. doi: 10.1038/35067088. [DOI] [PubMed] [Google Scholar]
- Wang G. L., Semenza G. L. Characterization of hypoxia-inducible factor 1 and regulation of DNA binding activity by hypoxia. J. Biol. Chem. 1993;268:21513–21518. [PubMed] [Google Scholar]
- Wang Z., Mandell K. J., Parkos C. A., Mrsny R. J., Nusrat A. The second loop of occludin is required for suppression of Raf1-induced tumor growth. Oncogene. 2005;24:4412–4420. doi: 10.1038/sj.onc.1208634. [DOI] [PubMed] [Google Scholar]
- Wang Z., Wade P., Mandell K. J., Akyildiz A., Parkos C. A., Mrsny R. J., Nusrat A. Raf 1 represses expression of the tight junction protein occludin via activation of the zinc-finger transcription factor slug. Oncogene. 2007;26:1222–1230. doi: 10.1038/sj.onc.1209902. [DOI] [PubMed] [Google Scholar]
- Wolburg H., Lippoldt A. Tight junctions of the blood-brain barrier: development, composition and regulation. Vascul. Pharmacol. 2002;38:323–337. doi: 10.1016/s1537-1891(02)00200-8. [DOI] [PubMed] [Google Scholar]
- Yang M. H., Wu M. Z., Chiou S. H., Chen P. M., Chang S. Y., Liu C. J., Teng S. C., Wu K. J. Direct regulation of TWIST by HIF-1alpha promotes metastasis. Nat. Cell Biol. 2008;10:295–305. doi: 10.1038/ncb1691. [DOI] [PubMed] [Google Scholar]
- Yoshikawa M., Hishikawa K., Marumo T., Fujita T. Inhibition of histone deacetylase activity suppresses epithelial-to-mesenchymal transition induced by TGF-beta1 in human renal epithelial cells. J. Am. Soc. Nephrol. 2007;18:58–65. doi: 10.1681/ASN.2005111187. [DOI] [PubMed] [Google Scholar]
- Young A. P., Schlisio S., Minamishima Y. A., Zhang Q., Li L., Grisanzio C., Signoretti S., Kaelin W. G., Jr VHL loss actuates a HIF-independent senescence programme mediated by Rb and p400. Nat. Cell Biol. 2008;10:361–369. doi: 10.1038/ncb1699. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.