Abstract
A third staphylococcal enterotoxin C (C3) has been identified, purified, and characterized. Staphylococcal enterotoxin C3 was identified from a Staphylococcus aureus isolated received from England. The purified toxin was determined by gel permeation chromatography and sodium dodecyl sulfate-polyacrylamide gel electrophoresis to be a simple protein with a molecular weight of 26,900. The isoelectric point of the major band was determined by isoelectric focusing in polyacrylamide gels to be 8.15. The reaction of enterotoxin C3 with its specific antibody was not affected by tryptic digestion at pH 8.0 or peptic digestion at pH 4.5. The enterotoxin C3 consisted of 236 amino acid residues. Serine was shown to be the NH2-terminal amino acid residue by end group analysis. The protein was highly emetic in cynomolgus monkeys both per os and intravenously.
Full text
PDF

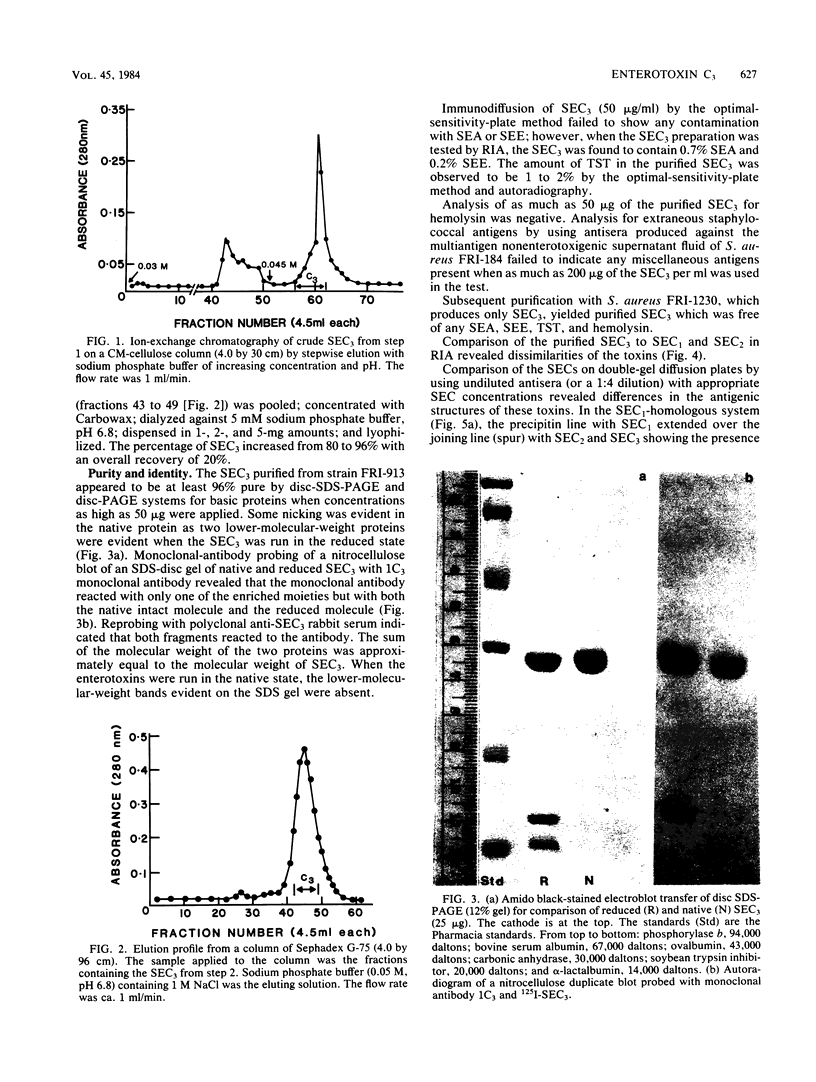
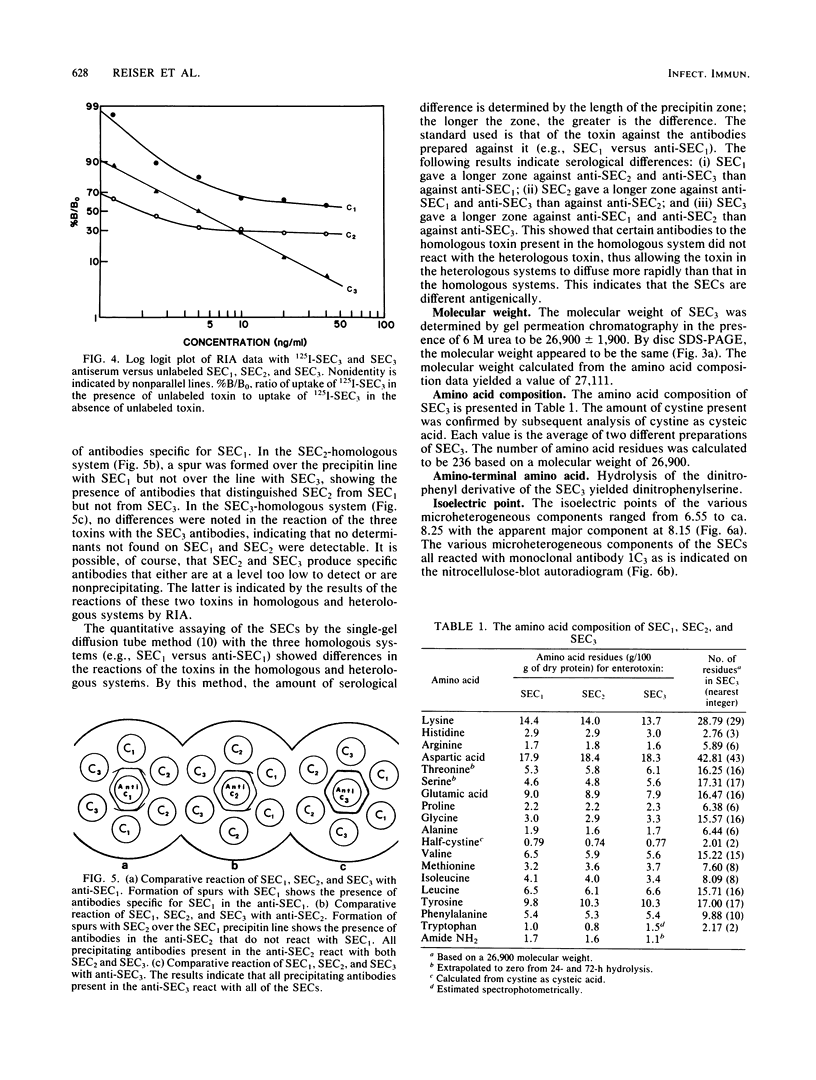

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avena R. M., Bergdoll M. S. Purification and some physicochemical properties of enterotoxin C, Staphylococcus aureus strain 361. Biochemistry. 1967 May;6(5):1474–1480. doi: 10.1021/bi00857a033. [DOI] [PubMed] [Google Scholar]
- Bergdoll M. S., Borja C. R., Avena R. M. Identification of a new enterotoxin as enterotoxin C. J Bacteriol. 1965 Nov;90(5):1481–1485. doi: 10.1128/jb.90.5.1481-1485.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borja C. R., Bergdoll M. S. Purification and partial characterization of enterotoxin C produced by Staphylococcus aureus strain 137. Biochemistry. 1967 May;6(5):1467–1473. doi: 10.1021/bi00857a032. [DOI] [PubMed] [Google Scholar]
- Change P. C., Yano Y., Dighton M., Dickie N. Fractionation of staphylococcal enterotoxin C 2 by isoelectric focusing. Can J Microbiol. 1971 Nov;17(11):1367–1372. doi: 10.1139/m71-218. [DOI] [PubMed] [Google Scholar]
- Chu F. S., Thadhani K., Schantz E. J., Bergdoll M. S. Purification and characterization of staphylococcal enterotoxin A. Biochemistry. 1966 Oct;5(10):3281–3289. doi: 10.1021/bi00874a030. [DOI] [PubMed] [Google Scholar]
- Edelhoch H. Spectroscopic determination of tryptophan and tyrosine in proteins. Biochemistry. 1967 Jul;6(7):1948–1954. doi: 10.1021/bi00859a010. [DOI] [PubMed] [Google Scholar]
- FRAENKEL-CONRAT H., HARRIS J. I., LEVY A. L. Recent developments in techniques for terminal and sequence studies in peptides and proteins. Methods Biochem Anal. 1955;2:359–425. doi: 10.1002/9780470110188.ch12. [DOI] [PubMed] [Google Scholar]
- Kato E., Khan M., Kujovich L., Bergdoll M. S. Production of enterotoxin a. Appl Microbiol. 1966 Nov;14(6):966–972. doi: 10.1128/am.14.6.966-972.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee A. C., Robbins R. N., Reiser R. F., Bergdoll M. S. Isolation of specific and common antibodies to staphylococcal enterotoxins B, C1, and C2. Infect Immun. 1980 Feb;27(2):431–434. doi: 10.1128/iai.27.2.431-434.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger J. F., Johnson A. D., Spero L. Intrinsic and chemically produced microheterogeneity of Staphylococcus aureus enterotoxin type C. Infect Immun. 1975 Jul;12(1):93–97. doi: 10.1128/iai.12.1.93-97.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller B. A., Reiser R. F., Bergdoll M. S. Detection of staphylococcal enterotoxins A, B, C, D, and E in foods by radioimnunoassay, using staphyloccal cells containing protein A as immunoadsorbent. Appl Environ Microbiol. 1978 Sep;36(3):421–426. doi: 10.1128/aem.36.3.421-426.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REISFELD R. A., LEWIS U. J., WILLIAMS D. E. Disk electrophoresis of basic proteins and peptides on polyacrylamide gels. Nature. 1962 Jul 21;195:281–283. doi: 10.1038/195281a0. [DOI] [PubMed] [Google Scholar]
- Reiser R. F., Robbins R. N., Khoe G. P., Bergdoll M. S. Purification and some physicochemical properties of toxic-shock toxin. Biochemistry. 1983 Aug 2;22(16):3907–3912. doi: 10.1021/bi00285a028. [DOI] [PubMed] [Google Scholar]
- Robbins R., Gould S., Bergdoll M. Detecting the enterotoxigenicity of Staphylococcus aureus strains. Appl Microbiol. 1974 Dec;28(6):946–950. doi: 10.1128/am.28.6.946-950.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SURGALLA M. J., BERGDOLL M. S., DACK G. M. Some observations on the assay of staphylococcal enterotoxin by the monkey-feeding test. J Lab Clin Med. 1953 May;41(5):782–788. [PubMed] [Google Scholar]
- Sanger F. The free amino groups of insulin. Biochem J. 1945;39(5):507–515. doi: 10.1042/bj0390507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt J. J., Spero L. The complete amino acid sequence of staphylococcal enterotoxin C1. J Biol Chem. 1983 May 25;258(10):6300–6306. [PubMed] [Google Scholar]
- Spero L., Griffin B. Y., Middlebrook J. L., Metzger J. F. Effect of single and double peptide bond scission by trypsin on the structure and activity of staphylococcal enterotoxin C. J Biol Chem. 1976 Sep 25;251(18):5580–5588. [PubMed] [Google Scholar]
- Symington J., Green M., Brackmann K. Immunoautoradiographic detection of proteins after electrophoretic transfer from gels to diazo-paper: analysis of adenovirus encoded proteins. Proc Natl Acad Sci U S A. 1981 Jan;78(1):177–181. doi: 10.1073/pnas.78.1.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson N. E., Ketterhagen M. J., Bergdoll M. S. Monoclonal antibodies to staphylococcal enterotoxins B and C: cross-reactivity and localization of epitopes on tryptic fragments. Infect Immun. 1984 Jul;45(1):281–285. doi: 10.1128/iai.45.1.281-285.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]