Abstract
From yeast to mammals, two types of GTPase-activating proteins, ArfGAP1 and ArfGAP2/3, control guanosine triphosphate (GTP) hydrolysis on the small G protein ADP-ribosylation factor (Arf) 1 at the Golgi apparatus. Although functionally interchangeable, they display little similarity outside the catalytic GTPase-activating protein (GAP) domain, suggesting differential regulation. ArfGAP1 is controlled by membrane curvature through its amphipathic lipid packing sensor motifs, whereas Golgi targeting of ArfGAP2 depends on coatomer, the building block of the COPI coat. Using a reporter fusion approach and in vitro assays, we identified several functional elements in ArfGAP2/3. We show that the Golgi localization of ArfGAP3 depends on both a central basic stretch and a carboxy-amphipathic motif. The basic stretch interacts directly with coatomer, which we found essential for the catalytic activity of ArfGAP3 on Arf1-GTP, whereas the carboxy-amphipathic motif interacts directly with lipid membranes but has minor role in the regulation of ArfGAP3 activity. Our findings indicate that the two types of ArfGAP proteins that reside at the Golgi use a different combination of protein–protein and protein–lipid interactions to promote GTP hydrolysis in Arf1-GTP.
INTRODUCTION
The COPI system of vesicular transport mediates trafficking in the endoplasmic reticulum (ER)-Golgi shuttle and is essential for the biogenesis/maintenance of the Golgi apparatus (for reviews, see Lee et al., 2004; Bethune et al., 2006). The best characterized function of this system is the retrograde trafficking of proteins between Golgi cisternae and from the Golgi to the ER. Membrane proteins that need to be transported in retrograde COPI vesicles contain signals on their cytosolic tails that are recognized by the heteroheptameric COPI coat (coatomer), whereas luminal proteins are sorted through transmembrane adaptors that recognize cargo at the luminal side and coatomer at the cytosolic side.
The key regulator of the COPI coat is the small G protein ADP-ribosylation factor (Arf) 1 (Donaldson and Honda, 2005; Kahn et al., 2005; D'Souza-Schorey and Chavrier, 2006; Gillingham and Munro, 2007). After its activation by the guanine nucleotide exchange factor GBF1, Arf1 initiates the recruitment of coatomer. ArfGAPs, initially thought to solely function as terminators of Arf activity, may also play a role during the buildup of the COPI coat and in the sorting of cargo that is transported through this system (Lewis et al., 2004; Lee et al., 2005; Yang et al., 2005; Frigerio et al., 2007). Three ArfGAPs belonging to two subfamilies, ArfGAP1 (Cukierman et al., 1995) and ArfGAP2/3 (Liu et al., 2001; Watson et al., 2004), are associated with the Golgi complex and are thought to function in the COPI mechanism. Most studies so far have dealt with ArfGAP1. This protein was found to interact with coatomer through both its amino terminal catalytic GTPase-activating protein (GAP) domain and the carboxy noncatalytic part (Lee et al., 2005). The noncatalytic part contains two motifs termed amphipathic lipid packing sensor (ALPS) that mediate the interaction of the protein with liposomes containing poorly packed lipids in vitro (Bigay et al., 2005; Mesmin et al., 2007) and that are required for Golgi targeting in vivo (Parnis et al., 2006; Levi et al., 2008). The ALPS motifs make the activity of ArfGAP1 on membrane-bound Arf1-GTP extremely sensitive to membrane curvature, suggesting a negative feedback loop based on the membrane distortion imposed by the COPI coat (Bigay et al., 2003; Bigay et al., 2005). In addition, the catalytic activity of ArfGAP1 is stimulated by coatomer (Goldberg, 1999), although this effect seems relatively modest when Arf1 is bound to lipid membranes (Szafer et al., 2000; Szafer et al., 2001). Less is known about the more recently discovered ArfGAP2 and ArfGAP3. These two proteins are highly related (58% identity) and show little similarity to ArfGAP1 outside the catalytic domain. Nevertheless, ArfGAP1 and ArfGAP2/3 display functional interplay, as indicated by synthetic lethality observed between these ArfGAPs in HeLa cells (Frigerio et al., 2007) and between their orthologues Gcs1 and Glo3 in Saccharomyces cerevisiae (Poon et al., 1999). The association of ArfGAP2 with the Golgi membrane seems to depend on its relatively strong interaction with coatomer (Eugster et al., 2000; Watson et al., 2004); whether coatomer regulates ArfGAP2/3 activity has not yet been reported, although GAP activity of yeast Glo3 on Golgi membranes was found to be coatomer dependent (Szafer et al., 2001). Studies in both mammalian cells and in S. cerevisiae clearly implicate ArfGAP2/3 and Glo3, respectively, in retrograde Golgi-to-ER trafficking (Dogic et al., 1999; Lewis et al., 2004; Frigerio et al., 2007).
To further understand the cellular function of ArfGAP2/3, we combined in vivo and in vitro assays to define their functional determinants, focusing on ArfGAP3. We report the characterization of motifs that are required for coatomer binding, for coatomer stimulation of ArfGAP3 activity, and for interaction with the Golgi membrane. Our findings indicate that multiple elements along the ArfGAP3 molecule act cooperatively to confer its cellular function and point to basic differences in structure and regulation between ArfGAP2/3 and ArfGAP1.
MATERIALS AND METHODS
DNA Constructs for Mammalian Cell Expression
CD4 constructs in pCDNA3 (Yuan et al., 2003) were kindly provided by Dr. Blanche Schwappach (University of Manchester, Manchester, United Kingdom). Polymerase chain reaction (PCR)-amplified ArfGAP3 fragments were cloned between the NotI and XhoI sites that are present at the 3′ side of the CD4 insert, and ArfGAP2 was similarly cloned using NotI and XbaI. Green fluorescent protein (GFP) fusion proteins were prepared by cloning PCR products into the pEGFP-C2 vector (Clontech, Mountain View, CA) by using the EcoRI and BamHI sites, except for ArfGAP2 cDNA, which was cloned using KpnI/BamHI. Mutations were introduced by a two-stage PCR. The authenticity of all constructs was verified by DNA sequencing.
Immunofluorescence Microscopy
Plasmids (0.2 μg each) were transfected into HeLa cells grown on 13-mm glass coverslips in 24-well plates by using 1 μl of FuGENE-6 (Roche Diagnostics, Mannheim, Germany) according to manufacturer's instructions. Cells were fixed with 4% paraformaldehyde 20–22 h after transfection. For immunostaining, cells were permeabilized by treatment with phosphate-buffered saline [PBS] containing 0.05% saponin and 0.2% bovine serum albumin, and incubated with a monoclonal antibody against CD4 (clone MT310; Santa Cruz Biotechnology, Santa Cruz, CA; 1:250) or GM130 (BD Biosciences Transduction Laboratories, Lexington, KY; 1:300) followed by Cy3-conjugated donkey anti-mouse immunoglobulin G (Jackson ImmunoResearch Laboratories, West Grove, PA; 1:400). Coverslips were mounted on glass slides using Mowiol as glue, and cells were visualized under a DMIRE2 inverted fluorescent microscope (Leica, Wetzlar, Germany) at a 40× or 63× magnification.
Preparation of Recombinant ArfGAP3 Fragments
For coatomer binding experiments, ArfGAP3 cDNAs were excised either from the corresponding GFP fusions with XhoI and BamHI or from the CD4 fusions with NotI and XhoI. The fragments were cloned into a pKM260 vector that introduces an amino-terminal hexahistidine tag. The vector was modified by insertion, from 5′ to 3′, of NotI, SmaI, XhoI, and Acc651 restriction sites between the vector NheI and BamHI sites. Proteins were expressed in BL21(DE3)pLysS Escherichia coli by induction for 3 h at 30°C in the presence of 0.1 mM isopropyl-β-d-thiogalactopyranoside. Bacterial pellets were resuspended in 6 M guanidine hydrochloride, 20 mM Tris, pH 8.0, and 5 mM 2-mercaptoethanol followed by centrifugation (20,000 × g for 20 min). Proteins in the supernatant were adsorbed to nickel beads (Adar Biotech, Rehovot, Israel). After a preliminary trial, the amount of bound proteins was adjusted to ∼3 mg/ml bead. The beads were washed with the guanidine solution followed by washing with binding buffer (150 mM NaCl, 25 mM Tris, pH 8.0, 10 mM imidazole-HCl, and 0.5% Triton X-100).
For in vitro activity measurements, full-length and fragments of ArfGAP3 were PCR amplified from a pET21d-ArfGAP3 construct (gift from R. Duden, University of Luebeck, Luebeck, Germany) by using NdeI and BamHI primers, and cloned into pET16b (Novagen, Madison, WI) to obtain fusion proteins with a 10× His N-terminal tag. Point mutations were introduced in the pET16b construct by using the QuikChange kit (Stratagene, La Jolla, CA). Proteins were expressed in BL21-Gold E. coli for 2 h at 37°C in the presence of 1 mM isopropyl-β-d-thiogalactopyranoside. Proteins were purified as described previously for ArfGAP1 (Bigay et al., 2005), except for the renaturation step that was modified as follows. The protein eluate was diluted 1:3 in guanidine buffer and dialyzed three times at 4°C for 2 h against 25 mM Tris, pH 7.5, 400 mM NaCl, 10% glycerol, 50 μM ZnCl2, 5 mM dithiothreitol (DTT), and 0.1 mM phenylmethylsulfonyl fluoride. The sample was then centrifugated at 100,000 × g for 15 min, and the supernatant was flash frozen in liquid nitrogen and stored at −80°C. Before use, each protein aliquot was centrifuged at 100,000 × g for 5 min to discard potential aggregates, and the supernatant was analyzed by SDS-polyacrylamide gel electrophoresis (PAGE) for control of protein concentration.
Coatomer Pull-Down Assays
Pull-down was assayed using rat liver cytosol prepared as described previously (Makler et al., 1995). Fifteen-microliter aliquots of nickel bead-immobilized ArfGAP3 were incubated under rotation for 1 h at 4°C in binding buffer with 0.5 ml of cytosol (2 mg protein/ml) and then washed with binding buffer. Bead pellets were treated with SDS sample buffer, resolved by SDS-PAGE (5–20% gradient gels), blotted onto nitrocellulose sheets, and coatomer was probed with the M3A5 anti-βCOP antibody and detected using the enhanced chemiluminescence system. The intensity of bands was quantified by the Tina program and corrected for the bead-only background.
Liposomes
The sonicated liposomes used for circular dichroism (CD) spectroscopy were prepared as follows. A dried film was prepared by evaporation of a lipid mixture (dioleoyl-phosphatidylcholine, 70 mol%/dioleoyl-phospatidylserine, 30 mol%) in chloroform and was resuspended in 150 mM KCl and 10 mM Tris, pH 8.0, to a final lipid concentration of 15 mM. The mixture was sonicated for 5 min by using a Microson sonicator set to low intensity and centrifuged at 100,000 × g for 20 min to remove debris. Average liposome radius as determined by dynamic light scattering was 26 nm. The liposomes used in the GAP assays were prepared by the extrusion method as described previously (Robbe and Antonny, 2003; Bigay and Antonny, 2005). Briefly, they were prepared from a lipid film containing 50 mol% egg phosphatidylcholine, 19 mol% egg phosphatidylethanolamine, 5 mol% brain phosphatidylserine, 10 mol% liver phosphatidylinositol, 16 mol% cholesterol, and 0.2 mol% N-(7-nitrobenz-2-oxa-1, 3-diazol-4-yl)-1,2-dihexadecanoyl-sn-glycero-3-phosphoethanolamine (NBD-PE). The lipid film was resuspended in HK buffer (50 mM HEPES, pH 7.2, and 120 mM K acetate) to a final lipid concentration of 2 mM. Liposomes were sequentially extruded through filters of defined pore size (0.2, 0.1, 0.05, and 0.03 μm), and their final size was determined by dynamic light scattering. The lipopeptide p23 (lp23), from a stock solution in dimethyl sulfoxide, was added to the liposomes at surface concentration of 2 mol%.
Circular Dichroism Measurements
CD spectroscopy was performed on a Chirascan spectropolarimeter (Applied Photophysics, Surrey, United Kingdom) by using a synthetic peptide containing the ArfGAP3 carboxy sequence DMAQFKQGVRSVAGKLSVFANGVVTSI (prepared by Sigma-Aldrich, St. Louis, MO). The experiments were performed at room temperature in buffer containing 150 mM KCl and 10 mM Tris, pH 8.0, in a Hellma quartz cell with an optical path length of 0.05 cm. Each spectrum was obtained by averaging several scans recorded from 200 to 260 nm, with a bandwidth of 1 nm, a step of 1 nm, and a scan speed of 50 nm/min. Data were analyzed by the CDPro software (http://lamar.colostate.edu/∼sreeram/CDPro/main.html).
ArfGAP Activity Measurements
GTP hydrolysis in Arf1 was followed by tryptophan fluorescence (excitation, 297.5 nm; emission, 340 nm) as described previously (Robbe and Antonny, 2003; Bigay and Antonny, 2005). In a preliminary stage, myristoylated Arf1-GDP (1 μM) was added to HK buffer supplemented with 1 mM MgCl2 and 1 mM DTT and containing extruded liposomes (0.2 mM; 0.03-μm filter pore size). Arf1 was activated by the addition of 50 μM GTP and 2 mM EDTA. After 15 min, 2 mM MgCl2 was added to stop nucleotide exchange. An aliquot of this reaction mixture was then transferred to the fluorescence cuvette, supplemented with coatomer where indicated, and GTP hydrolysis was initiated by the addition of ArfGAPs. All experiments were performed at 37°C.
Static Light Scattering Measurements
Light scattering (excitation and emission, 350 nm; bandwidth, 1.5 nm) was measured at right angle in a Shimadzu R5301 fluorimeter equipped with a small quartz cuvette (100 μl) (Bigay and Antonny, 2005). All experiments were performed at 37°C in HK buffer supplemented with 1 mM MgCl2 and 1 mM DTT and with liposomes (0.1 mM) containing 2 mol% of lp23 that had been extruded through various pore size filters (0.2, 0.1, 0.05, and 0.03 μm).
RESULTS
Previous studies have demonstrated that transplantation of coatomer-binding signals such as the K(X)KXX signal (Letourneur et al., 1994) into a reporter plasma membrane protein results in the diversion of the reporter to the ER, apparently due to its retrieval from the Golgi by the COPI mechanism. We tested whether this approach can be used to reveal COPI-interacting motifs on ArfGAPs. To this end, the ArfGAPs and their fragments/mutants were fused at the cytoplasmic side of the CD4 coreceptor, and the localization of the fusion constructs in transfected HeLa cells was determined using anti-CD4 antibodies. As shown in Figure 1A, wild-type CD4 was expressed at the cell surface, whereas CD4 tagged with a KKXX signal was localized to the ER, as described previously. Significantly, CD4 fusions of ArfGAP1, ArfGAP2, and ArfGAP3 displayed ER localization as evident from a reticular pattern and/or staining of the nuclear envelope, with no staining at the plasma membrane region. The reticular pattern of the ER was more apparent in some cells than in others, and both patterns were observed with the different constructs used (cf. Figure 1, A and C). The absence of surface expression of CD4-fused ArfGAPs was further confirmed when nonpermeabilized cells were stained with anti-CD4 antibodies to selectively reveal receptors at the cell surface; surface receptors were detected in wild-type CD4, but not in the ArfGAP fusions (Figure 1B).
Figure 1.
ER diversion of CD4 localization by its fusion to ArfGAPs. (A) This panel shows the switch of the localization of CD4 in HeLa cells from the plasma membrane to the ER upon fusion to ArfGAP1-3. Compartment markers used are cyan fluorescent protein (CFP)-SRβ (ER) and yellow fluorescent protein (YFP)-GPI anchor signal (plasma membrane). CD4-KKTN served as control for ER retrieval. (B) The cells were cotransfected with the CD4 fusions along with GFP used as transfection marker and were fixed and stained with anti-CD4 without permeabilization; note that only wild-type CD4 reacts under these conditions, which selectively detect the surface receptor. (C) The Golgi complex remains intact in CD4-ArfGAP–transfected cells. The Golgi was labeled by cotransfection with CFP-N-acetylgalactoseamine transferase.
Conceivably, ER localization of the CD4 fusions could be mediated by COPI-dependent as well as COPI-independent ER retrieval, and also by ER retention mechanisms. Another mechanism that could lead to ER localization of CD4-ArfGAP fusions is the dissociation of coatomer from Golgi cisternae due to increased catalytic GAP activity. This might lead to fusion of Golgi cisternae with the ER and a block of all anterograde traffic as observed previously in cells overexpressing ArfGAP1 (Huber et al., 1998). However, the Golgi apparatus seemed intact in CD4-ArfGAP-transfected cells (Figure 1C), ruling out such mechanism. A general block of ER-to-Golgi traffic is also unlikely as GFP-glycosylphosphatidylinositol (GPI) anchor cotransfected with CD4-ArfGAP2/3 was effectively transported to the plasma membrane (Figure 1A).
In most subsequent experiments, we focused on the characterization of the ArfGAP3 protein. To analyze putative COPI interaction determinants, we tested the localization of a series of CD4 fusions containing ArfGAP3 fragments (Figure 2A). The results showed a decrease in ER localization of CD4-fused ArfGAP3 fragments upon further deletion on either the amino or carboxy side, suggesting the presence of ER targeting determinants in the deleted regions. Unequivocal phenotypes were observed in a series containing amino fragments, where the fused ArfGAP3(1-267) fragment gave rise to complete ER localization, whereas truncation of 32 or more carboxy residues (1-139, 1-235) resulted in complete localization at the plasma membrane. Even though the catalytic part in itself (1-139) did not confer ER localization, this part was required for ER localization of a fragment ending in residue 267. This however was not due to catalytic activity, because mutation of the invariant arginine residue that is essential for catalytic activity of all ArfGAPs tested so far (Arg53 in ArfGAP3) had no effect on the ER localization of the 1-267 fragment (data not shown). Some truncation constructs like 360-516 showed mixed ER/plasma membrane (PM) phenotype, whereas a clear switch from ER-to-PM localization was obtained upon truncation of the last 33 residues of ArfGAP3 in a carboxy terminal fragment (compare 267-516 and 267-483). Comparison of other pairs of truncation constructs suggested the presence of additional determinants contributing the ER localization of CD4 fusions in the 235-267 and 303-435 regions. It is also apparent from the comparison of different constructs that none of the short stretches whose truncation abrogates ER localization could by itself confer this localization; instead, longer stretches were required, suggesting that ER diversion depends on cooperation between several determinants.
Figure 2.

Truncation analysis of ArfGAP3. (A) Truncation analysis of CD4 fusions of ArfGAP3. Bars summarize the localizations observed, and regions containing determinants that contribute to ER localization of CD4 fusions are indicated as black boxes in the bottom bar. (B) Coatomer pull-down from liver cytosol by using nickel-nitrilotriacetic acid-agarose-immobilized ArfGAP3 fragments.
A likely mechanism for causing ER localization of the CD4 fusions is their incorporation into retrograde COPI vesicles due to direct interaction of coatomer, reported previously to interact with ArfGAP2/3 and their noncatalytic part (Watson et al., 2004; Frigerio et al., 2007). This was tested by pull-down experiments from cytosol by using immobilized ArfGAP3 fragments similar to those tested in the CD4 assay. The pull-down results (Figure 2B) were in agreement with the CD4 data for most truncated constructs, indicating the presence of determinants contributing to coatomer binding in both catalytic and noncatalytic parts. Notably, a central fragment encompassing residues 204-360 interacted very efficiently with coatomer. By contrast, a carboxy fragment (residues 267-516) that caused ER localization of fused CD4 (Figure 2A) showed poor coatomer binding.
Identification of ArfGAP3 Residues Involved in Coatomer Interaction
Based on the combined results of the CD4 and pull-down assays, we decided to carry out detailed analysis of the central fragment (residues 204-360). This region was subjected to alanine scanning mutagenesis of residues whose conservation was indicated by multiple alignment of ArfGAP2/3 from different species. As summarized in Figure 3A and shown in Figure 3B, we identified several residues whose mutation strongly diminished ER localization. These mutations were spread along this central region (positions 228-229, 232, 235-237, 304-305, and 323-324) and implied amino acids of various classes (basic, hydrophobic, and polar). We noticed that dibasic KK motifs like the one at positions 228-229 whose mutation abrogated ER localization are also present in positions 215-216 and 222-223. Although mutation of each of these two KK pairs had little effect, ER localization of the CD4 fusion construct was abolished when all four residues were replaced by alanines (4K215/16/22/23). Mutation of F242 also significantly reduced ER localization. Together, these findings suggest that the basic stretch that includes positions 215-242 forms a hot spot for coatomer interaction and that this interaction involves the use of partially redundant KK motifs. Inspection of this region reveals high degree of evolutionary conservation of the residues that were found critical for ER localization of CD4-ArfGAP3(204-360) (Figure 3C).
Figure 3.
Identification of ArfGAP3 residues involved in ER diversion of CD4-ArfGAP3(204-360). (A) The sequence of the middle part of ArfGAP3 is displayed, with mutations that abrogated ER localization in red, mutations that had no effect in blue, and those with partial effect in orange. The corresponding images are displayed in B. Note the abundance of abrogating mutations in the basic stretch including residues 215-242. Multispecies alignment (C) shows the high conservation of these residues (Hs, Homo sapiens; Mm, Mus musculus; Xl, Xenopus laevis; Tn, Tetraodon nigroviridis; Gg, Gallus gallus; Dm, Drosophila melanogaster; Sc, S. cerevisiae).
To examine the predicted role of the basic stretch in coatomer binding, we tested the effect of mutations using the coatomer pull-down assay. As shown in Figure 4A, the effects of alanine replacements in the context of the 204-360 fragment mirrored those observed in the CD4 assay, with strongest effects of mutations in the 228-237 subregion. Mutations in this subregion also diminished significantly coatomer binding to the full-length ArfGAP3 (Figure 4B). By contrast, two mutations in a different region that were also found to abrogate ER diversion of CD4 fusions (R304A/L305A and D323A/M324A) had little effect in the pull-down assay. We also tested the effect of mutations in the basic stretch of ArfGAP2. Mutation of the KK sequence at position 228-229 decreased coatomer pull-down by ∼50%, whereas mutation of a QKV sequence that corresponds to the ArfGAP3 QKL at positions 235-237 strongly abrogated coatomer interaction (Supplemental Figure S1-A).
Figure 4.
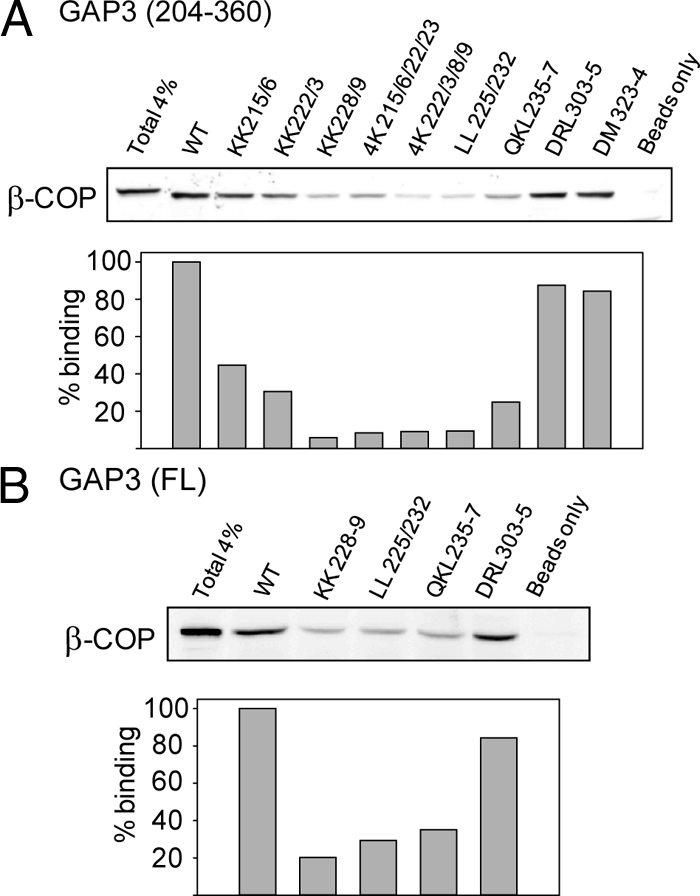
The basic stretch is involved in coatomer binding. Effect of alanine replacement mutations on coatomer pull-down by ArfGAP3(204-360) (A) and by full-length ArfGAP3 (B).
ArfGAP3 Regions Mediating Golgi Localization
ArfGAP2/3 are localized at the Golgi apparatus, and a previous study has suggested that this localization depends on coatomer interaction (Watson et al., 2004). To define Golgi localization regions, we tested the localization of a series of truncated, GFP-fused versions of ArfGAP3. As shown in Figure 5A, localization does not require the catalytic part, in agreement with previous results. The shortest construct showing good Golgi localization encompassed residues 204-516, whereas this localization was abrogated in a 267-516 construct that lacks the basic stretch found to be important for coatomer binding. Significantly, deletion of the carboxy-terminal segment (residue 484-516) strongly diminished Golgi localization of full-length ArfGAP3. This carboxy segment was also required for ER diversion of CD4-ArfGAP3(267-516) (Figure 2A).
Figure 5.
ArfGAP3 regions involved in Golgi targeting and effect of mutations that disrupt coatomer interaction. (A) Truncation analysis of GFP-ArfGAP3 fusions. (B) Mutations that abrogate coatomer interaction also diminish Golgi localization of GFP-full length ArfGAP3. Staining with antibodies against the cis-Golgi protein GM130 was used to mark the Golgi region.
The role of the basic stretch in Golgi targeting of ArfGAP3 was further dissected by point mutation analysis. As shown in Figure 5B, mutations in the basic stretch that were found to strongly abrogate coatomer interaction caused a decrease in the Golgi localization of full-length ArfGAP3, resulting in increased cytosolic distribution in comparison with the wild-type GFP fusion. Abrogation of Golgi localization was also observed when the corresponding residues of ArfGAP2 were mutated (Supplemental Figure S1-B).
Role of a Carboxy-Amphipathic Motif
Inspection of the sequence of the carboxy segment of ArfGAP3 that was found important for Golgi localization revealed that residues 485-510 can be portrayed as amphipathic helix with a high concentration of hydrophobic residues on one face and mostly hydrophilic residues on the other, a topology that is conserved in ArfGAP2 and in S. cerevisiae Glo3 (Figure 6A). Although quite different in length and amino acid composition from the ALPS motifs that are found in the middle of ArfGAP1 (Mesmin et al., 2007; Levi et al., 2008), this sequence also has the potential to interact with lipid membranes. We thus tested whether the ArfGAP3 carboxy segment could fold as an amphipathic helix at the surface of liposomes by using a synthetic peptide representing this region. Circular dichroism analysis (Figure 6B) revealed that the peptide partially folded to a helical structure in the presence of liposomes with typical minima at 210 and 225 nm; calculation of the folding efficiency indicated ∼15% helical transition. Attempts to detect helical transition of full-length ArfGAP3 and its noncatalytic fragment were unsuccessful, possibly due to this comparatively low folding propensity of the carboxy motif.
Figure 6.
Golgi localization depends on amphipathic motif at the carboxy terminus of ArfGAP3. (A) Helical wheel presentation of carboxy-terminal parts of ArfGAP2/3 and Glo3; part of the ALPS1 motif of ArfGAP1 is displayed for comparison. (B) Circular dichroism spectra of a synthetic peptide representing residues 484-510 of ArfGAP3 (70 μM) in the absence or presence of sonicated liposomes (2 mM), demonstrating liposome-induced transition to helical conformation. (C) Mutations expected to abrogate helix amphipathicity diminish Golgi localization of the full-length ArfGAP3. (D) Appending the ALPS1 motif (ArfGAP1 residues 198-240) to ArfGAP3(1-483) restores Golgi localization. This effect is abolished in the W211D mutant of the ALPS1 motif.
To investigate whether amphipathicity of this region plays a role in Golgi localization, we tested the effect of mutations that are expected to disrupt this feature. For this, we introduced a negative charge at the hydrophobic face (mutations F488D, L499D, or I510D), altered the helix register by adding two extra alanines after A496, or introduced a helix-disrupting residue (N504P). As shown in Figure 6C, these mutations reduced Golgi localization and increased cytosolic distribution of full-length ArfGAP3. By contrast, conservative mutation in the hydrophobic face (L499F) or aspartate replacement of the single hydrophobic residue (V501) that is predicted to point to the hydrophilic face had no effect.
To further examine the functional relationship between amphipathic motifs from ArfGAP1 and ArfGAP3, we replaced the ArfGAP3 C terminus by an ALPS1 peptide. As shown in Figure 6D, the GAP3-ALPS1 chimera showed normal Golgi localization, whereas a mutation (W211D) that strongly affects the amphipathic character of the ALPS1 motif diminished Golgi localization of the chimera.
Role of Coatomer Binding and Amphipathic Motifs in Conferring Golgi Localization on ArfGAP1 and ArfGAP3
The findings so far have suggested that Golgi localization of ArfGAP3 depends on two kinds of motifs, the coatomer binding stretch and the amphipathic carboxy part that presumably interacts with membranes. In ArfGAP1, only a role of amphipathic motifs in Golgi localization has been demonstrated so far (Parnis et al., 2006; Levi et al., 2008). We asked whether increasing the strength of the coatomer-dependent component could bypass the requirement for the amphipathic motif. This was tested using aluminum fluoride complexes, known to stabilize ternary complexes between Arf1, ArfGAP, and coatomer by mimicking the transition state of the GTPase reaction (Bigay et al., 2003; Liu et al., 2005). As shown in Figure 7A, addition of aluminum and fluoride ions to cells expressing GFP-fused ArfGAP3(1-303), which lacks much of the carboxy part, including the amphipathic motif, caused a strong shift from cytosolic to Golgi distribution. Fluoride treatment had a partial effect on ArfGAP3(1-267) but was without effect on ArfGAP3(1-219). A role of coatomer in the fluoride effect on the 1-303 construct is indicated by the finding that this effect was diminished in mutants that are defective in coatomer binding (4K222/223/228/229, QKL235-237).
Figure 7.

Effect of aluminum fluoride on the localization of ArfGAPs and their mutants. (A) Aluminum fluoride confers Golgi localization on ArfGAP3 fragments lacking the carboxy-amphipathic motif. Note the strong effect of fluoride on the 1-303 fragment, and its abrogation upon mutations in the coatomer binding motif. (B) Fluoride cannot confer Golgi localization on full-length ArfGAP1 containing mutations in the ALPS motifs. Cells that have been transfected with GFP-ArfGAP3 fragments and mutants thereof or with GFP-ArfGAP1 and its ALPS mutants (M204A-L207A-W211A for ALPS1; V279D for ALPS2) were incubated with 30 mM NaF plus 50 μM AlNH4(SO4)2 for 30 min at 37°C or left untreated (control).
For comparison, we carried out similar analysis on ArfGAP1 (Figure 7B). Previous studies have demonstrated that Golgi association of a fraction of GFP-tagged ArfGAP1 is stabilized in cells treated with fluoride ions, indicating a role for coatomer (Liu et al., 2005). By contrast, we found that addition of aluminum and fluoride to cells expressing GFP-fused, full-length ArfGAP1 with triple mutation in hydrophobic residues of the ALPS1 motif known to disrupt Golgi localization (Parnis et al., 2006) failed to restore Golgi localization. A mutant in the ALPS2 motif that shows partial decrease in Golgi distribution (Mesmin et al., 2007) also did not display increased localization after aluminum fluoride treatment. We concluded from these observations that the amphipathic motifs rather than coatomer are the most critical contributors to Golgi targeting of ArfGAP1, in contrast to what was found for ArfGAP3.
The Activity of ArfGAP2/3 is strictly coatomer-dependent
To directly address the role of the various protein–protein and protein–lipid interactions inferred from the above experiments, we next studied the activity of ArfGAP2/3 on Arf1-GTP bound to model liposomes. This system has been used in the past to reconstitute several steps of the GDP/GTP cycle of Arf1, including catalysis of GTP hydrolysis by ArfGAP1 (Robbe and Antonny, 2003; Bigay and Antonny, 2005). Myristoylated Arf1-GDP was incubated with liposomes of defined size and composition and converted to the GTP-bound and membrane-associated state. Thereafter, GTP hydrolysis was initiated by the addition of a catalytic amount of ArfGAP1 or ArfGAP3. The conversion of Arf1 from the GTP conformation to the GDP conformation was followed by tryptophan fluorescence. This assay revealed striking differences between ArfGAP2/3 and ArfGAP1.
The activity of ArfGAP1 on liposome-bound Arf1-GTP is highly sensitive to membrane curvature, reaching maximal value on small liposomes displaying a radius in the range of 30 nm (Bigay et al., 2003, 2005; Mesmin et al., 2007). In contrast, ArfGAP2 and ArfGAP3 displayed no detectable activity on small liposomes, suggesting that they do not function in an autonomous manner on isolated Arf1-GTP (Figure 8A). Coatomer was then added to the reaction. Its recruitment by Arf1-GTP was facilitated by the presence of a lipopeptide, lp23, that mimics the cytosolic tail of the Golgi protein p23 (Bremser et al., 1999). Strikingly, very fast time courses of GTP hydrolysis were now observed upon ArfGAP2 or ArfGAP3 addition, whereas the rate of ArfGAP1-mediated GTP hydrolysis in Arf1 was accelerated only approximately threefold. By varying the relative concentration of ArfGAP3 and coatomer, we observed that maximal activity required roughly equal amount of the two proteins, suggesting that the functional entity that promotes GTP hydrolysis in Arf1 is a one-to-one ArfGAP3:coatomer complex (Figure 8B). With 30 nM ArfGAP3 and 50 nM coatomer present (or 40 nM taking into account coatomer purity), GTP hydrolysis in 1 μM myrArf1 was complete within 60 s, suggesting that each round of GTP hydrolysis takes ∼2 s. This gives for the kcat of the reaction a lower limit of 0.5 s−1.
Figure 8.
The activity of ArfGAP2/3 but not ArfGAP1 is strictly coatomer-dependent. (A) Time course of GTP hydrolysis in liposome-bound myristoylated Arf1 and with the indicated concentration of coatomer. The liposomes were prepared by extrusion through 0.03-μm pore size filters (mean radius, 35 nm) and contained 2 mol% of lp23. GTP hydrolysis was initiated by the addition of 25 nM ArfGAP1 (top), ArfGAP2 (middle), or ArfGAP3 (bottom) and was followed by tryptophan fluorescence. Note the lack of GTP hydrolysis when ArfGAP2/3 were used in the absence of coatomer. (B) Dose–response curves. The rate of GTP hydrolysis in Arf1 was measured as in described in A, with various concentrations of ArfGAP3 and coatomer. Maximal rate of GTP hydrolysis was observed with roughly equal amounts of the two proteins (coatomer was ∼80% pure), suggesting a functional one-to-one complex. The bottom curves show the corresponding fluorescence recordings.
The different behavior of ArfGAP3 and ArfGAP1 with regard to membrane curvature was evident in a COPI assembly–disassembly assay where light scattering was used to follow the stepwise assembly of the COPI coat on liposomes and its subsequent disassembly upon GTP hydrolysis (Figure 9). With liposomes of large or intermediate size (radius = 118 or 64 nm, respectively), ArfGAP1 was less potent than ArfGAP3 in inducing COPI disassembly. In contrast, ArfGAP1 promoted much faster coat disassembly than ArfGAP3 on small (R = 38 nm) liposomes.
Figure 9.
Disassembly from liposomes of the COPI coat by ArfGAP1 and ArfGAP3. Light-scattering at right angle was used to follow the assembly–disassembly cycle of the COPI coat on liposomes of defined radii. In a first stage myrArf1 (0.75 μM) was activated by the addition of GTP and by lowering the concentration of free Mg2+ with EDTA. Next, addition of coatomer (0.3 μM) induced an instantaneous jump reflecting assembly of the coat. COPI disassembly was then initiated by the addition of ArfGAP1 (5 nM; red curves) or ArfGAP3 (10 nM; black curves). Note that ArfGAP3 was more efficient than ArfGAP1 in promoting COPI disassembly on large (top row) or medium-size (middle row) liposomes. In striking contrast ArfGAP1 promoted much faster coat disassembly than ArfGAP3 on small liposomes (bottom row). Complete coatomer disassembly was achieved after further addition of 95 nM ArfGAP1 (at t = 24 min).
We concluded that ArfGAP1 and ArfGAP2/3 are controlled by very different mechanisms. ArfGAP1 is very sensitive to the curvature of the membrane on which Arf1-GTP is bound, but it is only marginally affected by coatomer or by other effectors such as golgins (Drin et al., 2008), whereas ArfGAP3 requires coatomer for activity but is insensitive to membrane curvature.
Stimulation of ArfGAP3 Activity by Coatomer Relies on the Basic Stretch
Guided by the results of the CD4 diversion and coatomer-binding assays, we compared the activity of several ArfGAP3 constructs, all harboring an intact N-terminal GAP domain but displaying various truncations/mutations in the noncatalytic region. Deleting the C terminus at positions 510, 483, 410, and 303 gradually decreased the activity of ArfGAP3, which in all cases was detectable only in the presence of coatomer (Figure 10A and Supplemental Figure S2-A). The 1-303 construct still displayed 20% the activity of the full-length protein, suggesting that none of the regions downstream of amino acid 303, including the carboxy-amphipathic helix, plays a critical role in the formation of a catalytically active ArfGAP3–coatomer complex. However, further deletion of the C-terminal region at position 195 abolished the activity of ArfGAP3 (Figure 10A). Thus, in line with the various functional assays described above, the central region of ArfGAP3 plays an essential role in coatomer interaction. This was further confirmed by competition experiments: the activity of full-length ArfGAP3 on Arf1-GTP in the presence of coatomer was decreased by the addition of a peptide corresponding to the central (195-303) region of ArfGAP3 (Figure 10B).
Figure 10.
Localization of ArfGAP3 determinants involved in coatomer stimulation. In all panels, GTP hydrolysis in liposome-bound Arf1 was measured as in Figure 8A in the presence of 30 nM ArfGAP3 or the indicated truncated/mutated forms. (A) Activity measurements of truncated ArfGAP3 forms in presence of 50 nM coatomer. A gradual decrease in activity is observed upon shortening the C-terminal end of ArfGAP3. The last truncation caused the most severe effect suggesting a key role of the central (196-303) region. (B) Activity measurements of full-length ArfGAP3 with 30 nM coatomer and with the indicated concentration of the (195-303) ArfGAP3 fragment. Specificity of the inhibitory effect was checked by the addition of boiled peptide. (C) Effect of point mutations in the central basic stretch of ArfGAP3. The activity of the mutants was determined in the presence of 50 nM coatomer. 4K(1) and 4K(2) correspond to full-length ArfGAP3 4K(215/16/22/23)4A and 4K(222-223-228-229)4A mutants, respectively.
Next, we compared the activity of several point mutants of ArfGAP3. Mutation of the two ISS motifs that are present near the carboxy terminus, previously implicated in the functioning of the yeast Glo3 orthologue in vivo (Yahara et al., 2006), had no effect on ArfGAP3 activity on Arf1-GTP (Supplemental Figure S2-B). By contrast, mutation of the KK motifs whose importance was suggested by the CD4 and coatomer pull-down assays decreased the activity of ArfGAP3 by 10- to 20-fold (Figure 10C). Thus coatomer-binding to the noncatalytic basic stretch of ArfGAP3 is required for efficient stimulation of GTP hydrolysis in Arf1.
DISCUSSION
In this study, we have introduced a CD4 fusion approach for identifying trafficking signals on ArfGAPs, based on the diversion of fusion constructs from plasma membrane to ER localization. Although a reporter fusion approach has been widely used for identifying targeting functions of short signal peptides and for dissecting the role of different domains in transmembrane proteins by domain swap, our results suggest its usefulness for analyzing functional elements in cytosolic trafficking proteins that function in the ER–Golgi shuttle. In principle, ER localization may result not only from interaction of the CD4-fused protein with the COPI system but also from ER retention by quality control systems that detect misfolded domains. However, our analysis is based on the identification of mutations that abrogate ER localization of CD4 fusions and allow their transport to the plasma membrane, and it seems unlikely that mutations act by increasing protein folding as would be expected if ER localization was due to misfolding.
Among several ArfGAP3 regions that were found to contribute to ER diversion of CD4 fusions, we focused on two relatively short determinants. A basic stretch (residues 215-242) where multiple mutations were found to abrogate ER diversion of CD4 fusions was identified as a major contributor to coatomer binding and to coatomer stimulation of GAP activity (Figures 3, 4, and 10). The basic stretch was also required for Golgi localization in HeLa cells (Figure 5), indicating a role of coatomer interaction in Golgi targeting of ArfGAP3, a role previously demonstrated for the closely-related ArfGAP2 based on disruption of its localization upon overexpression of the γ-COP appendage (Watson et al., 2004). A second determinant initially identified by the CD4 assay at the carboxy-terminal part was also found crucial for Golgi localization of GFP fusion of the full-length ArfGAP3 protein (Figure 5). This determinant has amphipathic potential, and mutations that disrupt amphipathicity abrogated Golgi localization (Figure 6C). Moreover, CD spectroscopy with a model peptide representing this motif demonstrated its ability to partially fold into a helical structure in the presence of small liposomes (Figure 6B). This feature is reminiscent of ALPS motifs that are present in ArfGAP1 and are required for Golgi targeting of this protein (Mesmin et al., 2007; Levi et al., 2008). Even though the amphipathic motifs in ArfGAP1 and in ArfGAP2/3 differ in chemistry (particularly in terms of the hydrophilic residues) and in length, we found that the ALPS1 motif from ArfGAP1 could substitute for the function provided by the carboxy motif of ArfGAP3 in conferring Golgi localization (Figure 6D). This and the promiscuity of the hydrophobic residues of the ArfGAP3 amphipathic motif suggest that this motif is involved in hydrophobic interaction with the Golgi membrane. The membrane association of the carboxy helix may provide proper orientation to nearby motifs such as the previously described ISS repeats (Yahara et al., 2006).
Somewhat unexpectedly, ER localization was observed in CD4 fusions of certain ArfGAP3 carboxy fragments that did not show significant coatomer binding in pull-down assays, although the carboxy part did influence the magnitude of coatomer-dependent GAP activity (Figures 2, A and B, and 10A). We also identified two mutations in the central part, R304A-L305A and D323A-M324A, which abrogated ER diversion of CD4 fusions but had little effect in coatomer pull-down assays (Figures 3 and 4). Although the pull-down assay may fail to detect relatively weak coatomer binding sites, it is also possible that some determinants contributing to ER diversion of CD4 fusions of ArfGAP3 interact with other components of COPI vesicles such as cargo and soluble N-ethylmaleimide-sensitive factor attachment protein receptors, causing their trapping in retrograde transport vesicles. Further studies will be required to pinpoint the role of these additional determinants in the cellular function of ArfGAP3.
Our results point to fundamental differences between the ArfGAP1 and ArfGAP2/3 types of proteins. The coatomer-binding motif that we have identified in ArfGAP2/3 is absent in ArfGAP1. Interestingly, a position roughly equivalent to the coatomer-binding motif in ArfGAP3 (residues 215-242) is occupied by the amphipathic ALPS1 motif in ArfGAP1 (residues 199-259). By contrast, the amphipathic motif in ArfGAP3 that we found necessary for Golgi targeting is positioned near the carboxy terminus. These differences in topology are likely to account for the contrasting sensitivity of ArfGAP1 and ArfGAP2/3 to coatomer and to the lipid membrane. On artificial liposomes, the activity of ArfGAP1 on Arf1-GTP is moderately increased by coatomer (Figure 8A) but is increased up to 100-fold by membrane curvature, an effect that is mediated by the ALPS motifs (Bigay et al., 2003; Bigay et al., 2005; Mesmin et al., 2007). In contrast, ArfGAP2/3 are not sensitive to membrane curvature, but their activity strictly depends on engagement in a complex with coatomer through the basic stretch (Figures 8–10). This difference in regulation is further suggested by experiments that tested the modulation of the Golgi localization of various constructs of ArfGAP1 and ArfGAP3 by aluminum fluoride, a reagent known to stabilize ternary complexes containing GDP-bound Arf1, ArfGAP, and coatomer (Bigay et al., 2003). Fluoride could induce Golgi localization in ArfGAP3 fragments lacking large segments of the carboxy part, including the amphipathic motif. By contrast, the contribution of the ALPS motifs to Golgi localization of ArfGAP1 could not be bypassed by fluoride treatment (Figure 7).
Although it is clear that ArfGAP1 senses membrane curvature through its central ALPS motifs, whereas ArfGAP3 senses the presence of coatomer through its central basic stretch, the mechanisms by which these sensors are coupled to the catalytic N-terminal GAP domain remain to be fully investigated. The paucity of structural information on ArfGAP proteins and on coatomer, the likely involvement of flexible ArfGAP regions and the fact that GTP hydrolysis occurs at the surface of a lipid membrane makes this issue very challenging. Nevertheless, several types of mechanisms can be invoked. In the first mechanism, the sensor regions help to concentrate the GAP domain near Arf1-GTP. Thus, if the affinity of the GAP domain for Arf1-GTP is very low, additional interactions between the basic stretch of ArfGAP3 and coatomer or between the ALPS motifs of ArfGAP1 and the membrane should help to confine the GAP domain near its substrate. In the second mechanism, the GAP domain is autoinhibited through interaction with regulatory regions. The binding of the sensor region to its target (a curved membrane in the case of the ALPS motifs; coatomer in case of the basic stretch of ArfGAP3) could relieve this inhibition. This type of mechanism helps to create sharp (high signal/noise) responses and is recurrent in enzymology; it applies for some G proteins effectors and regulators (Torres and Rosen, 2006; DiNitto et al., 2007). In the third mechanism, the GAP domain is not a complete enzymatic machinery and requires contribution of residues from flanking regions or from other proteins to efficiently hydrolyze GTP in Arf1. Thus, membrane by causing structuring of the ALPS motifs of ArfGAP1 or coatomer by structuring the central region of ArfGAP2/3 could generate an extended folded region, leading to increased catalytic efficiency. Coatomer could also contribute to the reaction in a manner akin to the stimulation of sec23 GAP activity on Sar1 by the sec31 component of the COPII coat (Bi et al., 2007). With our present knowledge, none of these mechanisms, which in fact are complementary, can be ruled out, but their relative importance in the regulation of the two types of ArfGAP is likely to be different owing to the different organization of their noncatalytic region.
Another complex issue is the relative role of ArfGAP1 and ArfGAP3 at the Golgi. The strict dependency of ArfGAP2/3 and S. cerevisiae Glo3 on coatomer (Figure 8; Szafer et al., 2001) suggests that these proteins are intimately associated with COPI vesicles, possibly serving as essential coat components as proposed previously (Lewis et al., 2004; Frigerio et al., 2007). Because its activity is only modestly affected by effectors, ArfGAP1 may have a more general role, acting as a terminator of Arf1 activity in conjunction with the various Arf targets, such as golgins, that are known to interact with the Golgi complex (Drin et al., 2008). Last, the formation of a transport vesicle is a complex task and it may be advantageous to combine two ArfGAPs having the ability to sense different features associated with the formation of a proper coated vesicle, including the incorporation of cargo molecules and the degree of membrane deformation.
While this manuscript was under revision, a paper by Weimer et al. (2008) comparing the properties of the Golgi-associated ArfGAPs was published. Although relying to a large extent on different experimental approaches, the two studies reach similar conclusions with regard to the functional differences between ArfGAP1 and the ArfGAP2/3.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by grants from the Israel Science Foundation (171/08) and from the Agence Nationale de la Recherche (program blanc). We thank Anne Spang and Felix Wieland for sharing results, all members of the Cassel and Antonny laboratories for discussions, and Blanch Schwappach, Rainer Duden, and Koret Hirschberg for plasmids. B. M. was supported by a fellowship from the Fondation pour la Recherche Médicale.
Abbreviations used:
- Arf
ADP-ribosylation factor
- ALPS
amphipathic lipid packing sensor
- GAP
GTPase-activating protein.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E08-10-1010) on December 24, 2008.
REFERENCES
- Bethune J., Wieland F., Moelleken J. COPI-mediated transport. J. Membr. Biol. 2006;211:65–79. doi: 10.1007/s00232-006-0859-7. [DOI] [PubMed] [Google Scholar]
- Bi X., Mancias J. D., Goldberg J. Insights into COPII coat nucleation from the structure of Sec23.Sar1 complexed with the active fragment of Sec31. Dev. Cell. 2007;13:635–645. doi: 10.1016/j.devcel.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigay J., Antonny B. Real-time assays for the assembly-disassembly cycle of COP coats on liposomes of defined size. Methods Enzymol. 2005;404:95–107. doi: 10.1016/S0076-6879(05)04010-3. [DOI] [PubMed] [Google Scholar]
- Bigay J., Casella J. F., Drin G., Mesmin B., Antonny B. ArfGAP1 responds to membrane curvature through the folding of a lipid packing sensor motif. EMBO J. 2005;24:2244–2253. doi: 10.1038/sj.emboj.7600714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigay J., Gounon P., Robineau S., Antonny B. Lipid packing sensed by ArfGAP1 couples COPI coat disassembly to membrane bilayer curvature. Nature. 2003;426:563–566. doi: 10.1038/nature02108. [DOI] [PubMed] [Google Scholar]
- Bremser M., Nickel W., Schweikert M., Ravazzola M., Amherdt M., Hughes C. A., Sollner T. H., Rothman J. E., Wieland F. T. Coupling of coat assembly and vesicle budding to packaging of putative cargo receptors. Cell. 1999;96:495–506. doi: 10.1016/s0092-8674(00)80654-6. [DOI] [PubMed] [Google Scholar]
- Cukierman E., Huber I., Rotman M., Cassel D. The ARF1 GTPase-activating protein: zinc finger motif and Golgi complex localization. Science. 1995;270:1999–2002. doi: 10.1126/science.270.5244.1999. [DOI] [PubMed] [Google Scholar]
- D'Souza-Schorey C., Chavrier P. ARF proteins: roles in membrane traffic and beyond. Nat. Rev. Mol. Cell Biol. 2006;7:347–358. doi: 10.1038/nrm1910. [DOI] [PubMed] [Google Scholar]
- DiNitto J. P., Delprato A., Gabe Lee M. T., Cronin T. C., Huang S., Guilherme A., Czech M. P., Lambright D. G. Structural basis and mechanism of autoregulation in 3-phosphoinositide-dependent Grp1 family Arf GTPase exchange factors. Mol. Cell. 2007;28:569–583. doi: 10.1016/j.molcel.2007.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dogic D., de Chassey B., Pick E., Cassel D., Lefkir Y., Hennecke S., Cosson P., Letourneur F. The ADP-ribosylation factor GTPase-activating protein Glo3p is involved in ER retrieval. Eur. J. Cell Biol. 1999;78:305–310. doi: 10.1016/s0171-9335(99)80064-8. [DOI] [PubMed] [Google Scholar]
- Donaldson J. G., Honda A. Localization and function of Arf family GTPases. Biochem. Soc. Trans. 2005;33:639–642. doi: 10.1042/BST0330639. [DOI] [PubMed] [Google Scholar]
- Drin G., Morello V., Casella J. F., Gounon P., Antonny B. Asymmetric tethering of flat and curved lipid membranes by a golgin. Science. 2008;320:670–673. doi: 10.1126/science.1155821. [DOI] [PubMed] [Google Scholar]
- Eugster A., Frigerio G., Dale M., Duden R. COP I domains required for coatomer integrity, and novel interactions with ARF and ARF-GAP. EMBO J. 2000;19:3905–3917. doi: 10.1093/emboj/19.15.3905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frigerio G., Grimsey N., Dale M., Majoul I., Duden R. Two human ARFGAPs associated with COP-I-coated vesicles. Traffic. 2007;8:1644–1655. doi: 10.1111/j.1600-0854.2007.00631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillingham A. K., Munro S. The small G proteins of the Arf family and their regulators. Annu. Rev. Cell Dev. Biol. 2007;23:579–611. doi: 10.1146/annurev.cellbio.23.090506.123209. [DOI] [PubMed] [Google Scholar]
- Goldberg J. Structural and functional analysis of the ARF1-ARFGAP complex reveals a role for coatomer in GTP hydrolysis. Cell. 1999;96:893–902. doi: 10.1016/s0092-8674(00)80598-x. [DOI] [PubMed] [Google Scholar]
- Huber I., Cukierman E., Rotman M., Aoe T., Hsu V. W., Cassel D. Requirement for both the amino-terminal catalytic domain and a noncatalytic domain for in vivo activity of ADP-ribosylation factor GTPase-activating protein. J. Biol. Chem. 1998;273:24786–24791. doi: 10.1074/jbc.273.38.24786. [DOI] [PubMed] [Google Scholar]
- Kahn R. A., Volpicelli-Daley L., Bowzard B., Shrivastava-Ranjan P., Li Y., Zhou C., Cunningham L. Arf family GTPases: roles in membrane traffic and microtubule dynamics. Biochem. Soc. Trans. 2005;33:1269–1272. doi: 10.1042/BST0331269. [DOI] [PubMed] [Google Scholar]
- Lee M. C., Miller E. A., Goldberg J., Orci L., Schekman R. Bi-directional protein transport between the ER and Golgi. Annu. Rev. Cell Dev. Biol. 2004;20:87–123. doi: 10.1146/annurev.cellbio.20.010403.105307. [DOI] [PubMed] [Google Scholar]
- Lee S. Y., Yang J. S., Hong W., Premont R. T., Hsu V. W. ARFGAP1 plays a central role in coupling COPI cargo sorting with vesicle formation. J. Cell Biol. 2005;168:281–290. doi: 10.1083/jcb.200404008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letourneur F., Gaynor E. C., Hennecke S., Demolliere C., Duden R., Emr S. D., Riezman H., Cosson P. Coatomer is essential for retrieval of dilysine-tagged proteins to the endoplasmic reticulum. Cell. 1994;79:1199–1207. doi: 10.1016/0092-8674(94)90011-6. [DOI] [PubMed] [Google Scholar]
- Levi S., Rawet M., Kliouchnikov L., Parnis A., Cassel D. Topology of amphipathic motifs mediating Golgi localization in ArfGAP1 and its splice isoforms. J. Biol. Chem. 2008;283:8564–8572. doi: 10.1074/jbc.M709738200. [DOI] [PubMed] [Google Scholar]
- Lewis S. M., Poon P. P., Singer R. A., Johnston G. C., Spang A. The ArfGAP Glo3 is required for the generation of COPI vesicles. Mol. Biol. Cell. 2004;15:4064–4072. doi: 10.1091/mbc.E04-04-0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W., Duden R., Phair R. D., Lippincott-Schwartz J. ArfGAP1 dynamics and its role in COPI coat assembly on Golgi membranes of living cells. J. Cell Biol. 2005;168:1053–1063. doi: 10.1083/jcb.200410142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Zhang C., Xing G., Chen Q., He F. Functional characterization of novel human ARFGAP3. FEBS Lett. 2001;490:79–83. doi: 10.1016/s0014-5793(01)02134-2. [DOI] [PubMed] [Google Scholar]
- Makler V., Cukierman E., Rotman M., Admon A., Cassel D. ADP-ribosylation factor-directed GTPase-activating protein. Purification and partial characterization. J. Biol. Chem. 1995;270:5232–5237. doi: 10.1074/jbc.270.10.5232. [DOI] [PubMed] [Google Scholar]
- Mesmin B., Drin G., Levi S., Rawet M., Cassel D., Bigay J., Antonny B. Two lipid-packing sensor motifs contribute to the sensitivity of ArfGAP1 to membrane curvature. Biochemistry. 2007;46:1779–1790. doi: 10.1021/bi062288w. [DOI] [PubMed] [Google Scholar]
- Parnis A., Rawet M., Regev L., Barkan B., Rotman M., Gaitner M., Cassel D. Golgi localization determinants in ArfGAP1 and in new tissue-specific ArfGAP1 isoforms. J. Biol. Chem. 2006;281:3785–3792. doi: 10.1074/jbc.M508959200. [DOI] [PubMed] [Google Scholar]
- Poon P. P., Cassel D., Spang A., Rotman M., Pick E., Singer R. A., Johnston G. C. Retrograde transport from the yeast Golgi is mediated by two ARF GAP proteins with overlapping function. EMBO J. 1999;18:555–564. doi: 10.1093/emboj/18.3.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbe K., Antonny B. Liposomes in the study of GDP/GTP cycle of Arf and related small G proteins. Methods Enzymol. 2003;372:151–166. doi: 10.1016/S0076-6879(03)72009-6. [DOI] [PubMed] [Google Scholar]
- Szafer E., Pick E., Rotman M., Zuck S., Huber I., Cassel D. Role of coatomer and phospholipids in GTPase-activating protein-dependent hydrolysis of GTP by ADP-ribosylation factor-1. J. Biol. Chem. 2000;275:23615–23619. doi: 10.1074/jbc.M003171200. [DOI] [PubMed] [Google Scholar]
- Szafer E., Rotman M., Cassel D. Regulation of GTP hydrolysis on ADP-ribosylation factor-1 at the Golgi membrane. J. Biol. Chem. 2001;276:47834–47839. doi: 10.1074/jbc.M106000200. [DOI] [PubMed] [Google Scholar]
- Torres E., Rosen M. K. Protein-tyrosine kinase and GTPase signals cooperate to phosphorylate and activate Wiskott-Aldrich syndrome protein (WASP)/neuronal WASP. J. Biol. Chem. 2006;281:3513–3520. doi: 10.1074/jbc.M509416200. [DOI] [PubMed] [Google Scholar]
- Watson P. J., Frigerio G., Collins B. M., Duden R., Owen D. J. gamma-COP appendage domain—structure and function. Traffic. 2004;5:79–88. doi: 10.1111/j.1600-0854.2004.00158.x. [DOI] [PubMed] [Google Scholar]
- Weimer C., Beck R., Eckert P., Reckmann I., Moelleken J., Brugger B., Wieland F. Differential roles of ArfGAP1, ArfGAP2, and ArfGAP3 in COPI trafficking. J. Cell Biol. 2008;183:725–735. doi: 10.1083/jcb.200806140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yahara N., Sato K., Nakano A. The Arf1p GTPase-activating protein Glo3p executes its regulatory function through a conserved repeat motif at its C-terminus. J. Cell Sci. 2006;119:2604–2612. doi: 10.1242/jcs.02997. [DOI] [PubMed] [Google Scholar]
- Yang J. S., Lee S. Y., Spano S., Gad H., Zhang L., Nie Z., Bonazzi M., Corda D., Luini A., Hsu V. W. A role for BARS at the fission step of COPI vesicle formation from Golgi membrane. EMBO J. 2005;24:4133–4143. doi: 10.1038/sj.emboj.7600873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan H., Michelsen K., Schwappach B. 14-3-3 dimers probe the assembly status of multimeric membrane proteins. Curr. Biol. 2003;13:638–646. doi: 10.1016/s0960-9822(03)00208-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.









