Abstract
Follicle stem cells (SCs) residing in the bulge region of a hair follicle (HF) can give rise to multiple lineages during the hair cycle and wound healing. The activation and self-renewal of follicle SCs must be tightly regulated to maintain the HF and epidermal homeostasis. Here we show that, in young mice, disruption of epidermal Smad4, the common mediator of transforming growth factor-β (TGF-β) signaling, stimulated the activation of follicle SCs, leading to hyperplasia of interfollicular epidermis (IFE), HFs, and sebaceous glands (SGs). Increased proliferation of follicle SCs ultimately exhausted the SC niche, indicated by the loss of bromodeoxyuridine (BrdU) label–retaining cells (LRCs), loss of keratin 15 (K15), and CD34 expression. In addition, the colony-forming efficiency of Smad4 mutant keratinocytes was significantly decreased. Increased nuclear localization of β-catenin and increased expression of c-Myc were correlated with the overactivation and depletion of follicle SCs. We concluded that Smad4 plays a pivotal role in follicle SC maintenance.
INTRODUCTION
Follicle stem cells (SCs) residing in the bulge of hair follicles (HFs) continuously self-renew and differentiate into interfollicular epidermis (IFE), HFs, and sebaceous glands (SGs) and are thus necessary for the maintenance of skin homeostasis and for hair regeneration (Blanpain and Fuchs, 2006). Follicle SCs derived cells can also be recruited to regenerate the various epithelial cell types of hairy skin after injury (Blanpain et al., 2004; Morris et al., 2004; Tumbar et al., 2004; Levy et al., 2005). The bulge area serves as a niche in which the self-renewal and activation of multipotent follicle SCs are tightly regulated. Although the Wnt/β-catenin, Sonic hedgehog, and Notch signaling pathways have been shown to regulate the activation and differentiation of follicle SCs, the molecular mechanism underlying the follicle SC maintenance remains poorly understood (Fuchs, 2007).
Bone morphogenetic protein (BMP) signaling has been shown to play essential roles in HF morphogenesis, as well as in regulating follicle SC activation and the differentiation of postnatal HFs. BMPs are differentially expressed in various HF cell lineages, with constitutive BMP type 1a receptor (BMPR1A) expression seen in all the HF epithelial cells including follicle SCs (Kobielak et al., 2003). Ectopic overexpression of BMP4 or targeted disruption of the BMP antagonist Noggin results in disturbed HF induction and progressive hair loss. Overexpression of Noggin reveals that BMP signaling plays important roles in induction of the anagen phase (Botchkarev et al., 1999), hair shaft differentiation (Kulessa et al., 2000), and regulation of HF size (Sharov et al., 2006). Blockade of BMP signaling by deletion of the Bmpr1A leads to aberrant de novo HF morphogenesis accompanied by hair matrix cell hyperplasia and the formation of follicular tumors (Andl et al., 2004; Ming Kwan et al., 2004). Recently, several lines of evidence have suggested that BMP signaling in the follicle SC niche inhibits follicle SC activation and HF anagen induction (Zhang et al., 2006; Kobielak et al., 2007). The dynamic expression of Noggin in follicle SCs during the HF cycling triggers the cyclic inactivation of BMP signaling which correlates with the activation of follicle SCs during the early anagen phase (Zhang et al., 2006). A very recent study reveals that cyclic dermal BMP signaling regulates SC homeostasis during hair regeneration by the interorgan macroenvironment (Plikus et al., 2008).
The Wnt/β-catenin pathway has been well characterized and shown to function in regulating follicle SC proliferation and fate determination. In the resting follicle, SCs are in a quiescent, Wnt-inhibited state (DasGupta and Fuchs, 1999; Tumbar et al., 2004). Activation of Wnt/β-catenin signaling is critical for HF morphogenesis and plays a key role in driving follicle SCs along hair differentiation lineages (Merrill et al., 2001; Zhang et al., 2006). β-Catenin stabilization is essential for promoting the transition from quiescent follicle SCs into proliferating transit-amplifying cells (Lowry et al., 2005). Recent studies suggest that BMP signaling could inhibit β-catenin stabilization in the follicle SC niche through regulation of PTEN/phosphatidylinositol 3′-kinase (PI3K)/Akt signaling pathway (Zhang et al., 2006; Kobielak et al., 2007).
BMP/transforming growth factor-β (TGF-β) signals transduce through transmembrane receptors and intracellular mediator Smads (Shi and Massague, 2003). Microarray profiling of bulge SCs has revealed that a variety of genes in the BMP and TGF-β pathways are preferentially expressed in the bulge relative to the proliferating basal cells of the epidermis (Blanpain et al., 2004; Morris et al., 2004; Tumbar et al., 2004). Overexpression of Smad2 results in disorganized epidermis, indicating an important role of Smad2 in regulating TGF-β–mediated epidermal homeostasis (Ito et al., 2001). The up-regulated BMP/TGF-β signals in bulge SCs are correlated with nuclear localization of phospho-Smad1 and phospho-Smad2 together with activated target genes and/or Smad-interacting proteins (Tumbar et al., 2004; Mou et al., 2006), indicating a role for Smad-mediated BMP/TGF-β signals in maintaining the special quiescent nature of resting follicle SCs. However, the function of Smads in follicle SC maintenance remains largely undefined.
We and others have previously reported that targeted ablation of Smad4 in the epidermis and outer root sheath of HFs results in progressive hair loss and skin tumor formation (Yang et al., 2005; Qiao et al., 2006). A recent study has shown that Smad4 loss associated depletion of desmoglein-4 expression contributes to hair follicle degeneration in Smad4 mutants (Owens et al., 2008). In the current study, we reported that Smad4 disruption in the epidermis leads to activation of follicle SCs, which caused hypertrophic of IFE, HFs, and SGs. This hypertrophic phenotype is transient, because activated SCs are eventually exhausted in the follicle. We further provide evidence that an increase in nuclear β-catenin and up-regulation of c-Myc may be responsible for the depletion of follicle SCs in Smad4 mutant mice.
MATERIALS AND METHODS
Mouse Strains and Genotyping
Mouse genotyping of the keratinocyte specific Smad4 gene knockout mice was performed as described in Yang et al. (2005). All animal studies were approved by the Review Board of Institute of Biotechnology.
Oil Red O and LacZ Staining
Oil Red O staining was performed on frozen skin sections from Smad4 mutants and control littermates aged 3, 6, and 12 mo. Slides were stained in 0.5% Oil Red O in 100% propylene glycol for 30 min and counterstained with Mayer's hematoxylin. Skin tissues of postnatal day 45 (P45) mouse were fixed in 4% paraformaldehyde for 2 h and then subjected to LacZ staining.
Histological Analysis and Immunofluorescence
Dorsal skin was embedded in Cryomatrix (Thermo Scientific, Waltham, MA) and sectioned at 10 μm. Frozen sections were fixed in 4% paraformaldehyde, and immunofluorescence was performed as described in Yuan et al. (2008). Primary antibodies used in this study were K14 at 1:1000, K1 at 1:1000, involucrin at 1:1000 (Covance Laboratories, Madison, WI), Ki67 at 1:1000 (Abcam, Cambridge, MA), bromodeoxyuridine (BrdU) at 1:100 (Abcam), CD34 at 1:200 (eBioscience, San Diego, CA), K15 at 1:100 (Chemicon, Temecula, CA), active-β-catenin at 1:100 (Upstate Biotechnology, Lake Placid, NY), and p-Akt at 1:200 (Cell Signaling, Beverly, MA). Antigens were visualized with FITC or TRITIC-conjugated secondary antibodies (Zymed Laboratories, South San Francisco, CA). Nuclear DNA was stained with the DAPI reagent (Sigma, St. Louis, MO).
BrdU Label–retaining Analyses
Ten-day-old mice were injected intraperitoneally with 50 μg/g body weight BrdU (Sigma) every 12 h for 48 h. Mice were killed at least 2 mo after the last injection. Whole mounts of mouse tail epidermis preparation and immunofluorescence were performed as previously described (Braun et al., 2003). A laser scanning confocal microscope (Bio-Rad, Hercules, CA; Radiance 2100) was used to obtain fluorescence images.
Clonogenicity Assays and Immunofluorescence
Keratinocytes from back skin of 6–8-wk-old mice were obtained as described previously (Frye et al., 2003); although the Smad4 mutant HFs resisted degeneration into catagen, we could peel off the epidermis and hypertrophic anagen HFs thoroughly from the dermis at ∼2 mo old (Supplemental Figure S1). Cells, 1 × 105, were plated per 60-mm dish on mitomycin C–treated 3T3 feeder layer. After 32 d, colonies were photographed and stained with 0.5% rhodamine B. For immunofluorescence, cells were subsequently fixed in formaldehyde 4% and then immunostained and stained with DAPI for DNA before mounting as described in Sun et al. (2008).
Western Blot Analysis
Tissue proteins were obtained from the extracts of epidermis of mice at P42 and Western blot performed as described in Yang et al. (2005). Antibody reaction was done with antibodies against active-β-catenin (Upstate), β-catenin (Santa Cruz Biotechnology, Santa Cruz, CA), c-Myc (Santa Cruz), PTEN (Cell Signaling), p-Akt (Cell Signaling), GSK-3β (Cell Signaling), β1-integrin (Santa Cruz), and β-actin (Sigma).
Flow Cytometry
Primary mouse keratinocytes were isolated from back skin of 72-d-old mice as described previously (Blanpain et al., 2004). Cells were labeled with anti-α6-integrin antibody conjugated to phycoerithrin (eBioscience) and anti-CD34 conjugated to FITC (eBiosciences) for 45 min at room temperature with rotation. Cells were then analyzed using BD FACScalibur sorter and CellQuest FACS analysis system.
RESULTS
The Hypertrophy Phenotypes in Smad4 Mutant Epidermis Are Transient
In our previous study, we generated keratinocyte-specific Smad4 knockout mice (Smad4co/co;K5-Cre) using the K5-Cre transgenic mice (Yang et al., 2005). The Smad4co/co;K5-Cre mutant mice began to exhibit progressive hair loss after P20. By 12 mo of age, 70% of the mutant mice developed visible skin tumors (Yang et al., 2005). We further observed that the majority of (22/25) tumors occurred in mice younger than 9 mo (Supplemental Figure S2). About 10% of mutant mice survived as long as 15 mo without any skin tumors.
We performed Oil Red O staining to analyze the histological changes in the epidermis of 3-, 6-, and 12-mo-old Smad4co/co;K5-Cre and control mice (Figure 1). At 3 mo, the Smad4 mutant epidermis displayed significantly thickened IFE, hypertrophic HFs, and enlarged SGs compared with wide type littermates (Figure 1, A and D). Surprisingly, we found the cellularity of the IFE, HFs and SGs to be decreased in Smad4 mutants at 6 mo of age compared with that at 3 mo (Figure 1, D and E). By 12 mo, the thickness of mutant epidermis was significantly reduced compared with controls, and all HF infundibula were found to have degenerated into cysts that contained a large amount of keratose debris (Figure 1, C and F).
Figure 1.
Hypertrophy phenotypes withered in aged Smad4 mutant mice. (A–F) Histological analysis by Oil Red O staining performed on dorsal skin of 3- (A and D), 6- (B and E), and 12-mo-old (C and F) wild-type (Cre/+/+, A–C) and Smad4 mutant mice (Cre/Co/Co, D–F). Bar, 50 μm.
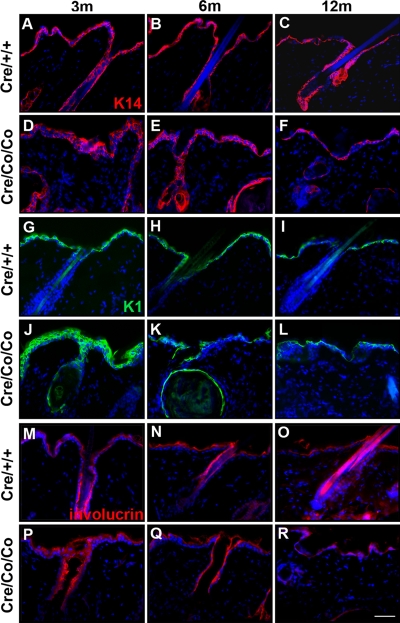
We also performed immunofluorescence in Smad4 mutant and control epidermis to determine the expression levels of keratin14 (K14, Figure 2, A–F), keratin1 (K1, Figure 2, G–L), and involucrin (Figure 2, M–R), which are markers, respectively, for the basal layer of the epidermis and the HF outer root sheaths, the spinous layers of the epidermis and inner root sheaths of HFs, and the granular layers of the epidermis. The results showed that IFE and HF hypertrophy diminished gradually in Smad4 mutants (Figure 2, A–R; Supplemental Figure S3).
Figure 2.
Dynamic changes of epidermis markers expression in postnatal Smad4 mutant epidermis and HFs. Immunofluorescence staining with K14 (A–F), K1 (G–L), and involucrin (M–R) on dorsal skin of 3- (A, D, G, J, M, and P), 6- (B, E, H, K, N, and Q), and 12-mo-old (C, F, I, L, O, and R) wild-type (A–C, G–I, and M–O) and Smad4 mutant mice (D–F, J–L, and P–R). Bar, 40 μm.
Loss of Smad4 Resulted in a Transient Increase in Epidermal Cellular Proliferation
We next used Ki67 immunofluorescence to examine proliferation in the skin of mouse back (Figure 3, A–F) and tail (Figure 3, G–L) at 3, 6, and 12 mo, respectively. At 3 mo, there was a significantly increased proliferation in Smad4 mutant epidermis (Figure 3D) compared with wild-type (Figure 3A). The number of Ki67-positive cells in Smad4 mutants (Figure 3E) had decreased to control epidermal levels by 6 mo (Figure 3B). By 12 mo, the number of Ki67-positive cells in Smad4 mutants was further declined (Figure 3F). Whole mount Ki67 labeling of mouse tail epidermis (Figure 3, G–L) confirmed the transient increased proliferation in Smad4 mutant mice (Figure 3, J and M).
Figure 3.
Loss of Smad4 resulted in a transient increased proliferation in epidermis and HFs. (A–F) Ki67 immunofluorescence on mice dorsal skin sections at 3 (A and D), 6 (B and E), and 12 mo (C and F), respectively. In Smad4 mutant epidermis there was an increase in the number of Ki67-positive keratinocytes at 3 mo (D) compared with that of wide-type mice (A), whereas it decreased gradually at 6 (E) and 12 mo (F). (G–L) Ki67 immunofluorescence of whole mount mouse tail epidermis at 3 (G and J), 6 (H and K), and 12 mo (I and L) confirmed the transient increased proliferation in Smad4 mutants. (M) Percentage of Ki67-positive cells; n = 6 for each time point and genotype. **p < 0.01. Bar, (A–F) 50 μm; (G–L) 100 μm.
Smad4 Deletion Resulted in Overactivation of Follicle SCs
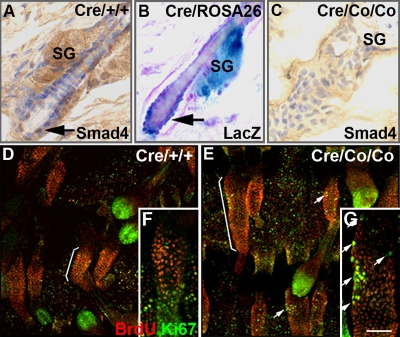
The withering of the hypertrophy phenotypes after a transient increase in proliferation suggested that the follicle SCs may have been induced to exit from their compartment. To test this hypothesis, we first examined the expression pattern of Smad4 in the bulge. As shown in Figure 4A, Smad4 is normally expressed in the bulge SCs of HF at telogen (P45). β-Galactosidase staining done in the P45 K5-Cre;ROSA26 double transgenic mice revealed Cre-mediated recombination in the bulge SCs at telogen (Figure 4B). No Smad4 expression was detected in P45 Smad4Co/Co;K5-Cre mutant HFs (Figure 4C). These data indicated that the Smad4 expression was successfully disrupted in the follicle SC compartment. We further investigated whether BrdU label–retaining cells (LRCs; Cotsarelis et al., 1990) in the HF bulge were affected in Smad4 mutants. Ten-day-old mice received an injection of BrdU every 12 h for a total of four injections. After a 60-d chase, keratinocytes in the bulge of wild-type tail epidermis retained the BrdU label (Figure 4D, bracket). Ki67 labeling was confined to the lower bulb region of the HF, indicating that most of the follicle SCs in wild-type tail epidermis was quiescent. In contrast, the LRC zones were expanded in Smad4-deleted mice (Figure 4E, bracket). Increased numbers of Ki67-positive LRCs were found in Smad4 mutant tail HFs (Figure 4, E and G, arrows, 11.2 ± 5.0% in Smad4 mutants (n = 16) vs. 2.9 ± 2.5% in controls, (n = 10), p < 0.01), indicating that more Smad4 mutant LRCs were dividing.
Figure 4.
Smad4 deletion result in overactivation of follicle SCs. Immunohistochemistry staining of Smad4 in HFs of wild-type (A) and Smad4 mutants (C). (B) LacZ staining of a HF in a K5-Cre;ROSA26 double transgenic mouse. (D–G) Costaining of BrdU and Ki67 were performed after a 60-d chase. LRCs concentrated in the bulge of wild-type tail epidermis (D and F, brackets), whereas Ki67-labeled cells were concentrated most in the bulb (D and F, arrows). In Smad4 mutants, the LRC zones were expanded (G, brackets), and increased numbers of LRCs were colabeled with Ki67 (E and G, arrows). SG, sebaceous gland. Bar, (A–C) 25 μm; (D and E) 100 μm; (F and G) 40 μm.
Smad4 Deletion Led to Depletion of the Follicle SC Compartment
Continued proliferation of LRCs eventually resulted in loss of the label after a 77-d chase, scarcely any LRCs were evident in the back skin of Smad4 mutant mice (Figure 5B), whereas in wild-type back skin, many LRCs could be found in the bulge (Figure 5A, arrow).
Figure 5.
Smad4 deletion led to depletion of the follicle SC compartment. (A and B) Costaining of BrdU and Ki67 after a 77-d chase. Note that LRCs were detected in the bulge of HFs of wild-type controls (A, bracket), but barely detected in Smad4 mutants (B). (C–N) Immunofluorescence of CD34 and K15 marks the HF bulge of wild-type mice (C, E, G, I, K, and M), whereas in Smad4 mutant mice they were gradually lost (D, F, H, J, L, and N). Bar, (A, C, G, and N) 35 μm; (B and D–F) 70 μm.
To confirm that Smad4 deletion led to depletion of the follicle SC compartment, we next examined the expression of the HF bulge marker CD34. CD34 was detected in the bulge of P72 wild-type mice but not in that of their mutant littermates (Figure 5, C and D). The expression of another bulge marker, keratin 15 (K15), was also found to have been lost in the mutant tail epidermis (Figure 5, E and F). We subsequently examined the expression of K15 in mutant epidermis between P22 and P72. Comparable expression of K15 was detected in P22 and P29 mutants (Figure 5, G–J). The expression of K15 was significantly decreased at P40 (Figure 5, K and L) and was completely absent in mutants at P72 (Figure 5, M and N). The gradually diminishing expression of CD34 in the bulge is also illustrated in Supplemental Figure S4. The number of CD34-positive cells that expressed high levels of a6-integrin was significantly decreased in Smad4 mutants at P72 (13.2 ± 5.5% in controls vs. 2.0 ± 1.3% in Smad4 mutants, n = 4; Figure 6, A and B).
Figure 6.
Significantly decreased colony-forming efficiency of Smad4 mutant keratinocytes. (A and B) Flow cytometry of α6-integrin and CD34 double-labeled keratinocytes from dorsal epidermis of wild-type and Smad4 mutant mice at P72. (C–J) Clonal forming assay with 1 × 105 total keratinocytes from 6- to 8-wk-old control (C, E, G, and I) and Smad4 mutant mice (D, F, H, and J). Microscopic view of wild-type (E) and Smad4 mutant (F) colonies. Immunofluorescence on wild-type and Smad4 mutant keratinocytes in culture for K14 (G and H), involucrin (I and J), and Ki67 (K–R). Bar, (E–J) 20 μm; (K and L) 25 μm; (M–R) 50 μm.
To further study the effect of Smad4 inactivation on SC function, we carried out assays of clone formation. After a 32-d culture, wild-type SCs gave rise to large, round clones (Figure 6C) containing tightly arranged, small, round cells (Figure 6E). In contrast, Smad4 mutant cells produced small, irregularly shaped, and fully differentiated colonies (Figure 6, D and F). Immunofluorescent detection of K14 revealed that clones derived from both wild-type and Smad4 mutant keratinocytes were K14 positive (Figure 6, G and H). Notably, Smad4 mutant, but not wild-type, keratinocytes expressed the terminal differentiation marker involucrin (Figure 6, I and J). Mutant keratinocytes contained a greater percentage of Ki67-positive cells at early stages of culture compared with controls (day 10, Figure 6, K, L, and S). A comparable percentage of Ki67-positive cells was detected in mutant and control keratinocytes cultured for 15 and 22 d (Figure 6, M–P and S). When cultured for 30 d, the majority of mutant keratinocytes were differentiated, and the percentage of proliferating cells was lower than controls (Figure 6, Q—S). These results suggested that the SC compartment might be reduced in Smad4 mutant epidermis.
The Increased Nuclear Localization of β-Catenin and Increased c-Myc Expression in Smad4 Mutant Epidermis
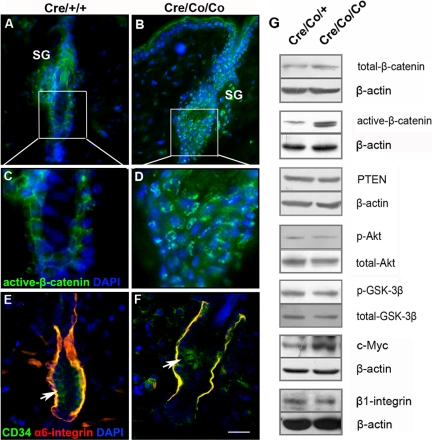
Stabilization of β-catenin is essential for promoting the transition from quiescent bulge SCs into transit-amplifying cells (Lowry et al., 2005). Increased nuclear staining of active β-catenin was observed in bulge keratinocytes of Smad4 mutants at telogen (Figure 7, A–D). Western blots confirmed the increased level of active β-catenin in Smad4 mutants, whereas total level β-catenin was not altered (Figure 7G). Pten, phospho-Akt (the active form of Akt), and phospho-GSK-3β were at normal levels in Smad4 mutant epidermis compared with that in controls (Figure 7G). C-Myc has been reported as a downstream target of β-catenin/TCF–signaling pathways (He et al., 1998), and both increased and decreased c-Myc levels have been shown to deplete epidermal SCs by modulating adhesive interactions with the local microenvironment (Waikel et al., 2001; Braun et al., 2003; Frye et al., 2003; Zanet et al., 2005). We found an increase in c-Myc expression in Smad4 mutant epidermis, whereas β1-integrin expression was not altered (Figure 7G); in contrast, α6-integrin levels were diminished in Smad4 mutant SC niche where CD34 was expressed (Figure 7, E and F).
Figure 7.
The increased nuclear localization of β-catenin and increased c-Myc expression in Smad4 mutant epidermis. (A–D) The immunofluorescence of active-β-catenin in the HFs of the control (A and C) and Smad4 mutant mice (B and D) at P42. (E and F) The immunofluorescence of α6-integrin and CD34 in HFs showed that α6-integrin was down-regulated in SC niche of Smad4 mutants at P42. (G) The expression of total β-catenin, active-β-catenin, PTEN, p-Akt, p-GSK-3β, c-Myc, and β1-integrin in epidermis of P42 Smad4 mutant and control mice were detected by Western blot analyses. Bar, (A and B) 35 μm; (C and D) 15 μm; (E and F) 25 μm.
DISCUSSION
This study revealed an essential role for Smad4 in the regulation of follicle SCs. We have shown that disruption of Smad4 in mouse epidermis, including bulge follicle SCs, results in persistent activation of follicle SCs, ultimately leading to their depletion. We further show that both β-catenin and c-Myc are up-regulated in Smad4 mutants, independent of overactivation of the PI3K/AKT signaling pathway.
Many studies have indicated that TGF-β–signaling pathways, in particular BMP signals, play crucial roles in the regulation of follicle SC activation. Microarray profiling of follicle SCs has led to the identification of genes in the TGF-β pathways that are preferentially expressed in the bulge (Fuchs, 2007). Latent TGF-β–binding protein 1 and p-Smad2 expression in the bulge are confirmed by immunofluorescence (Morris et al., 2004; Tumbar et al., 2004). Recent studies revealed that cyclic dermal BMP signals control follicle SC activation during hair regeneration (Plikus et al., 2008), whereas dynamic expression of Noggin, a BMP antagonist leads to activation of follicle SCs and initiation of the anagen phase (Zhang et al., 2006). Conditional ablation of BMPR1A resulted in activation of epidermal SCs and loss of a quiescent niche (Zhang et al., 2006; Kobielak et al., 2007). In this study, we showed that Smad4 was expressed in bulge SCs and that targeted disruption of Smad4 caused overactivation of follicle SCs, leading to significantly thickened IFE and hypertrophic HFs and SGs. The exit from the SC compartment was accelerated in young Smad4 mutants, indicated by an expanded LRC zone and a transit increase in proliferation. Several lines of evidence suggested that conditional deletion of Smad4 resulted in the depletion of follicle SCs. In relatively young Smad4 mutant mice (P72), some keratinocytes in the expanded LRC zone were also marked by Ki67. Continued proliferation of LRCs eventually led to the loss of main features of follicle SCs, BrdU label retention, and K15 expression. The expression of CD34 which were important for bulge SC tumorigenesis (Trempus et al., 2007) were also lost. Together, these data indicate that, in Smad4 mutants, overactivated SCs generated more transient amplifying cells, at the expense of their self-renewal. As the population of SCs was exhausted, the number of transient amplifying cells was gradually decreased in Smad4 mutants. Consequently, the hypertrophic phenotypes in IFE, HFs, and SGs withered in aged Smad4 mutant mice (6 and 12 mo) and there was scarcely any skin tumorigenesis in Smad4 mutant older than 9 mo (Supplemental Figure S2). Additional evidence for follicle SC depletion came from the colony-forming experiments. Smad4 mutant keratinocytes gave rise to abortive clones consisting of large, terminally differentiated keratinocytes after a 32-d culture. These findings suggest an important role of Smad4 in maintaining homeostasis of follicle SCs. Surprisingly, wound healing experiments showed that 10-mo-old Smad4 mutants were able to heal the wound at a rate comparable to that of controls (Supplemental Figure S6, G and H), although the reepithelialization rate was significantly reduced compared with that at 2 mo (Supplemental Figure S6, F, H, and J). This finding suggests that follicle SCs are not necessary for cutaneous wound healing, which is supported by a recent observation that an extended epidermal response heals cutaneous wounds in the absence of a follicular SC contribution (Langton et al., 2008).
Our findings may not completely concur with the reports that induced conditional inactivation of BMPR1A leads to overproduction of HF stem/progenitor cells (Zhang et al., 2006) or expansion of early progenitors of bulge cells (Kobielak et al., 2007). The deletion of Smad4 would be expected to disrupt the cellular responses to both TGF-β and BMP signals, resulting in a discrepancy between Smad4 and BMPR1A in epithelial differentiation as well as follicle SC homeostasis maintenance. In our previous study, we found that Smad4 mutants showed defective programmed regression of HFs, which is in line with the observations that loss of TGF-β1 results in failure of catagen induction (Foitzik et al., 2000; Yang et al., 2005). Moreover, the Smad4 mutant mice exhibit failure in hair cycling and skin tumor formation, which resemble the phenotypes of BMPR1A-deficient mice (Andl et al., 2004; Ming Kwan et al., 2004; Yang et al., 2005; Zhang et al., 2006). Deletion of Smad4 may intercept BMP in repressing follicle SCs activation; however, we could not exclude the possibility that Smad4-mediated TGF-β signaling is also required for the maintenance of the follicle SCs. How BMP signals are orchestrated with other TGF-β signals or antagonists to regulate the activation, proliferation, and maintenance of follicle SCs needs to be further investigated.
We have provided evidence showing that overactivation of β-catenin might contribute to the depletion of follicle SCs in Smad4 mutants. Transient increased levels of β-catenin or transient activation of β-catenin leads to accelerated transition from the resting to the growth phase of the hair cycle and new follicle formation (Van Mater et al., 2003; Lo Celso et al., 2004; Silva-Vargas et al., 2005). Activated β-catenin is usually restricted to in the bulge region when follicle SCs are activated during the early anagen phase (DasGupta and Fuchs, 1999; Zhang et al., 2006). Our previous study showed that cyclin D1 and c-Myc, the direct targets of Wnt/β-catenin signaling, are both significantly up-regulated in Smad4 mutants (Yang et al., 2005). In this study, we further showed that increased nuclear staining of β-catenin in the bulge region was correlated with the overactivation of follicle SCs. A recent study has provided genetic evidence showing that cyclic dermal BMP signaling prevents the activation of bulge SCs, a process usually involving periodic β-catenin activity (Plikus et al., 2008). Consistently, enhanced β-catenin stabilization is presented in BMPR1A-deficient mice and is accompanied by the activation of the PI3K/AKT pathway (Zhang et al., 2006; Kobielak et al., 2007). However, no alteration of Pten, p-Akt and p-GSK-3β were detected in Smad4 mutants, suggesting that Smad4 functions differently from BMPR1A in maintaining follicle SCs homeostasis.
Balanced c-Myc expression appears to be crucial for the maintenance of follicle SCs, because its overexpression in transgenic mice depletes follicle SCs and drives them to terminal differentiation (Arnold and Watt, 2001; Waikel et al., 2001). Conditional loss of endogenous c-Myc also leads to a loss of epidermal SCs and precocious differentiation of basal epidermal cells (Zanet et al., 2005). Previous studies have shown that expression of c-Myc could be activated by β-catenin in colorectal cancer cells (He et al., 1998). In this study, we showed that up-regulation of c-Myc was correlated with increased nuclear localization of β-catenin in Smad4 mutants. The consistently up-regulated c-Myc led to transient epidermal hyperproliferation and skin tumor formation (Yang et al., 2005) and eventually caused the depletion of follicle SCs. Up-regulation of c-Myc in Smad4 mutants could also be due to blocked cellular responsiveness to TGF-β, because in vitro studies have demonstrated that TGF-β inhibits cell growth through transcriptional repression of c-Myc (Frederick et al., 2004).
Previous studies have suggested that c-Myc activation depletes the epidermal SC niche by reducing adhesive interactions with the local microenvironment (Frye et al., 2003). In this study, we showed that c-Myc activation was associated with down-regulation of α6-integrin expression, suggesting a potential role for c-Myc–induced suppression of adhesion in the stimulation of follicle SCs to differentiate. A c-Myc binding site has been found about 350–360 base pairs upstream of the transcription start site of human α6-integrin promoter (Nishida et al., 1997). Coexpression of H-Ras and c-Myc in a hematopoietic cell line significantly inhibits the expression of α6-integrin and leads to a loss of adhesiveness to laminin (Nagashima et al., 2001). Notably, sustained activation of c-Myc together with down-regulation of α6-integrin expression leads to apoptosis and differentiation of human embryonic SCs (Sumi et al., 2007).
In summary, we have shown that targeted ablation of Smad4 in epidermis leads increased β-catenin nuclear localization independent of overactivation of the PI3K/Akt pathway. It also leads to c-Myc activation, which reduces α6-integrin expression and results in the depletion of follicle SCs. These data suggest a critical role for Smad4 in the normal maintenance of follicle SCs and potentially in related disorders.
Supplementary Material
ACKNOWLEDGMENTS
We thank Chuxia Deng (National Institutes of Health) for Smad4-floxed mouse; Zhiyi Zhang (National Center of Biomedical Analysis, Beijing, China) for obtaining fluorescence images by a laser scanning confocal microscope; Wei Zhou (Institute of Biotechnology, Beijing, China) for flow cytometry analysis; and Yu Lan, Youliang Wang, Qiang Sun, and Cuihua Xu (Institute of Biotechnology, Beijing, China) for helpful discussion and technical assistance. This work was supported by Chinese National Key Program on Basic Research (2005CB522506, 2006CB943501, and 2006BAI23B01-3), National Natural Science Foundation of China (30430350), National High-Tech Research and Development Program (2006AA02Z168) and Grant Z0006303041231.
Abbreviations used:
- BMP
bone morphogenetic protein
- BMPR1A
BMP type 1a receptor
- BrdU
bromodeoxyuridine
- HF
hair follicle
- IFE
interfollicular epidermis
- K1, K14, and K15
keratin 1, 14, and 15, respectively
- LRC
label-retaining cell
- P20
postnatal day 20
- SC
stem cell
- SG
sebaceous gland
- TGF-β
transforming growth factor-β.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E08-07-0731) on December 10, 2008.
REFERENCES
- Andl T., et al. Epithelial Bmpr1a regulates differentiation and proliferation in postnatal hair follicles and is essential for tooth development. Development. 2004;131:2257–2268. doi: 10.1242/dev.01125. [DOI] [PubMed] [Google Scholar]
- Arnold I., Watt F. M. c-Myc activation in transgenic mouse epidermis results in mobilization of stem cells and differentiation of their progeny. Curr. Biol. 2001;11:558–568. doi: 10.1016/s0960-9822(01)00154-3. [DOI] [PubMed] [Google Scholar]
- Blanpain C., Fuchs E. Epidermal stem cells of the skin. Annu. Rev. Cell Dev. Biol. 2006;22:339–373. doi: 10.1146/annurev.cellbio.22.010305.104357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanpain C., Lowry W. E., Geoghegan A., Polak L., Fuchs E. Self-renewal, multipotency, and the existence of two cell populations within an epithelial stem cell niche. Cell. 2004;118:635–648. doi: 10.1016/j.cell.2004.08.012. [DOI] [PubMed] [Google Scholar]
- Botchkarev V. A., et al. Noggin is a mesenchymally derived stimulator of hair-follicle induction. Nat. Cell Biol. 1999;1:158–164. doi: 10.1038/11078. [DOI] [PubMed] [Google Scholar]
- Braun K. M., Niemann C., Jensen U. B., Sundberg J. P., Silva-Vargas V., Watt F. M. Manipulation of stem cell proliferation and lineage commitment: visualisation of label-retaining cells in wholemounts of mouse epidermis. Development. 2003;130:5241–5255. doi: 10.1242/dev.00703. [DOI] [PubMed] [Google Scholar]
- Cotsarelis G., Sun T. T., Lavker R. M. Label-retaining cells reside in the bulge area of pilosebaceous unit: implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell. 1990;61:1329–1337. doi: 10.1016/0092-8674(90)90696-c. [DOI] [PubMed] [Google Scholar]
- DasGupta R., Fuchs E. Multiple roles for activated LEF/TCF transcription complexes during hair follicle development and differentiation. Development. 1999;126:4557–4568. doi: 10.1242/dev.126.20.4557. [DOI] [PubMed] [Google Scholar]
- Foitzik K., et al. Control of murine hair follicle regression (catagen) by TGF-beta1 in vivo. FASEB J. 2000;14:752–760. doi: 10.1096/fasebj.14.5.752. [DOI] [PubMed] [Google Scholar]
- Frederick J. P., Liberati N. T., Waddell D. S., Shi Y., Wang X. F. Transforming growth factor beta-mediated transcriptional repression of c-myc is dependent on direct binding of Smad3 to a novel repressive Smad binding element. Mol. Cell. Biol. 2004;24:2546–2559. doi: 10.1128/MCB.24.6.2546-2559.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye M., Gardner C., Li E. R., Arnold I., Watt F. M. Evidence that Myc activation depletes the epidermal stem cell compartment by modulating adhesive interactions with the local microenvironment. Development. 2003;130:2793–2808. doi: 10.1242/dev.00462. [DOI] [PubMed] [Google Scholar]
- Fuchs E. Scratching the surface of skin development. Nature. 2007;445:834–842. doi: 10.1038/nature05659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He T. C., Sparks A. B., Rago C., Hermeking H., Zawel L., da Costa L. T., Morin P. J., Vogelstein B., Kinzler K. W. Identification of c-MYC as a target of the APC pathway. Science. 1998;281:1509–1512. doi: 10.1126/science.281.5382.1509. [DOI] [PubMed] [Google Scholar]
- Ito Y., et al. Over expression of Smad2 reveals its concerted action with Smad4 in regulating TGF-beta-mediated epidermal homeostasis. Dev. Biol. 2001;236:181–194. doi: 10.1006/dbio.2001.0332. [DOI] [PubMed] [Google Scholar]
- Kobielak K., Pasolli H. A., Alonso L., Polak L., Fuchs E. Defining BMP functions in the hair follicle by conditional ablation of BMP receptor IA. J. Cell Biol. 2003;163:609–623. doi: 10.1083/jcb.200309042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobielak K., Stokes N., de la Cruz J., Polak L., Fuchs E. Loss of a quiescent niche but not follicle stem cells in the absence of bone morphogenetic protein signaling. Proc. Natl. Acad. Sci. USA. 2007;104:10063–10068. doi: 10.1073/pnas.0703004104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulessa H., Turk G., Hogan B. L. Inhibition of Bmp signaling affects growth and differentiation in the anagen hair follicle. EMBO J. 2000;19:6664–6674. doi: 10.1093/emboj/19.24.6664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langton A. K., Herrick S. E., Headon D. J. An extended epidermal response heals cutaneous wounds in the absence of a hair follicle stem cell contribution. J. Invest. Dermatol. 2008;128:1311–1318. doi: 10.1038/sj.jid.5701178. [DOI] [PubMed] [Google Scholar]
- Levy V., Lindon C., Harfe B. D., Morgan B. A. Distinct stem cell populations regenerate the follicle and interfollicular epidermis. Dev. Cell. 2005;9:855–861. doi: 10.1016/j.devcel.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Lo Celso C., Prowse D. M., Watt F. M. Transient activation of beta-catenin signalling in adult mouse epidermis is sufficient to induce new hair follicles but continuous activation is required to maintain hair follicle tumours. Development. 2004;131:1787–1799. doi: 10.1242/dev.01052. [DOI] [PubMed] [Google Scholar]
- Lowry W. E., Blanpain C., Nowak J. A., Guasch G., Lewis L., Fuchs E. Defining the impact of beta-catenin/Tcf transactivation on epithelial stem cells. Genes Dev. 2005;19:1596–1611. doi: 10.1101/gad.1324905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill B. J., Gat U., DasGupta R., Fuchs E. Tcf3 and Lef1 regulate lineage differentiation of multipotent stem cells in skin. Genes Dev. 2001;15:1688–1705. doi: 10.1101/gad.891401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming Kwan K., Li A. G., Wang X. J., Wurst W., Behringer R. R. Essential roles of BMPR-IA signaling in differentiation and growth of hair follicles and in skin tumorigenesis. Genesis. 2004;39:10–25. doi: 10.1002/gene.20021. [DOI] [PubMed] [Google Scholar]
- Morris R. J., Liu Y., Marles L., Yang Z., Trempus C., Li S., Lin J. S., Sawicki J. A., Cotsarelis G. Capturing and profiling adult hair follicle stem cells. Nat. Biotechnol. 2004;22:411–417. doi: 10.1038/nbt950. [DOI] [PubMed] [Google Scholar]
- Mou C., Jackson B., Schneider P., Overbeek P. A., Headon D. J. Generation of the primary hair follicle pattern. Proc. Natl. Acad. Sci. USA. 2006;103:9075–9080. doi: 10.1073/pnas.0600825103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagashima G., Asai J., Suzuki R., Fujimoto T. Different distribution of c-myc and MIB-1 positive cells in malignant meningiomas with reference to TGFs, PDGF, and PgR expression. Brain Tumor Pathol. 2001;18:1–5. doi: 10.1007/BF02478918. [DOI] [PubMed] [Google Scholar]
- Nishida K., Kitazawa R., Mizuno K., Maeda S., Kitazawa S. Identification of regulatory elements of human alpha 6 integrin subunit gene. Biochem. Biophys. Res. Commun. 1997;241:258–263. doi: 10.1006/bbrc.1997.7808. [DOI] [PubMed] [Google Scholar]
- Owens P., Bazzi H., Engelking E., Han G., Christiano A. M., Wang X. J. Smad4-dependent desmoglein-4 expression contributes to hair follicle integrity. Dev. Biol. 2008 doi: 10.1016/j.ydbio.2008.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plikus M. V., Mayer J. A., de la Cruz D., Baker R. E., Maini P. K., Maxson R., Chuong C. M. Cyclic dermal BMP signalling regulates stem cell activation during hair regeneration. Nature. 2008;451:340–344. doi: 10.1038/nature06457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao W., Li A. G., Owens P., Xu X., Wang X. J., Deng C. X. Hair follicle defects and squamous cell carcinoma formation in Smad4 conditional knockout mouse skin. Oncogene. 2006;25:207–217. doi: 10.1038/sj.onc.1209029. [DOI] [PubMed] [Google Scholar]
- Sharov A. A., Sharova T. Y., Mardaryev A. N., Tommasi di Vignano A., Atoyan R., Weiner L., Yang S., Brissette J. L., Dotto G. P., Botchkarev V. A. Bone morphogenetic protein signaling regulates the size of hair follicles and modulates the expression of cell cycle-associated genes. Proc Natl. Acad. Sci. USA. 2006;103:18166–18171. doi: 10.1073/pnas.0608899103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y., Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- Silva-Vargas V., Lo Celso C., Giangreco A., Ofstad T., Prowse D. M., Braun K. M., Watt F. M. Beta-catenin and Hedgehog signal strength can specify number and location of hair follicles in adult epidermis without recruitment of bulge stem cells. Dev. Cell. 2005;9:121–131. doi: 10.1016/j.devcel.2005.04.013. [DOI] [PubMed] [Google Scholar]
- Sumi T., Tsuneyoshi N., Nakatsuji N., Suemori H. Apoptosis and differentiation of human embryonic stem cells induced by sustained activation of c-Myc. Oncogene. 2007;26:5564–5576. doi: 10.1038/sj.onc.1210353. [DOI] [PubMed] [Google Scholar]
- Sun Q., et al. Transforming growth factor-beta-regulated miR-24 promotes skeletal muscle differentiation. Nucleic Acids Res. 2008;36:2690–2699. doi: 10.1093/nar/gkn032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trempus C. S., et al. CD34 expression by hair follicle stem cells is required for skin tumor development in mice. Cancer Res. 2007;67:4173–4181. doi: 10.1158/0008-5472.CAN-06-3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumbar T., Guasch G., Greco V., Blanpain C., Lowry W. E., Rendl M., Fuchs E. Defining the epithelial stem cell niche in skin. Science. 2004;303:359–363. doi: 10.1126/science.1092436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Mater D., Kolligs F. T., Dlugosz A. A., Fearon E. R. Transient activation of beta-catenin signaling in cutaneous keratinocytes is sufficient to trigger the active growth phase of the hair cycle in mice. Genes Dev. 2003;17:1219–1224. doi: 10.1101/gad.1076103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waikel R. L., Kawachi Y., Waikel P. A., Wang X. J., Roop D. R. Deregulated expression of c-Myc depletes epidermal stem cells. Nat. Genet. 2001;28:165–168. doi: 10.1038/88889. [DOI] [PubMed] [Google Scholar]
- Yang L., et al. Targeted disruption of Smad4 in mouse epidermis results in failure of hair follicle cycling and formation of skin tumors. Cancer Res. 2005;65:8671–8678. doi: 10.1158/0008-5472.CAN-05-0800. [DOI] [PubMed] [Google Scholar]
- Yuan C., et al. The up-regulation of 14-3-3 proteins in Smad4 deficient epidermis and hair follicles at catagen. Proteomics. 2008;8:2230–2243. doi: 10.1002/pmic.200700760. [DOI] [PubMed] [Google Scholar]
- Zanet J., Pibre S., Jacquet C., Ramirez A., de Alboran I. M., Gandarillas A. Endogenous Myc controls mammalian epidermal cell size, hyperproliferation, endoreplication and stem cell amplification. J. Cell Sci. 2005;118:1693–1704. doi: 10.1242/jcs.02298. [DOI] [PubMed] [Google Scholar]
- Zhang J., He X. C., Tong W. G., Johnson T., Wiedemann L. M., Mishina Y., Feng J. Q., Li L. Bone morphogenetic protein signaling inhibits hair follicle anagen induction by restricting epithelial stem/progenitor cell activation and expansion. Stem Cells. 2006;24:2826–2839. doi: 10.1634/stemcells.2005-0544. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.