Abstract
Sirtuin family of proteins possesses NAD-dependent deacetylase and ADP ribosyltransferase activities. They are found to respond to nutrient deprivation and profoundly regulate metabolic functions. We have previously reported that caloric restriction increases the expression of one of the seven mammalian sirtuins, SIRT2, in tissues such as white adipose tissue. Because adipose tissue is a key metabolic organ playing a critical role in whole body energy homeostasis, we went on to explore the function of SIRT2 in adipose tissue. We found short-term food deprivation for 24 h, already induces SIRT2 expression in white and brown adipose tissues. Additionally, cold exposure elevates SIRT2 expression in brown adipose tissue but not in white adipose tissue. Intraperitoneal injection of a β-adrenergic agonist (isoproterenol) enhances SIRT2 expression in white adipose tissue. Retroviral expression of SIRT2 in 3T3-L1 adipocytes promotes lipolysis. SIRT2 inhibits 3T3-L1 adipocyte differentiation in low-glucose (1 g/l) or low-insulin (100 nM) condition. Mechanistically, SIRT2 suppresses adipogenesis by deacetylating FOXO1 to promote FOXO1's binding to PPARγ and subsequent repression on PPARγ transcriptional activity. Overall, our results indicate that SIRT2 responds to nutrient deprivation and energy expenditure to maintain energy homeostasis by promoting lipolysis and inhibiting adipocyte differentiation.
INTRODUCTION
Sirtuin family of proteins possess NAD-dependent deacetylase and ADP ribosyltransferase activities (Frye, 1999). They regulate many biological functions, such as longevity and metabolism (Guarente, 2006; Michan and Sinclair, 2007). SIRT2 is the mammalian ortholog of the yeast Hst2 gene, which has been shown to play a complementary role to the yeast Sir2 gene in mediating life span extension by caloric restriction (Lamming et al., 2005). SIRT2 proteins are distributed throughout the cytoplasm (Yang et al., 2000; North and Verdin, 2007), mainly colocalizing with microtubules and functioning as an α-tubulin deacetylase (North et al., 2003). A recent report showed SIRT2 can transiently migrate to nuclei in the G2/M transition during mitosis to deacetylate histone H4Lys16 (Vaquero et al., 2006). SIRT2 plays a role in the control of G2/M transition, through its increase in expression and phosphorylation during the G2/M phase. Cells overexpressing SIRT2 have an extended mitotic phase (Dryden et al., 2003). In addition, SIRT2 expression is down-regulated in gliomas, suggesting a potential role in the control of cell proliferation (Hiratsuka et al., 2003). We have previously discovered that the expression of SIRT2 is induced by caloric restriction in several mouse tissues, most prominently, the white adipose tissue (Wang et al., 2007). In addition, SIRT2 level is elevated by oxidative stress and consequently deacetylates FOXO3a to activate FOXO3a-mediated anti-oxidative stress response to reduce cellular levels of reactive oxygen species (Wang et al., 2007).
Adipose tissue plays a major role for regulating metabolism by storing excess energy and mobilizing the stored lipids for energy supply in case of need. Moreover, adipose tissue also functions as an endocrine organ, secreting various adipokines and cytokines, such as leptin and adiponectin, to influence metabolism (Nawrocki and Scherer, 2005). Adipose tissue mass is tightly regulated according to nutritional and physiological conditions. Many factors participate in the up- or down-regulation of adipose tissue formation. Among these factors, PPARγ transcription factor plays a central role (Rosen and MacDougald, 2006; Gesta et al., 2007). Mice deficient of PPARγ gene (Barak et al., 1999; Kubota et al., 1999; Rosen et al., 1999) or with an adipose specific deletion of PPARγ (Jones et al., 2005) have adipogenesis defects. In addition to PPARγ, C/EBP family of transcription factors are also major regulators of adipocyte differentiation (Christy et al., 1989; Lane et al., 1999). On stimulation with proadipogenic signals, the expression of C/EBP family of transcription factors and PPARγ are elevated in a sequential manner. They then regulate the expression of genes associated with the adipocyte phenotype (Rosen et al., 2002). Conversely, many factors suppress adipogenesis (Rosen and MacDougald, 2006). FOXO1 transcription factor is one of those negative regulators found to inhibit adipogenesis(Dowell et al., 2003; Armoni et al., 2006).
FOXO transcription factors are key component of the insulin/IGF-signaling cascade (Woods and Rena, 2002). This pathway is pivotal in controlling organism growth and metabolism, it also regulates life span (Daitoku and Fukamizu, 2007). It was found that Caenorhabditis elegans harboring a mutation of the insulin receptor-like gene daf-2, lives longer. This is dependent on the FOXO ortholog daf-16 (Kenyon et al., 1993). Interestingly, ablation of the insulin receptor gene just in the adipose is enough to delay aging (Bluher et al., 2003), indicating the importance of both the insulin-signaling pathway and the adipose in longevity determination. FOXO family of transcription factors regulate metabolism (Burgering and Kops, 2002; Accili and Arden, 2004) and confer stress resistance (Kajihara et al., 2006). In adipose, it was shown that FOXO1 transcription factor interacted with PPARγ and negatively regulated its transcriptional activity (Dowell et al., 2003). FOXO1 can also bind to PPARγ promoter region and suppress PPARγ expression(Armoni et al., 2006). Meanwhile, FOXO1 up-regulates p21 expression to suppress adipogenesis by inhibiting clonal expansion at the early stage of adipocyte differentiation (Morrison and Farmer, 1999; Nakae et al., 2003).
In this study, we investigated the effect of short-term fasting and cold exposure on the expression of SIRT2 in adipose tissues. We also studied the effect of SIRT2 on 3T3-L1 differentiation. We further explored the mechanism underlying SIRT2's action on adipogenesis and found that SIRT2 deacetylates FOXO1 to repress the transcriptional activity of PPARγ.
MATERIALS AND METHODS
Animals and Reagents
Sixteen-week-old C57BL/6 male mice were fasted for 24 h starting at 6 pm, followed by 3 or 24 h refeeding. For cold exposure, 4-wk-old C57BL/6 male mice were exposed to 5°C for 12 h. In addition, 3–4-mo-old C57BL/6 male mice were exposed to either room temperature at 23 or 27.5°C. Lastly, isoproterenol was injected intraperitoneally to 2-wk-old male C57BL/6 mice at a dose of 10 mg/kg. Saline (0.85%) was used as control. Six hours after injection, tissues were collected for protein extraction and immunoblotting.
Cell Culture and Differentiation
3T3-L1 preadipocytes were maintained in high-glucose (4.5g/L) DMEM with 10% calf serum. To induce differentiation, 2 d after confluence, cells were treated for 3 d with DMEM containing either a high (4.5 g/l) or low (1.0 g/l) concentration of glucose, supplemented with 10% fetal bovine serum (FBS), 1 μM dexamethasone, 0.5 mM isobutyl methyl xanthine (IBMX), and 5 μg/ml (872 nM) insulin. The cells were then maintained in DMEM with 10% FBS and 5 μg/ml insulin for another 4 d for the high-glucose condition or 6–8 d for the low-glucose condition. During the differentiation process, media were changed daily for the low-glucose condition and every other day for high-glucose condition.
Plasmids
pBabe puro-SIRT2 and pCMV-SIRT2 were previously described (Wang et al., 2007). pCMV-SIRT2N168A was generated by a PCR-based mutagenesis (Makarova et al., 2000) with the following primer: 5′-GCGCTGCTACACGCAGGCCATAGACACGCTGGAAC-3′.
Transient Transfection
HEK293T cells were cultured in high-glucose DMEM with 10% calf serum. For transient transfection, 1–5 μg of plasmid DNA was transfected into six-well plates by the calcium phosphate method (Jordan et al., 1996). Twenty-four hours after transfection, cells were lysed in RIPA buffer (Wang et al., 2007).
Retroviral and Lentiviral Infection
Retroviral expression of SIRT2 in 3T3-L1 cells was conducted similarly as previously described (Shi et al., 2005). Briefly, Bosc23 cells were transfected with 5 μg pBabe-puro plasmids carrying a SIRT2 or SIRT2N168A mutant by the calcium phosphate method in T25 flasks. Two days after transfection, packaged retroviral particles in the supernatant were collected and filtrated through 0.45-μm filters. Viral suspension (3 ml) were then mixed with 1 ml culture medium containing 16 μg polybrene to infect 3T3-L1 cells in T25 flasks. Forty-eight hours after infection, 2 μg/ml puromycin was added to the medium to select for infected cells for 5–7 d.
Lentiviral-mediated SIRT2 short hairpin RNA (shRNA) knockdown 3T3-L1 cells were generated as described in our previous work (Wang et al., 2007). Packaged lentivirus produced from 293T cells were used to infect 3T3-L1 cell. Three days later, lentiviral infected 3T3-L1 cells coexpressing green fluorescent protein (GFP) were selected by fluorescence-activated cell sorter. SIRT2 knockdown efficiency was confirmed by immunoblotting with anti-SIRT2 antibodies.
Northern Blot Analysis
Total RNA was isolated from cells or tissues using TRIzol Reagent (Invitrogen, Carlsbad, CA) following the manufacturer's instruction. Ten micrograms of RNA of each sample was separated on 1% agarose gels and then transferred to nylon membrane. cDNA fragments for mouse SIRT2, PPARγ, adipsin, and Glut4 were used as templates to synthesize probes labeled with [α-32P]dCTP to detect the expression of corresponding genes.
Western Blot Analysis
Cells were lysed in lysis buffer (50 mM Tris, 50 mM KCl, 20 mM NaF, 1 mM Na3VO4, 10 mM EDTA, 1% NP-40, 10 mM nicotinamide (NAM), 1 mM tricostatin A (TSA; Cayman, Ann Arbor, MI), 1 mM PMSF, 5 μg/ml leupeptin, pH 8.0). Protein concentration was determined with BCA protein assay kit (Pierce, Rockford, IL). Fifteen micrograms of protein of each sample were separated by 10% SDS-PAGE and electro-transferred to nitrocellulose membrane for immunoblot anaylsis. The following antibodies were used: anti-SIRT2 (Santa Cruz Biotechnology, Santa Cruz, CA; sc-20966, 1:1000), anti-actin (Santa Cruz, sc-1616, 1:1000), anti-FOXO1 (Santa Cruz, sc-11350, 1:1000), anti-PPARγ (Santa Cruz, sc-7196, sc-7273, 1:500), anti-GAPDH (Ambion, Austin, TX; 4300, 1:500,000), anti-Lamin A/C (Cell Signaling, Beverly, MA; 4056, 1:1000), anti-acetylated lysine (Cell Signaling, 9441, 1:1000), anti-α-tubulin (Sigma, St. Louis, MO; T5168, 1:100,000), HRP-conjugated anti-mouse (Bio-Rad, Richmond, CA; 170-6516, 1:3000), anti-rabbit (Bio-Rad, 170-6515, 1:3000), and anti-rabbit IgG-native (Sigma, R3155, 1:2000) antibodies. In addition, anti-Flag-HRP (Sigma, A8592, 1:2000) was also used. The SuperSignal West Pico Chemiluminescent kit (Pierce) was used as substrates.
Immunoprecipitation
Cell lysate (200 μl) was supplemented with 300 μl lysis buffer to get the appropriate protein concentration and volume. Forty microliters of anti-Flag M2 agarose affinity gel (Sigma, A2220) were added to the lysate and rocked in 4°C overnight. After washing for five times with lysis buffer, protein was eluted with 2× loading buffer for Western blot analysis. For endogenous protein–protein interaction, 10 mg total 3T3-L1 adipocyte lysates were incubated with 4 μg antibodies against SIRT2 or PPARγ overnight and then captured by 1 ml goat anti-rabbit or goat anti-mouse IgG magnetic beads (Polysciences, Warrington, PA) for 4 h. After washing four times with lysis buffer, proteins were eluted with 2× loading buffer for Western blot analysis.
Luciferase Assay
PPARγ-responding luciferase construct PPRE-Luc was transfected into HEK293T cells along with plasmids expressing the PPARγ, SIRT2, FOXO1, or FOXO1-3KA mutant using the calcium phosphate method. Renilla luciferase reporter (pRL-TK) was included as an internal control for transfection efficiency. Eight hours after transfection, cells were treated with PPARγ ligand rosiglitazone (1 μM) for 15 h. Cells were then lysed, and luciferase activity was measured with the dual luciferase kit (Promega, Madison, WI) according to the manufacturer's instructions. Firefly luciferase activity was normalized by the Renilla luciferase activity.
Real-Time PCR
Real-time PCR was performed by using FastStart SYBR Green Master reagent (Roche, Indianapolis, IN) or Power SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA) according to manufacturer's instructions. Primers used were as follows: C/EBPα: AGTCGGTGGACAAGAACAGC and ACTCCAGCACCTTCTGTTGC; PPARγ: GCTGTTATGGGTGAAACTCT and TGGCATCTCTGTGTCAACCA; Adipsin: ATGGATGGAGTGACGGATGAC and ATACCATCGCTTGTAGGGTTCAG; SIRT2: CTCATCAGCAAGGCACCACTAG and CCATCATCATGCCCAGGAA; Cyclophilin: CTGTTTGCAGACAAAGTTCCA and AGGATGAAGTTCTCATCCTCA; 18sRNA: AACGAGACTCTGGCATGCTAACTAG and CGCCACTTGTCCCTCTAAGAA.
Cytoplasmic/Nuclear Fractionation
Cytosol and nuclear protein fractionation was performed using the NE-PER Nuclear and Cytoplasmic Extraction kit (Pierce) according to the manufacturer's instructions.
Lipolysis Assay
3T3-L1 cells with SIRT2 overexpression were stimulated for differentiation in high-glucose medium for 7 d. Cells were then cultured in medium with or without insulin for 48 h. Glycerol content in the medium was measured by the glycerol assay kit (Randox; Crumlin, Co. Antrim, United Kingdom) following the manufacturer's instructions. Briefly, 10 μl supernatant of culture was incubated with 1 ml assay reagent for 5 min at 37°C. The absorbance was measured by spectrometer at 520 nm.
Statistics
Statistical significance was determined by the Student's t test. Differences between groups were considered statistically significant if p < 0.05.
RESULTS
SIRT2 Expression in Adipose Tissue Is Up-Regulated by Fasting
Our previous study found an increase of SIRT2 expression in kidney and adipose tissue when mice were dietary restricted for 18 mo (Wang et al., 2007). In this study, we decided to investigate the influence on SIRT2 expression by shorter-term nutrient deprivation in the form of 24-h fasting. As shown in Figure 1A, fasting increased SIRT2 mRNA levels as detected by quantitative RT-PCR in white adipose tissue. SIRT2 protein level is also elevated by 24-h fasting (Figure 1B). Refeeding for 24 h restored SIRT2 mRNA and protein levels to the baseline. Fasting also induced the expression of SIRT2 markedly in brown adipose tissues (Supplemental Figure S1). However, this up-regulation of SIRT2 by fasting was not seen in liver or skeletal muscle (data not shown).
Figure 1.
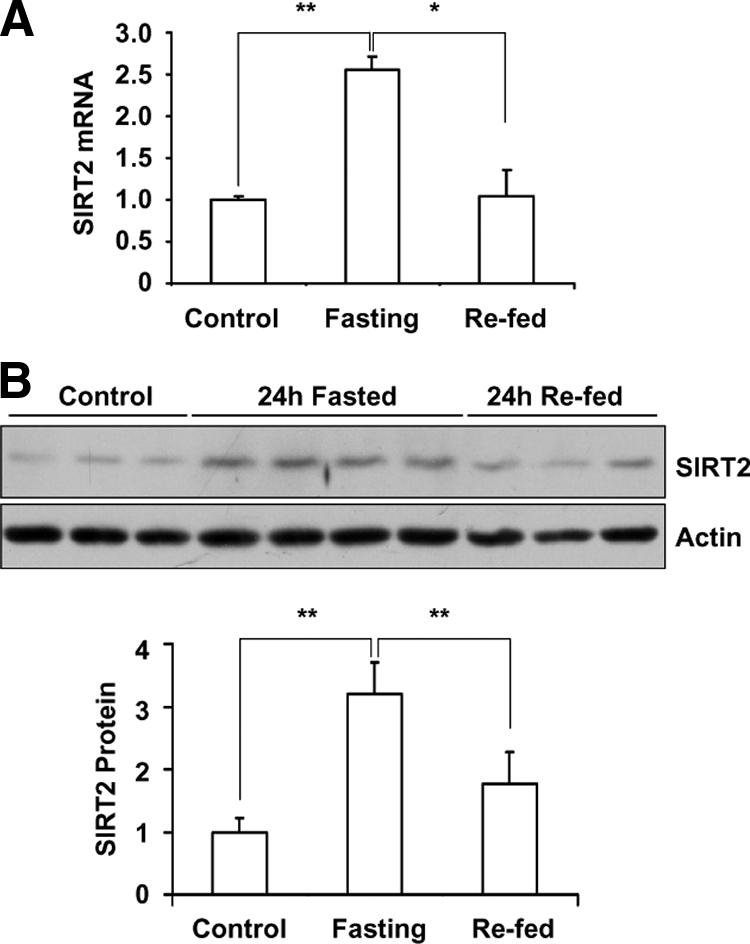
Fasting induce SIRT2 mRNA and protein expression in white adipose tissue. (A) C57BL/6 male mice were deprived of food at the onset of dark cycle (6:00 pm). Twenty-four hours later, mice were refed for 24 h. SIRT2 mRNA level in white adipose tissue was examined by real time RT-PCR analysis and normalized by the level of cyclophilin. (B) SIRT2 protein levels in white adipose from fasted mice were detected by Western blotting, SIRT2 protein amount was quantified and normalized by actin level. *p < 0.05; **p < 0.01.
SIRT2 Expression in Brown Adipose Tissue Is Up-Regulated by Cold Exposure
Because rodent brown adipose is responsible for cold-stimulated nonshivering thermogenesis (Lowell and Spiegelman, 2000; Spiegelman and Flier, 2001), we investigated the relationship between cold exposure and SIRT2 expression in adipose tissues. The mice were exposed to 5°C temperature for 12 h (Shi et al., 2005). As shown in Figure 2A, the RNA levels of SIRT2 were increased in brown adipose by cold exposure. In contrast, the SIRT2 expression level in white adipose tissue is not regulated by cold (data not shown). When we subjected mice to a thermo-neutral temperature of 27.5°C for 16 h, we found that SIRT2 expression in brown adipose tissue was down-regulated, compared with the mice kept at ambient room temperature of 23°C (Figure 2B). We also tested the SIRT2 protein levels in brown adipose tissue from mice exposed to these temperatures. As shown in Figure 2C, SIRT2 protein levels is elevated in brown adipose by cold exposure and dampened in higher ambient temperature. These results suggest that the expression of SIRT2 in the brown adipose is regulated in response to environmental temperature.
Figure 2.
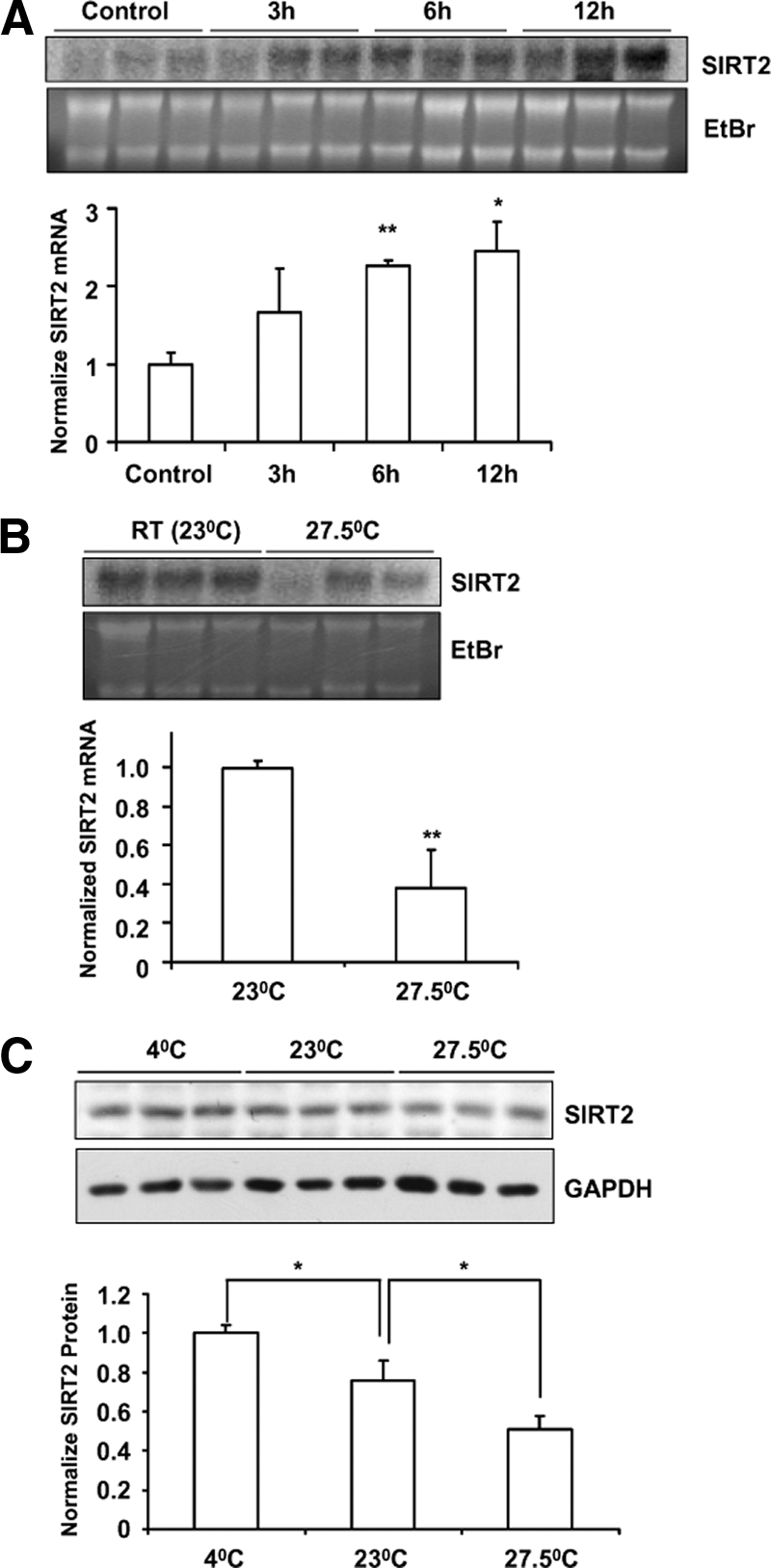
Regulation of SIRT2 expression in brown adipose by environmental temperature. (A) Four-week-old C57BL/6 male mice were exposed to 5°C for 3, 6, or 12 h, whereas the control mice were kept at room temperature (23°C). Northern blot analysis was used to detect SIRT2 expression in brown adipose. (B) Mice were exposed to 27.5°C for 16 h, whereas the control mice were kept at 23°C. SIRT2 expression in brown adipose tissue was detected by Northern blot analysis. Ethidium bromide (EtBr) staining of the RNA was included to show the loading and the integrity of samples. (C) SIRT2 protein levels in the brown adipose tissues from mice exposed to 5, 23, or 27.5°C for 16 h were detected by Western blot analysis. SIRT2 protein amount was quantified and normalized by GAPDH levels. *p < 0.05; **p < 0.01.
SIRT2 Expression in Adipocytes Is Stimulated by β-Adrenergic Agonist (Isoproterenol)
Because cold exposure and starvation cause the release of sympathetic and glucocorticoid hormones, we tested whether β-adrenergic agonist (isoproterenol) or glucocorticoid (dexamethasone) participated in the stimulation of SIRT2 expression. We treated differentiated 3T3-L1 cells with isoproterenol and dexamethasone for 6 h. SIRT2 protein levels were detected by Western blot analysis. As shown in Figure 3A, isoproterenol treatment elevated SIRT2 protein level, whereas dexamethasone had no effect. To test if isoproterenol elevates SIRT2 protein level in vivo, isoproterenol or saline control was injected into C57BL/6 male mice intraperitoneally. Six hours after injection, tissues were collected for protein extraction, and SIRT2 protein levels were detected via Western blot analysis. We found that the expression of SIRT2 in white adipose tissue is stimulated by isoproterenol (Figure 3B). These results indicate that SIRT2 expression in adipocytes is stimulated by β-adrenergic agonist but not glucocorticoids.
Figure 3.
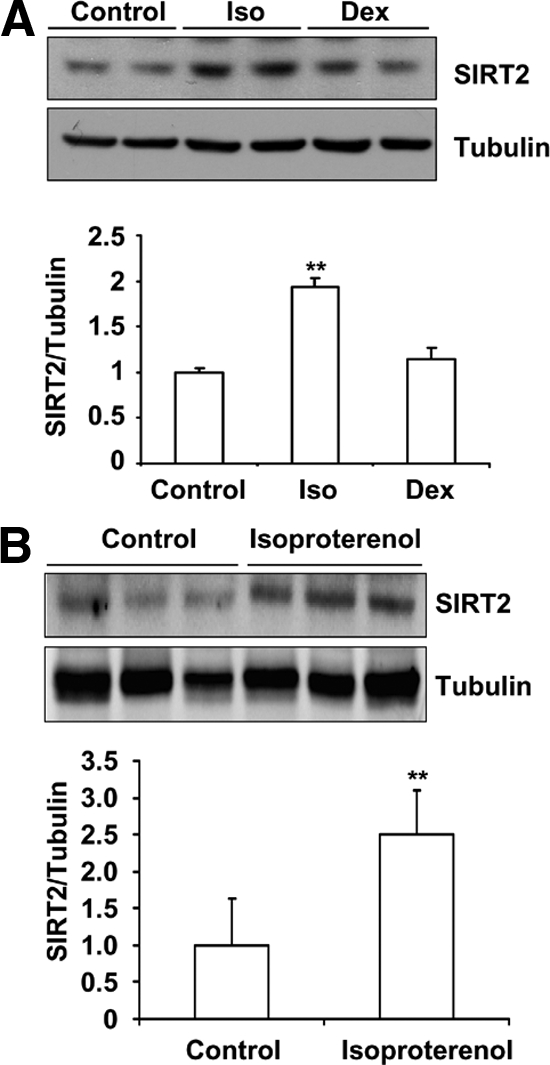
Isoproterenol stimulates SIRT2 expression in 3T3-L1 adipocytes and adipose tissue. (A) 3T3-L1 cells are differentiated according to standard protocol with high-glucose (4.5 g/l) and high-insulin (5 μg/ml) medium. Differentiated 3T3-L1 cells were treated with 10 μM isoproterenol or 1 μM dexamethasone for 6 h, and SIRT2 protein levels were detected by Western blotting. (B) Twenty-one-week-old C57BL/6 male mice were injected with 10 mg/kg isoproterenol intraperitoneally. Saline (0.85%) was used as the control. Six hours after injection, white adipose tissue was collected for protein extraction. SIRT2 expression was detected via Western blotting. **p < 0.01.
SIRT2 Increases Lipolysis in Mature Adipocytes
Because SIRT2 expression is specifically regulated in adipose by fasting and cold exposure, we detected the expression pattern of SIRT2 during adipocyte differentiation by Northern blot analysis. As shown in Figure 4A, SIRT2 expression was increased when the cells reached confluence. SIRT2 level decreased after the initiation of differentiation, and gradually increased from day 2, suggesting SIRT2 may play a role in regulating adipocyte differentiation and function in mature adipocytes. We established 3T3-L1 cells with constitutive SIRT2 expression using a retroviral system and tested adipocyte differentiation in the presence of SIRT2 overexpression under the conventional differentiation condition with high glucose (4.5 g/l) and high insulin (5 μg/ml). Under this condition, adipocyte differentiation was not affected by overexpression of SIRT2 or a SIRT2 deacetylase mutant with asparagine 168 to alanine substitution (SIRT2-N168A; Finnin et al., 2001; data not shown). We then measured lipolysis of these cells, by measuring glycerol release to the culture medium in the presence or absence of insulin. As expected, insulin treatment suppressed adipocyte lipolysis, whereas SIRT2 increases glycerol release with or without insulin treatment (Figure 4B).
Figure 4.
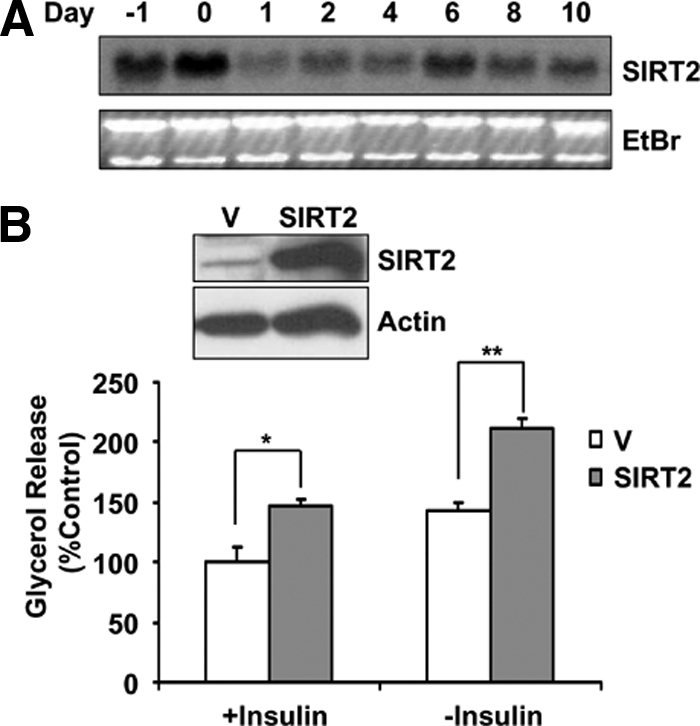
SIRT2 increases lipolysis in mature adipocytes. (A) 3T3-L1 cells are differentiated according to standard protocol with high-glucose (4.5 g/l) and high-insulin (5 μg/ml) medium. RNA was isolated at indicated time points with the initiation of differentiation by adding differentiation medium as day 0. The mRNA levels of SIRT2 were detected by Northern blot analysis. (B) 3T3-L1 cells with retroviral-mediated SIRT2 expression were established. These cells were differentiated with standard high-glucose, high-insulin medium. Glycerol release in the culture medium with or without insulin treatment from differentiated 3T3-L1 cells was measured. The SIRT2 protein levels in these cells were shown in the inset. V, vector control cells. *p < 0.05; **p < 0.01.
SIRT2 Inhibits Adipocyte Differentiation
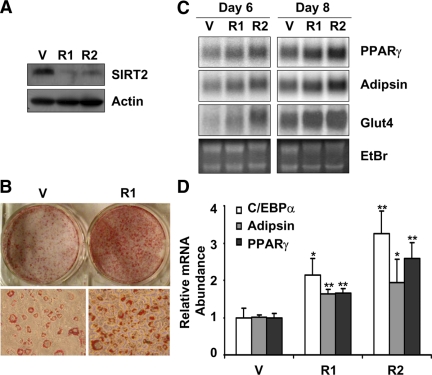
To further investigate the function of SIRT2 in adipocytes, 3T3-L1 cells with SIRT2 knockdown were generated, using two lentiviral mediated shRNA targeting two different regions of SIRT2. SIRT2 protein level was detected by Western blot analysis to confirm the down-regulation of SIRT2 in these cells (Figure 5A). Under conventional adipocyte differentiation procedure, where cells were differentiated in media containing high glucose (4.5 g/l) and high insulin (5 μg/ml, 872 nM), both SIRT2 knockdown cells and vector control cells were well differentiated and show no difference (data not shown). It was reported that 3T3-L1 adipocytes differentiated in the presence of high glucose are insulin resistant, compared with the cells differentiated in medium with lower glucose (Lin et al., 2005). Therefore, we conducted adipocyte differentiation in low-glucose medium (5.6 mM, 1 g/l glucose), which is close to the normal physiological glucose level. Under this condition, the SIRT2 knockdown 3T3-L1 cells differentiated better than the vector control cells. Oil red-O staining of the differentiated cells revealed SIRT2 knockdown cells accumulated more lipid than vector control cells (Figure 5B). In addition, SIRT2 knockdown cells also had higher expression of markers of adipocyte differentiation, such as Glut4, PPARγ, and adipsin as detected by Northern blot analysis (Figure 5C) and C/EBPα, PPARγ and adipsin as detected by quantitative RT-PCR (Figure 5D). However, SIRT2-overexpressing cells showed no difference in terms of adipogenesis, compared with vector control cells, when differentiated with either high- or low-glucose medium (data not shown). A recent study (Jing et al., 2007) reported that SIRT2 overexpression inhibits 3T3-L1 cell differentiation in high-glucose medium (4 g/l) with low-insulin concentration (100 nM). Therefore, we repeated the differentiation of 3T3-L1 cells with SIRT2 knockdown or SIRT2 overexpression under a similar condition (4.5 g/l glucose and 100 nM insulin). We confirmed that under this condition, SIRT2 knockdown promotes adipogenesis (Supplemental Figure S2A), whereas SIRT2 over expression inhibits 3T3-L1 differentiation (Supplemental Figure S2B). In addition, we found SIRT2 deacetylase mutant SIRT2-N168A lose this effect (Supplemental Figure S2B), suggesting SIRT2 deacetylase activity is required for its action. Furthermore, PPARγ agonist rosiglitazone rescues adipocyte differentiation in SIRT2 over expressing cells, implying that SIRT2 inhibits adipogenesis through repression of the key adipogenic factor PPARγ (Supplemental Figure S2B). Comparing adipocyte differentiation under various conditions, we conclude that SIRT2's inhibitory effect on adipocyte differentiation can be abrogated by high-insulin or high-glucose concentration or rescued by PPARγ ligand treatment.
Figure 5.
SIRT2 regulates 3T3-L1 adipocyte differentiation. (A) 3T3-L1 cells with SIRT2 shRNA knockdown were generated and SIRT2 knockdown efficiency was detected by Western blot analysis. (B) 3T3-L1 cells with SIRT2 knockdown were stimulated and differentiated in low-glucose DMEM (1 g/l glucose) for 8 d. Lipid accumulation in cells was visualized by Oil red O staining. Top panel, photograph of cells in a six-well plate. Bottom panel, micrograph of representative areas of corresponding cells. (C) The expression of adipocyte markers (adipsin, PPARγ, and Glut4) in SIRT2 knockdown cells differentiated as in B for indicated days was detected by Northern blot analysis. (D) The expression of adipocyte markers (C/EBPα, adipsin, and PPARγ) in SIRT2 knockdown cells differentiated for 6 d were detected by real-time PCR. V, vector control cells. R1 and R2, cells expressing two different shRNA against SIRT2.
SIRT2 Deacetylates FOXO1
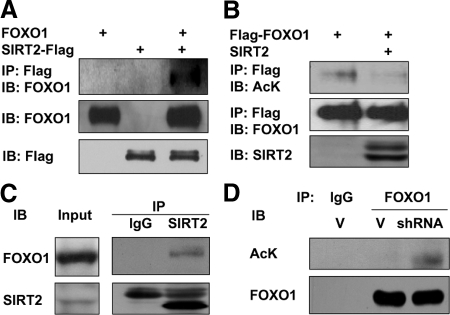
Because we have found SIRT2 deacetylates FOXO3a (Wang et al., 2007) and it was known that FOXO1 regulates adipogenesis (Nakae et al., 2003), it is likely that SIRT2 also deacetylates FOXO1 to regulate its function. To investigate SIRT2 interaction with FOXO1, we transfected 293T cells with FOXO1 and SIRT2-Flag. SIRT2 proteins were immunoprecipitated with anti-Flag agarose beads. FOXO1 interaction with SIRT2 protein was detected by immunoblot analysis with anti-FOXO1 antibodies. As shown in Figure 6A, FOXO1 is coimmunoprecipitated with SIRT2-Flag. Furthermore, we express Flag-FOXO1 with SIRT2 in 293T cells, after immunoprecipitation of FOXO1 by anti-Flag agarose beads, FOXO1 acetylation levels were detected by anti-acetylated lysine antibodies. As shown in Figure 6B, FOXO1 acetylation level is decreased in SIRT2 coexpressing cells, suggesting that SIRT2 deacetylates FOXO1. To detect endogenous interaction between SIRT2 and FOXO1 in adipocytes, we immunoprecipitated SIRT2 from 3T3-L1 adipocyte lysate, and the presence of FOXO1 in the precipitate can be detected by anti-FXOX1 Western blot analysis (Figure 6C). Furthermore, in 3T3-L1 adipocytes with SIRT2 knocking down, endogenous FOXO1 acetylation level is elevated (Figure 6D). These results indicate SIRT2 binds and deacetylates FOXO1 in adipocytes.
Figure 6.
SIRT2 interacts with FOXO1 and deacetylates FOXO1. (A) HEK293 cells were transiently transfected with FOXO1, SIRT2-Flag, or both by the calcium phosphate method. Forty hours after transfection, cells were lysed, and SIRT2-Flag proteins were immunoprecipitated with agarose beads and conjugated with anti-Flag antibodies. The precipitated proteins were washed five times and analyzed by Western blotting as indicated. IP, immunoprecipitation. IB, immunoblotting. (B) HEK293 cells were transfected with Flag-FOXO1 and SIRT2. Cells were treated with 1 μM tricostatin A (TSA) for 2 h before harvest. Flag-FOXO1 was immunoprecipitated with anti-Flag beads, and acetylated FOXO1 was detected with anti-acetylated lysine antibodies. (C) Endogenous SIRT2 in 3T3-L1 adipocyte lysate was immunoprecipitated with anti-SIRT2 antibody, and coprecipitation of FOXO1 was detected by Western blot assay. (D) SIRT2 knockdown or vector control adipocytes were treated with 1 μM TSA for 2 h. Cells were lysed and endogenous FOXO1 was immunoprecipitated with anti-FOXO1 antibodies, and the acetylation level was detected by anti-acetyl lysine antibodies. AcK, acetylated lysine.
Deacetylation of FOXO1 by SIRT2 Increases Its Binding to PPARγ
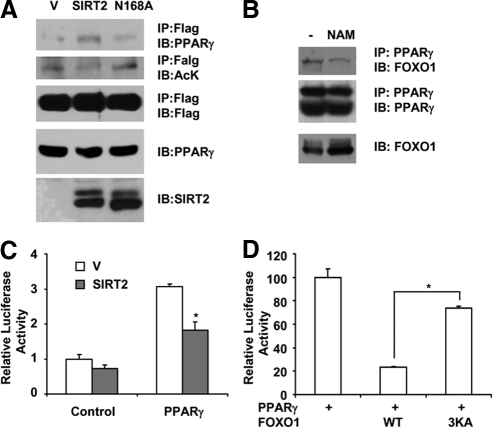
It was reported that FOXO1 binds to PPARγ and inhibits its transcriptional activity (Dowell et al., 2003). However, it was not known whether the acetylation modification of FOXO1 affects its interaction with PPARγ. To this end, HEK293 cells were transfected with Flag-FOXO1 and PPARγ, together with SIRT2 or SIRT2-N168A deacetylase mutant. Cell lysates were collected and flag-tagged FOXO1 proteins were immunoprecipitated with anti-Flag antibodies conjugated to agarose beads. As shown in Figure 7A, in the presence of wild-type SIRT2 expression, FOXO1 acetylation level is decreased, whereas the interaction between PPARγ and Flag-FOXO1 is increased. SIRT2 deacetylase mutant SIRT2-N168A loses the ability to promote the interaction between PPARγ and FOXO1, indicating deacetylation of FOXO1 is important for its ability to bind to PPARγ. To test if modulating FOXO1 acetylation affects endogenous FOXO1–PPARγ interaction in adipocytes, we treated 3T3-L1 adipocytes with sirtuin inhibitor nicotinamide. We found the interaction between FOXO1 and PPARγ is reduced upon nicotinamide treatment (Figure 7B), supporting the notion that acetylation of FOXO1 diminishes its binding to PPARγ in adipocytes. Nicotinamide inhibits not only SIRT2, but also other sirtuins, including SIRT1, which was also shown to be able to deacetylate FOXO1 (Daitoku et al., 2004; Motta et al., 2004; Yang et al., 2005). Because it was found that the expression of SIRT2 is more abundant than SIRT1 in white adipose tissue (Jing et al., 2007), SIRT2 is more likely to play a major role.
Figure 7.
SIRT2 increases FOXO1 repressive binding to PPARγ. (A) HEK293T cells were transfected with Flag-FOXO1 and pSV-PPARγ, pCMV-SIRT2, or pCMV-SIRT2N168A. Cells were treated with 1 μM tricostatin A for 4 h before harvest. FOXO1 was immunoprecipitated with anti-Flag beads. Coprecipitation of PPARγ was detected with anti-PPARγ antibodies. IP, immunoprecipitation; IB, immunoblotting. (B) Differentiated 3T3-L1 adipocytes were treated with 5 mM nicotinamide (NAM) for 4 h. Interaction between FOXO1 and PPARγ was detected by immunoprecipitation of PPARγ followed by anti-FOXO1 Western blot analysis. The levels of FOXO1 in the lysates were also examined by direct Western blot analysis. (C) HEK293T cells were transfected with PPRE-Luciferase construct, together with pSV-PPARγ and SIRT2. Cells were harvest for luciferase assay as described in Materials and Methods. (D) HEK293T cells were transfected with PPRE-Luciferase, PPARγ, and FOXO1 or FOXO1-3KA. Luciferase activity was measured. *p < 0.05.
In agreement with the finding that SIRT2 promotes FOXO1 binding to PPARγ, PPARγ transcriptional activity was reduced in the presence of SIRT2, revealed by luciferase assay experiments using PPAR response element (PPRE)-driven luciferase reporter construct (Figure 7C). Furthermore, the FOXO1–3KA mutant, which has the lysine residues at 242, 245, and 262 substituted with alanines and mimicking the acetylated form of FOXO1 (Matsuzaki et al., 2005), lose the ability to inhibit PPARγ, indicating deacetylation of FOXO1 is required for its repression on PPARγ (Figure 7D). These results suggest that SIRT2 deacetylation of FOXO1 directly increases its repressive binding to PPARγ. As PPARγ is a key proadipogenic factor, SIRT2 may inhibit adipogenesis through the FOXO1-mediated repression on PPARγ.
DISCUSSION
We have previously reported that caloric restriction stimulates SIRT2 expression in adipose tissue and kidney. Here we have found SIRT2 expression in adipose tissue also responded to short-term nutrient deprivation (24-h fasting) or cold exposure. These conditions may trigger many physiological responses in tissues to cope with nutritional or environmental changes. Among them, hormonal changes play important roles. Fasting and cold exposure increase the secretion of both adrenergic and glucocorticoid hormones. We found isoproterenol, but not dexamethasone, stimulated SIRT2 expression in cultured 3T3-L1 adipocytes, indicating SIRT2 expression is regulated by catecholamine but not glucocorticoids. Furthermore, we have demonstrated that intraperitoneal injection of isoproterenol also elevated SIRT2 level in white adipose, confirming the regulation of SIRT2 expression by adrenergic signaling in vivo.
Our findings demonstrate that SIRT2 level in adipose tissue is regulated by fasting and environmental temperature changes. SIRT2 suppresses adipogenesis, fitting the scenario that during times of energy deficiency (caloric restriction or fasting) or energy needs (cold exposure), SIRT2 expression is elevated in adipose to promote fuel availability, as we have found that SIRT2 increases adipocyte lipolysis. This implies SIRT2 level changes in adipose tissues in response to food availability and seasonal environmental changes may result in the regulation of adipose function.
The inhibitory effect of SIRT2 on adipocyte differentiation is only shown when the cells are differentiated under low-glucose or low-insulin conditions. The reason for this is not clear at this point. Given the fact that SIRT2 expression is induced in adipose by caloric restriction (Wang et al., 2007) and fasting, it is possible that SIRT2's enzymatic activity is only activated under low-glucose or low-insulin states. On the other hand, we found the overall adipocyte differentiation rate is lower with either low glucose or low insulin. It is possible that the 3T3-L1 cells are already maximally differentiated under the high-glucose and high-insulin condition that the SIRT2 knockdown is not able to further enhance adipocyte differentiation. When 3T3-L1 cells are differentiated in a less optimal condition with low-glucose or low-insulin medium, SIRT2 knockdown is able to show its effect on adipogenesis. As for cells with SIRT2 over expression, high glucose or high insulin may abrogate the action of SIRT2 on adipogenesis.
A previous publication has demonstrated FOXO1 binds to PPARγ and inhibits its transcription activity (Dowell et al., 2003). In addition, FOXO1 was also found to inhibit PPARγ gene promoter in primary adipocytes (Armoni et al., 2006). Our result furthered these findings by identifying FOXO1 deacetylation as a necessary step for FOXO1's repressive interaction with PPARγ. Under our experiment condition, SIRT2 decreases the acetylation level of FOXO1 and increases its binding to PPARγ (Figure 7A), without affecting FOXO1 subcellular localization (Supplemental Figure S3). The interaction of FOXO1 and PPARγ happens endogenously in 3T3-L1 adipocytes and sirtuin inhibitor nicotinamide treatment diminishes this interaction in 3T3-L1 adipocytes (Figure 7B). Furthermore, we found PPARγ ligand (rosiglitazone) treatment rescued adipocyte differentiation in SIRT2-overexpressing cells (Supplemental Figure S2B), supporting our hypothesis that SIRT2 inhibits adipogenesis through the inhibition of PPARγ.
It is interesting to note that SIRT2 and SIRT1 share a similar function in adipose tissue. It was revealed that caloric restriction elevates SIRT1 level in several rat tissues, including adipose tissue, brain, liver, and kidney (Cohen et al., 2004). In adipose tissue, SIRT1 attenuates adipogenesis and promotes lipolysis (Picard et al., 2004). As SIRT1 is already known to deacetylate FOXO1, the SIRT1 function in inhibiting adipocyte differentiation may also be mediated by regulation of FOXO1—PPARγ interaction.
Dietary caloric restriction delays aging and the onset of age-related diseases, including obesity, diabetes (Hansen and Bodkin, 1993), tumors (Fernandes et al., 1976; Sarkar et al., 1982), kidney diseases (Fernandes and Good, 1984), and several types of neurodegenerative disorders (Duan and Mattson, 1999; Duan et al., 2003; Mattson, 2003). The underlying mechanism is not clear. It is conceivable that the metabolic changes rendered by caloric restriction may mediate the beneficial effects on delaying aging and the on set of age-related diseases. It was shown that sirtuins mediate the action of caloric restriction in lower organisms, such as yeast, C. elegans and Drosophila (Kaeberlein et al., 1999; Tissenbaum and Guarente, 2001; Wood et al., 2004; Lamming et al., 2005). In addition, we and others have shown that the expression of mammalian sirtuins is also responsive to caloric restriction (Cohen et al., 2004; Shi et al., 2005; Wang et al., 2007). Therefore, sirtuins may mediate the action of caloric restriction in higher organisms. The metabolic regulation function of SIRT2 described here and similar functions for other sirtuins (Picard et al., 2004; Shi et al., 2005) may be the obligatory pathways underlying caloric restriction's effect on aging.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. Wenlong Bai (University of South Florida) for providing pcDNA-Flag-FOXO1, Dr. Akiyoshi Fukamizu (University of Tsukuba, Japan) for providing pcDNA-Flag-FOXO1-3KA plasmids. F.W. is supported by American Federation for Aging Research senior postdoctoral fellowship. This work was also supported by US Department of Agriculture Grant 6250-51000-049 and National Institutes of Health Grant RO1DK075978 to Q.T.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E08-06-0647) on November 26, 2008.
REFERENCES
- Accili D., Arden K. C. FoxOs at the crossroads of cellular metabolism, differentiation, and transformation. Cell. 2004;117:421–426. doi: 10.1016/s0092-8674(04)00452-0. [DOI] [PubMed] [Google Scholar]
- Armoni M., Harel C., Karni S., Chen H., Bar-Yoseph F., Ver M. R., Quon M. J., Karnieli E. FOXO1 represses peroxisome proliferator-activated receptor-gamma1 and -gamma2 gene promoters in primary adipocytes. A novel paradigm to increase insulin sensitivity. J. Biol. Chem. 2006;281:19881–19891. doi: 10.1074/jbc.M600320200. [DOI] [PubMed] [Google Scholar]
- Barak Y., Nelson M. C., Ong E. S., Jones Y. Z., Ruiz-Lozano P., Chien K. R., Koder A., Evans R. M. PPAR gamma is required for placental, cardiac, and adipose tissue development. Mol. Cell. 1999;4:585–595. doi: 10.1016/s1097-2765(00)80209-9. [DOI] [PubMed] [Google Scholar]
- Bluher M., Kahn B. B., Kahn C. R. Extended longevity in mice lacking the insulin receptor in adipose tissue. Science. 2003;299:572–574. doi: 10.1126/science.1078223. [DOI] [PubMed] [Google Scholar]
- Burgering B. M., Kops G. J. Cell cycle and death control: long live Forkheads. Trends Biochem. Sci. 2002;27:352–360. doi: 10.1016/s0968-0004(02)02113-8. [DOI] [PubMed] [Google Scholar]
- Christy R. J., Yang V. W., Ntambi J. M., Geiman D. E., Landschulz W. H., Friedman A. D., Nakabeppu Y., Kelly T. J., Lane M. D. Differentiation-induced gene expression in 3T3–L1 preadipocytes: CCAAT/enhancer binding protein interacts with and activates the promoters of two adipocyte-specific genes. Genes Dev. 1989;3:1323–1335. doi: 10.1101/gad.3.9.1323. [DOI] [PubMed] [Google Scholar]
- Cohen H. Y., Miller C., Bitterman K. J., Wall N. R., Hekking B., Kessler B., Howitz K. T., Gorospe M., de Cabo R., Sinclair D. A. Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. Science. 2004;305:390–392. doi: 10.1126/science.1099196. [DOI] [PubMed] [Google Scholar]
- Daitoku H., Fukamizu A. FOXO transcription factors in the regulatory networks of longevity. J. Biochem. 2007;141:769–774. doi: 10.1093/jb/mvm104. [DOI] [PubMed] [Google Scholar]
- Daitoku H., Hatta M., Matsuzaki H., Aratani S., Ohshima T., Miyagishi M., Nakajima T., Fukamizu A. Silent information regulator 2 potentiates Foxo1-mediated transcription through its deacetylase activity. Proc. Natl. Acad. Sci. USA. 2004;101:10042–10047. doi: 10.1073/pnas.0400593101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowell P., Otto T. C., Adi S., Lane M. D. Convergence of peroxisome proliferator-activated receptor gamma and Foxo1 signaling pathways. J. Biol. Chem. 2003;278:45485–45491. doi: 10.1074/jbc.M309069200. [DOI] [PubMed] [Google Scholar]
- Dryden S. C., Nahhas F. A., Nowak J. E., Goustin A. S., Tainsky M. A. Role for human SIRT2 NAD-dependent deacetylase activity in control of mitotic exit in the cell cycle. Mol. Cell. Biol. 2003;23:3173–3185. doi: 10.1128/MCB.23.9.3173-3185.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan W., Guo Z., Jiang H., Ware M., Li X. J., Mattson M. P. Dietary restriction normalizes glucose metabolism and BDNF levels, slows disease progression, and increases survival in huntingtin mutant mice. Proc. Natl. Acad. Sci. USA. 2003;100:2911–2916. doi: 10.1073/pnas.0536856100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan W., Mattson M. P. Dietary restriction and 2-deoxyglucose administration improve behavioral outcome and reduce degeneration of dopaminergic neurons in models of Parkinson's disease. J. Neurosci. Res. 1999;57:195–206. doi: 10.1002/(SICI)1097-4547(19990715)57:2<195::AID-JNR5>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Fernandes G., Good R. A. Inhibition by restricted-calorie diet of lymphoproliferative disease and renal damage in MRL/lpr mice. Proc. Natl. Acad. Sci. USA. 1984;81:6144–6148. doi: 10.1073/pnas.81.19.6144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes G., Yunis E. J., Good R. A. Suppression of adenocarcinoma by the immunological consequences of calorie restriction. Nature. 1976;263:504–507. doi: 10.1038/263504b0. [DOI] [PubMed] [Google Scholar]
- Finnin M. S., Donigian J. R., Pavletich N. P. Structure of the histone deacetylase SIRT2. Nat. Struct. Biol. 2001;8:621–625. doi: 10.1038/89668. [DOI] [PubMed] [Google Scholar]
- Frye R. A. Characterization of five human cDNAs with homology to the yeast SIR2 gene: Sir2-like proteins (sirtuins) metabolize NAD and may have protein ADP-ribosyltransferase activity. Biochem. Biophys. Res. Commun. 1999;260:273–279. doi: 10.1006/bbrc.1999.0897. [DOI] [PubMed] [Google Scholar]
- Gesta S., Tseng Y. H., Kahn C. R. Developmental origin of fat: tracking obesity to its source. Cell. 2007;131:242–256. doi: 10.1016/j.cell.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Guarente L. Sirtuins as potential targets for metabolic syndrome. Nature. 2006;444:868–874. doi: 10.1038/nature05486. [DOI] [PubMed] [Google Scholar]
- Hansen B. C., Bodkin N. L. Primary prevention of diabetes mellitus by prevention of obesity in monkeys. Diabetes. 1993;42:1809–1814. doi: 10.2337/diab.42.12.1809. [DOI] [PubMed] [Google Scholar]
- Hiratsuka M., et al. Proteomics-based identification of differentially expressed genes in human gliomas: down-regulation of SIRT2 gene. Biochem. Biophys. Res. Commun. 2003;309:558–566. doi: 10.1016/j.bbrc.2003.08.029. [DOI] [PubMed] [Google Scholar]
- Jing E., Gesta S., Kahn C. R. SIRT2 regulates adipocyte differentiation through FoxO1 acetylation/deacetylation. Cell Metab. 2007;6:105–114. doi: 10.1016/j.cmet.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones J. R., Barrick C., Kim K. A., Lindner J., Blondeau B., Fujimoto Y., Shiota M., Kesterson R. A., Kahn B. B., Magnuson M. A. Deletion of PPARgamma in adipose tissues of mice protects against high fat diet-induced obesity and insulin resistance. Proc. Natl. Acad. Sci. USA. 2005;102:6207–6212. doi: 10.1073/pnas.0306743102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan M., Schallhorn A., Wurm F. M. Transfecting mammalian cells: optimization of critical parameters affecting calcium-phosphate precipitate formation. Nucleic Acids Res. 1996;24:596–601. doi: 10.1093/nar/24.4.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaeberlein M., McVey M., Guarente L. The SIR2/3/4 complex and SIR2 alone promote longevity in Saccharomyces cerevisiae by two different mechanisms. Genes Dev. 1999;13:2570–2580. doi: 10.1101/gad.13.19.2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajihara T., et al. Differential expression of FOXO1 and FOXO3a confers resistance to oxidative cell death upon endometrial decidualization. Mol. Endocrinol. 2006;20:2444–2455. doi: 10.1210/me.2006-0118. [DOI] [PubMed] [Google Scholar]
- Kenyon C., Chang J., Gensch E., Rudner A., Tabtiang R. A C. elegans mutant that lives twice as long as wild type. Nature. 1993;366:461–464. doi: 10.1038/366461a0. [DOI] [PubMed] [Google Scholar]
- Kubota N., et al. PPAR gamma mediates high-fat diet-induced adipocyte hypertrophy and insulin resistance. Mol. Cell. 1999;4:597–609. doi: 10.1016/s1097-2765(00)80210-5. [DOI] [PubMed] [Google Scholar]
- Lamming D. W., Latorre-Esteves M., Medvedik O., Wong S. N., Tsang F. A., Wang C., Lin S. J., Sinclair D. A. HST2 mediates SIR2-independent life-span extension by calorie restriction. Science. 2005;309:1861–1864. doi: 10.1126/science.1113611. [DOI] [PubMed] [Google Scholar]
- Lane M. D., Tang Q. Q., Jiang M. S. Role of the CCAAT enhancer binding proteins (C/EBPs) in adipocyte differentiation. Biochem. Biophys. Res. Commun. 1999;266:677–683. doi: 10.1006/bbrc.1999.1885. [DOI] [PubMed] [Google Scholar]
- Lin Y., et al. The hyperglycemia-induced inflammatory response in adipocytes: the role of reactive oxygen species. J. Biol. Chem. 2005;280:4617–4626. doi: 10.1074/jbc.M411863200. [DOI] [PubMed] [Google Scholar]
- Lowell B. B., Spiegelman B. M. Towards a molecular understanding of adaptive thermogenesis. Nature. 2000;404:652–660. doi: 10.1038/35007527. [DOI] [PubMed] [Google Scholar]
- Makarova O., Kamberov E., Margolis B. Generation of deletion and point mutations with one primer in a single cloning step. Biotechniques. 2000;29:970–972. doi: 10.2144/00295bm08. [DOI] [PubMed] [Google Scholar]
- Matsuzaki H., Daitoku H., Hatta M., Aoyama H., Yoshimochi K., Fukamizu A. Acetylation of Foxo1 alters its DNA-binding ability and sensitivity to phosphorylation. Proc. Natl. Acad. Sci. USA. 2005;102:11278–11283. doi: 10.1073/pnas.0502738102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson M. P. Gene-diet interactions in brain aging and neurodegenerative disorders. Ann. Intern. Med. 2003;139:441–444. doi: 10.7326/0003-4819-139-5_part_2-200309021-00012. [DOI] [PubMed] [Google Scholar]
- Michan S., Sinclair D. Sirtuins in mammals: insights into their biological function. Biochem. J. 2007;404:1–13. doi: 10.1042/BJ20070140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison R. F., Farmer S. R. Role of PPARgamma in regulating a cascade expression of cyclin-dependent kinase inhibitors, p18(INK4c) and p21(Waf1/Cip1), during adipogenesis. J. Biol. Chem. 1999;274:17088–17097. doi: 10.1074/jbc.274.24.17088. [DOI] [PubMed] [Google Scholar]
- Motta M. C., Divecha N., Lemieux M., Kamel C., Chen D., Gu W., Bultsma Y., McBurney M., Guarente L. Mammalian SIRT1 represses forkhead transcription factors. Cell. 2004;116:551–563. doi: 10.1016/s0092-8674(04)00126-6. [DOI] [PubMed] [Google Scholar]
- Nakae J., Kitamura T., Kitamura Y., Biggs W. H., 3rd, Arden K. C., Accili D. The forkhead transcription factor Foxo1 regulates adipocyte differentiation. Dev. Cell. 2003;4:119–129. doi: 10.1016/s1534-5807(02)00401-x. [DOI] [PubMed] [Google Scholar]
- Nawrocki A. R., Scherer P. E. Keynote review: the adipocyte as a drug discovery target. Drug Discov. Today. 2005;10:1219–1230. doi: 10.1016/S1359-6446(05)03569-5. [DOI] [PubMed] [Google Scholar]
- North B. J., Marshall B. L., Borra M. T., Denu J. M., Verdin E. The human Sir2 ortholog, SIRT2, is an NAD+-dependent tubulin deacetylase. Mol. Cell. 2003;11:437–444. doi: 10.1016/s1097-2765(03)00038-8. [DOI] [PubMed] [Google Scholar]
- North B. J., Verdin E. Interphase nucleo-cytoplasmic shuttling and localization of SIRT2 during mitosis. PLoS ONE. 2007;2:e784. doi: 10.1371/journal.pone.0000784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard F., Kurtev M., Chung N., Topark-Ngarm A., Senawong T., Machado De Oliveira R., Leid M., McBurney M. W., Guarente L. Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-gamma. Nature. 2004;429:771–776. doi: 10.1038/nature02583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen E. D., Hsu C. H., Wang X., Sakai S., Freeman M. W., Gonzalez F. J., Spiegelman B. M. C/EBPalpha induces adipogenesis through PPARgamma: a unified pathway. Genes Dev. 2002;16:22–26. doi: 10.1101/gad.948702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen E. D., MacDougald O. A. Adipocyte differentiation from the inside out. Nat. Rev. Mol. Cell Biol. 2006;7:885–896. doi: 10.1038/nrm2066. [DOI] [PubMed] [Google Scholar]
- Rosen E. D., Sarraf P., Troy A. E., Bradwin G., Moore K., Milstone D. S., Spiegelman B. M., Mortensen R. M. PPAR gamma is required for the differentiation of adipose tissue in vivo and in vitro. Mol. Cell. 1999;4:611–617. doi: 10.1016/s1097-2765(00)80211-7. [DOI] [PubMed] [Google Scholar]
- Sarkar N. H., Fernandes G., Telang N. T., Kourides I. A., Good R. A. Low-calorie diet prevents the development of mammary tumors in C3H mice and reduces circulating prolactin level, murine mammary tumor virus expression, and proliferation of mammary alveolar cells. Proc. Natl. Acad. Sci. USA. 1982;79:7758–7762. doi: 10.1073/pnas.79.24.7758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi T., Wang F., Stieren E., Tong Q. SIRT3, a mitochondrial sirtuin deacetylase, regulates mitochondrial function and thermogenesis in brown adipocytes. J. Biol. Chem. 2005;280:13560–13567. doi: 10.1074/jbc.M414670200. [DOI] [PubMed] [Google Scholar]
- Spiegelman B. M., Flier J. S. Obesity and the regulation of energy balance. Cell. 2001;104:531–543. doi: 10.1016/s0092-8674(01)00240-9. [DOI] [PubMed] [Google Scholar]
- Tissenbaum H. A., Guarente L. Increased dosage of a sir-2 gene extends lifespan in Caenorhabditis elegans. Nature. 2001;410:227–230. doi: 10.1038/35065638. [DOI] [PubMed] [Google Scholar]
- Vaquero A., Scher M. B., Lee D. H., Sutton A., Cheng H. L., Alt F. W., Serrano L., Sternglanz R., Reinberg D. SirT2 is a histone deacetylase with preference for histone H4 Lys 16 during mitosis. Genes Dev. 2006;20:1256–1261. doi: 10.1101/gad.1412706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F., Nguyen M., Qin F. X., Tong Q. SIRT2 deacetylates FOXO3a in response to oxidative stress and caloric restriction. Aging Cell. 2007;6:505–514. doi: 10.1111/j.1474-9726.2007.00304.x. [DOI] [PubMed] [Google Scholar]
- Wood J. G., Rogina B., Lavu S., Howitz K., Helfand S. L., Tatar M., Sinclair D. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature. 2004;430:686–689. doi: 10.1038/nature02789. [DOI] [PubMed] [Google Scholar]
- Woods Y. L., Rena G. Effect of multiple phosphorylation events on the transcription factors FKHR, FKHRL1 and AFX. Biochem. Soc. Trans. 2002;30:391–397. doi: 10.1042/bst0300391. [DOI] [PubMed] [Google Scholar]
- Yang Y., Hou H., Haller E. M., Nicosia S. V., Bai W. Suppression of FOXO1 activity by FHL2 through SIRT1-mediated deacetylation. EMBO J. 2005;24:1021–1032. doi: 10.1038/sj.emboj.7600570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y. H., Chen Y. H., Zhang C. Y., Nimmakayalu M. A., Ward D. C., Weissman S. Cloning and characterization of two mouse genes with homology to the yeast Sir2 gene. Genomics. 2000;69:355–369. doi: 10.1006/geno.2000.6360. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.