Abstract
Background
Post-weaning social isolation in rats produces profound and long-lasting cognitive and behavioral deficits in adult animals. Importantly, this housing manipulation alters sensitivity to a number of drugs of abuse including ethanol. However, most studies with ethanol have utilized continuous or limited home cage access to examine interactions between juvenile social experience and drinking. More recently, social isolation was shown to increased ethanol responding in a ‘dipper’ model of self-administration (Deehan et al. 2007 Alcohol. Clin. Exper. Res. 31: 1692−1698). In the current study, we utilize a ‘sipper’ operant self-administration model to distinguish the effects of isolation rearing on ethanol seeking- and drinking-related behaviors.
Methods
Post-weaning juvenile male Long-Evans rats were placed into two housing groups for six weeks: one group consisted of individually-housed animals; the second group was housed four animals per cage. Following the isolation period, anxiety-like behavior was assessed to confirm the efficacy of the isolation procedure. In some animals, ethanol drinking in the home cage was assessed using a continuous access, two-bottle choice paradigm. All animals were then individually housed and trained to lever-press for a sipper tube containing either an ethanol solution or a sucrose solution.
Results
Post-weaning social isolation increased the expression of anxiety-like behavior in the elevated plus maze but not the light-dark box. Ethanol consumption was also increased during continuous home-cage access with the two-bottle choice paradigm. During operant self-administration, isolation housing increased the response rate and increased ethanol consumption but did not alter responding for or consumption of sucrose. The housing manipulation did not change the total number of lever responses during extinction sessions. Paired-pulse inhibition deficits that are characteristic of juvenile isolation remained intact after prolonged experience with sucrose self-administration.
Discussion
The effects of post-weaning social isolation on ethanol drinking in the home cage are also manifest during operant self-administration. Importantly, these alterations in adult operant self-administration are ethanol-specific.
Keywords: social isolation, appetitive behavior, consumption, plus maze, light/dark box
INTRODUCTION
The post-weaning development period in male rats is critical for establishing normal adult behaviors. During this period, rats establish social hierarchies and gender-appropriate affiliative and agonistic behaviors (Meaney and Stewart, 1981). Isolation rearing during this period produces long-term behavioral alterations in adults and is characterized by increased expression of anxiety-like behavior (Hall et al., 1998a; Wright et al., 1991), and, in some cases, altered locomotor activity in response to novel environments (Heidbreder et al., 2000; Larsson et al., 2002). Likewise, social isolation increases food-hoarding behavior (Heidbreder et al., 2000), decreases cognitive function (Larsson et al., 2002), and produces deficits in sensory-motor gating (Heidbreder et al., 2000; Larsson et al., 2002). Importantly, post-weaning social isolation increases adult sensitivity/self-administration of amphetamine (Bardo et al., 2001) and cocaine (Howes et al., 2000). Conversely, isolated animals appear to have reduced sensitivity to opioids (Wongwitdecha and Marsden, 1996). Thus, the cognitive effects of juvenile social experience may differentially modulate adult sensitivity to drugs of abuse.
The effects of social isolation on voluntary ethanol intake appear to vary across different strains of rats and drinking paradigms. For example, using a home-cage, two-bottle choice paradigm, Fahlke and colleagues have shown that social isolation decreases continuous-access ethanol consumption/preference in Wistar rats that were weaned early (post-natal day 16) and given ethanol access during the isolation procedure (Fahlke et al., 1997). In contrast, social isolation increased ethanol consumption/preference in adult Wistar (Hall et al., 1998b), Fawn-hooded (Hall et al., 1998b; Lodge and Lawrence, 2003), and Long-Evans rats (Deehan et al., 2007) that do not receive a juvenile ethanol experience. Interestingly, post-weaning isolation did not produce any effects on continuous-access, home-cage ethanol consumption in the Wistar-derived P and NP selectively-bred rat strains (Ehlers et al., 2007). Two studies have tested the effects of juvenile social isolation on limited-access drinking. One study (Ehlers et al., 2007) used ‘home-cage’-like environments and offered ethanol during a brief 20min drinking session. Social isolation increased ethanol consumption/preference during this limited access, but only for P rats and not for NP individuals. In a second study (Deehan et al., 2007), post-weaning, isolation-reared male Long-Evans rats were compared to socially-housed and enrichment-housed individuals using a two-lever ‘dipper’-type self-administration model. Here, isolation rearing increased ethanol-specific responding, ethanol preference relative to water, and ethanol-seeking during reversal learning sessions. Importantly, it is unclear if the effects of social isolation on ethanol consumption/preference are related to appetitive- or consumption-related behaviors or some other parameter.
Since ethanol has a relatively slow-onset and long duration of action, consumption of the drug can itself alter the behavioral and neurobiological mechanisms regulating ethanol-seeking and –consumption during a given drinking episode or self-administration session. For this reason, Samson and colleagues (Samson et al., 1998) designed an operant self-administration paradigm that procedurally separates ethanol-seeking and –taking, essentially segregating the lever-response (seeking) phase from the consumption (drinking) phase. Importantly, pharmacological analyses of this model have indeed shown that the seeking and drinking behaviors are governed by distinct neurobiological processes (Sharpe and Samson, 2001; Slawecki and Roth, 2003). These data together suggest that this model might be used to help define potential mechanisms contributing to multiple aspects of ethanol drinking behavior following post-weaning social isolation.
MATERIALS & METHODS
Social Isolation
Male Long-Evans rats were purchased from Harlan at post-natal day 21−25 (46.2±0.5g, n=59). Animals were housed for one week under standard conditions (4−5 animals/cage, food/water ad libitum, 12hr light/dark cycle) to recover from shipping and were then randomly divided into two experimental groups. One group was housed individually (social isolation or SI; n=31) in rat cages (20.3cm × 26.7cm). The other group was maintained with four animals/cage (group housing or GH; n=28, 7 groups of 4) in guinea pig-cages (33cm × 59.7cm; Nalgene). Both groups were maintained in these housing conditions for six weeks, tested for anxiety-like behavior (see below), and then individually housed for the remainder of the study. All procedures were approved by the WFUSM ACUC.
Anxiety Assays
To measure anxiety-like behavior, we used the light/dark box and the elevated plus maze as previously described (McCool and Chappell, 2007). Assays followed a 20min habituation to the experimental room. For the light/dark box, we initially placed rats from different housing groups into the brightly-lit ‘light’ chamber (400 lux) facing away from the door leading to the adjacent ‘dark’ chamber. Infrared sensors recorded the animal's geometric center within the apparatus (Colbourn Inst.) over the 5min duration of the test. ‘Anxiety’-related dependent variables included the time to egress from the light-side, the latency (in seconds) to reenter the light-side after initial egress into the dark-side, the number of transitions between light and dark compartments, and total amount of time spent in the ‘light’-side. ‘Locomotor’-related variables (Crawley and Goodwin, 1980), including the total distance traveled in the entire apparatus during the 5min trial, the total time spent moving during the trial, and total number of movements during this time, were also measured. Between animals, the apparatus was cleaned with a warm water rinse, 70% ethanol, a repeated warm water rinse, and thoroughly dried.
Three days after the light/dark test, animals were placed in the junction of the plus-maze apparatus facing an open arm. Room lights were dimmed to ∼40lux to encourage open-arm exploration. During each 300sec trial, infrared beam-breaks at the opening of each arm were recorded using a computer-assisted automated system (Med Associates). Dependent variables included open-arm time, closed-arm time, junction time, and open- and closed-arm entries. Clinically relevant anxiolytics increase the amount of time animals spend in the open arms and can increase the number of open-arm entries (Pellow et al., 1985). Closed-arm entries can reflect locomotor behavior in this assay (Canto-de-Souza et al., 2002). Between animals, the apparatus was cleaned with a warm water rinse, 70% ethanol, a repeated warm water rinse, and thoroughly dried. All data are reported as mean ± SEM and analyzed with standard student's t-test.
Forced Ethanol Exposure and Two-bottle Choice
Following the anxiety assays, all animals were housed individually. Animals that were to be used in the ethanol self-administration study (below; n=16 for GH and n=19 for SI) were then given continuous home-cage access to 10% (v/v) ethanol solution as their sole liquid source for three consecutive 24hr periods. The total amount consumed was monitored every day.
Following this forced exposure, these animals were subjected to a two-bottle home cage procedure consisting of continuous access to bottles containing either 10% (v/v) ethanol solution or water presented for five consecutive days (Hall et al., 1998b). Water and ethanol solutions were presented in graduated drinking tubes (Med Associates); and, the position of the bottles was alternated daily to reduce the influence of side preference by individual animals. Ethanol and water intake (milliliters) were measured daily when fresh bottles were placed on the home cage. All data are reported as mean ± SEM and analyzed using two-way ANOVA with Bonferroni's posttest or standard t-test as indicated.
Limited Access Sucrose and Ethanol Self-Administration
Following the forced- and two-bottle ethanol drinking, animals were trained to self-administer ethanol using a modified sucrose-fade procedure (Samson, 1986). Daily sessions were performed in sound-attenuated, commercially-available operant chambers (Med Associates, East Fairfield VT) as previously described (Samson et al., 1999). Each chamber contains a house light to signal the beginning of a session, a retractable lever that extends into the chamber at the initiation of each session and retracts upon completion of the response requirement, and a motorized sipper tube that extends into the chamber upon completion of the response requirement. The entire system was computer controlled; and response/drinking data were collected at 2Hz and analyzed using Med PC software (Med Associates). 20sec analysis bins were used to define bout size and number throughout a session. All data are reported as mean ± SEM and analyzed with standard student's t-test.
To establish operant ethanol self-administration, we used an abbreviated standard sucrose-substitution protocol based on the method of Samson (Samson, 1986). Briefly, in the initial four-hour session, animals were shaped to lever press on a Fixed Ratio 1 (FR1) that allowed 40seconds access to a sipper tube connected to a 10% sucrose solution. During subsequent sessions, total session time was gradually decreased and the fixed ratio/sipper tube access time gradually increased until, after one week, animals had a single daily 20 minute time limit to fulfill the desired response requirement followed by a single 20 minute drinking period. For ethanol-drinking animals, the 10% sucrose was gradually replaced with increasing concentrations of ethanol such that animals had access to 2% sucrose/10% ethanol at the end of the first week. Over the next seven days, the remaining sucrose was slowly decreased until animals stably responded with a response requirement of thirty lever presses to gain access to 10% ethanol alone. For sucrose drinking animals, the concentration of sucrose was gradually decreased to 3% since this amount has been shown to generate appetitive behaviors similar to 10% ethanol (Samson et al., 1998).
Lever press-related (appetitive) and drinking (consumption) behaviors were continuously monitored using commercially-available software (Med Associates). Baseline response- and consumption-related behaviors are reported from the first two weeks that animals responded with 30 lever presses for the 10% ethanol or 3% sucrose solutions. During the third and fifth weeks of self-administration, animals were probed with a single ‘extinction’ trial (Samson et al., 2001) during which animals could press the lever for 20minutes without receiving access to the sipper tube. The two extinction trials were separated by a single week of standard self-administration. Separating these sessions by one week has been shown to produce stable extinction responding in this model (Samson et al., 2001).
Pre-pulse Inhibition
In a cohort of sucrose-drinking animals, paired-pulse inhibition experiments were carried out at the end of the self-administration study to determine if training or self-administration experience disrupted any of the characteristic behavioral deficits produced by the social isolation. Startle responses to an auditory stimulus were determined in commercially-available, sound-attenuated startle chambers (San Diego Inst.). Animals were exposed to the apparatus on three consecutive ‘sham’ days to customize them to the handling and startle chamber. On days four and five, sessions consisted of twenty randomized ‘startle only’ or ‘pre-pulse’ trials. During startle trials, animals were exposed to a single 50msec auditory stimulus (111dB); and, movement in the startle chamber was recorded continuously. The maximal amplitude during these trials was averaged to yield a startle baseline for any given individual animal. For the pre-pulse trials, the startle stimulus was preceded by a 50msec auditory stimulus of lesser intensity. Two separate pre-pulse intensities, 50 and 65dB, were performed on separate days. The inter-trial interval ranged between 15 and 60 seconds and was randomized for each animal. Pre-pulse inhibition for an individual animal was calculated as the mean startle amplitude during pre-pulse trials divided by the mean startle amplitude during ‘startle-only trials. Data were analyzed on individual days with standard t-tests.
RESULTS
Social isolation
Animals were housed individually (socially isolated or SI) or in groups of four/cage (group housed or GH) for six weeks beginning around post-natal day 25. Over the first five weeks under these housing conditions, animals from both housing groups gained weight at a similar rate: 49.9±0.6g/week for all GH animals and 49.9±0.5g/week for all SI animals (t=0.05, P>0.05, t-test). On the sixth and final week, GH animals weighed 328±4g and SI animals weighed 329±3g (t=0.16, P>0.05, t-test).
Anxiety-like Behavior
At the end of six weeks in the ‘group’ and ‘isolated’ environments, we measured anxiety-like behavior using two different assays to probe unique aspects of the behavior. In the light/dark box (Table 1), the different housing conditions influenced neither anxiety-related dependent variables (egress latency, time in the light-side, light-dark transitions, or re-entry latency) nor locomotor-related behaviors (number of moves, move distance, move time). These findings suggest that post-weaning group- or isolation-housing does not influence anxiety-like behaviors expressed in the light/dark box.
Table 1.
Light/Dark Box Behaviora
| Group Housedb | Socially Isolatedb | t-test | |
|---|---|---|---|
| Egress Latency (sec) | 17.7±2.5 | 26.8±4.4 | n.s. (P<0.1) |
| Time in Light (sec) | 121.4±9.0 | 127.2±5.6 | n.s. |
| Light-Dark Transitions | 6.5±0.4 | 6.3±0.5 | n.s. |
| Re-entry Latency (sec) | 70.8±15.1 | 48.1±7.3 | n.s. |
| Number of Moves | 77.8±1.7 | 81.9±1.7 | n.s. |
| Move Distance (cm) | 1335±39 | 1299±32 | n.s. |
| Move Time (sec) | 228±3 | 223±3 | n.s. |
Animals were placed in the light-side of the light/dark box. Their position was remotely monitored for 300 seconds. b - Group housed, n=28 (7 groups of 4); socially isolated n=31.
Group housed, n=28 (7 groups of 4); socially isolated n=31.
In contrast, when animals were examined on the elevated plus maze, we noted a significant increase in anxiety-like behavior expressed by the SI animals. For example, the time spent in the open arms (Fig. 1A) was significantly lower in SI animals compared to their GH counterparts (t=2.25, P<0.05, t-test). Correspondingly, SI animals entered the open arms significantly less than did GH animals (Fig. 1C; t=2.16, P<0.05, t-test). The amount of time spent in the junction between the open- and closed-arms was not significantly different between housing treatments (not shown). Likewise, the number of closed-arm entries (Fig. 1D) was not different between GH and SI animals. These latter findings, together with the light/dark box dataset, suggest that locomotor behaviors are not dramatically altered by the environmental manipulation, at least in these behavioral assays. Thus, post-weaning housing experience can alter anxiety-like behavior in an assay-dependent fashion.
Figure 1.
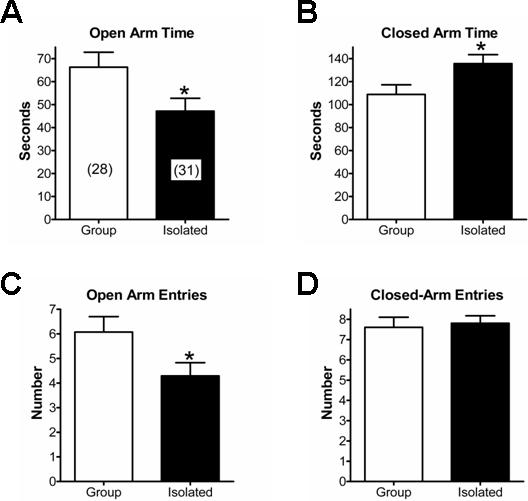
Social isolation of male Long-Evans rats increases expression of anxiety-like behavior in the plus maze. Following six weeks in each housing condition, group-housed (“Group”, □) and socially-isolated (“Isolated”, ■) were examined in the plus maze. (A) Social isolation significantly decreased time spent in the open arms. Numbers in parentheses indicate the number of individual animals in each group. Isolation also increased the time spent in the closed arms (B) and decreased the number of open-arm entries (C). Note the measures of locomotor activity, like the number of closed-arm entries (D), were not significantly affected by the housing experience. * – P<0.05, t-test
Home-cage ethanol drinking
Following the anxiety assays, all animals were individually housed. For a subset of animals (n=16 GH and 19 SI), a 10% ethanol solution was used as the sole liquid source for three consecutive days. The total grams ethanol per kilogram weight consumed by GH and SI animals during this forced exposure was not significantly different during this period (Fig. 2A; t=0.75, P>0.05, t-test). Importantly, the body weight of GH (360±6g) and SI (356±5g) was also not significantly different during this period (t=0.66, P>0.05, t-test). These results suggest that post-weaning housing experience does not influence forced ethanol consumption in male Long-Evans rats.
Figure 2.
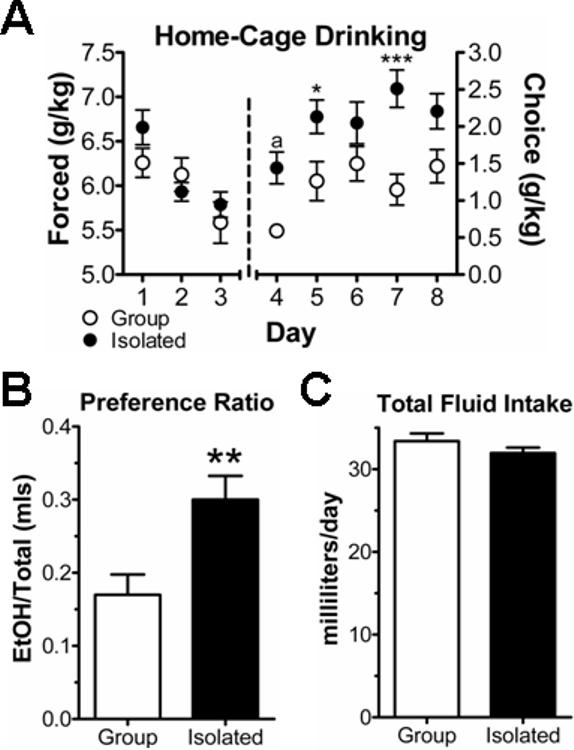
Social isolation of male Long-Evans rats increases ethanol consumption and preference in a continuous-access, two-bottle choice paradigm. (A) Home-cage ethanol consumption was divided into an initial three-day forced exposure to 10% ethanol as the only drinking liquid followed by a five-day period of two-bottle choice between 10% ethanol and water (see text for details). Two-way ANOVA analysis of the choice consumption period indicated significant effects of housing (P<0.0001) and consumption day (P<0.01) but no significant interaction. Bonferroni's posttests indicated that isolated rats (n=16, •) drank significantly more ethanol than group-housed animals (n=11, ○) on two out of five days. a – P<0.1, * – P<0.05, *** – P<0.001 (B) The preference ratio (volume of 10% ethanol consumed divided by the total volume of fluid consumed, both in milliliters) was significantly greater in the isolated animals (** – P<0.01, t-test) compared to group-housed individuals across the five-day choice-drinking period. (C) The total volume of fluid consumed during each day of the two-bottle drinking was not significantly affected by the housing experience.
Immediately following the forced exposure, we placed two bottles, one bottle with water and one bottle with 10% ethanol in water, on the home-cage of GH and SI animals for five consecutive days. Two-way analysis of variance across the two-bottle choice 5-day drinking period indicated a significant effect of housing (P<0.0001, F=35.32) and a significant effect of day (P<0.01, F= 4.45). Bonferroni's posttests found significant between-group differences on two out of five days with a trend towards significance on a third day (Fig. 2A). When collapsed across the entire five-day drinking period, the ethanol preference ratio (milliliters ethanol solution consumed divided by total amount of fluid consumed) was significantly larger in the SI animals (Fig. 2B; t=2.98, P<0.01, t-test). Importantly, neither the total amount of fluid consumed (Fig. 2C; t=1.28, P>0.05, t-test) nor the weight of the GH and SI animals (381±6g and 375±5g, respectively) was significantly different between the different housing conditions (t=0.50, P>0.05, t-test). These data indicate that post-weaning social isolation of male Long-Evans rats increases home-cage ethanol preference and consumption in a continuous-access model.
Operant ethanol self-administration
Following the two-bottle home-cage ethanol drinking, animals were trained to complete thirty (30) lever-presses to gain 20min access to a sipper tube connected to 10% ethanol in water (Samson et al., 1999). Eleven out of sixteen GH rats and sixteen out of nineteen SI animals successfully achieved this response requirement. Data from the GH and SI animals that did not learn the response requirement of 30 lever presses are not included in the self-administration data shown in the manuscript. Following the establishment of a stable baseline (typically 2−3days), lever-pressing (appetitive) and drinking (consumption) behaviors were assessed for two weeks.
When collapsed over the entire two-week period, both the response latency (time to begin lever pressing; Fig. 3A2; t=2.17, P<0.05, t-test) and the response rate (presses/sec; Fig. 3A3; t=2.18, P<0.05, t-test) were significantly greater in SI animals compared to GH rats. Time versus lever-press relationships for exemplar GH and SI animals are shown in Figure 3A1. Likewise, the number of response bouts (periods of lever pressing without a 20sec pause) was significantly less in SI animals (2.8±0.3) compared to GH (3.9±0.4; t=2.36, P<0.05, t-test). These differences were evident during the remainder of the self-administration period (not shown). There was also a tendency for SI animals to complete the response requirement more quickly from the beginning of the session (4.1±0.6min) than GH rats (5.5±0.8min), but this did not reach statistical significance. These response-related data suggest that, while the SI animals were slow to initiate lever pressing at the onset of a session, animals with a history of post-weaning social isolation completed the response requirement at a faster rate and with fewer pauses compared to group-housed individuals. Thus, animals from both treatment groups completed the response requirement at similar times.
Figure 3.
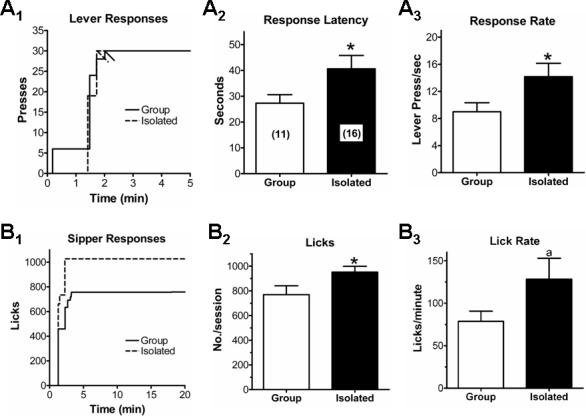
Social experience affects ethanol self-administration in a limited-access, operant paradigm. (A) Early housing experience modifies appetitive behaviors during operant self-administration. A cumulative ‘lever-press versus time’ plot (A1) for individual animals during a single session illustrate the characteristics of both housing groups. Vertical deflections indicate ‘runs’ of responding without a delay between responses of >20seconds. Diagonal hash-marks indicate the completion of the response requirement and extension of the sipper tube into the operant chamber (see text). Relative to group-housed animals, isolated rats took longer to begin lever-pressing once the operant session began (A2) but pressed at a greater rate (A3). There was no difference between treatment groups with respect to the time it took to complete the response requirement. (B) Social isolation increases ethanol consumption during operant self-administration. A cumulative ‘sipper-response versus time’ plot (B1) for individual animals from both housing groups illustrate the effects of housing experience. Vertical deflections indicate ‘runs’ of licking on the sipper tube without a delay between licks of >20seconds. Note that all the ethanol ingested during this session was consumed within a five minute window regardless of housing experience. The total number of licks on the sipper tube (B2) was significantly greater for isolation rats compared to group-housed. Similarly, ethanol consumption (g/kg) in each session was greater in isolated animals compared to group-housed (B3). Weeks one and two occurred before the first extinction trial while week three was between the first and second extinction trials. * – P<0.05, ** – P<0.01, t-test
In addition to lever press-related behaviors, several consumption-associated dependent variables were altered by the housing manipulation. For example, during the initial two-week assessment period, the total number of licks (Fig. 3B2; t=2.24, P<0.05, t-test) was significantly larger in SI animals compared to GH. The lick rate (Fig. 3B3) also tended to be greater in SI animals, but this was not significantly different between the housing groups (t=1.81, P<0.1, t-test). “Time versus lick” relationships are shown for exemplar GH and SI animals in Fig. 3B1. Note that SI animals weighed 497±10g and GH animals weighed 515±14g (t=0.95, P>0.05, t-test) at the end of the self-administration period. The effects of post-weaning isolation on ethanol consumption-related behaviors were specific for the number of licks and the amount of ethanol consumed. The number of lick-bouts (licking without a pause >20sec) during a 20min session was 4.5±0.7 in GH animals and 4.8±0.6 in SI individuals (t=0.32, P>0.05, t-test). The latency to begin licking after completing the response requirement was also not significantly different between SI (1.4±0.1sec) and GH (1.4±0.1sec) animals (t=0.32, P>0.5, t-test). Both groups of ethanol-drinking animals completed 90% of the total number of licks in less than three minutes (not shown). These data indicated that post-weaning social isolation increased the consumption of 10% ethanol but did not dramatically alter the microstructure of drinking behaviors per se.
To address whether the response-related differences reflect distinct ethanol-seeking behaviors in GH and SI animals, appetitive behaviors were also assessed using two separate, single-session ‘extinction’ trials where animals could lever press un-interrupted for a 20min session but never gained access to the ethanol sipper tube. Single-session extinction trials were performed on Wednesdays of weeks three and five of the self-administration period. Data were collapsed here across the sessions. Housing experience did not influence the number of lever ‘extinction’ lever presses, the overall rate of responding (not shown), or number of response bouts during the session (Table 2). However, like the baseline trial, the latency to begin responding at the onset of an extinction trial was significantly greater in SI animals than in GH animals (t=2.09, P<0.05, t-test). Likewise, the lever press rate during the first responding bout (initial phase of lever pressing without a pause >20sec) was significantly larger in SI rats than in GH rats (t=2.13, P<0.05, t-test). Thus, while some response-related behaviors in the extinction trial were not altered by the housing experience, those related to the initial expression of ethanol seeking (i.e. first bout size and rate) were modestly affected. Importantly, for the week of self-administration (week 4) between the two weeks that contained extinction trials, total ethanol consumption (g/kg), the number of licks, and the response latency were still significantly different SI compared to GH animals (not shown). This suggests that the experience of the first extinction session did not alter those differences in self-administration that were apparent during the initial two weeks of self-administration (see Fig. 3).
Table 2.
Extinction Responding in Group-Housed and Socially Isolated Long- Evans Ratsa
| Group Housedb | Socially Isolated | Statisticsc | |
|---|---|---|---|
| Ethanol Self-Administration | |||
| Total Lever Responses | 123.3 ± 9.1 | 127.8 ± 12.5 | n.s. |
| Response Bouts | 9.2 ± 0.9 | 9.1 ± 0.6 | n.s. |
| 1st Bout Responses | 15.7 ± 4.6 | 33.4 ± 8.9 | n.s. (P<0.1) |
| 1st Bout Response Rate (press/min) | 28.5 ±4.4 | 54.7 ± 11.4 | t=2.13, P<0.05 |
| Response Latency (sec) |
26.4 ± 6.1 |
45.2 ± 6.2 |
t=2.09, P<0.05 |
| Sucrose Self-Administration | |||
| Total Lever Responses | 141.8 ± 9.6 | 141.0 ± 20.0 | n.s. |
| Response Bouts | 4.2 ± 0.8 | 4.8 ± 0.9 | n.s. |
| 1st Bout Responses | 30.3 ± 11.9 | 24.7 ± 8.3 | n.s. |
| 1st Bout Response Rate (press/min) | 5.6 ± 0.3 | 6.1 ± 0.3 | n.s. |
| Response Latency (sec) | 40.5 ± 9.6 | 36.4 ± 9.2 | n.s. |
Extinction responding was measured with two separate individual trials separated by a week of standard self administration. Averages for individual animals were used for the group comparisons.
For the ethanol self-administration study, n=11 for the group housed animals and n=16 for the socially isolated animals (see text). For the sucrose self-administration study, n= 11 for both the group housed and socially isolated animals.
Statistical comparisons were made using Student's t-test. P<0.05 was considered significant. “n.s.” = not significant
Operant sucrose self-administration
Separate cohorts of SI (n=11) and GH animals (n=11, see Methods) were trained to self-administer a 3% sucrose solution using the same 30 lever-press response requirement and sipper-tube presentation as in the ethanol self-administration study. One animal from each group failed to learn responding to this response requirement and was excluded from the study. The results of these studies are shown in Table 3. Surprisingly, none of the response- or consumption-related differences noted in the ethanol study were present during sucrose self-administration. Similarly, there were no housing effects on response-related behaviors measured during the extinction sessions (Table 2).
Table 3.
Effects of Juvenile Housing Environment on Operant Sucrose Self-Administrationa
| Group Housedb | Socially Isolatedb | t-test | |
|---|---|---|---|
| Response Latencyc (sec) | 42.3±10.9 | 39.4±7.0 | n.s. |
| Response Rate (press/sec) | 11.5±2.0 | 9.8±1.6 | n.s. |
| Response Bouts (per session) | 3.1±0.3 | 3.7±0.5 | n.s. |
| Lick Latency (sec) | 1.4±0.2 | 1.1±0.1 | n.s. |
| Lick Rate (licks/min) | 133.7±10.5 | 138.4±9.0 | n.s. |
| Number of Licks (per session) | 2253±172 | 2367±168 | n.s. |
| Consumption (g/kg) | 1.07±0.08 | 1.10±0.07 | n.s. |
All animals were required to complete 30 lever presses to gain access to a 3% sucrose solution. When this response requirement was completed, animals had free access to a sipper tube for 20minutes (Samson & Chappell, 2001).
group housed, n=11; socially isolated, n=11
Data represents mean session data collected over the first two ‘baseline’ weeks (a total of 10 operant sessions; see text).
Two-way analysis of variance using the type of drink (10% ethanol or 3% sucrose) and housing (GH or SI) as the main effects revealed a few differences in response-related behaviors. For example, there was a significant interaction between drink and housing for the number of response bouts (P<0.05, F=5.228); but, post-hoc analysis with Bonferroni's post-test did not reveal any significant differences between the main variables. Response latency, response rate, and extinction responding were not significantly different between sucrose- and ethanol-drinkers. Not surprisingly, there were more dramatic differences with respect to consumption related behaviors. The number of licks was significantly greater in sucrose-drinking animals (P<0.0001, F=147.2) compared to ethanol drinkers regardless of housing condition (P>0.05, Bonferroni's post-test). Similarly, on a g/kg basis across the entire baseline period, rats consumed significantly more sucrose than ethanol (P<0.01, F=10.0); but, this appeared to be related specifically to differential consumption between ethanol- and sucrose-drinking GH animals (P<0.05, Bonferroni's post-test) rather than their SI counterparts (P>0.05).
Prepulse inhibition
One possible confound for both the ethanol and sucrose self-administration data is that the training experience and weeks of self-administration might act as an environmental enrichment. Since environmental enrichment has been shown to ‘reverse’ some behaviors related to post-weaning isolation syndrome (Hellemans et al., 2004), we examined prepulse inhibition in cohorts of both group-housed and socially-isolated animals. To avoid potential interactions between this measure and ethanol exposure, prepulse inhibition was measured only in sucrose-drinking animals at the end of the 5 week self-administration period. Baseline acoustic startle reactivity to a 50msec, 100dB acoustic stimulus was not significantly different between GH and SI animals (Fig. 4A; t=1.66, P>0.05, t-test). Conversely, the level of prepulse inhibition in SI animals was significantly less than GH animals at the highest intensity prepulse tested (Fig. 4B; t=2.21, P<0.05, t-test). These data suggest that the deficits in prepulse inhibition which characterize post-weaning isolation syndrome (Geyer et al., 1993) are not dramatically attenuated by adult self-administration experience.
Figure 4.
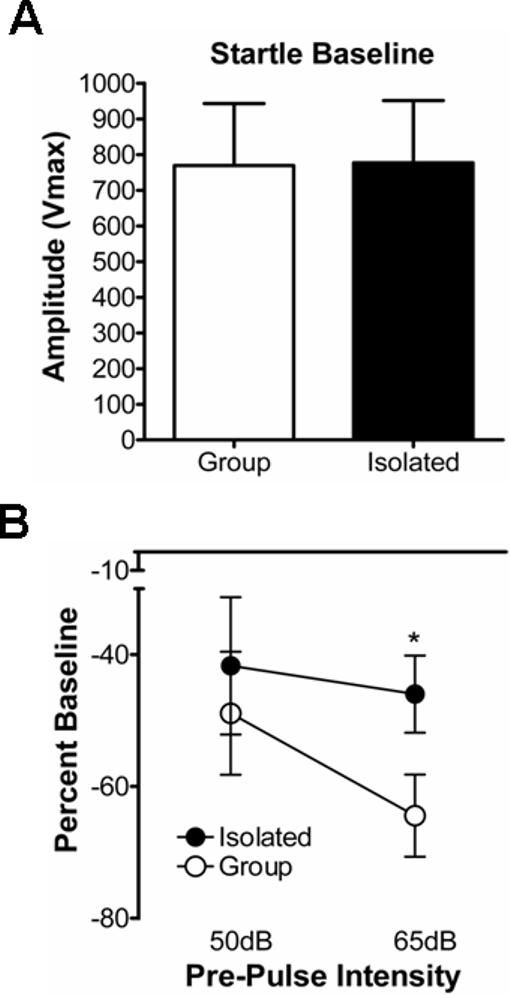
Social isolation impairs prepulse inhibition in rats with a sucrose self-administration history. (A) Baseline startle responses to an auditory stimulus were not significantly different between group- and isolation-housed animals following five weeks of operant self-administration experience. (B) Attenuation of the startle response by a pre-stimulus auditory cue was significantly less in isolated animals compared to group-housed individuals. * – P<0.05, t-test
DISCUSSION
Our results demonstrate that the post-weaning housing environment alters a number of behaviors that might increase the risk related to alcohol abuse. For example, the increased anxiety-like behavior in SI animals measured in the elevated plus maze was subsequently accompanied by increased ethanol drinking in both a continuous access, two-bottle choice paradigm in the home cage as well as a limited-access, operant self-administration procedure. Antecedent anxiety in naïve individuals is frequently seen as a risk-factor for alcoholism in humans (reviewed in (Kushner et al., 2000)) and is enhanced in some, but not all, rodent strains bred for increased alcohol preference/consumption (Colombo et al., 1995; Izidio and Ramos, 2007). We also found that early social experience modulates pre-pulse inhibition in Long-Evans rats, consistent with previous studies in other rat strains (Ehlers et al., 2007; Varty and Geyer, 1998). Our study extends previous work in selectively bred ‘P’ rats (Ehlers et al., 2007) to show that the effect is specific for ethanol (e.g. not found in sucrose-drinking animals) and generalizes to a genetically heterogeneous outbred strain of rat.
The increase in anxiety-like behaviors associated with the post-weaning environmental manipulation appears to depend upon the context in which the behaviors are measured. For example, there was little indication that SI influenced most behaviors expressed in the light-dark box. The one finding was that SI animals tended to be slower to leave the light-side of the apparatus following their initial placement. This delay in the initial egress from the light-side of the apparatus is typically seen when animals are treated with anxiolytics like benzodiazepines (Costall et al., 1989). One possible interpretation is that isolation housing produced a modest anxiolytic effect in this apparatus. However, neither the total time spent in the light-side nor the re-entry latency nor the number of light/dark transitions, all measures of anxiety-like behavior (Costall et al., 1989), differed between SI and GH animals. Importantly, across both groups, animals spent almost 40% of the time in the light-side of the light/dark apparatus. Assuming an absence of side-preference would be indicated by a 50% side-to-side distribution, neither group of animals appeared to find the light/dark box particularly anxiogenic under our assay conditions. A more parsimonious interpretation for the delay in egress latency in SI rats would be that the well-characterized sensory-motor gating deficits characteristic of these animals (Geyer et al., 1993) delays the animal's initial movement from the novel environment represented by the light-side of the apparatus into the dark-side immediately behind the animal. Similarly, in the plus maze, animals were placed in the apparatus facing an open arm; and, there was a trend for SI animals to first enter a closed arm more slowly (25.9 ± 3.7 sec) than GH animals (18.9 ± 3.4 sec) although this did not reach significance (P>0.05, t-test).
Regardless, the light/dark box and plus maze clearly provide unique measures of anxiety-like behavior (see (Griebel et al., 2000)). Indeed, SI animals expressed more prominent anxiety-like behavior in the plus maze relative to the GH group. Across both housing groups, animals spent only 20% of their time on the open arms of the plus maze apparatus. This suggests that the plus maze has more anxiogenic potential than the light-dark box in this study. These data are consistent with previous observations from our lab with this rat strain (McCool and Chappell, 2007). However, since animals were first exposed to the light/dark apparatus and then three days later tested in the plus maze, we must acknowledge that an order-effect might contribute to the plus-maze dataset. It is critical to note that the light/dark and plus maze data for the GH animals in the current study (Figure 1 and Table 1) are very similar to findings previously published by our lab (see Tables 1 & 2 in (McCool and Chappell, 2007)) using ‘adult’ male Long-Evans rats obtained commercially. In this latter study, animals were subjected to the light/dark box and plus maze on different weeks. And, since these historical data are almost identical to the data on GH animals in the current study, it is unlikely a significant order-effect would have contributed to the plus-maze dataset for this housing group. We cannot rule-out the possibility that an order-effect might be expressed solely in the SI animals. Regardless, the data are consistent with a more robust response by the SI animals to a more anxiogenic environment (e.g. the plus maze).
Importantly, the anxiety-related effects of early social experience may be strain dependent. In this study, we show increased anxiety-like elevated plus maze behavior in socially-isolated male Long-Evans rats. These data suggest that the much longer (12 week) isolation period used by another study (Hellemans et al., 2004) is not necessary to generate the anxiety-related phenotype in this strain. Indeed, the six week isolation used in the current study more precisely spans a critical period of social and sexual development in male Long-Evans rats (Meaney and Stewart, 1981). Previous work also demonstrated increased anxiety in socially isolated Wistar rats that did not generalize to isolated Fawn-hooded rats (Hall et al., 1998a). Furthermore, the anxiogenic response in Wistar animals was also dependent upon the lighting condition of the test area, with differential lighting conditions unveiling anxiogenic responses in the isolated animals from each strain. Similarly, under low-light conditions, social isolation did not produce an anxiogenic effect in either the “P” or “NP” rat lines (Ehlers et al., 2007), both derived from Wistar rats (Murphy et al., 1982). Thus, the ‘anxiogenic potential’ of a given assay may dramatically influence anxiety-related outcome measures in isolated animals. Finally, it is worth noting that both the Hall et al. and Ehlers et al. study utilized smaller groups of socially-housed animals (two/cage). Therefore, any inconsistencies among studies might also reflect different housing conditions in the group-housed animals.
As with anxiety-like behavior, the effect of social isolation on home-cage ethanol consumption may also be strain-dependent. We report here that social isolation increased both ethanol intake (g/kg) and ethanol preference in male Long-Evans rats using a continuous access, two-bottle choice paradigm yet did not alter forced (one-bottle) consumption in the same animals (Fig. 2). Since individual housing is a well-known acute stressor in rats (Armando et al., 1989; Parker and Radow, 1974), we cannot exclude the possibility that the level of ethanol intake by GH animals during the forced consumption reflects their acute response to being recently individually housed. However, the differences between housing groups with respect to ethanol consumption in the two-bottle choice paradigm are suggestive by the first day of the assay (Fig. 2A). In support of this, Hall et al (Hall et al., 1998b) likewise observed increased two-bottle, home-cage intake by Fawn-hooded and Wistar rats following early social isolation. However, in these strains, the increased ethanol consumption was found only at the highest concentration tested (16% but not 8%) and was not associated with any change in ethanol preference relative to water. Forced ethanol consumption was not tested in this study. Conversely, Ehlers et al. (Ehlers et al., 2007) recently reported that social isolation of P and NP rats increased only forced (one-bottle) ethanol consumption but not continuous-access two-bottle drinking. When these P and NP animals were given limited one-bottle access in the home cage, social isolation increased ethanol intake in the P rats, but not the NP rats. Regardless, it can be concluded that social isolation generally increases home-cage ethanol intake across several distinct rat strains. The precise mechanism may still differ across strains. For example, social isolation increased the ethanol preference ratio (relative to water) only during the two-bottle preference test in the current study.
In addition to the home-cage ethanol consumption, social isolation increased ethanol consumption in a limited-access, operant self-administration model. This model separates ethanol consumption from appetitive (operant) behaviors to allow assessment of the latter in the absence of the pharmacological effects of ethanol (Samson et al., 1999). Along these lines, we observed an increase in the latency to begin responding in the ethanol-drinking socially-isolated animals. While it is tempting to speculate that this was related to the sensory-motor gating deficits in isolated rats, it was not evident in the sucrose drinking animals regardless of housing history. In fact, group-housed ethanol-drinking animals tended to begin responding more quickly than any other group. This suggests an interaction between ethanol-reinforced responding and housing condition for this particular variable. Since there were no significant effects of isolation on single-session extinction-responding for either ethanol- or sucrose-drinking animals, it is unlikely these response-related behaviors are related exclusively to ethanol- or sucrose-seeking behavior.
Our results also suggest that there are dramatic differences between sucrose and ethanol consumption-related behaviors in group-housed and socially isolated animals. Specifically, social isolation increased ethanol consumption but not sucrose intake. This suggests that the effects of social isolation in the operant self-administration model may be specific for ethanol, at least compared to sucrose. However, our data also indicate that sucrose and ethanol engender distinct patterns of consumption. For example, the first drinking bout (first period of licking without a pause >20sec) represents 76±3% of the total number of licks in ethanol-drinking animals but only 46±4% in sucrose-drinking rats. This difference is even more impressive when one considers that the rate of consumption during this first bout was significantly slower in sucrose-drinking animals (4.9±0.5 licks/sec) compared to ethanol drinking rats (7.4±0.3 licks/sec). Neither measure was altered by the housing experience. Regardless, these data are consistent with previous reports illustrating the unique consumption patterns associated with sucrose and ethanol ingestion (Freedland et al., 2001). We must therefore conclude that the differential sensitivity of ethanol and sucrose consumption to social isolation is related to the unique mechanisms governing the intake of these distinct solutions.
Our dataset also provide an opportunity to assess the relationship between anxiety-like behavior and ethanol consumption, at least in the context of the housing manipulation. Surprisingly, correlational analysis between anxiety-related dependent variables in the plus maze and ethanol consumption in both the home cage and during self administration were suggestive of an inverse relationship between these behavioral characteristics in SI animals. For example, the Pearson correlation statistic between the open-arm time and the g/kg consumed during the two-bottle choice paradigm was r = 0.60 (R2=0.36, P<0.05). A similar trend was noted for this anxiety-related variable and the g/kg consumed during the self-administration period (r = 0.42) although this correlation only approached statistical significance (R2 = 0.18, P<0.1). Given the relatively small numbers of animals in our study, we cannot provide a definitive conclusion with respect to these relationships. However, our findings might suggest that increased antecedent anxiety resulting from early housing experience is not necessarily causally related to ethanol consumption with these models. We should emphasize that results in the literature have been inconsistent in this respect. For example, rat lines selectively bred for increased anxiety-like behavior have either increased (Izidio and Ramos, 2007) or decreased (Henniger et al., 2002) ethanol consumption in the home cage. However, rat lines selectively bred for increased ethanol consumption more consistently present with higher levels of anxiety-like behavior relative to ethanol non-preferring strains (Colombo et al., 1995; Stewart et al., 1993). Similarly, there is a positive relationship between anxiety-like behavior and ethanol consumption in out-bred Wistar rats (Spanagel et al., 1995). At the very least, these findings together suggest that the relationship between ethanol preference/consumption and anxiety-like behavior, especially when it reflects more ‘trait’ rather than ‘state’ anxiety, is complex.
In conclusion, we have shown that post-weaning social isolation increases anxiety-like behavior, disrupts pre-pulse inhibition, and increases home-cage and operant ethanol self-administration in adult male Long-Evans rats. These data extend previous results by demonstrating that line-dependent increases in home-cage ethanol consumption (Ehlers et al., 2007; Hall et al., 1998b) can be extended to outbred animals using an operant model that separates appetitive/seeking behaviors from consumption-related behaviors. Importantly, early-life housing experience increased consumption-related behaviors in this model. Since stimulus-control behaviors contribute to consumption in this model (Czachowski et al., 2001; Sharpe and Samson, 2001), the well studied sensory-motor gating deficits that characterize social isolation may interfere with neurobehavioral processes that serve to evaluate interoceptive cues associated with ethanol pharmacology and ultimately contribute to increased ethanol consumption.
Acknowledgements
We are grateful to Dr. Hank Samson for his encouragement during our pursuit of these studies. We are also indebted to Drs. Jeff Weiner and Cristine Czachowski for their helpful discussions during the early phases of the study and their constructive comments on the manuscript.
Supported by: R01 AA014445, U01 AA016671, and Project 3 of P01 AA017056
REFERENCES
- Armando I, Lemoine AP, Ferrini M, Segura ET, Barontini M. Repeated (isolation) stress increases tribulin-like activity in the rat. Cell Mol Neurobiol. 1989;9(1):115–22. doi: 10.1007/BF00711448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardo MT, Klebaur JE, Valone JM, Deaton C. Environmental enrichment decreases intravenous self-administration of amphetamine in female and male rats. Psychopharmacology (Berl) 2001;155(3):278–84. doi: 10.1007/s002130100720. [DOI] [PubMed] [Google Scholar]
- Canto-de-Souza A, Luiz Nunes-de-Souza R, Rodgers RJ. Anxiolytic-like effect of way-100635 microinfusions into the median (but not dorsal) raphe nucleus in mice exposed to the plus-maze: influence of prior test experience. Brain Res. 2002;928(1−2):50–9. doi: 10.1016/s0006-8993(01)03354-6. [DOI] [PubMed] [Google Scholar]
- Colombo G, Agabio R, Lobina C, Reali R, Zocchi A, Fadda F, Gessa GL. Sardinian alcohol-preferring rats: a genetic animal model of anxiety. Physiol Behav. 1995;57(6):1181–5. doi: 10.1016/0031-9384(94)00382-f. [DOI] [PubMed] [Google Scholar]
- Costall B, Jones BJ, Kelly ME, Naylor RJ, Tomkins DM. Exploration of mice in a black and white test box: validation as a model of anxiety. Pharmacol Biochem Behav. 1989;32(3):777–85. doi: 10.1016/0091-3057(89)90033-6. [DOI] [PubMed] [Google Scholar]
- Crawley J, Goodwin FK. Preliminary report of a simple animal behavior model for the anxiolytic effects of benzodiazepines. Pharmacol Biochem Behav. 1980;13(2):167–70. doi: 10.1016/0091-3057(80)90067-2. [DOI] [PubMed] [Google Scholar]
- Czachowski CL, Legg BH, Samson HH. Effects of acamprosate on ethanol-seeking and self-administration in the rat. Alcohol Clin Exp Res. 2001;25(3):344–50. [PubMed] [Google Scholar]
- Deehan GA, Jr., Cain ME, Kiefer SW. Differential rearing conditions alter operant responding for ethanol in outbred rats. Alcohol Clin Exp Res. 2007;31(10):1692–8. doi: 10.1111/j.1530-0277.2007.00466.x. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Walker BM, Pian JP, Roth JL, Slawecki CJ. Increased alcohol drinking in isolate-housed alcohol-preferring rats. Behav Neurosci. 2007;121(1):111–9. doi: 10.1037/0735-7044.121.1.111. [DOI] [PubMed] [Google Scholar]
- Fahlke C, Hard E, Eriksson CJ. Effects of early weaning and social isolation on subsequent alcohol intake in rats. Alcohol. 1997;14(2):175–80. doi: 10.1016/s0741-8329(96)00141-3. [DOI] [PubMed] [Google Scholar]
- Freedland CS, Sharpe AL, Samson HH, Porrino LJ. Effects of SR141716A on ethanol and sucrose self-administration. Alcohol Clin Exp Res. 2001;25(2):277–82. [PubMed] [Google Scholar]
- Geyer MA, Wilkinson LS, Humby T, Robbins TW. Isolation rearing of rats produces a deficit in prepulse inhibition of acoustic startle similar to that in schizophrenia. Biol Psychiatry. 1993;34(6):361–72. doi: 10.1016/0006-3223(93)90180-l. [DOI] [PubMed] [Google Scholar]
- Griebel G, Belzung C, Perrault G, Sanger DJ. Differences in anxiety-related behaviours and in sensitivity to diazepam in inbred and outbred strains of mice. Psychopharmacology (Berl) 2000;148(2):164–170. doi: 10.1007/s002130050038. [DOI] [PubMed] [Google Scholar]
- Hall FS, Huang S, Fong GW, Pert A, Linnoila M. Effects of isolation-rearing on locomotion, anxiety and responses to ethanol in Fawn Hooded and Wistar rats. Psychopharmacology (Berl) 1998a;139(3):203–9. doi: 10.1007/s002130050705. [DOI] [PubMed] [Google Scholar]
- Hall FS, Huang S, Fong GW, Pert A, Linnoila M. Effects of isolation-rearing on voluntary consumption of ethanol, sucrose and saccharin solutions in Fawn Hooded and Wistar rats. Psychopharmacology (Berl) 1998b;139(3):210–6. doi: 10.1007/s002130050706. [DOI] [PubMed] [Google Scholar]
- Heidbreder CA, Weiss IC, Domeney AM, Pryce C, Homberg J, Hedou G, Feldon J, Moran MC, Nelson P. Behavioral, neurochemical and endocrinological characterization of the early social isolation syndrome. Neuroscience. 2000;100(4):749–68. doi: 10.1016/s0306-4522(00)00336-5. [DOI] [PubMed] [Google Scholar]
- Hellemans KG, Benge LC, Olmstead MC. Adolescent enrichment partially reverses the social isolation syndrome. Brain Res Dev Brain Res. 2004;150(2):103–15. doi: 10.1016/j.devbrainres.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Henniger MS, Spanagel R, Wigger A, Landgraf R, Holter SM. Alcohol self-administration in two rat lines selectively bred for extremes in anxiety-related behavior. Neuropsychopharmacology. 2002;26(6):729–36. doi: 10.1016/S0893-133X(01)00408-0. [DOI] [PubMed] [Google Scholar]
- Howes SR, Dalley JW, Morrison CH, Robbins TW, Everitt BJ. Leftward shift in the acquisition of cocaine self-administration in isolation-reared rats: relationship to extracellular levels of dopamine, serotonin and glutamate in the nucleus accumbens and amygdala-striatal FOS expression. Psychopharmacology (Berl) 2000;151(1):55–63. doi: 10.1007/s002130000451. [DOI] [PubMed] [Google Scholar]
- Izidio GS, Ramos A. Positive association between ethanol consumption and anxiety-related behaviors in two selected rat lines. Alcohol. 2007;41(7):517–24. doi: 10.1016/j.alcohol.2007.07.008. [DOI] [PubMed] [Google Scholar]
- Kushner MG, Abrams K, Borchardt C. The relationship between anxiety disorders and alcohol use disorders: a review of major perspectives and findings. Clin Psychol Rev. 2000;20(2):149–71. doi: 10.1016/s0272-7358(99)00027-6. [DOI] [PubMed] [Google Scholar]
- Larsson F, Winblad B, Mohammed AH. Psychological stress and environmental adaptation in enriched vs. impoverished housed rats. Pharmacol Biochem Behav. 2002;73(1):193–207. doi: 10.1016/s0091-3057(02)00782-7. [DOI] [PubMed] [Google Scholar]
- Lodge DJ, Lawrence AJ. The CRF1 receptor antagonist antalarmin reduces volitional ethanol consumption in isolation-reared fawn-hooded rats. Neuroscience. 2003;117(2):243–7. doi: 10.1016/s0306-4522(02)00793-5. [DOI] [PubMed] [Google Scholar]
- McCool BA, Chappell A. Strychnine and taurine modulation of amygdala-associated anxiety-like behavior is ‘state’ dependent. Behav Brain Res. 2007;178(1):70–81. doi: 10.1016/j.bbr.2006.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaney MJ, Stewart J. A descriptive study of social development in the rat (Rattus norvegicus). Anim Behav. 1981;29:34–45. [Google Scholar]
- Murphy JM, McBride WJ, Lumeng L, Li TK. Regional brain levels of monoamines in alcohol-preferring and -nonpreferring lines of rats. Pharmacol Biochem Behav. 1982;16(1):145–9. doi: 10.1016/0091-3057(82)90026-0. [DOI] [PubMed] [Google Scholar]
- Parker LF, Radow BL. Isolation stress and volitional ethanol consumption in the rat. Physiol Behav. 1974;12(1):1–3. doi: 10.1016/0031-9384(74)90060-2. [DOI] [PubMed] [Google Scholar]
- Pellow S, Chopin P, File SE, Briley M. Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods. 1985;14(3):149–67. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- Samson HH. Initiation of ethanol reinforcement using a sucrose-substitution procedure in food- and water-sated rats. Alcohol Clin Exp Res. 1986;10(4):436–42. doi: 10.1111/j.1530-0277.1986.tb05120.x. [DOI] [PubMed] [Google Scholar]
- Samson HH, Chappell A, Czachowski C, Sharpe A. Measuring ethanol-seeking behavior: the effect of using repeated extinction trials. Alcohol. 2001;24(3):205–9. doi: 10.1016/s0741-8329(01)00157-4. [DOI] [PubMed] [Google Scholar]
- Samson HH, Sharpe AL, Denning C. Initiation of ethanol self-administration in the rat using sucrose substitution in a sipper-tube procedure. Psychopharmacology (Berl) 1999;147(3):274–9. doi: 10.1007/s002130051167. [DOI] [PubMed] [Google Scholar]
- Samson HH, Slawecki CJ, Sharpe AL, Chappell A. Appetitive and consummatory behaviors in the control of ethanol consumption: a measure of ethanol seeking behavior. Alcohol Clin Exp Res. 1998;22(8):1783–7. [PubMed] [Google Scholar]
- Sharpe AL, Samson HH. Effect of naloxone on appetitive and consummatory phases of ethanol self-administration. Alcohol Clin Exp Res. 2001;25(7):1006–11. [PubMed] [Google Scholar]
- Slawecki CJ, Roth J. Neurokinin type-3 receptor stimulation impairs ethanol-associated appetitive behavior in Wistar rats. Alcohol Clin Exp Res. 2003;27(12):1962–70. doi: 10.1097/01.ALC.0000102412.53561.C6. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Montkowski A, Allingham K, Stohr T, Shoaib M, Holsboer F, Landgraf R. Anxiety: a potential predictor of vulnerability to the initiation of ethanol self-administration in rats. Psychopharmacology (Berl) 1995;122(4):369–73. doi: 10.1007/BF02246268. [DOI] [PubMed] [Google Scholar]
- Stewart RB, Gatto GJ, Lumeng L, Li TK, Murphy JM. Comparison of alcohol-preferring (P) and nonpreferring (NP) rats on tests of anxiety and for the anxiolytic effects of ethanol. Alcohol. 1993;10(1):1–10. doi: 10.1016/0741-8329(93)90046-q. [DOI] [PubMed] [Google Scholar]
- Varty GB, Geyer MA. Effects of isolation rearing on startle reactivity, habituation, and prepulse inhibition in male Lewis, Sprague-Dawley, and Fischer F344 rats. Behav Neurosci. 1998;112(6):1450–7. doi: 10.1037//0735-7044.112.6.1450. [DOI] [PubMed] [Google Scholar]
- Wongwitdecha N, Marsden CA. Effect of social isolation on the reinforcing properties of morphine in the conditioned place preference test. Pharmacol Biochem Behav. 1996;53(3):531–4. doi: 10.1016/0091-3057(95)02046-2. [DOI] [PubMed] [Google Scholar]
- Wright IK, Upton N, Marsden CA. Resocialisation of isolation-reared rats does not alter their anxiogenic profile on the elevated X-maze model of anxiety. Physiol Behav. 1991;50(6):1129–32. doi: 10.1016/0031-9384(91)90572-6. [DOI] [PubMed] [Google Scholar]


