Abstract
Objectives
Video-based delivery of HIV pre-test information might assist in streamlining HIV screening and testing efforts in the emergency department (ED). The objectives of this study were to determine if the video “Do you know about rapid HIV testing?” is an acceptable alternative to an in-person information session on rapid HIV pre-test information, in regards to comprehension of rapid HIV pre-test fundamentals; and to identify patients who might have difficulties in comprehending pre-test information.
Methods
This was a non-inferiority trial of 574 participants in an ED opt-in rapid HIV screening program who were randomly assigned to receive identical pre-test information from either an animated and live-action 9.5-minute video, or an in-person information session. Pre-test information comprehension was assessed using a questionnaire. The video would be accepted as not inferior to the in-person information session if the 95% confidence interval (CI) of the difference (Δ) in mean scores on the questionnaire between the two information groups was less than a 10% decrease in the in-person information session arm's mean score. Linear regression models were constructed to identify patients with lower mean scores based upon study arm assignment, demographic characteristics, and history of prior HIV testing.
Results
The questionnaire mean scores were 20.1 (95% CI = 19.7 to 20.5) for the video arm and 20.8 (95% CI = 20.4 to 21.2) for the in-person information session arm. The difference in mean scores compared to the mean score for the in-person information session met the non-inferiority criterion for this investigation (Δ = 0.68; 95% CI = 0.18 to 1.26). In a multivariable linear regression model, Blacks/African Americans, Hispanics, and those with Medicare and Medicaid insurance exhibited slightly lower mean scores, regardless of the pre-test information delivery format. There was a strong relationship between fewer years of formal education and lower mean scores on the questionnaire. Age, gender, type of insurance, partner/marital status, and history of prior HIV testing were not predictive of scores on the questionnaire.
Conclusions
In terms of patient comprehension of rapid HIV pre-test information fundamentals, the video was an acceptable substitute to pre-test information delivered by an HIV test counselor. Both the video and in-person information session were less effective in providing pre-test information for patients with fewer years of formal education.
Keywords: HIV, videotape, emergency services, counseling/methods, HIV testing
INTRODUCTION
The Centers for Disease Control and Prevention (CDC) and emergency medicine (EM) clinicians have called for expanded HIV screening and diagnostic testing in U.S. emergency departments (EDs).1-4 The support for this expansion comes from studies showing that some U.S. EDs administer medical care to persons at high risk for HIV,5,6 that the prevalence of HIV in some EDs is higher than at other settings in the surrounding communities,7-12 and that EDs can successfully conduct HIV screening programs, identify HIV-infected patients, and link them to definitive care.13-19
The provision of HIV pre-test information to patients in a uniform, efficient, and effective manner poses a challenge to the implementation of widespread ED-based HIV screening. As recommended by the CDC, HIV pre-test information, as opposed to HIV prevention or risk-reduction counseling, is information about the definition, nature, transmission, and prevention of HIV and AIDS; the benefits and potential adverse consequences of HIV testing; and the interpretation of HIV test results.20 Although the CDC currently recommends that HIV pre-test information can be delivered orally or in writing to test receipients,1 video might be a useful alternative. Video provides uniform information in a consistent manner and might be more efficient for use in the ED setting where staff demands are high and the delivery of oral pre-test information might be suboptimal. In addition, because of the ability of video to present and highlight information in oral, textual, and graphical forms, video might be more effective than oral presentations or written materials.
In this study, we evaluated the effectiveness of the video “Do you know about rapid HIV testing?” in an ED-based opt-in, rapid HIV screening program. The primary objective of this investigation was to determine in a randomized, controlled, non-inferiority trial, if the video is an adequate substitute for an in-person information session in regards to patient comprehension of rapid HIV pre-test information fundamentals. The non-inferiority trial was intended to determine if the video was an equivalent method of delivering HIV pre-test information as assessed by how well patients understood the information presented to them. This investigation followed a pilot study that suggested that patient comprehension of rapid HIV pre-test fundamentals was equivalent for those who watched this video compared to those who underwent an in-person information session with an HIV test counselor.21 In an exploratory analysis, the investigators also aimed to detect topics from the content of the pre-test information that patients did not understand well, whether presented by the video or through an in-person information session. The secondary objective was to identify patients who had greater difficulties in understanding the information presented to them via either information delivery method, based upon their demographic characteristics and HIV testing history.
METHODS
Study Design
From July 2005 to July 2006, 18 to 55-year-old ED patients with a subcritical illness or injury were randomly selected for possible inclusion in an opt-in, rapid HIV screening program. The hospital institutional review board approved the study.
Study Setting and Population
The HIV screening program and this trial occurred at an urban, academic/teaching, not-for-profit, adult ED in New England that has over 95,000 adult patient visits annually. At the time of this investigation, there were no HIV screening programs being conducted at this ED.
A random sample of approximately 70% of the patients in the ED during each shift was assessed for possible inclusion in the HIV screening program. With the assistance of a computerized random number generator program, the research assistants (RAs) selected a seven-digit sequence of integers in advance of each shift. These integers were used to randomly select patients to assess according to the terminal digit of the patients' medical record number. The medical record number is assigned sequentially and permanently to patients when they first enter the hospital system in the inpatient, outpatient, or ED setting. The number is not associated with their demographic characteristics or the nature of their medical condition or illness. During each shift, one RA conducted a primary assessment of the ED paper medical records of the patients in the ambulatory and urgent care areas of the ED whose terminal medical digit had been randomly selected for that shift. For those patients whose ED medical record indicated that they might be eligible for the HIV screening program, the RA performed an in-person secondary assessment to confirm their eligibility. Eligible patients were English-speaking, 18 to 55 years old, were not presenting for a psychiatric illness, were not prison inmates, not pregnant, not critically ill or injured, were not known to be HIV infected, were not in an HIV vaccine study, and did not have a physical disability or mental impairment that prevented them from participating in the screening program. Patients eligible for the screening program were asked if they would like to be tested for HIV in the ED using a rapid HIV test. Those who agreed to undergo HIV testing were then invited to enroll in this randomized, controlled, non-inferiority trial. Participants completed this trial prior to being tested for HIV.
Study Protocol
As described in detail elsewhere,22 the HIV screening program was conducted on randomly selected dates and eight-hour shifts, 24 hours per day, 7 days per week, except for 8 hospital holidays. According to the results of this screening program, approximately 2% of the 18 to 55 year old ED patients are known to be HIV infected.22 Trained research assistants (RAs) randomly selected patients in the ambulatory care and urgent care areas of the ED to be assessed for possible inclusion in the opt-in, rapid HIV screening program. The HIV screening program was non-targeted or universal, such that patients were not tested for HIV based upon their demographic characteristics, risk profiles, or any other criterion (except study inclusion criteria, as described above). The program used an opt-in approach in that participants were asked if they wanted to be tested for HIV instead of being informed that they would be tested for HIV, unless they declined (opt-out).
Video and Questionnaire Development and Composition
The development and content of the video “Do you know about rapid HIV testing?” and the “Rapid HIV pre-test information comprehension” questionnaire employed in this study have been described in detail previously.21 Briefy, the video development included reviewing videos on HIV and HIV testing, composing a script that addressed CDC-recommended components for HIV pre-test information,20 creating animated characters and images for the video, selecting live-action sequences to accompany the script, producing the video, conducting cognitive assessments of the video through intensive patient interviews of ED patients, and performing formal testing of the video in a pilot study. The questionnaire development included reviewing surveys assessing knowledge of HIV and HIV testing, drafting the questionnaire, evaluating the discriminatory power of the questionnaire through pilot testing, conducting cognitive-based assessments of the questionnaire through intensive patient interviews of ED patients, and performing a pilot study. The video and the survey questions were modified based upon the results of these evaluations.
The animated and live-action 9.5-minute video “Do you know about rapid HIV testing?” includes information in five subject areas: CDC-recommended information on the definition and nature of HIV and AIDS; HIV transmission, prevention, and testing methods20; and information on rapid HIV testing procedures and the meaning of test results using the OraQuick rapid HIV test (OraSure Technologies, Bethlehem, PA). The “Rapid HIV pre-test information comprehension” questionnaire is comprised of 26 questions, each in the form of a statement that cover these five subject areas (Table 2). The possible responses for each question are “True,” “False,” and “I don't know.” Each correctly answered item on the questionnaire is scored as one point out of a total of 26 points for the entire questionnaire. The questionnaire and survey instructions were at a Flesch-Kincaid seventh grade reading comprehension level.
Table 2.
Percentage of Correct and “I Don't Know” Responses on the Rapid HIV Testing Comprehension Questionnain
| Correct responses |
Comparison of proportions of correct answers |
“I don't know” responses |
Comparison of proportions of “I don't know” responses |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| All participants | In-person arm |
Video arm |
Δ in% | p-value | All participants | In-person arm |
Video arm |
Δin% | p-value | |
| n=674 | n=304 | n=270 | n=674 | n=304 | n=270 | |||||
| HIV/AIDS Definition | % | % | % | Δ | p≤ | % | % | % | Δ | p≤ |
| Q1 If you were HIV infected, current drug treatments would let you live longer. (T | 90.2 | 90.5 | 90.0 | 0.5 | 0.85 | 2.8 | 3.3 | 2.2 | 1.1 | 0.44 |
| Q2 People can get AIDS without getting HIV. (F) | 79.1 | 76.3 | 82.2 | -5.9 | 0.08 | 5.6 | 7.9 | 3.0 | 4.9 | 0.01 |
| Q3 Being infected with HIV does not mean you have AIDS. (T | 86.8 | 87.8 | 85.6 | 2.2 | 0.42 | 1.9 | 2.0 | 1.9 | 0.1 | 0.92 |
| Q4 A person can be infected with HIV for 5 years or more without getting AIDS. (T | 91.3 | 93.1 | 89.3 | 3.8 | 0.10 | 4.0 | 4.3 | 3.7 | 0.6 | 0.35 |
| HIV Transmission | ||||||||||
| Q5 A person cannot get HIV by donating blood. (T) | 58.2 | 58.6 | 57.8 | 0.8 | 0.85 | 5.4 | 5.3 | 5.6 | -0.3 | 0.88 |
| Q6 A woman with HIV can give HIV to her baby during breastfeeding. (T) | 97.7 | 99.3 | 95.9 | 3.4 | 0.01 | 1.2 | 0.0 | 2.6 | -2.6 | 0.00 |
| Q7 If someone gets HIV through needle sharing, that person can only spread HIV by sharing needles with other peopl | 78.1 | 78.0 | 78.1 | -0.1 | 0.96 | 0.5 | 0.0 | 1.1 | -1.1 | 0.07 |
| Q8 Coins, such as quarters or nickels, can carry HIV. (F) | 93.9 | 94.1 | 93.7 | 0.4 | 0.86 | 3.1 | 2.3 | 4.1 | -1.8 | 0.22 |
| Q9 A person cannot get HIV by putting their tongue in the mouth of someone who has HIV. (T) | 77.5 | 80.3 | 74.4 | 5.9 | 0.10 | 1.9 | 1.3 | 2.6 | -1.3 | 0.27 |
| HIV Prevention | ||||||||||
| Q10 HIV is destroyed by bleach. (T) | 59.9 | 70.4 | 48.2 | 22.2 | 0.00 | 0.2 | 0.3 | 0.0 | 0.3 | 0.35 |
| Q11 If you use injection drugs, the only way to prevent getting HIV is to quit using them. (F) | 62.5 | 65.1 | 59.6 | 5.5 | 0.17 | 1.6 | 1.3 | 1.9 | -0.6 | 0.61 |
| Q12 Wearing insect repellant to keep away mosquitoes will help prevent you from getting HIV. (F) | 90.1 | 88.2 | 92.2 | -4.0 | 0.10 | 4.2 | 4.3 | 4.1 | 0.2 | 0.90 |
| Q13 Not having sex is the only way to reduce your risk of getting HIV. (F) | 79.3 | 84.2 | 73.7 | 10.5 | 0.00 | 0.2 | 0.3 | 0.0 | 0.3 | 0.35 |
| Q14 You can prevent getting HIV after sex by washing your genitals or private parts. (F) | 94.8 | 95.1 | 94.4 | 0.7 | 0.74 | 0.9 | 1.0 | 0.7 | 0.3 | 0.75 |
| HIV Testing | ||||||||||
| Q15 HIV makes antibodies which harm a person's body. (F) | 32.4 | 37.8 | 26.3 | 11.5 | 0.00 | 4.2 | 4.6 | 3.7 | 0.9 | 0.59 |
| Q16 Having blood drawn for an HIV test will make you anemic. (F) | 93.4 | 94.8 | 92.1 | 2.7 | 0.19 | 5.8 | 7.2 | 4.1 | 3.1 | 0.10 |
| Q17 The HIV antibody test will help strengthen your antibodies to keep you from getting infected with HIV. (F) | 80.7 | 79.9 | 81.5 | -1.6 | 0.64 | 3.0 | 3.6 | 2.2 | 1.4 | 0.32 |
| Q18 If you were infected with HIV one week ago, your HIV test will be negative. (T) | 78.4 | 66.8 | 69.6 | -2.8 | 0.54 | 1.6 | 1.6 | 1.5 | 0.1 | 0.88 |
| Q19 The HIV antibody test will not tell you if you have AIDS. (T) | 69.7 | 69.4 | 70.0 | -0.6 | 0.88 | 7.3 | 8.2 | 6.3 | 1.9 | 0.38 |
| Q20 If your HIV test is negative, it must be repeated within a week to confirm the results. (F) | 68.1 | 79.3 | 77.4 | 1.9 | 0.92 | 5.4 | 6.9 | 3.7 | 3.2 | 0.09 |
| Rapid HIV Testing | ||||||||||
| Q21 It takes one to two days to perform a rapid HIV test. (F) | 90.2 | 89.8 | 90.7 | -0.9 | 0.71 | 2.1 | 3.3 | 0.7 | 2.6 | 0.03 |
| Q22 An invalid rapid HIV test result means you've been infected with HIV for fewer than 3 months. (F) | 83.3 | 84.5 | 81.9 | 2.6 | 0.39 | 6.1 | 8.2 | 3.7 | 4.5 | 0.02 |
| Q23 If your rapid HIV test is positive, then you will need a test to confirm this. (T) | 97.9 | 97.7 | 98.2 | -0.5 | 0.71 | 0.5 | 0.7 | 0.4 | 0.3 | 0.63 |
| Q24 The rapid HIV test with OraQuick uses a sample of your urine. (F) | 90.1 | 89.5 | 90.7 | -1.2 | 0.61 | 6.5 | 7.9 | 4.8 | 3.1 | 0.13 |
| Q25 A needle can be used to take blood from your arm for the OraQuick rapid HIV test. (T) | 48.3 | 54.3 | 41.5 | 12.8 | 0.00 | 4.2 | 5.9 | 2.2 | 3.7 | 0.03 |
| Q26 Even if your rapid HIV test is positive, you may not have HIV. (T) | 74.4 | 76.3 | 72.2 | 4.1 | 0.26 | 5.2 | 5.6 | 4.8 | 0.8 | 0.68 |
Rapid HIV pre-test information
Participants were assigned to one of two study arms (video vs. in-person information session) using their terminal medical record number digit. Patients with an odd terminal medical record number digit were assigned to the video arm, and those with an even terminal medical record number digit were assigned to the in-person information session arm.
The content of the video and in-person information session was the same and has been described elsewhere.21 Study participants watched the video or received the in-person information session in their ED examination room prior to being tested for HIV. Participants watched the video on a hand-held tablet personal computer and listened to the audio using headphones. The RAs monitored the video usage and were available to assist as needed. The RAs were not permitted to clarify or answer any questions about the video's content.
The RAs presented the in-person information session extemporaneously according to a defined script and did not use visual aids. As per the defined script, the RA asked each participant an introductory question about each pre-test information topic, acknowledged the participant's response, and then gave a short discourse on each topic. The RAs provided the same information to all participants regardless of the participants' responses to the introductory questions for each topic. During the trial, the median time elapsed to deliver the in-person information session was 10 minutes. The RAs underwent extensive training that included over 40 hours of mock interviews to ensure that they correctly presented the HIV pre-test information according to the defined script. The principal investigator observed the RAs directly during a patient encounter twice monthly and critiqued their performances. Deviations from the protocol and script were corrected. The RAs were certified by the state to perform HIV counseling and testing. In the state where this study was performed, test providers are required to provide all HIV test recipients with written information about HIV and HIV testing. The RAs provided this written information to all trial participants after they were tested for HIV, and hence after they completed this trial.
Data collection
The RAs administered the “Rapid HIV pre-test information comprehension” questionnaire to participants in the ED examining rooms after the in-person information session or viewing of the video. During the study consent process, participants were informed that they would be administered a questionnaire regarding their comprehension of the pre-test information they received as part of the study. RAs read the survey instructions and questions aloud to the participants. The RAs could repeat questions and instructions as needed, but were not permitted to clarify questions or terms. The study data were recorded in a QDS (Nova Research, Bethesda, MD) database. All entries were made in duplicate with immediate data entry verification. Following the questionnaire, the RAs debriefed participants about the questions and provided correct responses and additional information as needed. Participants then underwent rapid HIV testing and were offered personalized, HIV risk-reduction counseling by the RAs.
Data Analysis
All analyses were performed using STATA 9.2 (STATA Corporation, College Station, TX). Patients who dropped out of the study were not included in the analyses. Summary statistics on enrollment, patient demography, and HIV testing history were calculated for all patients and by study arm random assignment (video vs. in-person information session). The study arms were compared using Wilcoxon rank-sum test for continuous variables, and Pearson's χ2 test for categorical variables. Differences at the α = 0.05 level were considered significant.
We compared the mean scores on the “Rapid HIV testing comprehension questionnaire” by study arm to address the primary objective of determining if the video is not inferior to the in-person information session. We used the 95% confidence interval (CI) approach to assess non-inferiority as recommended by Piaggio, et al.23 In the absence of an established standard for this assessment, we chose a value of 10% as an acceptable difference between mean scores. The 10% acceptable difference criterion was based upon the results of our pilot study,21 the work by Calderon, et al. with a standard HIV testing video,24 and the typical values for “non-inferiority” used in other medical trials. By this a priori criterion, the video would not be inferior to the in-person information session if the 95% CI for the difference in means between the two groups was less than or equal to a 10% reduction in the in-person information session arm mean score.
In an exploratory analysis, we sought to identify rapid HIV pre-test information concepts that were not well understood by participants, or were not well presented by either or both information delivery methods. For the exploratory analysis, we calculated the percentage of correct responses and the percentage of “I don't know” responses for each item on the questionnaire. These percentages were calculated for all participants and by study arm assignment. For each item, the absolute differences between study arms were then calculated for the percentages of correct responses and “I don't know” responses. Two-sample tests for binomial proportions were used to compare study arms and identify questions that yielded relatively larger proportions of correct responses and “I don't know” responses. A significance level of α = 0.05 was used. To be maximally sensitive to potential differences between study arms, we did not adjust for multiple comparisons in the exploratory analysis.
Linear regression modeling was employed for satisfying the secondary objective of identifying patients with difficulties in comprehending rapid HIV pre-test fundamentals, based upon their demographic characteristics (age, gender, ethnicity/race, marital or partner status, insurance status, years of formal education), HIV testing history (history of ever being previously tested for HIV, time since last HIV test), study arm assignment, and the RA assigned to deliver the pre-test information. The outcome for the linear regression models was the mean score on the questionnaire. β-coefficients with corresponding 95% CIs were estimated. A preliminary multivariable model (Model 1) included all covariates from the univariable analyses that were significant at the α = 0.05 level. A final multivariable model (Model 2) was chosen that included only covariates from Model 1 that were significant at the α = 0.05 level. Two- and three-way interactions among significant variables were considered.
Using the final model (Model 2), predicted mean scores on the questionnaire were calculated and plotted using combinations of covariates that were statistically associated with lesser or greater mean scores. The purposes of these predictions were to determine what the scores on the questionnaire are expected to be for specified groups of ED patients, and illustrate which covariates have the strongest impact on questionnaire scores. The predicted scores take into account the influence of covariates shown to be important in the multivariable linear regression model.
RESULTS
Demographics and HIV Testing History of the Trial Participants
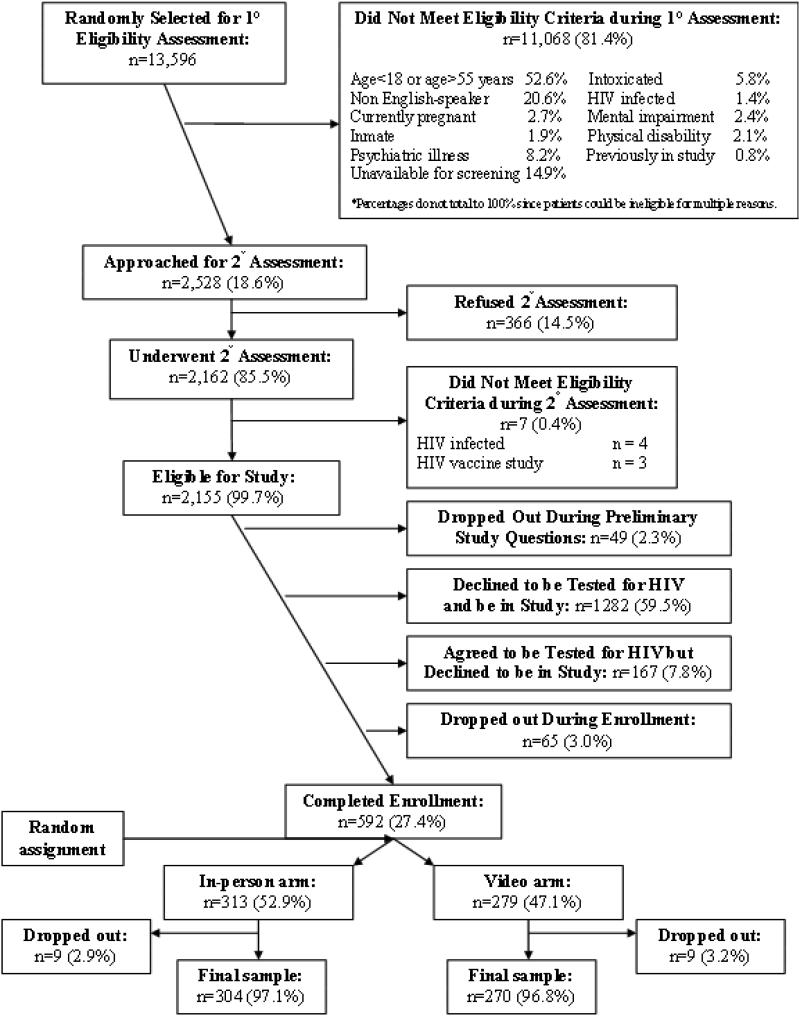
Figure 1 depicts the enrollment schema for the opt-in, rapid HIV screening program and the non-inferiority trial. Of 592 patients initially enrolled in the non-inferiority trial, 3.0% dropped-out. The chief reason for leaving the study was discharge from the ED prior to completing the study. Table 1 provides the demographic characteristics and the HIV testing histories of the 574 trial participants. The study arms did not differ by demographic characteristics or by HIV testing history. Random assignment using the medical record number, although unbiased, did not produce equal size study arms. Given the large sample size, there was no loss of power due to the imbalance.
Figure 1.
Table 1.
Demographic and HIV Testing History Profiles
| All participants |
In-person informational session arm |
Video arm |
p- value |
|
|---|---|---|---|---|
| n=574 | n=304 | n=270 | p≤ | |
| Median age, years (Range) | 30 (18-55) % |
30 (18-54) % |
29 (18-55) % |
0.45 |
| Gender | 0.14 | |||
| Female | 54.2 | 51.3 | 57.4 | |
| Male | 45.8 | 48.7 | 42.6 | |
| Ethnicity/Race | 0.24 | |||
| Black/African American | 19.9 | 19.7 | 20.0 | |
| Hispanic | 14.1 | 15.8 | 12.2 | |
| White | 64.1 | 63.5 | 64.8 | |
| Other | 1.9 | 1.0 | 3.0 | |
| Partner status | 0.07 | |||
| Single/never married | 48.4 | 43.4 | 54.1 | |
| Married | 18.6 | 20.7 | 16.3 | |
| Divorced/Separated/Widowed | 17.8 | 20.1 | 15.2 | |
| Unmarried couple | 15.2 | 15.8 | 14.4 | |
| Insurance status | 0.35 | |||
| Private | 36.9 | 36.2 | 37.8 | |
| Governmental | 35.7 | 33.9 | 37.8 | |
| Private/Governmental | 1.9 | 1.6 | 2.2 | |
| None | 25.5 | 28.3 | 22.2 | |
| Years of education | 0.35 | |||
| Grades 1-8 | 3.8 | 3.6 | 4.1 | |
| Grades 9-11 | 22.7 | 22.1 | 23.3 | |
| Grade 12 or equivalent | 34.2 | 34.5 | 33.7 | |
| College 1-3 years | 27.3 | 25.3 | 29.6 | |
| College 4 years | 12.0 | 14.5 | 9.3 | |
| Ever tested for HIV* | 0.93 | |||
| Previously HIV tested | 62.4 | 63.2 | 61.5 | |
| Never tested for HIV | 36.2 | 35.5 | 37.0 | |
| Unsure if ever tested | 1.4 | 1.3 | 1.5 | |
| Time elapsed since last HIV test* | n=357 | 0.19 | ||
| > 5 years ago | 19.6 | 21.9 | 17.0 | |
| >2 years but ≤5 years | 19.0 | 15.1 | 23.6 | |
| >1 year but ≤2 years | 17.1 | 19.3 | 14.6 | |
| >6 months but ≤1 year | 21.6 | 22.4 | 20.6 | |
| ≤6 months | 22.7 | 21.3 | 24.2 |
Excludes persons tested for HIV through blood donations
Comparison of Mean Scores on the “Rapid HIV Testing Comprehension Questionnaire”
The mean scores on the questionnaire were 20.1 (95% CI = 19.7 to 20.5) for the video arm and 20.8 (95% CI = 20.4 to 21.2) for the in-person information session arm. The 95% CI of the difference in mean scores between the two study arms (Δ = 0.68; 95% CI = 0.18 to 1.26) satisfied the non-inferiority criterion for the trial. In other words, the 95% CI of the difference was less than a 10% reduction in the mean score of the in-person information session arm (< 2.08). The absolute difference between the means of the two study arms of 0.68 indicates that the mean scores differed on average by less than one correctly answered question.
Responses to the “Rapid HIV Testing Comprehension Questionnaire”
Table 2 provides the results of the exploratory analysis identifying rapid HIV pre-test information concepts that appeared to be less understood by all participants, or were potentially less well presented through the in-person information session, the video, or both methods. As shown in Table 2, there were five questions that were correctly answered by fewer than 75% of all participants (Table 2: Q5, Q10, Q11, Q15, and Q25) and two by fewer than 50% of all participants (Q15 and Q25). There were eight questions for which more than 5% of all participants chose “I don't know” as an answer (Q2, Q5, Q16, Q19, Q20, Q22, Q24, and Q26).
As shown by the results of the two-sample tests of binomial proportions, there were five questions for which the in-person information session arm had a higher proportion of correct responses. The absolute difference in proportions of correctly answered questions was >10 percentage points for four of these questions (Q10, Q13, Q15, and Q25). More participants in the in-person information session than in the video arm answered “I don't know” to questions Q2, Q21, Q22, and Q25. More participants in the video arm answered Q6 as “I don't know” than the in-person information session arm.
Predictors of Mean Scores on the “Rapid HIV Testing Comprehension Questionnaire”
Table 3 provides the results of the linear regression analyses that aimed to identify demographic characteristics and HIV testing history factors that were predictive of lower mean scores on the questionnaire. The univariable analyses indicated that mean scores did not differ by age, gender, RA, or time elapsed since the participant's last HIV test (for those previously tested for HIV). As shown in the preliminary multivariable model (Model 1), marital or partner status and a history of previously being tested for HIV were not predictive of scores on the questionnaire.
Table 3.
Predictors of Higher Total Mean Scores on the Rapid HIV Testing Comprehension Questionnaire
| Univariable Models |
Multivariable Model 1 |
Multivariable Model 2 |
|
|---|---|---|---|
| n=565 | β (95% CI) | β (95% CI) | β (95% CI) |
| Age groups (years) | |||
| 18-24 | Reference | ||
| 25-29 | 0.23 (-0.63, 1.09) | ||
| 30-34 | 0.14 (-0.78, 1.07) | ||
| 35-39 | -0.20 (-1.20, 0.80) | ||
| 40-44 | -0.87 (-1.90, 0.16) | ||
| 45-49 | -0.56 (-1.72, 0.61) | ||
| 50-55 | -0.46 (-1.76, 0.84) | ||
| Gender | |||
| Male | Reference | ||
| Female | 0.07 (-0.51, 0.66) | ||
| Ethnicity/Race | |||
| White | Reference | Reference | Reference |
| Black/African American | -1.28 (-2.01, -0.55) | -0.79 (-1.49, -0.09) | -0.77 (-1.45, -0.09) |
| Hispanic | -1.64 (-2.49, -0.80) | -1.19 (-1.98,-0.39) | -1.19 (-1.98,-0.40) |
| Other | -2.17 (-4.24, -0.09) | -0.87 (-2.82, 1.08) | -0.84 (-2.78, 1.10) |
| Partner status | |||
| Married | Reference | Reference | |
| Divorced/Widowed/Separated | -1.24 (-2.20, -0.28) | -0.53 (-1.43, 0.37) | |
| Single/Never married | -1.24 (-2.03, -0.44) | -0.22 (-0.98, 0.54) | |
| Unmarried couple | -1.06 (-2.06, -0.06) | 0.02 (-0.92, 0.97) | |
| Insurance status | |||
| Private | Reference | Reference | Reference |
| Governmental | -2.39 (-3.04, -1.73) | -1.06 (-1.75,-0.37) | -1.12 (-1.80,-0.44) |
| Private/Governmental | -0.58 (-2.63,1.47) | 0.36 (-1.59, 2.32) | 0.36 (-1.59, 2.31) |
| None | -1.73 (-2.45,-1.01) | -0.55 (-1.30, 0.21) | -0.62 (-1.36,0.11) |
| Years of education | |||
| Grades 1-8 | Reference | Reference | Reference |
| Grades 9-11 | 2.09 (0.63, 3.56) | 1.94 (0.48,3.41) | 1.94 (0.48,3.39) |
| Grade 12 or equivalent | 3.47 (2.04, 4.89) | 3.01 (1.57, 4.46) | 2.99 (1.55, 4.42) |
| College 1-3 years | 4.78 (3.33, 6.22) | 4.16 (2.68, 5.63) | 4.13 (2.67, 5.60) |
| College 4 years | 5.96 (4.41, 7.52) | 4.94 (3.31, 6.58) | 4.90 (3.30, 6.50) |
| Research assistant | |||
| RA1 | Reference | ||
| RA 2 | 0.67 (-0.24, 1.58) | ||
| RA3 | 0.78 (-0.25, 1.81) | ||
| RA4 | 0.37 (-0.56, 1.31) | ||
| RA5 | 0.99 (0.15, 1.83) | ||
| Study arm | |||
| Video | Reference | Reference | Reference |
| In-person informational session | 0.73 (0.14, 1.31) | 0.65 (0.12, 1.18) | 0.65 (0.12-1.18) |
| Ever tested for HIV* | |||
| Never tested for HIV | Reference | Reference | |
| Previously HIV tested | -0.6 (-1.21, -0.00) | 0.02 (-0.55, 0.58) | |
| Time elapsed since last HIV test* | n=358 | ||
| > 5 years ago | Reference | ||
| >2 years but ≤5 years | -0.45 (-1.63, 0.73) | ||
| >1 year but ≤2 years | 0.03 (-1.18, 1.24) | ||
| >6 months but ≤1 year | -0.92 (-2.06, 0.22) | ||
| ≤6 months | -0.78 (-1.91, 0.34) | ||
| Not recalled | 5.33 (-1.63, 12.29) |
As shown in the final model (Model 2), the mean scores were slightly lower for Black/African American or Hispanic participants, those with governmental insurance (Medicare, Medicaid, or both), and those in the video arm. In contrast, participants with more years of formal education had higher mean scores. Two- and three-way interactions involving race, education, and study arm assignment were not significant (data not shown), which suggested that these factors were independently associated with scores on the questionnaire.
Figure 2 presents plots of the mean scores on the questionnaire that can be predicted by Model 2. Predicted scores are displayed according to three factors: years of formal education (in five groups), race or ethnicity (two groups), and study arm (two groups). White and “other race” participants, and Black/African American and Hispanic participants had similar mean scores, and so were combined into two groups, respectively. Across increasing years of formal education, white and “other race” participants had 1-2 point higher predicted mean scores than Black/African American and Hispanic patients, regardless of study arm. Participants who received pre-test information by an in-person information session had < 1 point higher scores than those who watched the video. The plot demonstrates that participants with more years of formal education had higher scores regardless of study arm and race or ethnicity. Participants with a college degree on average had two-point higher scores compared with high school graduates, and high school graduates on average had a three-point advantage or higher in scores over those with eight or fewer years of formal education.
Figure 2.
Although the difference among RAs' influence on scores was not significant in the univariable analyses, we were concerned that the RAs might have been subtly adjusting their in-person information session with patients over the course of the study. Despite the script and safeguards to reduce drift, the RAs could have been modifying their session based upon topics for which patients appeared to understand less well. In a univariable analysis, we found that there was a slight increase in scores over the course of the study for the in-person information session arm (β=0.005 [95% CI = 0.001 to 0.010]) but not for the video group (β=0.001 [95% CI = -0.003 to 0.006]). The differences did not persist in a multivariable model with the covariates from Model 2 (data not shown).
DISCUSSION
In this investigation, the video “Do you know about rapid HIV testing?” was an acceptable substitute for an in-person information session on rapid HIV pre-test fundamentals. The video can be used to deliver HIV pre-test information in EDs and perhaps other settings for patients preparing to undergo rapid HIV testing with OraQuick. This video, which is freely available on the internet, has a number of potential advantages that can streamline the testing process.25 We believe that the video can be shown at any time or place testing is conducted and video equipment are available, can free staff from in-person information sessions so they can conduct testing on more patients, and can be used for individual or group viewing.
HIV advocates critical of the CDC's recent HIV testing guidelines have voiced concern that some demographic groups may be adversely affected by streamlining of the HIV testing process.26-28 Advocates are concerned that traditionally disadvantaged racial and minority groups may need more rather than less information about HIV-related topics and more risk-reduction counseling. In this study, we assessed participant comprehension of pre-test information, but not HIV risk-reduction counseling. We do find evidence that patients who are Black/African American, Hispanic, or have governmental insurance scored less well on our questionnaire, independent of the pre-test information delivery method (in-person information session or video). However, this observed difference by race/ethnicity and insurance status was small, and was even smaller when scores were adjusted for years of formal education.
Greater comprehension of rapid HIV pre-test fundamentals was strongly related to years of formal education. This finding suggests that test providers should be mindful that patients with fewer years of formal education might not comprehend pre-test fundamentals as well as other patients, regardless of pre-test information format. These patients might benefit from other approaches to pre-test information that were not assessed in this trial, or by further augmentation of the modalities evaluated in this trial. On the other hand, the lower scores of these patients might simply reflect their test-taking skills, or perhaps the reading comprehension level of the questionnaire (although it was read aloud to participants). Patients with more years of formal education have more practice in testing than those with fewer years of education. We must add that because this study does not attempt to measure “competency” of patients to undergo HIV testing, it cannot be determined if participants had enough knowledge to engage in the testing process regardless of their score on the questionnaire. The content and nature of the information that test recipients require before being tested for HIV is not precisely known, is the subject of controversy, and is regulated differently across states and HIV testing venues. Research is needed to determine what information test recipients should receive, while taking into account the complex ethical, practical, and public health concerns that inform this determination. Of note, in the final multivariable model, a history of prior HIV testing did not confer greater knowledge about rapid HIV pre-test information. This finding suggests the need for providing HIV pre-test information at every testing encounter, with the caveat that the level of understanding of these fundamentals has not yet been established.
Although the difference in scores between the video and in-person information session study arms met the non-inferiority standard chosen for the trial, the mean score in the video arm was slightly less than the mean score for the in-person information session study arm. The results of the exploratory analysis that analyzed the difference in proportions of correct and “I don't know” responses by study arm indicate that a few key concepts can account for differences in mean scores. For HIV prevention, the concepts of using bleach to clean needles after injection-drug use (Q10), and the employment of “safer sexual practices” other than abstinence (Q13), appeared to be better presented in the in-person information session than the video. For rapid HIV testing information, participants receiving the in-person information session appeared to better understand that phlebotomy could be used to obtain blood samples for the rapid HIV testing (Q25). This difference can be explained by recognizing that the video emphasized the fingerstick method of sample collection. In addition, the low percentage of correct answers and the relatively high percentage of “I don't know” responses to questions about blood donation (Q5), phlebotomy for the rapid HIV test (Q25), and HIV antibodies as a marker of an infection in an HIV test (Q15), suggests that these concepts might not have been presented well to, or at least not well understood by, either group. We have made a few improvements to the video based upon these findings, and the revised video is posted on the internet.29
Some of the difference in scores by study arm could be due to subtle improvements in the RA information session over time, given that in comparison the video did not change during the life of the study. This effect appeared to be small. It should be noted that the RAs delivering the in-person information session were highly trained and provided a polished, well-rehearsed, idealized version of rapid HIV pre-test information. A more realistic analysis of the video's effectiveness would be to compare scores of patients who watch the video to those who receive information from clinicians or HIV counselors who are not a part of this study. We hope that future studies can demonstrate the revised video's better performance in such settings.
LIMITATIONS
Because the study was based in one ED in a single hospital, the findings are not necessarily generalizable to the broader U.S. population, or even other EDs. However, the random sampling scheme respective to the influx of patients to the ED helped to obtain a representative sample of patients presenting with sub-critical illness or injury. Those are the patients most likely to be tested in an HIV screening program in the ED.
We acknowledge that the findings about differences in HIV testing along demographic characteristics might not be applicable to the groups excluded from the study. We are hopeful that we can adapt the video and questionnaire for other demographic groups, and for use in other settings and in different languages.
Although it seems apparent that a video would streamline HIV testing efforts, this study did not assess the impact of the video on reducing the efforts, costs, and time expended in conducting rapid HIV testing in the ED or elsewhere. Furthermore, the need for this level of and the required content of pre-test information for rapid HIV testing in the ED or other testing venues is not yet known. The answers to these questions impact assessments of the value of using this method versus others to streamline HIV screening and testing efforts.
Despite efforts to develop a valid questionnaire for this trial, it is possible that some of the questions were not good measures of patient knowledge of rapid HIV pre-test information. As such, the exploratory analysis should be considered suggestive of areas in which comprehension was low, or an indication of questions that were problematic. However, for the primary objective of comparing the two information delivery methods, we do not believe that potentially misunderstood questions compromised the study findings, given the randomized controlled trial design.
Finally, we did not assess if the video and in-person information session could be used jointly to provide rapid HIV pre-test information. This approach might be a subject for future research.
CONCLUSIONS
The video “Do you know about rapid HIV testing?” is an acceptable substitute for an in-person information session in the ED setting, in regards to patient comprehension of rapid HIV pre-test information. Black/African Americans, Hispanics, and patients with Medicare and Medicaid insurance demonstrated slightly lower understanding of the rapid HIV pre-test fundamentals, regardless of whether pre-test information was delivered via an in-person information session or by video. Study patients with fewer years of formal education showed notably reduced understanding of the rapid HIV pre-test fundamentals compared to other patients. This finding suggests that test providers should be mindful that patients with fewer years of formal education might not comprehend pre-test fundamentals as well as other patients, regardless of information delivery format. Future research should consider methods of improving delivery of rapid HIV pre-test information to these patients.
Supplementary Material
Acknowledgments
Dr. Merchant and this study were supported by a career development grant from the National Institute for Allergy and Infectious Diseases (K23 A1060363). The study was also supported by a cooperative agreement grant from the Centers for Disease Control and Prevention (U65/CCU124504). Dr. Mayer was supported by the Center for AIDS Research at Lifespan/Tufts/Brown (P30 AI42853).
Footnotes
Presented at the Society for Academic Emergency Medicine annual meeting in Chicago, IL, May 19, 2007.
EDITOR'S NOTE: The use of Black/African American is approved for this manuscript.
REFERENCES
- 1.Branson BM, Handsfield HH, Lampe MA, et al. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep. 2006;55(RR-14):1–17. [PubMed] [Google Scholar]
- 2.Babcock Irvin C, Wyer PC, Gerson LW. Preventive care in the emergency department, Part II: Clinical preventive services--an emergency medicine evidence-based review. Society for Academic Emergency Medicine Public Health and Education Task Force Preventive Services Work Group. Acad Emerg Med. 2000;7:1042–54. doi: 10.1111/j.1553-2712.2000.tb02098.x. [DOI] [PubMed] [Google Scholar]
- 3.Rothman RE, Ketlogetswe KS, Dolan T, Wyer PC, Kelen GD. Preventive care in the emergency department: should emergency departments conduct routine HIV screening? a systematic review. Acad Emerg Med. 2003;10:278–85. doi: 10.1111/j.1553-2712.2003.tb02004.x. [DOI] [PubMed] [Google Scholar]
- 4.Rothman RE. Current Centers for Disease Control and Prevention guidelines for HIV counseling, testing, and referral: critical role of and a call to action for emergency physicians. Ann Emerg Med. 2004;44(1):31–42. doi: 10.1016/j.annemergmed.2004.01.016. [DOI] [PubMed] [Google Scholar]
- 5.Alpert PL, Shuter J, DeShaw MG, Webber MP, Klein RS. Factors associated with unrecognized HIV-1 infection in an inner-city emergency department. Ann Emerg Med. 1996;28(2):159–64. doi: 10.1016/s0196-0644(96)70056-2. [DOI] [PubMed] [Google Scholar]
- 6.Shuter J, Alpert PL, DeShaw MG, Greenberg B, Chang CJ, Klein RS. Gender differences in HIV risk behaviors in an adult emergency department in New York City. J Urban Health. 1999;76(2):237–46. doi: 10.1007/BF02344679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goggin MA, Davidson AJ, Cantril SV, O'Keefe LK, Douglas JM. The extent of undiagnosed HIV infection among emergency department patients: results of a blinded seroprevalence survey and a pilot HIV testing program. J Emerg Med. 2000;19(1):13–9. doi: 10.1016/s0736-4679(00)00175-x. [DOI] [PubMed] [Google Scholar]
- 8.Sturm JT. HIV prevalence in a midwestern emergency department. Ann Emerg Med. 1991;20(3):276–8. doi: 10.1016/s0196-0644(05)80939-4. [DOI] [PubMed] [Google Scholar]
- 9.Baraff LJ, Talan DA, Torres M. Prevalence of HIV antibody in a noninner-city university hospital emergency department. Ann Emerg Med. 1991;20(7):782–6. doi: 10.1016/s0196-0644(05)80842-x. [DOI] [PubMed] [Google Scholar]
- 10.Baker JL, Kelen GD, Sivertson KT, Quinn TC. Unsuspected human immunodeficiency virus in critically ill emergency patients. JAMA. 1987;257(19):2609–11. [PubMed] [Google Scholar]
- 11.Kelen GD, DiGiovanna T, Bisson L, Kalainov D, Sivertson KT, Quinn TC. Human immunodeficiency virus infection in emergency department patients. Epidemiology, clinical presentations, and risk to health care workers: the Johns Hopkins experience. JAMA. 1989;262(4):516–22. doi: 10.1001/jama.262.4.516. [DOI] [PubMed] [Google Scholar]
- 12.Jui J, Modesitt S, Fleming D, et al. Multicenter HIV and hepatitis B seroprevalence study. J Emerg Med. 1990;8(3):243–51. doi: 10.1016/0736-4679(90)90001-c. [DOI] [PubMed] [Google Scholar]
- 13.Kelen GD, Hexter DA, Hansen KN, et al. Feasibility of an emergency department-based, risk-targeted voluntary HIV screening program. Ann Emerg Med. 1996;27(6):687–92. doi: 10.1016/s0196-0644(96)70184-1. [DOI] [PubMed] [Google Scholar]
- 14.Kelen GD, Shahan JB, Quinn TC. Emergency department-based HIV screening and counseling: experience with rapid and standard serologic testing. Ann Emerg Med. 1999;33(2):147–55. doi: 10.1016/s0196-0644(99)70387-2. [DOI] [PubMed] [Google Scholar]
- 15.Lyons MS, Lindsell CJ, Ledyard HK, Frame PT, Trott AT. Emergency department HIV testing and counseling: an ongoing experience in a low-prevalence area. Ann Emerg Med. 2005;46(1):22–8. doi: 10.1016/j.annemergmed.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 16.Lyons MS, Lindsell CJ, Ledyard HK, Frame PT, Trott AT. Health department collaboration with emergency departments as a model for public health programs among at-risk populations. Public Health Rep. 2005;120(3):259–65. doi: 10.1177/003335490512000307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Silva A, Glick NR, Lyss SB, et al. Implementing an HIV and sexually transmitted disease screening program in an emergency department. Ann Emerg Med. 2007;49(5):564–72. doi: 10.1016/j.annemergmed.2006.09.028. [DOI] [PubMed] [Google Scholar]
- 18.Lyss SB, Branson BM, Kroc KA, Couture EF, Newman DR, Weinstein RA. Detecting unsuspected HIV infection with a rapid whole-blood HIV test in an urban emergency department. J Acquir Immune Defic Syndr. 2007;44(4):435–42. doi: 10.1097/QAI.0b013e31802f83d0. [DOI] [PubMed] [Google Scholar]
- 19.Brown J, Shesser R, Simon G, et al. Routine HIV screening in the emergency department using the new US Centers for Disease Control and Prevention Guidelines: results from a high-prevalence area. J Acquir Immune Defic Syndr. 2007;46(4):395–401. doi: 10.1097/qai.0b013e3181582d82. [DOI] [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention Revised guidelines for HIV counseling, testing, and referral. MMWR Recomm Rep. 2001;50(RR-19):1–57. [PubMed] [Google Scholar]
- 21.Merchant R, Gee E, Clark M, Mayer K, Seage GI, DeGruttola V. Comparison of patient comprehension of rapid HIV pre-test fundamentals by information delivery format in an emergency department setting. BMC Public Health. 2007;7:238. doi: 10.1186/1471-2458-7-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Merchant R, Seage G, Mayer K, Clark M, DeGruttola V, Becker B. Emergency department patient acceptance of opt-in, universal, rapid HIV screening. Public Health Rep. 2008;123(supp 3):27–40. doi: 10.1177/00333549081230S305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Piaggio G, Elbourne DR, Altman DG, Pocock SJ, Evans SJ. Reporting of noninferiority and equivalence randomized trials: an extension of the CONSORT statement. JAMA. 2006;295(10):1152–60. doi: 10.1001/jama.295.10.1152. [DOI] [PubMed] [Google Scholar]
- 24.Calderon Y, Haughey M, Bijur PE, et al. An educational HIV pretest counseling video program for off-hours testing in the emergency department. Ann Emerg Med. 2006;48(1):21–7. doi: 10.1016/j.annemergmed.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 25.Merchant R, Gee E, Christie SI. Do you know about rapid HIV testing? Available at: http://www.brown.edu/brunap/aids_video.html. Accessed Nov 4, 2008.
- 26.American Civil Liberties Union ACLU Says New CDC HIV Testing Recommendations Raise Health and Civil Liberties Concerns. Available at: http://www.aclu.org/hiv/testing/26819prs20060921.html. Accessed Nov 2, 2008.
- 27.Community HIV/AIDS Mobilization Project (CHAMP) Coalition Comments on CDC's Draft Revised HIV Testing Guidelines. Released March 31, 2006. Available at: http://www.champnetwork.org/media/Testing-Letter.pdf. Accessed Nov 4, 2008.
- 28.Community HIV/AIDS Mobilization Project (CHAMP) Federal HIV Testing Initiatives Can Only Succeed with Expanded Healthcare, Patient and Provider Education. Available at: http://www.champnetwork.org/media/Testing-Statement-092106.pdf. Accessed Nov 2, 2008.
- 29.Merchant RC, Gee EM, Sykes DA, Goishi D, Christie SI. What do you know about HIV and HIV testing? Available at: http://www.brown.edu/brunap/aids-video.html. Accessed Nov 4, 2008.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.