Summary
Multiple signaling pathways initiate and specify the formation of synapses in the central nervous system. General principles that organize nascent synapses have emerged from studies in multiple model organisms. These include the synapse-organizing roles of dedicated synaptic adhesion molecules, synaptic signaling following receptor-ligand interactions, and the regulation of synapse formation by secreted molecules. Intracellularly, a range of effectors subsequently regulates signaling steps and cytoskeletal changes. Together, a blueprint of synapse formation is emerging into which these distinct signaling steps will need to be integrated temporally and spatially.
Introduction
Synapse formation is a key process in brain development. It occurs subsequent to the birth and migration of neurons and their initial differentiation, and is central to the formation of neuronal networks. Synaptogenesis remains important in the adult brain for the activity-dependent reorganization of neuronal networks. Understanding these processes on the molecular level not only provides insights into a fundamental problem of cellular neuroscience. It is also biomedically relevant, as aberrations in synapse-organizing molecules are linked to autism-spectrum disorders, mental retardation, and neurological disorders.
Synaptic structures develop in consecutive assembly steps [1,2]. Cell-cell interactions mediate the initial contact of apposed neuronal membranes. This is followed by the differentiation of these membranes into pre- and postsynaptic specializations, a process shaped by cytoskeletal changes. Later steps include the pruning of synapses and finally their elimination. Along this path, different signals assemble protein complexes to give rise to the diverse types of central synapses, which vary in their target specificity, neurotransmitter use, and morphology.
This review highlights the progress made in the last two years in our molecular understanding of synapse formation. For a general overview, we would like to refer the reader to recent reviews [3,4].
Adhesive interactions of neurexins and neuroligins organize developing synapses
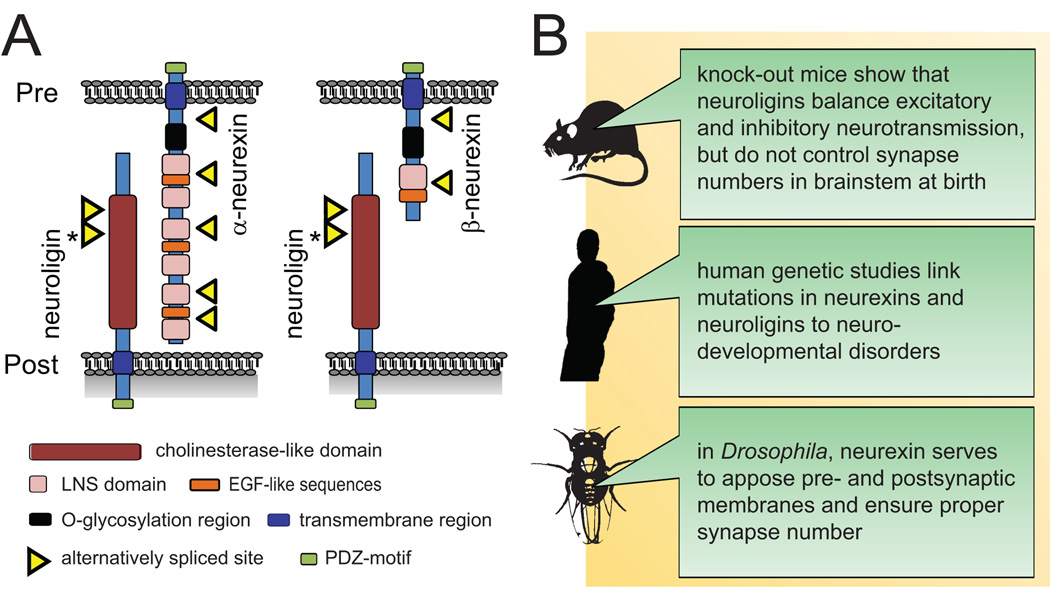
Trans-synaptic adhesion molecules can control the initial differentiation of nascent synapses. This was first demonstrated for neuroligins, postsynaptic membrane proteins that bind the presynaptic neurexins [5,6] (Fig.1). Three neuroligin genes are predominantly expressed in mouse brain, while three genes encode the neurexins. Each neurexin gene has two different promoters, giving rise to a long α- or a short β-isoform that differ only in their extracellular sequences. Through their transsynaptic interactions with neurexins, neuroligins induce neurons to form presynaptic terminals. In turn, neurexins induce the assembly of neuroligin-containing postsynaptic specializations.
Figure 1.
A. Structure of neuroligins and neurexins. The neuroligin 1 splice site that regulates its α over β-neurexin binding is highlighted by an asterisk. Pre, presynaptic membrane; post, postsynaptic membrane.
B. Key insights recently gained from in vivo studies of neurexins and neuroligins.
Corresponding to early roles of neuroligins in synapse formation, they are part of mobile dendritic protein complexes that are stabilized during synapse formation [7] and mark sites where axons form terminals [8]. In addition, neuroligins contribute to synapse specification: Neuroligins 1 and 2 differ in their relative propensity to promote excitatory and inhibitory synaptic specializations, respectively, consistent with their differential localization to these two synapse types [9–12]. Neuronal activity is required to achieve this enhancement of excitatory and inhibitory transmission by neuroligins [12]. With respect to postsynaptic specification through trans-synaptic binding, α-neurexin co-clusters neuroligin 2 with inhibitory, but not excitatory markers [13]. Neurexins and neuroligins also affect synaptic physiology, notably presynaptic release probability [14].
These interactions of the neuroligin/neurexin adhesion molecules are prominently regulated by alternative splicing, which modulates their binding in trans. A short, splicing-controlled insert in the extracellular sequence of neuroligin 1 that encodes an N-glycosylation site negatively regulates its binding to α-neurexin [15] and decreases [11] or abolishes [10,15] neuroligin binding to another splice isoform of β-neurexin. Further, the splicing of neuroligins at this site controls their sorting to excitatory and inhibitory postsynaptic specializations [10]. Several findings demonstrate that alternative splicing also regulates neurexin and neuroligin activities. First, splicing of β-neurexin can alter its ability to induce excitatory postsynaptic assemblies preferentially [11] or specifically [10], without affecting β-neurexin’s parallel induction of inhibitory sites. Neuronal activity regulates β-neurexin splicing, further pointing to dynamic roles of splicing [13]. Second, the neuroligin 1 splice form capable of interacting with α-neurexin promotes pre- and postsynaptic growth in addition to synapse formation [15]. Third, splicing in the extracellular neuroligin 1 site referred to above has been reported to switch its activity from promiscuously inducing excitatory and inhibitory postsynaptic sites to being a specific excitatory synaptogenic molecule [10]. This effect was not observed in another study [12], perhaps due to differences in expression levels or culturing conditions. In addition to splicing, interactions in cis constitute another regulatory mechanism. A fraction of neurexins was identified in postsynaptic membranes, where they can bind laterally to neuroligins to silence them [16].
These studies in dissociated neuronal cultures helped to develop the concept of neuroligins as synapse-inducing molecules with different roles in excitatory and inhibitory synapse formation. From a general point of view, studies in vivo support a general synapse-organizing role. Neuroligins specifically function in excitatory and inhibitory synaptic transmission, as shown in single knock-out mice [12]. These combined activities are of vital importance: Neuroligin triple knock-out mice die soon after birth due to imbalanced excitatory and inhibitory transmission in brainstem and ensuing respiratory failure [17••]. However, neuroligins do not affect synapse number or morphology in brainstem at the time of birth, pointing to roles in synapse maturation. This discrepancy with the synaptogenic functions of neuroligins in vitro remains to be resolved. It may involve redundancy with other synaptogenic systems in vivo, as well as potential developmental changes in the synaptic functions of neuroligins. Future studies using conditional neuroligin knock-out mice could address these points by analyzing the acute loss of neuroligins in higher brain regions at later postnatal stages, when most synaptogenesis occurs.
Invertebrates offer less redundant systems to investigate synaptic adhesion molecules in vivo. Two studies in Drosophila, which has only one α-neurexin and no β-isoform, now report effects of neurexin on synapse ultrastructure and number in vivo [18,19]. They demonstrate that α-neurexin is presynaptic at the fly neuromuscular junction (NMJ) and is required for the proper apposition of active zones to postsynaptic densities, normal synapse density, and synaptic transmission. In addition, Drosophila neurexin is sufficient to promote overall numbers of presynaptic boutons [18].
Human genetic studies support the importance of neurexins and neuroligins in brain development. Following previous studies of human neuroligin mutations in neurodevelopmental disorders, mouse models with altered expression of neuroligins now corroborate changes in synapse organization and autism-spectrum disorders (ASD)-linked behavior [20•,21]. Recent linkage analyses also implicate imbalanced neurexin gene dosage in ASD [22,23].
Together, neurexins and neuroligins have intriguing and essential synaptic functions. However, the facts that synapses form normally in mice lacking neurexins and neuroligins at birth, and that members of both families have overlapping synapse-specifying roles, point to the importance of parallel synaptogenic interactions.
Synapse organization by Ig- and LRR-domain containing adhesion molecules
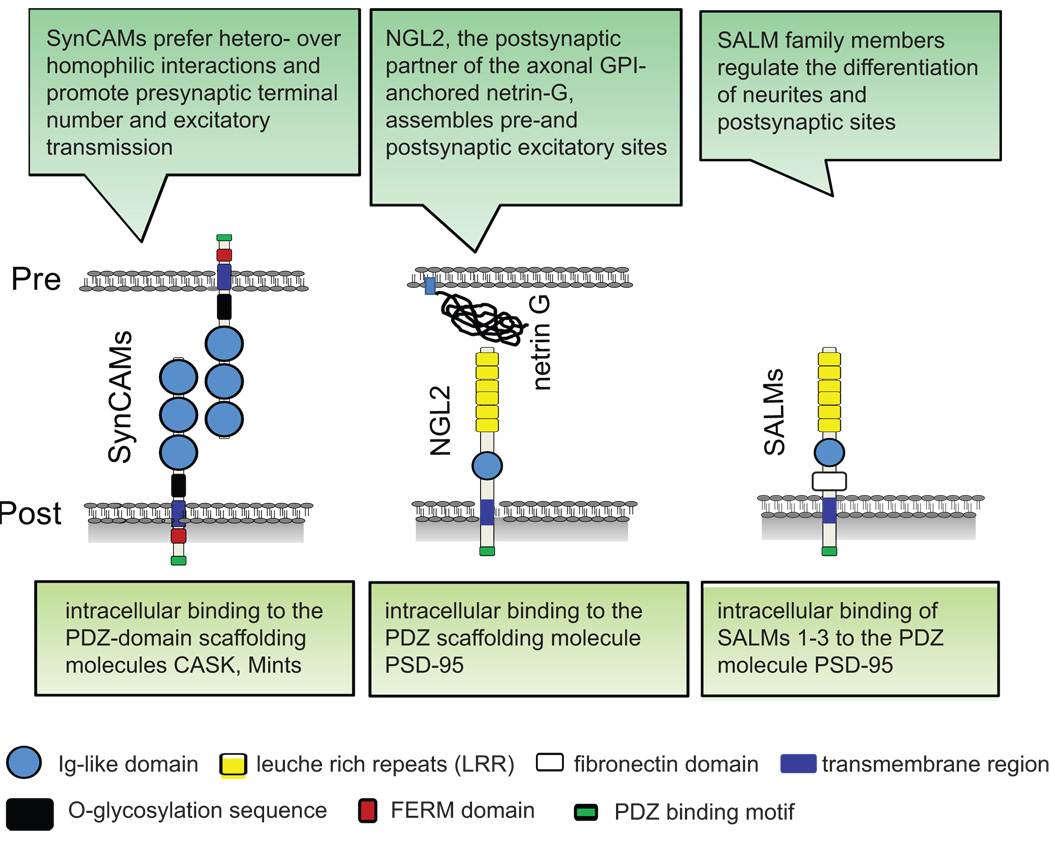
Adhesion molecules of the immunoglobulin (Ig) superfamily and proteins containing extracellular leucine rich-repeats (LRR) additionally mediate the pre- and postsynaptic differentiation of cultured hippocampal neurons (Fig.2).
Figure 2.
Overview of members of the Ig superfamily and proteins containing extracellular leucine-rich-repeats (LRR) that are involved in synapse differentiation.
The synaptic Ig-containing membrane protein SynCAM 1 (also named nectin-like 2) induces neurons to form functional excitatory presynaptic specializations [24] similar to neuroligin 1 [25]. While capable of homophilic binding, SynCAM 1 preferentially interacts with the related SynCAM 2 to form a trans-synaptic adhesion complex, and both proteins promote excitatory synapse number and function [26•]. The differential neuronal expression and heterophilic adhesion profiles of SynCAMs are reminiscent of an adhesive code and indicate distinct roles in synapse organization and specification [27]. All four family members share intracellular motifs binding to FERM domains of cytoskeletal adaptors and PDZ domains of scaffolding molecules, pointing to these interactions as synaptogenic steps downstream of SynCAM adhesion.
Other studies identified the LRR- and Ig-domain containing membrane proteins NGL2 (netrin G-ligand 2) and SALMs (synaptic adhesion-like molecules). NGL2 is a postsynaptic partner of the axonal, GPI-anchored protein netrin-G [28•]. Intracellularly, it binds to a PDZ domain of the scaffolding molecule PSD-95 to assemble postsynaptic proteins of excitatory synapses. Through its extracellular interactions, NGL2 in turn initiates presynaptic terminals [28•]. This activity presumably involves both interactions with netrin-G and other, yet unknown presynaptic transmembrane proteins that can signal into the terminal. Similar to NGL2, several SALM family members interact intracellularly with PSD-95, but differ in their developmental functions. At later stages of neuronal differentiation, SALM2 affects the clustering of postsynaptic molecules and increases the number of excitatory synapses [29], while SALM1 promotes neurite outgrowth at early stages [30]. No effects of SALMs on presynaptic organization are known. However, SALMs form distinct homo- and heterophilic interactions [31], suggesting adhesive roles on both sides of synapses.
Ig superfamily members also specify the localization of nascent synapses in vertebrates. This was shown in cerebellum, where the axons of stellate interneurons are guided by the Ig protein CHL1 (close homolog of L1) on Bergman glial fibers towards Purkinje cell dendrites [32]. Consequently, the interactions of CHL1 are required for the positioning and formation of these GABAergic synapses.
How are adhesion molecules signaling across membranes to initiate synapses?
The pathways downstream of synaptic adhesion remain insufficiently understood. Progress was made for neurexins and SynCAMs with the finding that their induction of presynaptic specializations involves the kinase Cdk5 [33•]. This indicates that both adhesion proteins engage overlapping signaling pathways, consistent with their similar intracellular sequences. Cdk5 also phosphorylates the adaptor molecule CASK, thereby regulating its interaction with neurexins [33•]. Cdk5-mediated phosphorylation of CASK may provide a direct presynaptic link from sites of neurexin-mediated adhesion to the CASK binding partner liprin-α, which organizes active zone formation in C. elegans [34]. However, identifying the signaling pathways of synapse-inducing adhesion molecules remains a critical open question.
Adhesion molecules also modulate synaptogenesis
Synaptic adhesion can not only signal the formation of synaptic specializations, it additionally modulates nascent synapses. Cadherins, among the best studied synaptic adhesion molecules, are not synaptogenic but set the pace of synaptic maturation [35,36]. This is in keeping with their subsynaptic re-localization in development [37]. Cadherin signaling engages multiple pathways on both sides of the developing synapse. Presynaptically, crosstalk of neurotrophin and cadherin signaling occurs [38]. Neurotrophins, which regulate synapse formation, mobilize synaptic vesicles and subsequently promote excitatory synapse numbers by disrupting the interaction of cadherins with β-catenin, a multifactorial adaptor for signaling molecules and transcription factors [38]. Postsynaptically, the cadherin partner p120-catenin controls Rho family GTPases, whose functions include the regulation of the actin cytoskeleton as well as cadherin levels themselves. Through these interactions, p120 modulates postsynaptic spine differentiation and synapse density in the developing brain [39•].
Integrins are another prominent class of adhesion molecules that transduce signals from the extracellular matrix. Recent evidence shows that they shape postsynaptic sites through controlling tyrosine kinases and G proteins. The α5 integrin subunit regulates spine and synapse formation through the non-receptor tyrosine kinase Src and the G protein regulator GIT1 (G protein–coupled receptor kinase–interacting protein 1) [40]. Integrins also activate the non-receptor tyrosine kinase Arg, which in turn inhibits the RhoGAP p190 [41]. Consequently, Arg signaling modulates synapse maintenance and spine maturation in the maturing brain.
Signaling receptors in synaptogenesis
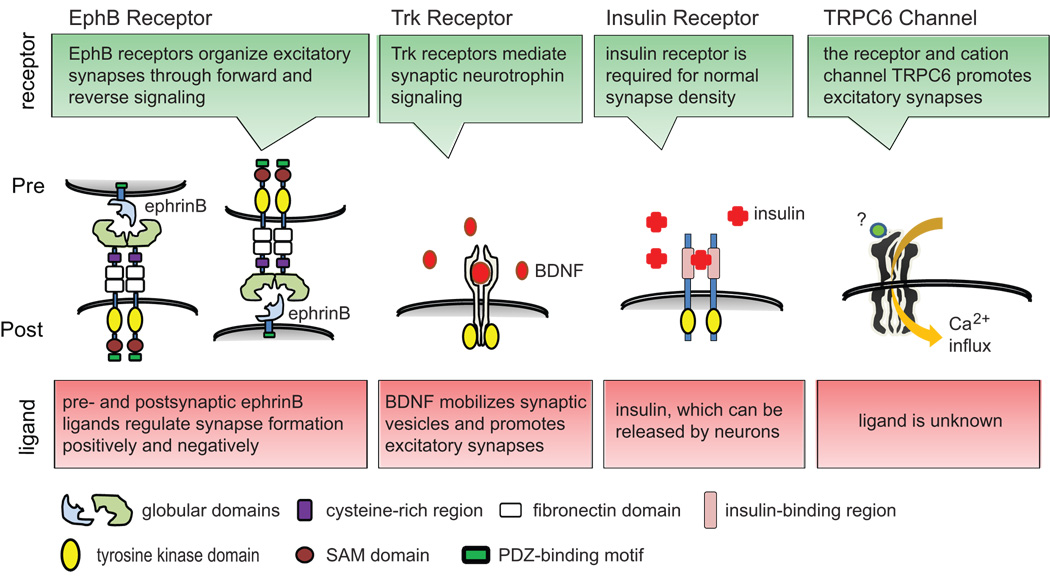
In contrast to adhesion molecules, transmembrane receptors can directly transduct synaptogenic signals across synaptic membranes (Fig.3). Several receptor tyrosine kinases, including EphB and Trk receptors, localize to synapses and help to instruct synaptogenesis. EphB receptors, which are mostly postsynaptic, signal intracellularly through a tyrosine kinase domain upon extracellular binding of their ephrinB ligands. The deletion of multiple EphB receptors in mice reduces synapse density and alters spine morphology [42], demonstrating that ephrin-to-EphB forward signaling controls excitatory synapses in vivo. A synaptogenic role was also confirmed for presynaptic terminals in cultured hippocampal neurons. Here, reverse EphB2 signaling from postsynaptic sites through ephrin binding into axons triggers presynaptic differentiation [43•]. This occurs in parallel to EphB receptor-mediated postsynaptic glutamate receptor assembly [43•]. Similarly, in the optic tectum of Xenopus, EphB2 receptors engage presynaptic ephrinB ligands to trigger their reverse signaling, which increases the formation and maturation of retinotectal synapses and enhances synaptic transmission and potentiation [44••].
Figure 3.
Roles of signaling receptors in synaptic differentiation.
What are the intracellular pathways of ephrin and EphB receptor signaling at the synapse? The kinase activity of Eph receptors is known to signal through several small GTPases, including Rho and Rac family members, thereby remodeling the actin cytoskeleton. Recent studies expand this signaling repertoire. Tiam1, a guanine nucleotide exchange factor (GEF) that activates Rac1, interacts postsynaptically with the EphB2 receptor after ephrin stimulation to promote excitatory spine density [45]. In a parallel pathway, stimulated EphB receptors bind focal adhesion kinase to activate RhoA through an intracellular signaling complex that shapes postsynaptic sites [46].
However, ephrin ligands are not only presynaptic, but can also be present in excitatory postsynaptic membranes where they mediate reverse EphB-to-ephrin signaling. Postsynaptic ephrinB3 was identified to promote spine density and maturation after stimulation by the EphB receptor, forming a complex with the G protein regulator GIT1 [47] that also functions downstream of integrin signaling [40]. Postsynaptic ephrinB3 independently affects the subset of excitatory synapses that are directly formed on the dendritic shaft, controlling the number of these shaft synapses [48]. Reverse ephrin signaling is highly versatile, and includes the negative regulation of synapse numbers in addition to the synaptogenic roles described above. This was observed in mice lacking ephrinB3, which display an increase in excitatory synapses in hippocampal neurons [49]. The pathways determining these contrasting effects of reverse ephrinB signaling remain to be identified.
Important functions in synapse differentiation are shared by other transmembrane receptor tyrosine kinases. These include the Trk receptors, which mediate neurotrophin signaling in neuronal and synapse differentiation [50]. Signaling by the insulin receptor also regulates synapse number and function. Studies in the Xenopus optic tectum identified that a dominant-negative insulin receptor strongly reduces the density of functional synapses along the dendritic tree, as well as the experience-dependent shaping of dendrites [51••]. This is consistent with a synapse-promoting function of the insulin receptor in vivo. Another family of receptor tyrosine kinases, the ErbB receptors, is already known to act in the formation of NMJs. Their roles in central synapses are now emerging, too, with ErbB4 promoting excitatory transmission in hippocampus [52] and its ligand neuregulin-1 enhancing GABA release from cortical interneurons [53]. As neuregulin is a schizophrenia susceptibility gene, and as synaptic alterations occur in this disorder, it will be of interest whether aberrations in this signaling pathway underlie the disorganization of central synapses in schizophrenia.
Synaptic transmembrane signaling is of course not limited to receptor tyrosine kinases. Yet, the finding that the ligand-gated cation channel TRPC6 (transient receptor potential canonical), which belongs to the TRP family of calcium-permeable channels, mediates synapse-organizing signaling across dendritic membranes was unexpected [54]. TRPC6 is localized to excitatory postsynaptic sites and promotes synapse densities in hippocampal neurons via stimulating CREB-controlled transcription, and alters hippocampus-dependent behavior. This opens up functions for the diverse family of TRP channels and their yet unknown synaptogenic ligands in the brain.
Soluble signals secreted by neurons and glia locally shape presynaptic sites
Control of synaptic differentiation is not restricted to the very short range that surface molecules provide. Soluble signaling molecules, such as morphogens that pattern tissues, also affect synapse formation and differentiation. One case are the synaptic functions of morphogenetic Wnt signaling. In cerebellum and hippocampus, Wnt7 positively regulates the assembly of presynaptic sites after its release by target neurons [55]. But at least in invertebrates, retrograde Wnt signaling also can inhibit synapse formation to ensure synaptic target specificity. This was observed both at the Drosophila NMJ [56] and in C. elegans, where Wnt morphogens directly control their receptor localization in axons to restrict presynaptic bouton formation to discrete sites [57•].
Morphogens are not the only soluble signals that regulate synapse formation, and neither is signaling limited to neuronderived factors. This is exemplified by synaptic netrin signaling in C. elegans. Subsequent to its roles in axon guidance, netrin is locally secreted by glia cells to promote the formation of presynaptic boutons by a specific neuron [58]. In vertebrates, the glial cell line-derived neurotrophic factor (GDNF) promotes hippocampal synaptogenesis through a less familiar type of receptor interaction. GDNF binding causes apposed receptor molecules to homophilically bridge pre- and postsynaptic membranes, which initiates presynaptic terminal differentiation [59]. Correspondingly, the lack of GDNF reduces synapse density in vivo. These findings underline the importance of neuron-glia signaling in synaptogenesis [60].
Cytoskeletal dynamics in synapse differentiation
Cytoskeletal changes underlie the differentiation of local plasma membrane surfaces into synaptic specializations. These structural transformations of nascent synapses not only involve the GEFs and GAPs for small G protein regulators of the actin cytoskeleton referred to above. Actin dynamics at postsynaptic excitatory specializations are also regulated by NWASP, which activates the actin-nucleating Arp2/3 complex to enhance the local formation of excitatory spines and synapses by hippocampal neurons [61]. Correspondingly, a dominant-negative form of the actin-binding protein spectrin interferes with postsynaptic assembly [62]. The presynaptic cytoskeleton is regulated as well, as shown for ankyrin, a spectrin-binding protein [63,64]. At the Drosophila NMJ, ankyrin forms a presynaptic lattice that organizes microtubules and adhesion proteins to restrict bouton size and control synapse number.
Activity-dependence of synapse organization
A key question is how activity affects synaptogenic signaling, as this can underlie synaptic homeostasis. The identification of activity-dependent functions of neuroligins represents one advance in addressing this question [12]. But neurons alter synapse density globally to adjust to activity levels, and insights into this process are being gained as well. The transcriptional regulator MeCP2, which is mutated in the neurodevelopmental disorder Rett syndrome, was identified as an activity-dependent positive regulator of excitatory synapse formation and function [65•]. Conversely, the transcription factor MEF2 represses excitatory synapse density in activated neurons [66]. While GABAergic synapse formation is less well understood than excitatory synaptogenesis [6], its activity-dependence can be surprisingly direct: Changes in the levels of GABA itself, which fluctuate in an activity-dependent manner in inhibitory neurons, regulate inhibitory synapse formation in cortex [67].
Screens for synaptogenic molecules
To gain more insight into synaptogenic signaling in vertebrate neurons, non-biased approaches need to extend the common analyses of candidate proteins. A beginning was made with a study that combined transcriptional profiling with RNAi in hippocampal neurons [68]. It identified that postsynaptic cadherins and membrane-bound semaphorins differentially control the alignment of synaptic sites at nascent synapses. This approach can now be pursued in larger-scale screens. Another approach was used in a genome-wide screen for molecules expressed during synapse formation [69]. Such approaches in vertebrate neurons will be likely to complement the genetic studies of synapse organization in invertebrates.
Outlook: how do synaptic signaling mechanisms come together?
Synapse formation requires multiple signaling mechanisms that demarcate future synaptic sites, align and specify them, and differentiate these nascent synapses to maturity. As reviewed above, a number of proteins have recently been identified to contribute to these signaling processes. Shared principles are emerging, such as the instructive roles of trans-synaptic interactions by adhesion molecules, the synaptogenic functions of receptor tyrosine kinases, and the modulation of synapse formation by secreted signaling molecules. However, it is now a key task to define the temporal and spatial interplay of signaling molecules in synapse assembly, maturation, and maintenance. This will lead to understanding how synapse development is instructed and specified at different synapse types and across brain regions.
Future studies will also need to consider the differential contribution of signaling to synapse formation or maintenance. The net outcome – an increase in synapse numbers – is the same, but these two aspects of synapse organization likely employ very different pathways which need to be elucidated. Additionally, the mechanisms that link activity-dependent changes to synaptic differentiation remain to be characterized in detail. Another important goal will be to better understand the signals that coordinate the converse process to synaptogenesis, namely synapse elimination, which is unlikely to be just the reverse of synapse formation.
In summary, a range of molecular interactions provide for synapse formation. On the molecular and cellular level, ongoing and future studies will identify both the signaling pathways that are fundamentally shared in synaptogenesis and those that specify it. Ultimately, these processes will have to be understood within the context of the brain itself [70].
Acknowledgments
We thank members of the Biederer group for comments and apologize for omitted citations due to space limitations. Work in our laboratory is supported by the National Institutes of Health Grant R01 DA018928.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
• • of outstanding interest
- 1.Vaughn JE. Fine structure of synaptogenesis in the vertebrate central nervous system. Synapse. 1989;3:255–285. doi: 10.1002/syn.890030312. [DOI] [PubMed] [Google Scholar]
- 2.McAllister AK. Dynamic aspects of CNS synapse formation. Annu Rev Neurosci. 2007;30:425–450. doi: 10.1146/annurev.neuro.29.051605.112830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gerrow K, El-Husseini A. Cell adhesion molecules at the synapse. Front Biosci. 2006;11:2400–2419. doi: 10.2741/1978. [DOI] [PubMed] [Google Scholar]
- 4.Dalva MB, McClelland AC, Kayser MS. Cell adhesion molecules: signalling functions at the synapse. Nat Rev Neurosci. 2007;8:206–220. doi: 10.1038/nrn2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Craig AM, Kang Y. Neurexin-neuroligin signaling in synapse development. Curr Opin Neurobiol. 2007;17:43–52. doi: 10.1016/j.conb.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang ZJ, Scheiffele P. GABA and neuroligin signaling: linking synaptic activity and adhesion in inhibitory synapse development. Curr Opin Neurobiol. 2008;18:77–83. doi: 10.1016/j.conb.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gerrow K, Romorini S, Nabi SM, Colicos MA, Sala C, El-Husseini A. A preformed complex of postsynaptic proteins is involved in excitatory synapse development. Neuron. 2006;49:547–562. doi: 10.1016/j.neuron.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 8.Sabo SL, Gomes RA, McAllister AK. Formation of presynaptic terminals at predefined sites along axons. J Neurosci. 2006;26:10813–10825. doi: 10.1523/JNEUROSCI.2052-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chih B, Engelman H, Scheiffele P. Control of excitatory and inhibitory synapse formation by neuroligins. Science. 2005;307:1324–1328. doi: 10.1126/science.1107470. [DOI] [PubMed] [Google Scholar]
- 10.Chih B, Gollan L, Scheiffele P. Alternative splicing controls selective trans-synaptic interactions of the neuroligin-neurexin complex. Neuron. 2006;51:171–178. doi: 10.1016/j.neuron.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 11.Graf ER, Kang Y, Hauner AM, Craig AM. Structure function and splice site analysis of the synaptogenic activity of the neurexin-1 beta LNS domain. J Neurosci. 2006;26:4256–4265. doi: 10.1523/JNEUROSCI.1253-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chubykin AA, Atasoy D, Etherton MR, Brose N, Kavalali ET, Gibson JR, Südhof TC. Activity-dependent validation of excitatory versus inhibitory synapses by neuroligin-1 versus neuroligin-2. Neuron. 2007;54:919–931. doi: 10.1016/j.neuron.2007.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kang Y, Zhang X, Dobie F, Wu H, Craig AM. Induction of GABAergic postsynaptic differentiation by alpha-neurexins. J Biol Chem. 2008;283:2323–2334. doi: 10.1074/jbc.M703957200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Futai K, Kim MJ, Hashikawa T, Scheiffele P, Sheng M, Hayashi Y. Retrograde modulation of presynaptic release probability through signaling mediated by PSD-95-neuroligin. Nat Neurosci. 2007;10:186–195. doi: 10.1038/nn1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boucard AA, Chubykin AA, Comoletti D, Taylor P, Südhof TC. A splice code for trans-synaptic cell adhesion mediated by binding of neuroligin 1 to alpha- and beta-neurexins. Neuron. 2005;48:229–236. doi: 10.1016/j.neuron.2005.08.026. [DOI] [PubMed] [Google Scholar]
- 16.Taniguchi H, Gollan L, Scholl FG, Mahadomrongkul V, Dobler E, Limthong N, Peck M, Aoki C, Scheiffele P. Silencing of neuroligin function by postsynaptic neurexins. J Neurosci. 2007;27:2815–2824. doi: 10.1523/JNEUROSCI.0032-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Varoqueaux F, Aramuni G, Rawson RL, Mohrmann R, Missler M, Gottmann K, Zhang W, Südhof TC, Brose N. Neuroligins determine synapse maturation and function. Neuron. 2006;51:741–754. doi: 10.1016/j.neuron.2006.09.003.This study of neuroligins in knock-out mice demonstrates vital functions in balancing excitatory and inhibitory transmission. Unexpectedly, no evidence for roles in synapse formation was obtained.
- 18.Li J, Ashley J, Budnik V, Bhat MA. Crucial role of Drosophila neurexin in proper active zone apposition to postsynaptic densities, synaptic growth, and synaptic transmission. Neuron. 2007;55:741–755. doi: 10.1016/j.neuron.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zeng X, Sun M, Liu L, Chen F, Wei L, Xie W. Neurexin-1 is required for synapse formation and larvae associative learning in Drosophila. FEBS Lett. 2007;581:2509–2516. doi: 10.1016/j.febslet.2007.04.068. [DOI] [PubMed] [Google Scholar]
- 20.Tabuchi K, Blundell J, Etherton MR, Hammer RE, Liu X, Powell CM, Sudhof TC. A neuroligin-3 mutation implicated in autism increases inhibitory synaptic transmission in mice. Science. 2007;318:71–76. doi: 10.1126/science.1146221.The authors analyse a mouse model carrying a neuroligin 3 mutation identified in human autism-spectrum disorders and observe a likely toxic gain-of-function effect on behavior. These findings link aberrant circuitry formation to autism.
- 21.Hines RM, Wu L, Hines DJ, Steenland H, Mansour S, Dahlhaus R, Singaraja RR, Cao X, Sammler E, Hormuzdi SG, et al. Synaptic imbalance, stereotypies, and impaired social interactions in mice with altered neuroligin 2 expression. J Neurosci. 2008;28:6055–6067. doi: 10.1523/JNEUROSCI.0032-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Szatmari P, Paterson AD, Zwaigenbaum L, Roberts W, Brian J, Liu XQ, Vincent JB, Skaug JL, Thompson AP, Senman L, et al. Mapping autism risk loci using genetic linkage and chromosomal rearrangements. Nat Genet. 2007;39:319–328. doi: 10.1038/ng1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim HG, Kishikawa S, Higgins AW, Seong IS, Donovan DJ, Shen Y, Lally E, Weiss LA, Najm J, Kutsche K, et al. Disruption of neurexin 1 associated with autism spectrum disorder. Am J Hum Genet. 2008;82:199–207. doi: 10.1016/j.ajhg.2007.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Biederer T, Sara Y, Mozhayeva M, Atasoy D, Liu X, Kavalali ET, Südhof TC. SynCAM, a synaptic adhesion molecule that drives synapse assembly. Science. 2002;297:1525–1531. doi: 10.1126/science.1072356. [DOI] [PubMed] [Google Scholar]
- 25.Sara Y, Biederer T, Atasoy D, Chubykin A, Mozhayeva MG, Südhof TC, Kavalali ET. Selective capability of SynCAM and neuroligin for functional synapse assembly. J Neurosci. 2005;25:260–270. doi: 10.1523/JNEUROSCI.3165-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fogel AI, Akins MR, Krupp AJ, Stagi M, Stein V, Biederer T. SynCAMs organize synapses through heterophilic adhesion. J Neurosci. 2007;27:12516–12530. doi: 10.1523/JNEUROSCI.2739-07.2007.This study identifies a heterophilic trans-synaptic adhesion complex constituted by the synaptic SynCAM Ig proteins that organizes nascent synapses to promote transmission, indicative of an adhesive code.
- 27.Thomas LA, Akins MR, Biederer T. Expression and adhesion profiles of SynCAM molecules indicate distinct neuronal functions. J Comp Neurol. 2008;510:47–67. doi: 10.1002/cne.21773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim S, Burette A, Chung HS, Kwon SK, Woo J, Lee HW, Kim K, Kim H, Weinberg RJ, Kim E. NGL family PSD-95-interacting adhesion molecules regulate excitatory synapse formation. Nat Neurosci. 2006;9:1294–1301. doi: 10.1038/nn1763.The authors introduce a trans-synaptic signaling pathway organized by the postsynaptic membrane protein NGL2 that differentiates glutamatergic synapses.
- 29.Ko J, Kim S, Chung HS, Kim K, Han K, Kim H, Jun H, Kaang BK, Kim E. SALM synaptic cell adhesion-like molecules regulate the differentiation of excitatory synapses. Neuron. 2006;50:233–245. doi: 10.1016/j.neuron.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 30.Wang CY, Chang K, Petralia RS, Wang YX, Seabold GK, Wenthold RJ. A novel family of adhesion-like molecules that interacts with the NMDA receptor. J Neurosci. 2006;26:2174–2183. doi: 10.1523/JNEUROSCI.3799-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seabold GK, Wang PY, Chang K, Wang CY, Wang YX, Petralia RS, Wenthold RJ. The SALM family of adhesion-like molecules forms heteromeric and homomeric complexes. J Biol Chem. 2008;283:8395–8405. doi: 10.1074/jbc.M709456200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ango F, Wu C, Van der Want JJ, Wu P, Schachner M, Huang ZJ. Bergmann glia and the recognition molecule CHL1 organize GABAergic axons and direct innervation of Purkinje cell dendrites. PLoS Biology. 2008;6:e103. doi: 10.1371/journal.pbio.0060103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Samuels BA, Hsueh Y-P, Shu T, Liang H, Tseng H-C, Hong C-J, Su SC, Volker J, Neve RL, Yue DT, et al. Cdk5 promotes synaptogenesis by regulating the subcellular distribution of the MAGUK family member CASK. Neuron. 2007;56:823–837. doi: 10.1016/j.neuron.2007.09.035.This study establishes that presynaptic signaling through the kinase Cdk5 participates in synapse formation and regulates synaptic scaffolds.
- 34.Dai Y, Taru H, Deken SL, Grill B, Ackley B, Nonet ML, Jin Y. SYD-2 Liprin-alpha organizes presynaptic active zone formation through ELKS. Nat Neurosci. 2006;9:1479–1487. doi: 10.1038/nn1808. [DOI] [PubMed] [Google Scholar]
- 35.Takeichi M. The cadherin superfamily in neuronal connections and interactions. Nat Rev Neurosci. 2007;8:11–20. doi: 10.1038/nrn2043. [DOI] [PubMed] [Google Scholar]
- 36.Kwiatkowski AV, Weis WI, Nelson WJ. Catenins: playing both sides of the synapse. Curr Opin Cell Biol. 2007;19:551–556. doi: 10.1016/j.ceb.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Elste AM, Benson DL. Structural basis for developmentally regulated changes in cadherin function at synapses. J Comp Neurol. 2006;495:324–335. doi: 10.1002/cne.20876. [DOI] [PubMed] [Google Scholar]
- 38.Bamji SX, Rico B, Kimes N, Reichardt LF. BDNF mobilizes synaptic vesicles and enhances synapse formation by disrupting cadherin-beta-catenin interactions. J Cell Biol. 2006;174:289–299. doi: 10.1083/jcb.200601087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Elia LP, Yamamoto M, Zang K, Reichardt LF. p120 catenin regulates dendritic spine and synapse development through Rho-family GTPases and cadherins. Neuron. 2006;51:43–56. doi: 10.1016/j.neuron.2006.05.018.This work examines postsynaptic signaling by studying the cadherin/p120-catenin pathway. p120 regulates both cadherin amounts and small GTPase signaling to maintain proper spine maturation and synapse numbers in vivo.
- 40.Webb DJ, Zhang H, Majumdar D, Horwitz AF. alpha5 integrin signaling regulates the formation of spines and synapses in hippocampal neurons. J Biol Chem. 2007;282:6929–6935. doi: 10.1074/jbc.M610981200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sfakianos MK, Eisman A, Gourley SL, Bradley WD, Scheetz AJ, Settleman J, Taylor JR, Greer CA, Williamson A, Koleske AJ. Inhibition of Rho via Arg and p190RhoGAP in the postnatal mouse hippocampus regulates dendritic spine maturation, synapse and dendrite stability, and behavior. J Neurosci. 2007;27:10982–10992. doi: 10.1523/JNEUROSCI.0793-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Henkemeyer M, Itkis OS, Ngo M, Hickmott PW, Ethell IM. Multiple EphB receptor tyrosine kinases shape dendritic spines in the hippocampus. Journal of Cell Biology. 2003;163:1313–1326. doi: 10.1083/jcb.200306033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kayser MS, McClelland AC, Hughes EG, Dalva MB. Intracellular and trans-synaptic regulation of glutamatergic synaptogenesis by EphB receptors. J Neurosci. 2006;26:12152–12164. doi: 10.1523/JNEUROSCI.3072-06.2006.This work demonstrates that the postsynaptic EphB2 receptor organizes glutamate receptor assembly and induces presynaptic terminals through interactions with its ephrinB ligand.
- 44.Lim BK, Matsuda N, Poo M-m. Ephrin-B reverse signaling promotes structural and functional synaptic maturation in vivo. Nat Neurosci. 2008;11:160–169. doi: 10.1038/nn2033.The authors show that ephrinB reverse signaling into axons promotes bouton numbers and synaptic plasticity, demonstrating that presynaptic ephrinB organizes both synaptic differentiation and function.
- 45.Tolias KF, Bikoff JB, Kane CG, Tolias CS, Hu L, Greenberg ME. The Rac1 guanine nucleotide exchange factor Tiam1 mediates EphB receptor-dependent dendritic spine development. Proceedings of the National Academy of Sciences. 2007;104:7265–7270. doi: 10.1073/pnas.0702044104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moeller ML, Shi Y, Reichardt LF, Ethell IM. EphB receptors regulate dendritic spine morphogenesis through the recruitment/phosphorylation of focal adhesion kinase and RhoA activation. J Biol Chem. 2006;281:1587–1598. doi: 10.1074/jbc.M511756200. [DOI] [PubMed] [Google Scholar]
- 47.Segura I, Essmann CL, Weinges S, Acker-Palmer A. Grb4 and GIT1 transduce ephrinB reverse signals modulating spine morphogenesis and synapse formation. Nat Neurosci. 2007;10:301–310. doi: 10.1038/nn1858. [DOI] [PubMed] [Google Scholar]
- 48.Aoto J, Ting P, Maghsoodi B, Xu N, Henkemeyer M, Chen L. Postsynaptic EphrinB3 promotes shaft glutamatergic synapse formation. J. Neurosci. 2007;27:7508–7519. doi: 10.1523/JNEUROSCI.0705-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rodenas-Ruano A, Perez-Pinzon MA, Green EJ, Henkemeyer M, Liebl DJ. Distinct roles for ephrinB3 in the formation and function of hippocampal synapses. Developm Biol. 2006;292:34–45. doi: 10.1016/j.ydbio.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 50.Reichardt LF. Neurotrophin-regulated signalling pathways. Philos Trans R Soc Lond B Biol Sci. 2006;361:1545–1564. doi: 10.1098/rstb.2006.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chiu SL, Chen CM, Cline HT. Insulin receptor signaling regulates synapse number, dendritic plasticity, and circuit function in vivo. Neuron. 2008;58:708–719. doi: 10.1016/j.neuron.2008.04.014.Synaptic signaling receives an intriguing additional player with this study, identifying roles of the insulin receptor in maintaining synapse density and neuronal network function.
- 52.Li B, Woo R-S, Mei L, Malinow R. The neuregulin-1 receptor ErbB4 controls glutamatergic synapse maturation and plasticity. Neuron. 2007;54:583–597. doi: 10.1016/j.neuron.2007.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Woo R-S, Li X-M, Tao Y, Carpenter-Hyland E, Huang YZ, Weber J, Neiswender H, Dong X-P, Wu J, Gassmann M, et al. Neuregulin-1 enhances depolarization-induced GABA release. Neuron. 2007;54:599–610. doi: 10.1016/j.neuron.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 54.Zhou J, Du W, Zhou K, Tai Y, Yao H, Jia Y, Ding Y, Wang Y. Critical role of TRPC6 channels in the formation of excitatory synapses. Nat Neurosci. 2008;11:741–743. doi: 10.1038/nn.2127. [DOI] [PubMed] [Google Scholar]
- 55.Ahmad-Annuar A, Ciani L, Simeonidis I, Herreros J, Fredj NB, Rosso SB, Hall A, Brickley S, Salinas PC. Signaling across the synapse: a role for Wnt and Dishevelled in presynaptic assembly and neurotransmitter release. J Cell Biol. 2006;174:127–139. doi: 10.1083/jcb.200511054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Inaki M, Yoshikawa S, Thomas JB, Aburatani H, Nose A. Wnt4 Is a local repulsive cue that determines synaptic target specificity. Curr Biol. 2007;17:1574–1579. doi: 10.1016/j.cub.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 57.Klassen MP, Shen K. Wnt signaling positions neuromuscular connectivity by inhibiting synapse formation in C. elegans. Cell. 2007;130:704–716. doi: 10.1016/j.cell.2007.06.046.The authors identify that Wnt signaling affects the localization of its receptor to negatively regulate synapse formation, and demonstrate with this a novel pathway that patterns synaptic connections.
- 58.Colon-Ramos DA, Margeta MA, Shen K. Glia promote local synaptogenesis through UNC-6 (netrin) signaling in C. elegans. Science. 2007;318:103–106. doi: 10.1126/science.1143762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ledda F, Paratcha G, Sandoval-Guzman T, Ibanez CF. GDNF and GFRalpha1 promote formation of neuronal synapses by ligand-induced cell adhesion. Nat Neurosci. 2007;10:293–300. doi: 10.1038/nn1855. [DOI] [PubMed] [Google Scholar]
- 60.Allen NJ, Barres BA. Signaling between glia and neurons: focus on synaptic plasticity. Curr Opin Neurobiol. 2005;15:542–548. doi: 10.1016/j.conb.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 61.Wegner AM, Nebhan CA, Hu L, Majumdar D, Meier KM, Weaver AM, Webb DJ. N-WASP and the Arp2/3 complex are critical regulators of actin in the development of dendritic spines and synapses. J. Biol. Chem. 2008 doi: 10.1074/jbc.M801555200. M801555200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sytnyk V, Leshchyns'ka I, Nikonenko AG, Schachner M. NCAM promotes assembly and activity-dependent remodeling of the postsynaptic signaling complex. J Cell Biol. 2006;174:1071–1085. doi: 10.1083/jcb.200604145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pielage J, Cheng L, Fetter RD, Carlton PM, Sedat JW, Davis GW. A presynaptic giant ankyrin stabilizes the NMJ through regulation of presynaptic microtubules and transsynaptic cell adhesion. Neuron. 2008;58:195–209. doi: 10.1016/j.neuron.2008.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Koch I, Schwarz H, Beuchle D, Goellner B, Langegger M, Aberle H. Drosophila ankyrin 2 Is required for synaptic stability. Neuron. 2008;58:210–222. doi: 10.1016/j.neuron.2008.03.019. [DOI] [PubMed] [Google Scholar]
- 65.Chao HT, Zoghbi HY, Rosenmund C. MeCP2 controls excitatory synaptic strength by regulating glutamatergic synapse number. Neuron. 2007;56:58–65. doi: 10.1016/j.neuron.2007.08.018.This study identifies that the transcriptional regulator MeCP2, which is mutated in Rett syndrome, regulates synapse number and transmission of glutamatergic neurons. This supports a link of this neurodevelopmental disorder to synaptogenesis.
- 66.Flavell SW, Cowan CW, Kim TK, Greer PL, Lin Y, Paradis S, Griffith EC, Hu LS, Chen C, Greenberg ME. Activity-dependent regulation of MEF2 transcription factors suppresses excitatory synapse number. Science. 2006;311:1008–1012. doi: 10.1126/science.1122511. [DOI] [PubMed] [Google Scholar]
- 67.Chattopadhyaya B, Di Cristo G, Wu CZ, Knott G, Kuhlman S, Fu Y, Palmiter RD, Huang ZJ. GAD67-mediated GABA synthesis and signaling regulate inhibitory synaptic innervation in the visual cortex. Neuron. 2007;54:889–903. doi: 10.1016/j.neuron.2007.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Paradis S, Harrar DB, Lin Y, Koon AC, Hauser JL, Griffith EC, Zhu L, Brass LF, Chen C, Greenberg ME. An RNAi-based approach identifies molecules required for glutamatergic and GABAergic synapse development. Neuron. 2007;53:217–232. doi: 10.1016/j.neuron.2006.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Valor LM, Charlesworth P, Humphreys L, Anderson CNG, Grant SGN. Network activity-independent coordinated gene expression program for synapse assembly. Proceedings of the National Academy of Sciences. 2007;104:4658–4663. doi: 10.1073/pnas.0609071104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Knott GW, Holtmaat A, Wilbrecht L, Welker E, Svoboda K. Spine growth precedes synapse formation in the adult neocortex in vivo. Nat Neurosci. 2006;9:1117–1124. doi: 10.1038/nn1747. [DOI] [PubMed] [Google Scholar]