Summary of Recent Advances
Neural cell adhesion molecules (CAMs) of the immunoglobulin superfamily engage in multiple neuronal interactions that influence cell migration, axonal and dendritic projection, and synaptic targeting. Their downstream signal transduction events specify whether a cell moves or projects axons and dendrites to targets in the brain. Many of the diverse functions of CAMs are brought about through homophilic and heterophilic interactions with other cell surface receptors. An emerging concept is that CAMs act as co-receptors to assist in intracellular signal transduction, and to provide cytoskeletal linkage necessary for cell and growth cone motility. Here we will focus on new discoveries that have revealed novel co-receptor functions for the best understood CAMs - L1, CHL1, and NCAM- important for neuronal migration and axon guidance. We will also discuss how dysregulation of CAMs may also bear on neuropsychiatric disease and cancer.
Introduction
Neural cell adhesion molecules of the immunoglobulin (Ig) superfamily are complex transmembrane proteins capable of multiple molecular interactions mediated by their large and divergent extracellular regions, while their shorter cytoplasmic domains link reversibly to the actin cytoskeleton [1]. The L1 family of cell adhesion molecules (L1-CAMs) is comprised of four structurally related transmembrane proteins in vertebrates: L1, Close Homolog of L1 (CHL1), NrCAM, and Neurofascin. L1-CAMs have 6 Ig-like domains and 4–5 fibronectin III repeats in their divergent extracellular regions and a more conserved cytoplasmic domain of ~100 residues that links to actin. Despite an overall similarity in structure, L1-CAMs share only 35–45% homology. The less closely related adhesion molecule, NCAM, was the first CAM identified, and contains 5 Ig-like and 2 fibronectin type III repeats followed by a variably spliced cytoplasmic domain that produces 2 major transmembrane isoforms (180 kDa,140 kDa) and a glycophosphatidyl inositol (GPI)-linked isoform (120 kDa). The cytoplasmic domains of transmembrane NCAM isoforms link to the actin cytoskeleton by direct binding to spectrin. L1-CAMs and NCAM have long been known to signal intracellularly, but recent studies demonstrate they act as co-receptors for integrins, growth factors, and receptors for repellent axon guidance molecules
L1 and CHL1 as Signaling Co-Receptors in Cortical Development
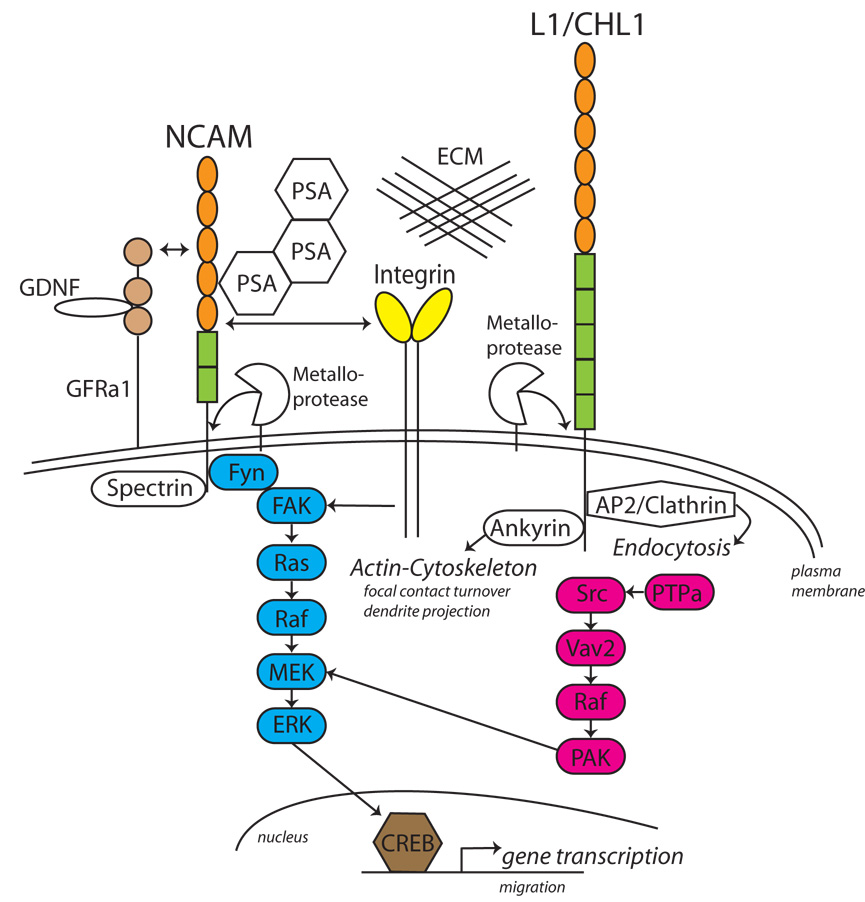
Cell migration and neurite outgrowth on extracellular matrix protein (ECM) substrates is potentiated by a functional interaction of L1 with β1 integrins. The interaction is mediated by a conserved RGD binding motif in the sixth immunoglobin-like domain and the third fibronectin type III repeat of L1 [2,3], which serves to strengthen adhesion of the cell to the ECM (Fig. 1). L1 and β1 integrins associate with low affinity on the cell surface, and activate a common intracellular signaling pathway. This pathway involves the sequential activation of the non-receptor tyrosine kinase c-Src, phosphotidylinositide 3-kinase (PI3 kinase), Vav2 guanine nucleotide exchange factor, Rac1 GTPase, p21-activated kinase (PAK1), MEK and the MAP kinases ERK1/2 [4,5] (Fig.1). The L1 and β1 integrin signaling pathway can converge with growth factor signaling pathways, culminating in increased ERK activation. For example, coincident activation of L1 and platelet-derived growth factor (PDGF) receptor causes sustained ERK activation, as opposed to transient activation in the case of L1 or PDGF signaling alone [6]. Sustained ERK signaling upregulates the expression of integrins and Rac1 to increase cell motility. Since ERK is downstream of many receptor tyrosine kinases, such as IGF1-, EGF-, and FGF-, and Trk receptors, L1 convergent signaling may be a widespread neurodevelopmental mechanism.
Fig. 1. NCAM and L1-CAMs as Co-Receptors in Integrin and GDNF Receptor Signaling.
Transmembrane isoforms of NCAM and L1/CHL1 activate distinct signaling pathways to regulate neuronal migration, axon growth, and dendrite projection, converging at the level of ERK1/2 MAP kinase. NCAM and L1-CAMs interact with β1 integrins to increase signaling and modulate adhesion to extracellular matrix proteins (ECM). NCAM also associates with, and transduces signals from, the GDNF receptor GFRα1. PSA can be added post-translationally to the NCAM Ig5 domain, reducing NCAM interactions. L1 is endocytosed by binding to the AP2 clathrin adaptor at a sequence (RSLE) in the cytoplasmic domain of a neuronal L1 isoform. NCAM, L1 and CHL1 are each cleaved by ADAM metalloproteases releasing ectodomain fragments that may downregulate or stimulate CAM function. Rectangles = FNIII domains, Ovals=Ig-like domains
ERK MAP kinases also feedback to L1 by modulating its linkage to the actin cytoskeleton. All L1-CAMs reversibly engage the actin cytoskeleton through a conserved motif FIGQ/AY in the cytoplasmic domain, which contains a critical tyrosine residue required for binding the spectrin-actin adaptor ankyrin [7]. ERK, a serine/threonine protein kinase, was shown to indirectly induce tyrosine phosphorylation in the FIGQY motif in the L1 intracellular domain [8], thereby dissociating ankyrin and uncoupling L1 from the actin cytoskeleton. The tyrosine kinase responsible for FIGQ/AY phosphorylation remains elusive. Dynamic adhesive interactions controlled by phosphorylation/dephosphorylation of the ankyrin motif in L1 family members may enable a cell or growth cone to cyclically attach and detach from the ECM substrate or from neighboring cells, thus facilitating migration. Such cytoskeletal coupling induced by L1-ankyrin interactions has a critical in vivo function in synaptic targeting. An L1 mutant mouse was engineered to contain a point mutation in the critical tyrosine residue of the ankyrin binding motif (Tyr1229His), which is also a human pathological mutation associated with mental retardation [9]. The L1-ankyrin deficient mouse displays defective topographic mapping of retinal axons to synaptic targets along the mediolateral axis of the superior colliculus regulated by the EphrinB/EphB guidance system. L1 cooperates with EphrinB/EphB to modulate adhesion of retinal cells to ECM in vitro, which could promote retinal axon branching important for mediolateral targeting or stabilize synaptic termination zones. Similarly, targeting of cerebellar granule cell axons to the Purkinje cell axon initial segment requires linkage to the cytoskeleton through interaction of ankyrin with Neurofascin [10].
CHL1 plays a key role in neuronal positioning in caudal neocortical areas (visual and somatosensory cortex), where CHL1 is enriched compared to rostral areas [11]. Analysis of CHL1-deficient mice revealed that CHL1 acts to position deep layer pyramidal neurons in appropriate cortical laminae by facilitating radial migration of neuronal precursors. CHL1 functions coordinately with β1-integrins during the migration process, since haptotactic migration of embryonic cortical neurons is inhibited in the presence of integrin blocking antibodies in vitro. Cell-based assays implicate c-Src, PI3 kinase, and ERK1/2 in the CHL1-integrin signaling pathway [12]. Integrins have been shown to participate in the mechanism of radial migration of embryonic cortical neurons [13] but appear to function when expressed on radial glia, rather than on neurons [14]. Thus, a trans heterophilic interaction between CHL1 on neurons, and β1 integrins on radial glia, may transduce signaling in migrating cortical neurons responsible for correct laminar positioning. Other L1-CAMs might contribute to radial migration of neurons in different brain regions. In this regard it is interesting that L1 and NrCAM double knockout mice exhibit a cerebellar phenotype consistent with impaired migration [15].
CHL1 is also required for directing apical dendrites of deep layer pyramidal neurons toward the pial surface in caudal cortical areas [11]. Important in this context, CHL1 associates with NB3, a glycophosphatidyl inositol-linked member of the TAG1/contactin/NB3 family of surface receptors, which like CHL1 is expressed in a low-rostral to high-caudal gradient in the developing neocortex [16]. Pyramidal neurons in NB3-deficient mice exhibit misoriented, often inverted, apical dendrites similar to those in CHL1 null mutants. However, NB3 mutant neurons do not show laminar displacement, suggesting that migration and dendritic orientation may be independently controlled. CHL1/NB3 clustering activates protein tyrosine phosphatase α (PTPα), which dephosphorylates and activates the Src family kinase Fyn. Both PTPα and Fyn are required for proper apical dendrite orientation of deep layer pyramidal neurons [17].
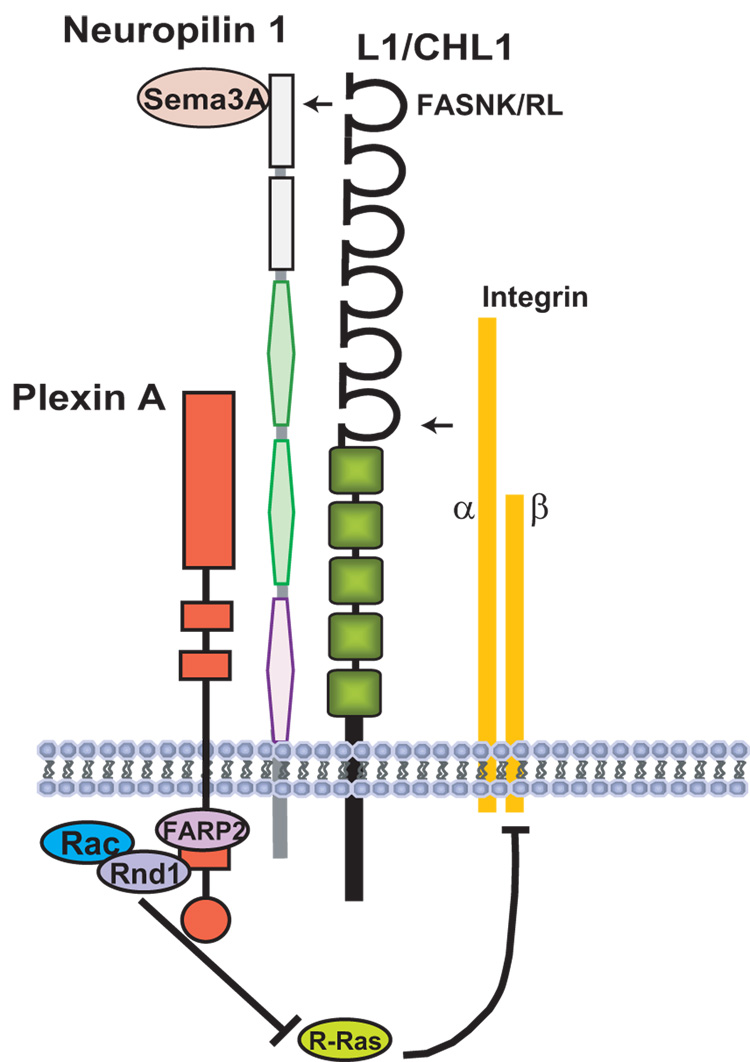
Semaphorin3A (Sema3A), a member of class-III Semaphorins, is a secreted protein that is involved in repulsive, and in some cases attractive, axon guidance and dendrite projection. Interestingly, a Sema3A gradient in the developing cortex is required to maintain radial migration of embryonic cortical neurons [18] and to attract their apical dendrites toward the pial surface [19]. However, Sema3A knock-out mice show no obvious cortical lamination defects, suggesting the involvement of compensatory factors. Both CHL1 [20] and L1[21] bind the Sema3A receptor, Neuropilin-1, via a conserved sequence in the Ig1 domain, and act as obligate co-receptors to mediate Sema3A-induced growth cone collapse and axon repulsion (Fig. 2). The repellent response of L1/Neuropilin-1 involves signaling through focal adhesion kinase (FAK) and ERK, leading to disassembly of focal adhesions [22]. However, Sema3A-induced repulsion can be converted to Sema3A-induced attraction by homophilic binding of L1 on an apposing cell in trans with L1 complexed to Neuropilin-1 in the responding neuron [21,23]. In this way, L1 and CHL1 acting as co-receptors with Neuropilin-1 in newly differentiating pyramidal neurons might promote attraction of apical dendrites in response to local homophilic binding in the presence of the cortical Sema3A gradient.
Fig. 2. L1-CAMs as Co-Receptors for Semaphorin3A Signaling in Axon Repulsion and Growth Cone Collapse.
L1 and CHL1 associate with the Sema3A receptor Neuropilin1 on the plasma membrane through conserved Ig1 domain motifs in L1 (FASNRL) and CHL1 (FASNKL) and with integrins through the Ig6 domain. PlexinAs are signaling co-receptors for Neuropilin-1. In response to Sema3A, the guanine nucleotide exchange factor FARP2 activates Rac1, which leads to inactivation of R-Ras and integrins through binding of Rnd1 to PlexinA. This mechanism contributes to growth cone collapse and axon repulsion from Sema3A.
NCAM Signaling and Post-translational Modification
Similar to L1-CAMs, NCAM regulates important aspects of integrin-dependent cell migration, axonal/dendritic outgrowth, and synaptic targeting. L1 and NCAM both interact functionally with integrins and activate convergent signaling pathways leading to ERK MAP kinase (Fig. 1). However, they have different proximal signaling intermediates that differentially influence the cellular outcome [1]. Whereas L1 activates ERK through Src in a FAK-independent manner, NCAM induces recruitment of tyrosine kinase Fyn to its intracellular domain, which in turn activates FAK, ERK, and the transcription factor cAMP response element binding protein (CREB), which stimulates neurite outgrowth (Fig. 1). FAK is a key intracellular regulator of migration in nonneuronal cells, acting by increasing turnover of focal adhesive contacts at the cell’s leading edge. In neurons, FAK is more important as a negative regulator of dendritic and axonal branching than migration outgrowth [24,25]. This may be due to the existence of multiple pathways that converge on FAK in vivo. NCAM signaling to ERK is more complex than L1-ERK signaling and involves interactions with different receptors. For example, NCAM signaling within lipid rafts is independent of the fibroblast growth factor (FGF) receptor, whereas a component of NCAM-ERK signaling in non-rafts is FGF receptor-dependent [26]. The NCAM-FGR receptor pathway further differs by signaling through phospholipase Cγ and protein kinase C [27].
NCAM is also a signaling co-receptor for the glial cell line-derived neurotrophic factor (GDNF) receptor GFRα1, and functions by potentiating Schwann cell migration and neurite growth [28]. GDNF signals in conjunction with NCAM and GFRα1 by activating FAK and Fyn. This activation decreases NCAM-dependent cell adhesion, illustrating a general principal that an optimal level of cell-substratum contact is needed for motility, with too much or too little impeding it. NCAM also mediates chemo-attraction of olfactory interneuron precursors to GDNF in the rostral migratory stream, but is not required for GDNF/GFRα1-mediated tangential migration of cortical interneurons [29].
An unusual mechanism that modulates NCAM-dependent cell adhesion during migration and synaptogenesis is provided by the polysialyltransferases PST and STX, which attach polysialic acid (PSA) to the fifth Ig-like domain of NCAM in a developmentally regulated manner. PSA is thought to act through steric repulsion between individual PSA clusters, thus increasing the distance between NCAM molecules on apposed cells. PSA attachment to NCAM globally regulates cellular responses to other factors by creating a permissive environment for cell migration. Deletion of PST and STX to prevent PSA attachment to NCAM inhibits the rostral migration of olfactory neuron precursors [30], and also affects both tangential and radial migration of cortical precursors, resulting in aberrant positioning of neuronal and glial cells [31]. PST/STX deficient mice also display more severe axonal defects than NCAM-deficient mice due to gain-of function effects [30]. Premature removal of PSA from NCAM by endoglycosidase N treatment promotes synaptogenesis between GABAergic interneuron subpopulations and pyramidal cells in the visual cortex and regulates the onset of the critical period of visual plasticity in adolescence [32]. On the other hand, surface levels of PSA-NCAM are upregulated in adult hippocampal neurons undergoing activity-dependent plasticity [33]. Thus, PSA modification of NCAM at different places and times may regulate molecular interactions leading to either synaptogenesis or synaptic remodeling.
NCAM function can be downregulated by other mechanisms such as ectodomain shedding by ADAM (a disintegrin and metalloprotease) proteases (see [34]) and endocytosis by NCAM ubiquitylation [35]. L1-CAMs are also subject to ADAM-mediated ectodomain shedding and endocytosis by a clathrin-dependent mechanism (Fig. 1). Ectodomain shedding of CAMs can downregulate CAM-dependent migration or neurite outgrowth [36], although in some cases it increases CAM activity by autocrine stimulation [37]. Downregulation of NCAM function by ADAM-mediated shedding in cortical neurons decreases branching and neurite outgrowth in vitro [36], and inhibits branching and synaptogenesis of cortical interneurons in a mouse transgenic model excessive NCAM ectodomain [38]. Dysregulation of NCAM shedding may contribute to neuronal pathology in schizophrenia. Elevated levels of the entire soluble NCAM extracellular fragment have been described in affected brain regions and cerebrospinal fluid in schizophrenia [39].
Neural Adhesion Molecules and Cancer
Neural cell adhesion molecules have gained much attention in cancer research due to their upregulation in human brain tumors. NCAM is strongly upregulated in many neuroblastomas, where it becomes post-translationally modified to PSA-NCAM, thus enhancing invasion of tumor cells [40]. Overexpression of L1 correlates with tumor progression and metastasis in certain neural tumors, most notably melanoma [41], while NrCAM is upregulated in high risk neuroblastomas without N-myc amplification [42]. Cell adhesion molecule upregulation is likely to stimulate tumor cell invasiveness by signaling mechanisms that enhance migration. This probably involves cycles of cell attachment and deattachment regulated through signaling by dynamic interactions with the cytoskeleton, since purely increased adhesion would be expected to reduce rather than promote migration. The well-documented ability of L1-CAMs and NCAM to promote integrin-dependent cell migration through ERK MAP kinase could contribute to tumor cell invasiveness. Accordingly, antibody-based therapeutic strategies are being pursued to functionally inhibit homophilic and heterophilic interactions of cell adhesion molecules to curb tumor invasiveness [43].
Current research into the basic mechanisms of neural cell adhesion molecule regulation and their functions as co-receptors for integrins, growth factors, and repellent axon guidance receptors may become instrumental in discovering new therapeutic approaches in cancer research. In turn, tumor models may increase our understanding of the function for neural cell adhesion molecules for migration and process outgrowth in the developing nervous system. Differences in L1-CAM and NCAM signaling pathways during development may generate distinct patterns of cortical connectivity in different neuronal populations or brain regions. Disruption of this signaling likely contributes to distinct developmental neuropsychiatric disorders that are associated with mutation or genetic polymorphisms in genes encoding L1 (X-linked mental retardation)[44], CHL1 (low IQ, speech and motor delay) [45], and NCAM (schizophrenia, bipolar disorder)[39,46,47].
Acknowledgements
This work was supported by NIH Grants NS049109 and NH064056 (Silvio O. Conte Center for Neuroscience of Mental Disorders), National Science Foundation Grant NSF0618176, and Autism Speaks Grant #1847.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Annotations
* of special interest: references 3, 6, 18, 20, 26, 33, 37
** of exceptional interest as follows:
** 10. The authors used BAC transgenic reporter mice expressing green fluorescent protein in an interneuron subpopulation to show that a subcellular gradient of the Neurofascin 186 isoform along the dendritic-somal-axon initial segment axis of cerebellar Purkinje cells is formed by direct interaction with an ankyrin B isoform, and is responsible for axon targeting of basket interneurons to the axon initial segment. AnkyrinG- associated Neurofascin186 is suggested to be required for the formation or stabilization of pinceau synapses at the axon initial segment.
** 11. This study demonstrated for the first time a function for a cell adhesion molecule, CHL1, in cortical neuron migration and laminar positioning. CHL1 knockout mice were crossed with a fluorescent reporter strain (Thy1-YFP) to label layer V pyramidal neurons to reveal malpositioning of neurons to deeper layers specifically in caudal cortical areas (somatosensory and visual cortex) where CHL1 is normally expressed.
**21. This important work revealed a novel function for L1 in mediating repellent axon guidance and growth cone collapse to Sema3A. Using co-cultures of cortical slices and spinal cord explants from wild type and L1-minus mice, the authors demonstrate that this mechanism underlies repellent guidance of cortico-spinal tract axons in response to Sema3A in the ventral spinal cord, which may contribute to guidance at the pyramidal decussation in vivo. It is also shown that L1 forms a stable cis-interacting complex with the Sema3A receptor Neuropilin-1, and that soluble L1 protein as a ligand converts the repulsive response into attraction.
**28. The authors find that NCAM can act as a co-receptor for the GDNF receptor GFRα1 to activate FAK and Fyn signaling without the known GDNF signaling receptor RET. The NCAM-GFRα1 interaction downregulates NCAM-NCAM homophilic binding, suggesting that cis-interacting GFRα1 induces conformation changes in NCAM that regulates its ability to interact with different ligands.
**30. The authors generated PSA-negative, NCAM -positive mice by deletion of two polysialyltransferase genes, STX and PST, to reveal for the first time that loss of a glycan can be more important than loss of the glyco-conjugated protein for brain development. Loss of PSA caused a more severe phenotype of axonal tract defects, hydrocephalus, and lethality, which were rescued in a triple knockouts lacking NCAM. Thus, a major function of PSA is to mask NCAM function at certain times and places enabling NCAM contacts to form in an orderly manner.
References
- 1.Maness PF, Schachner M. Neural recognition molecules of the immunoglobulin superfamily: signaling transducers of axon guidance and neuronal migration. Nat Neurosci. 2007;10:19–26. doi: 10.1038/nn1827. [DOI] [PubMed] [Google Scholar]
- 2.Thelen K, Kedar V, Panicker AK, Schmid RS, Midkiff BR, Maness PF. The neural cell adhesion molecule L1 potentiates integrin-dependent cell migration to extracellular matrix proteins. J Neurosci. 2002;22:4918–4931. doi: 10.1523/JNEUROSCI.22-12-04918.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Silletti S, Mei F, Sheppard D, Montgomery AM. Plasmin-sensitive dibasic sequences in the third fibronectin-like domain of L1-cell adhesion molecule (CAM) facilitate homomultimerization and concomitant integrin recruitment. J Cell Biol. 2000;149:1485–1502. doi: 10.1083/jcb.149.7.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schmid RS, Midkiff BR, Kedar VP, Maness PF. Adhesion molecule L1 stimulates neuronal migration through Vav2-Pak1 signaling. Neuroreport. 2004;15:2791–2794. [PubMed] [Google Scholar]
- 5.Schmid RS, Pruitt WM, Maness PF. A MAP kinase signaling pathway mediates neurite outgrowth on L1 and requires Src-dependent endocytosis. J Neurosci. 2000;11:4177–4188. doi: 10.1523/JNEUROSCI.20-11-04177.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Silletti S, Yebra M, Perez B, Cirulli V, McMahon M, Montgomery AM. Extracellular signal-regulated kinase (ERK)-dependent gene expression contributes to L1 cell adhesion molecule-dependent motility and invasion. J Biol Chem. 2004;279:28880–28888. doi: 10.1074/jbc.M404075200. [DOI] [PubMed] [Google Scholar]
- 7.Bennett V, Healy J. Organizing the fluid membrane bilayer: diseases linked to spectrin and ankyrin. Trends Mol Med. 2008;14:28–36. doi: 10.1016/j.molmed.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 8.Whittard JD, Sakurai T, Cassella MR, Gazdoiu M, Felsenfeld DP. MAP kinase pathway-dependent phosphorylation of the L1-CAM ankyrin binding site regulates neuronal growth. Mol Biol Cell. 2006;17:2696–2706. doi: 10.1091/mbc.E06-01-0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buhusi M, Schlatter MC, Demyanenko GP, Thresher R, Maness PF. L1 interaction with ankyrin regulates mediolateral topography in the retinocollicular projection. J Neurosci. 2008;28:177–188. doi: 10.1523/JNEUROSCI.3573-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ango F, di Cristo G, Higashiyama H, Bennett V, Wu P, Huang ZJ. Ankyrin-based subcellular gradient of neurofascin, an immunoglobulin family protein, directs GABAergic innervation at purkinje axon initial segment. Cell. 2004;119:257–272. doi: 10.1016/j.cell.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 11.Demyanenko GP, Schachner M, Anton E, Schmid R, Feng G, Sanes J, Maness PF. Close homolog of L1 modulates area-specific neuronal positioning and dendrite orientation in the cerebral cortex. Neuron. 2004;44:423–437. doi: 10.1016/j.neuron.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 12.Buhusi M, Midkiff BR, Gates AM, Richter M, Schachner M, Maness PF. Close homolog of L1 is an enhancer of integrin-mediated cell migration. J Biol Chem. 2003;278:25024–25031. doi: 10.1074/jbc.M303084200. [DOI] [PubMed] [Google Scholar]
- 13.Schmid RS, Jo R, Shelton S, Kreidberg JA, Anton ES. Reelin, Integrin and Dab1 Interactions during Embryonic Cerebral Cortical Development. Cereb Cortex. 2005 doi: 10.1093/cercor/bhi041. [DOI] [PubMed] [Google Scholar]
- 14.Belvindrah R, Graus-Porta D, Goebbels S, Nave KA, Muller U. Beta1 integrins in radial glia but not in migrating neurons are essential for the formation of cell layers in the cerebral cortex. J Neurosci. 2007;27:13854–13865. doi: 10.1523/JNEUROSCI.4494-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sakurai T, Lustig M, Babiarz J, Furley AJ, Tait S, Brophy PJ, Brown SA, Brown LY, Mason CA, Grumet M. Overlapping functions of the cell adhesion molecules Nr-CAM and L1 in cerebellar granule cell development. J Cell Biol. 2001;154:1259–1273. doi: 10.1083/jcb.200104122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ye H, Tan YL, Ponniah S, Takeda Y, Wang SQ, Schachner M, Watanabe K, Pallen CJ, Xiao ZC. Neural recognition molecules CHL1 and NB-3 regulate apical dendrite orientation in the neocortex via PTP alpha. Embo J. 2008;27:188–200. doi: 10.1038/sj.emboj.7601939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sasaki Y, Cheng C, Uchida Y, Nakajima O, Ohshima T, Yagi T, Taniguchi M, Nakayama T, Kishida R, Kudo Y, et al. Fyn and Cdk5 mediate semaphorin-3A signaling, which is involved in regulation of dendrite orientation in cerebral cortex. Neuron. 2002;35:907–920. doi: 10.1016/s0896-6273(02)00857-7. [DOI] [PubMed] [Google Scholar]
- 18.Chen G, Sima J, Jin M, Wang KY, Xue XJ, Zheng W, Ding YQ, Yuan XB. Semaphorin-3A guides radial migration of cortical neurons during development. Nat Neurosci. 2008;11:36–44. doi: 10.1038/nn2018. [DOI] [PubMed] [Google Scholar]
- 19.Polleux F, Morrow T, Ghosh A. Semaphorin 3A is a chemoattractant for cortical apical dendrites. Nature. 2000;404:567–573. doi: 10.1038/35007001. [DOI] [PubMed] [Google Scholar]
- 20.Wright AG, Demyanenko GP, Powell A, Schachner M, Enriquez-Barreto L, Tran TS, Polleux F, Maness PF. Close homolog of L1 and neuropilin 1 mediate guidance of thalamocortical axons at the ventral telencephalon. J Neurosci. 2007;27:13667–13679. doi: 10.1523/JNEUROSCI.2888-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Castellani V, Chedotal A, Schachner M, Faivre-Sarrailh C, Rougon G. Analysis of the L1-deficient mouse phenotype reveals cross-talk between Sema3A and L1 signaling pathways in axonal guidance [see comments] Neuron. 2000;27:237–249. doi: 10.1016/s0896-6273(00)00033-7. [DOI] [PubMed] [Google Scholar]
- 22.Bechara A, Nawabi H, Moret F, Yaron A, Weaver E, Bozon M, Abouzid K, Guan JL, Tessier-Lavigne M, Lemmon V, et al. FAK-MAPK-dependent adhesion disassembly downstream of L1 contributes to semaphorin3A-induced collapse. Embo J. 2008;27:1549–1562. doi: 10.1038/emboj.2008.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Castellani V, De Angelis E, Kenwrick S, Rougon G. Cis and trans interactions of L1 with neuropilin-1 control axonal responses to semaphorin 3A. EMBO J. 2002;21:6348–6357. doi: 10.1093/emboj/cdf645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beggs HE, Schahin-Reed D, Zang K, Goebbels S, Nave KA, Gorski J, Jones KR, Sretavan D, Reichardt LF. FAK deficiency in cells contributing to the basal lamina results in cortical abnormalities resembling congenital muscular dystrophies. Neuron. 2003;40:501–514. doi: 10.1016/s0896-6273(03)00666-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rico B, Beggs HE, Schahin-Reed D, Kimes N, Schmidt A, Reichardt LF. Control of axonal branching and synapse formation by focal adhesion kinase. Nat Neurosci. 2004;7:1059–1069. doi: 10.1038/nn1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Niethammer P, Delling M, Sytnyk V, Dityatev A, Fukami K, Schachner M. Cosignaling of NCAM via lipid rafts and the FGF receptor is required for neuritogenesis. J Cell Biol. 2002;157:521–532. doi: 10.1083/jcb.200109059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kolkova K, Novitskaya V, Pedersen N, Berezin V, Bock E. Neural cell adhesion molecule-stimulated neurite outgrowth depends on activation of protein kinase C and the Ras-mitogen-activated protein kinase pathway. J Neurosci. 2000;20:2238–2246. doi: 10.1523/JNEUROSCI.20-06-02238.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paratcha G, Ledda F, Ibanez CF. The neural cell adhesion molecule NCAM is an alternative signaling receptor for GDNF family ligands. Cell. 2003;113:867–879. doi: 10.1016/s0092-8674(03)00435-5. [DOI] [PubMed] [Google Scholar]
- 29.Pozas E, Ibanez CF. GDNF and GFRalpha1 promote differentiation and tangential migration of cortical GABAergic neurons. Neuron. 2005;45:701–713. doi: 10.1016/j.neuron.2005.01.043. [DOI] [PubMed] [Google Scholar]
- 30.Weinhold B, Seidenfaden R, Rockle I, Muhlenhoff M, Schertzinger F, Conzelmann S, Marth JD, Gerardy-Schahn R, Hildebrandt H. Genetic ablation of polysialic acid causes severe neurodevelopmental defects rescued by deletion of the neural cell adhesion molecule. J Biol Chem. 2005;280:42971–42977. doi: 10.1074/jbc.M511097200. [DOI] [PubMed] [Google Scholar]
- 31.Angata K, Huckaby V, Ranscht B, Terskikh A, Marth JD, Fukuda M. Polysialic acid-directed migration and differentiation of neural precursors are essential for mouse brain development. Mol Cell Biol. 2007;27:6659–6668. doi: 10.1128/MCB.00205-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Di Cristo G, Chattopadhyaya B, Kuhlman SJ, Fu Y, Belanger MC, Wu CZ, Rutishauser U, Maffei L, Huang ZJ. Activity-dependent PSA expression regulates inhibitory maturation and onset of critical period plasticity. Nat Neurosci. 2007;10:1569–1577. doi: 10.1038/nn2008. [DOI] [PubMed] [Google Scholar]
- 33.Dityatev A, Dityateva G, Sytnyk V, Delling M, Toni N, Nikonenko I, Muller D, Schachner M. Polysialylated neural cell adhesion molecule promotes remodeling and formation of hippocampal synapses. J Neurosci. 2004;24:9372–9382. doi: 10.1523/JNEUROSCI.1702-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brennaman LH, Maness PF. NCAM in Neuropsychiatric and Neurodegenerative Disorders. Neurochem Res. 2008 doi: 10.1007/978-1-4419-1170-4_19. [DOI] [PubMed] [Google Scholar]
- 35.Diestel S, Schaefer D, Cremer H, Schmitz B. NCAM is ubiquitylated, endocytosed and recycled in neurons. J Cell Sci. 2007;120:4035–4049. doi: 10.1242/jcs.019729. [DOI] [PubMed] [Google Scholar]
- 36.Brennaman LH, Maness PF. Developmental regulation of GABAergic interneuron branching and synaptic development in the prefrontal cortex by soluble neural cell adhesion molecule. Mol Cell Neurosci. 2008;37:781–793. doi: 10.1016/j.mcn.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mechtersheimer S, Gutwein P, Agmon-Levin N, Stoeck A, Oleszewski M, Riedle S, Fogel M, Lemmon V, Altevogt P. Ectodomain shedding of L1 adhesion molecule promotes cell migration by autocrine binding to integrins. J Cell Biol. 2001;155:661–673. doi: 10.1083/jcb.200101099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pillai-Nair N, Panicker AK, Rodriguiz RM, Gilmore KL, Demyanenko GP, Huang JZ, Wetsel WC, Maness PF. Neural cell adhesion molecule-secreting transgenic mice display abnormalities in GABAergic interneurons and alterations in behavior. J Neurosci. 2005;25:4659–4671. doi: 10.1523/JNEUROSCI.0565-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vawter MP. Dysregulation of the neural cell adhesion molecule and neuropsychiatric disorders. Eur J Pharmacol. 2000;405:385–395. doi: 10.1016/s0014-2999(00)00568-9. [DOI] [PubMed] [Google Scholar]
- 40.Zecchini S, Cavallaro U. Neural Cell Adhesion Molecule in Cancer: Expression and Mechanisms. Neurochem Res. 2008 doi: 10.1007/978-1-4419-1170-4_20. [DOI] [PubMed] [Google Scholar]
- 41.Meier F, Busch S, Gast D, Goppert A, Altevogt P, Maczey E, Riedle S, Garbe C, Schittek B. The adhesion molecule L1 (CD171) promotes melanoma progression. Int J Cancer. 2006;119:549–555. doi: 10.1002/ijc.21880. [DOI] [PubMed] [Google Scholar]
- 42.Liu X, Mazanek P, Dam V, Wang Q, Zhao H, Guo R, Jagannathan J, Cnaan A, Maris JM, Hogarty MD. Deregulated Wnt/beta-catenin program in high-risk neuroblastomas without MYCN amplifycation. Oncogene. 2008;27:1478–1488. doi: 10.1038/sj.onc.1210769. [DOI] [PubMed] [Google Scholar]
- 43.Novak-Hofer I, Cohrs S, Grunberg J, Friedli A, Schlatter MC, Pfeifer M, Altevogt P, Schubiger PA. Antibodies directed against L1-CAM synergize with Genistein in inhibiting growth and survival pathways in SKOV3ip human ovarian cancer cells. Cancer Lett. 2008;261:193–204. doi: 10.1016/j.canlet.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 44.Kenwrick S, Watkins A, Angelis ED. Neural cell recognition molecule L1: relating biological complexity to human disease mutations. Hum Mol Genet. 2000;9:879–886. doi: 10.1093/hmg/9.6.879. [DOI] [PubMed] [Google Scholar]
- 45.Frints SGM, Marynen P, Hartmann D, Fryns JP, Steyaert J, Schachner M, Rolf B, Craessaerts K, Snellinx A, Hollanders K, et al. CALL interrupted in a patient with nonspecific mental retardation: gene dosage-dependent alteration of murine brain development and behavior. Hum Mol Genet. 2003;12:1463–1474. doi: 10.1093/hmg/ddg165. [DOI] [PubMed] [Google Scholar]
- 46.Vawter MP, Freed WJ, Kleinman JE. Neuropathology of bipolar disorder. Biol Psychiatry. 2000;48:486–504. doi: 10.1016/s0006-3223(00)00978-1. [DOI] [PubMed] [Google Scholar]
- 47.Atz ME, Rollins B, Vawter MP. NCAM1 association study of bipolar disorder and schizophrenia: polymorphisms and alternatively spliced isoforms lead to similarities and differences. Psychiatr Genet. 2007;17:55–67. doi: 10.1097/YPG.0b013e328012d850. [DOI] [PMC free article] [PubMed] [Google Scholar]