Abstract
The low-density lipoprotein receptor-related protein 1 (LRP1) is a multifunctional endocytic receptor involved in the metabolism of various extracellular ligands including proteinases that play critical roles in tumor invasion. Although several studies have demonstrated an increased expression of LRP1 in cancer cells, its function in tumor development and progression remains largely unclear. Here, we reveal a novel mechanism by which LRP1 induces the expression of matrix metalloproteinase 2 (MMP2) and MMP9 and thereby promotes the migration and invasion of human glioblastoma U87 cells. Knockdown of LRP1 expression greatly decreased U87 cell migration and invasion, which was rescued by forced expression of a functional LRP1 minireceptor. Inhibition of ligand binding to LRP1 by a specific antagonist RAP also led to reduced cancer cell migration and invasion. Because MMPs play critical roles in cancer cell migration and invasion, we examined the expression of several MMPs and found that the expression of functional MMP2 and MMP9 was selectively decreased in LRP1-knockdown cells. More importantly, decreased cell migration and invasion of LRP1-knockdown cells were completely rescued by exogenous expression of MMP2 or MMP9, suggesting that these MMPs are likely downstream targets of LRP1-mediated signaling. We further show that the level of phosphorylated ERK was significantly decreased in LRP1-silenced cells, suggesting that ERK is a potential mediator of LRP1-regulated MMP2 and MMP9 expression in U87 cells. Together, our data strongly suggest that LRP1 promotes glioblastoma cell migration and invasion by regulating the expression and function of MMP2 and MMP9 perhaps via an ERK-dependent signaling pathway.
Keywords: LRP1, U87 glioblastoma, invasion, MMP2, MMP9
Introduction
Glioblastoma Multiforme (GBM-grade IV) is the most aggressive type of brain tumor with a strong ability to invade and migrate into surrounding normal brain tissue. These cells secrete various extracellular matrix (ECM) degrading enzymes including matrix metalloproteinases (MMP) to facilitate their migration and invasion (1, 2). In particular, MMP2 and MMP9 are highly expressed in gliomas as compared to normal brain tissues, and their mRNA and protein levels are further increased upon tumor progression (3–5). In addition, it is well established that MMP2 and MMP9 are closely associated with tumor invasion and metastasis in a variety of human tumors (6–8).
The low-density lipoprotein receptor (LDLR)-related protein 1 (LRP1) is a large endocytic receptor that belongs to the LDLR family. LRP1 binds and endocytoses over 30 structurally and functionally distinct ligands including apolipoprotein, proteinases, proteinase-inhibitor complexes, and extracellular matrix proteins such as MMPs and urokinase-type plasminogen activator (uPA) (9, 10). Although several studies have implicated LRP1 in tumorigenesis, its precise role and potential underlying mechanisms remain controversial. For example, several reports show that a low expression level of LRP1 is closely related to the aggressive phenotype of tumor cells derived from various tissues such as human prostate, thyroid, and breast (11–13). LRP1-deficient fibroblasts, which have a decreased rate of uPA:PAI-1:uPAR complex catabolism, show an increased cell migration rate (14). In addition, inhibiting LRP1 expression and function is commonly reported to increase cell migration and invasion (15, 16). However, Li et al. showed that high LRP1 expression promotes breast cancer cell invasiveness (17) and LRP1 neutralization could abrogate cell motility in both tumor and non-tumor cells (18, 19). Moreover, we previously reported that silencing LRP1 expression in human smooth muscle cells by LRP1 siRNA resulted in significantly decreased cell migration (20). Recently, Dedieu et al. (21) reported that LRP1 silencing prevents the invasion of a follicular thyroid carcinoma cell line despite the increased pericellular proteolytic activities of MMP2 and uPA. Collectively, these results demonstrate the complexity of LRP1’s function in tumor cell migration and invasion, which likely depends on the tumor cell type and the specific extracellular proteins involved in these processes.
In this study, we hypothesized that LRP1 regulates tumor cell migration and invasion by altering the expression and function of MMPs. First, several MMPs, including MMP2, MMP9, and MMP13, directly or indirectly interact with LRP1 which could alter MMP-mediated pericellular proteolysis (22–25). Second, several microarray studies have shown that LRP1 as well as MMP2 and MMP9, are highly up-regulated in human glioblastoma suggesting that their expression levels are likely coupled (4–6, 26). Herein, we present evidence that LRP1 regulates tumor cell migration and invasion by altering the expression of MMP2 and MMP9.
Materials and Methods
Materials and cDNA constructs
Human α2-macroglobulin (α2M) was purified from human plasma and activated with methylamine (α2M*) as described (27). Human recombinant receptor-associated protein (RAP) was expressed as a glutathione S-transferase fusion protein and was isolated as described previously (28). MMP inhibitors, OA-Hy and Inhibitor IV, were obtained from Calbiochem. All tissue culture media and serum were from Sigma. Rabbit polyclonal anti-LRP1 antibody has been described previously (29, 30). Peroxidase-labeled anti-rabbit antibody and ECL system were from GE Healthcare. Carrier-free Na 125I was purchased from Perkin Elmer Lifescience. Minireceptor of LRP1 mLRP4 was described in previous report (31). MMP2 and MMP9 promoter luciferase vectors were kindly provided by Dr. Christopher C.W. Hughes at the University of California, Irvine. MMP2-AAV and MMP9-AAV expression constructs were kindly provided by Dr. Jin-Moo Lee at Washington University in St. Louis.
LRP1 siRNA
The sense and antisense sequences for LRP1 siRNA were reported in our previous studies (20). Single-stranded, LRP1-specific sense and antisense RNA oligonucleotides were synthesized by Ambion, and double-stranded RNA molecules were generated according to the manufacturer’s instructions. The sequences of oligos are as follows; sense siRNA: GCAGUUUGCCUGCAGAGAUtt, and antisense siRNA: AUCUCUGCAGGCAAACUGCtt.
Cell culture and transfection
Human glioblastoma U87 cells were cultured in Dulbecco’s minimum essential medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 2 mM -glutamine, and 1 mM sodium pyruvate. For transfection, U87 cells were grown to 80% confluence, cells were transfected with LRP1 siRNA using Lipofectamine2000 (Invitrogen) according to the manufacturer’s specifications. After 48 h of transfection, cells were collected for migration and invasion assays, real-time PCR, and Western blotting.
Ligand degradation assay
The ligand degradation assay was performed as previously described (32). Briefly, 2×105 cells were seeded into 12-well dishes 1 day prior to assays. Pre-warmed binding buffer (DMEM containing 0.6% bovine serum albumin with radioligand, 0.6 ml/well) was added to cell monolayers in the presence or absence of unlabeled 500 nM RAP, followed by incubation for 4 h at 37°C. Thereafter, the media were collected and precipitated by addition of trichloroacetic acid (TCA, 20% of final conc.) and BSA (10 mg/ml). Degradation of radioligand was defined as the appearance of radioactive fragments in the overlying medium that were soluble in 20% of TCA. The protein concentration of each cell lysate was measured in parallel dishes that did not contain LRP1 ligands.
Quantitative Real-Time PCR
Quantitative RT-PCR was carried out using real-time PCR with the SYBR Green reporter. Total RNA isolated using the RNeasy Mini Kit (Qiagen) was subsequently reverse transcribed to cDNA with the SuperScript First-strand Synthesis System (Invitrogen). The reaction mix was subjected to quantitative real-time PCR to detect levels of the corresponding GAPDH, LRP1, and several MMPs. The GAPDH was used as an internal control for each specific gene. The relative levels of expression were quantified and analyzed by using Bio-Rad iCycler iQ software. Three independent experiments were performed to analyze the relative gene expression and each sample was tested in triplicate. The real-time value for each sample was averaged and compared using the CT method. The amount of target RNA (2−ΔΔCT) was normalized to the endogenous GAPDH reference (ΔCT) and related to the amount of target gene in control sample, which was set as the calibrator at 1.0.
Western blotting
U87 cells were lysed in lysis buffer (phosphate-buffered saline (PBS) containing 1% Triton X-100, protease inhibitor cocktail, and 1 mM phenylmethylsulfonyl fluoride (PMSF)) at 4°C for 30 min. Equal quantities of protein were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE). Following transfer to Immobilon-P transfer membrane, successive incubations with anti-LRP1 antibody or anti-actin antibody and horseradish peroxidase-conjugated secondary antibody were carried out. The immunoreactive proteins were then detected using the ECL system. Kodak Digital Science1D image analysis software was used for quantification.
Cell migration assay
Cell migration activities were examined by three-dimensional Boyden chamber assay and two-dimensional wound healing assay. Boyden chamber assay was carried out in 6.5-mm diameter transwell chambers with pore size of 8.0 μm. 24 h following LRP1 siRNA transfection, cells were resuspended in the migration medium of DMEM containing 0.1% BSA and 2 mM L-glutamine, and placed in the upper compartment of the Transwell chambers coated with collagen I on the lower surface (5×104 cells in 100 μl). The lower compartment was filled with 600 μl of the same medium. After incubation for 6 h at 37°C, cells on the lower surface of the filter were fixed and stained, and five random fields/filter were counted at ×200 magnifications. For wound healing assay, at 24 h after LRP1 siRNA transfection, a rectangular lesion was created using a cell scraper, and then the cells were rinsed twice with serum free medium and incubated. After the designated times, three randomly selected fields at the lesion border were acquired using a CCD camera (Olympus) on an inverted microscope. In each field, the distance from the margin of the lesion to the 10 most migrated cells were measured, and the mean value of the distances was taken as the mobility of cells in each culture dish.
In vitro invasion assay
Matrigel invasion assays were used to assess the effect of the LRP1 knockdown on the invasiveness of cancer cells. Invasion of cells through Matrigel was determined using 24-well BD invasion chambers (8.0 μm pore size with polycarbonate membrane; BD Biosciences, UK) according to the manufacturer’s instructions. BD invasion chambers were prehydrated with serum-free DMEM (500 μL/well) for 2 h at 37°C in 5% CO2. After harvest, cells were suspended in serum-free DMEM at a concentration of 1 × 106 cells/ml and immediately placed 100μl of the cell suspension onto the upper compartment of the plates. Subsequently, the lower compartment was filled with complete medium (500 μL). Following 24 h incubation, the non-invading cells remaining on the upper surface of the membrane were removed by wiping with cotton-tipped swabs. Cells on the lower surface of the membrane were stained with DiffQuick staining solution (IMEB Inc. CA) according to the manufacturer’s instructions. Five fields of adherent cells were counted randomly in each well under an inverted microscope at ×200 magnification, and the results were numerically averaged and counted.
Luciferase assay
U87 cells were co-transfected with the appropriate cDNAs: MMP2 or MMP9 promoter-Luc, and LRP1 siRNA or control siRNA. A β-gal reporter construct was also co-transfected for normalizing transfection efficiency. 24 h after transfection, the luciferase activity and β-gal activity were measured by the Luciferase Assay System and β-gal Assay System, respectively, following the manufacturer’s instructions (Promega).
Statistical Analysis
All quantified data represent an average of at least triplicate samples. Error bars represent standard deviation of the mean. Statistical significance was determined by Student’s t-test, and p < 0.05 was considered significant.
Results
LRP1 silencing inhibits U87 cell migration and invasion
To determine the specific role of LRP1 in U87 glioblastoma cell migration and invasion, we knocked down LRP1 expression by LRP1 siRNA. Previously we had tested four double-stranded, 21-nucleotide-long LRP1 siRNAs with TT dinucleotide 3′ overhangs against the coding sequence of human LRP1 (GI 4758685) (20). Among them, we chose the LRP1 siRNA most effective at silencing LRP1 expression. As seen in Figs. 1A and 1B, LRP1 expression levels were clearly decreased by >80% by LRP1 siRNA, indicating that this LRP1 siRNA is a powerful tool to modulate LRP1 expression in U87 cells. We found a similar LRP1 reduction in LN827 cells, another human glioblastoma, by the same LRP1 siRNA (Figs. 1A and 1B). We confirmed that the LRP1 siRNA significantly decreased LRP1 mRNA levels in both U87 cells and LN827 cells by quantitative real-time PCR (Fig. 1C). The reduction of LRP1 expression was sustained for > 72 h after transfection with LRP1 siRNA (data not shown). We then examined whether the LRP1 siRNA-mediated suppression of LRP1 expression results in reduced LRP1 function in ligand degradation using the LRP1-specific ligand, α2M. As expected, LRP1 knockdown greatly reduced α2M degradation compared to control cells (Fig. 1D). In addition, we examined the effect of LRP1 silencing on the survival and proliferative activity of U87 cells since a decrease in survival or proliferation after LRP1 silencing may also contribute to the observed decrease in migration and invasion of U87 cells. As shown in Supplementary Fig. S1, LRP1 silencing decreased the proliferation rate of U87 cells slightly, but significantly, compared to control cells. Survival of U87 cells were not changed significantly by MTT assay (data not shown). Given this finding, we normalized the altered migratory and invasive activity of U87 cells in the following experiments against the altered proliferation rate.
Figure 1.

LRP1 silencing in glioblastoma cells. U87 or LN827 glioblastoma cells were transfected with LRP1 siRNA or control siRNA using Lipofectamine2000 (LP2000). After 24 h transfection, cells were harvested for Western blotting, RNA extraction, or ligand degradation assays. A, LRP1 silencing by LRP1 siRNA in both U87 and LN827 glioblastoma cells. The same amounts of lysates were analyzed by Western blotting using an antibody against the 85-kDa subunit of LRP1. Anti-actin blot was used as a loading control. B, Densitometric analysis of Western blots from triplicate samples was performed. C, mRNA levels of LRP1 were quantified by real-time PCR after treatment control or LRP1 siRNAs. D, 125I-α2M (1nM) uptake and degradation assays were performed at 37°C for 4 h in the absence or presence of RAP (500nM). The degradation of 125I-α2M was analyzed as described in the Materials and Methods section. Values are the average of triple determinations with the S.D. indicated by error bars. **p < 0.01
To investigate whether LRP1 silencing affects U87 cell migration and invasion, we conducted three-dimensional cell migration assays using transwell chambers and invasion assays with Matrigel pre-coated Transwell chambers. We found that knockdown of LRP1 in U87 cells decreased cell migration and invasion approximately 70% and 80%, respectively, compared to controls (Fig. 2A). To confirm this result, a two-dimensional migration assays were performed. As shown in Fig. 2B, compared to control cells, the migrated distances of LRP1 siRNA-transfected U87 cells were decreased by 60% at 8 h and 40% at 24 h, after making the scratch. In addition, LRP1 siRNA significantly decreased both migration and invasion of LN827 cells by more than 60% (Supplementary Fig. S2A). To further confirm the effects of LRP1 silencing on the cell migration and invasion, we also examined the effects of short hairpin RNAs for LRP1, which silenced LRP1 expression by > 90% compared to the empty vector control. We found that LRP1 shRNA-transduced U87 cells exhibit significantly decreased 80% migration and invasion rates compared to control cells (Supplementary Fig. S2B).
Figure 2.

LRP1 silencing and functional blocking by RAP significantly decreased U87 cell migration and invasion A, After 24 h control or LRP1 siRNA transfection, three-dimensional migration assays were performed using Boyden chambers. Cell invasion was analyzed using Matrigel pre-coated transwell chambers. B, Two-dimensional migration assays were conducted with a modified wound healing assay. The migrated distance during the designated period was measured. Bar indicates 100 μm. C, LRP1 minireceptor mLRP4 was co-transfected with LRP1 siRNA into U87 cells and cellular properties of U87 cells were analyzed. LRP1 silencing and mLRP4 overexpression were analyzed by Western blotting. D, U87 cell migration and invasion were analyzed with or without 500 nM RAP in both chambers. Representative photos of migration and invasion are presented, which were taken at ×200 magnification under inverted microscopy. Values are the average of triple determinations with the S.D. indicated by error bars. *p < 0.05, **p < 0.01
To confirm that the effect of LRP1 silencing on the decrease of migration and invasion of U87 cells is specific, we restored LRP1 function by co-transfecting a minireceptor of LRP1 mLRP4 which possess the full functional activities of LRP1 (31). This mLRP4 does not contain LRP1 siRNA-targeting sequence and is therefore resistant to LRP1 siRNA knockdown. We observed that mLRP4 expression restored U87 cell migration and invasion (Fig. 2C). We have further tested the effects of LRP1 silencing on the invasion of several lung cancer cell lines: H292, H441, H520, and SK-LU-1, and found that the invasiveness of these lung cancer cells positively correlates with LRP1 expression levels (Supplementary Fig. S3A). Further, the invasion rate was decreased by 50% in LRP1 knockdown SK-LU-1 cells, which express high level of endogenous LRP1. These results demonstrated that the expression levels of LRP1 positively correlate with the migration and invasion rates of different cancer cell types.
LRP1 ligand-binding function is required for efficient cancer cell migration and invasion
To determine whether the ligand binding ability of LRP1 affects U87 cell migration and invasion, we pretreated U87 cells with receptor-associated protein (RAP), an antagonist that blocks all ligand binding to LRP1 (31). U87 cells treated with 500nM RAP showed significant decrease in migration and invasion, approximately 70% and 50%, respectively, compared to control cells (Fig. 2D). Treating SK-LU-1 lung cancer cells with 500nM RAP also resulted in a significant decrease, approximately 80% of invaded cells compared to control cells (Supplementary Fig. S3B), suggesting that ligand binding to LRP1 is required for LRP1’s function in tumor cell migration and invasion.
LRP1 modulates cell migration and invasion by regulating MMP2 and MMP9 expression
To examine whether MMP activity is required for U87 cell migration and invasion, we tested the effect of OA-Hy, a broad spectrum MMP inhibitor, and inhibitor IV, a specific inhibitor of MMP2 and MMP9. Fig. 3A shows that both inhibitors significantly blocked U87 cell migration and invasion, demonstrating that MMP activity is necessary for efficient migration and invasion of U87 cells.
Figure 3.

LRP1 silencing decreased MMP2 and MMP9 expression. A, Migration and invasion assays were performed with U87 cells in the absence or presence of 10 μM MMP inhibitor OA-Hy or 10nM MMP inhibitor IV. B, mRNA levels of LRP1, MMP2, MMP9, LDLR, and MMP7 were quantified by real-time PCR 48 h after treatment with LRP1 siRNA. Levels of GAPDH mRNA were used as internal standards. C, U87 cells were co-transfected with control or LRP1 siRNA and MMP2-Luc or MMP9-Luc. Cells were harvested 24 h later and analyzed for luciferase expression. β-gal expression vector was co-transfected for normalization of transfection efficiency. Values are the average of triple determinations with the S.D. indicated by error bars. *p < 0.05, **p < 0.01
To examine whether LRP1 regulates U87 cell migration and invasion by modulating MMP expression, we analyzed the mRNA levels of MMP2 and MMP9 upon LRP1 silencing. Quantitative real-time PCR results showed that the mRNA levels of MMP2 and MMP9 were decreased by > 30% and 50% respectively, in LRP1-silenced cells (Fig. 3B). As controls, the mRNA levels of the LDL receptor and MMP7 were not altered under these conditions, indicating that downregulation of MMP2 and MMP9 expression by LRP1 silencing is specific. To confirm that LRP1 regulates MMP expression at the transcriptional level, we performed a luciferase reporter assay using constructs containing promoter regions of MMP2 and MMP9. As shown in Fig. 3C, knockdown of LRP1 decreased the transcriptional activity of both MMP2 and MMP9 promoters by 30% and 60%, respectively. These results suggest that LRP1 is involved in transcriptional regulation of MMP2 and MMP9 in U87 cells.
To confirm the finding that LRP1 regulates MMP2 and MMP9 expression, we examined the mRNA levels of MMP2 and MMP9 in LRP1-deficient mouse embryonic fibroblasts (MEF2) and LRP1-expressing MEF cells (MEF1) by quantitative real-time PCR. Consistent with findings in LRP1 knockdown cells, mRNA levels of MMP2 and MMP9 in LRP1-deficient MEF2 cells are only ~50% and 30%, respectively, of WT MEF1 cells (Fig. 4A). Furthermore, we found that the mRNA levels of MMP2 and MMP9 in the brain of LRP1 forebrain knockout mice (33) are also significantly lower than those in the brain of littermate control mice (Fig. 4B). In both cases, the levels of LDLR and MMP7 were not changed, suggesting that LRP1 specifically regulates MMP2 and MMP9 expression.
Figure 4.

MMP2 and MMP9 are down-regulated in LRP1 deficient cells and brain tissues. Levels of MMP2 and MMP9 mRNA in MEF cells or mouse brain tissues were quantified by real-time PCR. A, mRNA levels of MMP2, MMP9, LRP1, LDLR, and MMP7 in WT MEF1 and LRP1-deficient MEF2 cells. B, mRNA levels of MMP2, MMP9, LRLP1, LDLR, and MMP7 in brain tissues from control and LRP1-forebrain knockout mice. Values are the average of triple determinations with the S.D. indicated by error bars. *p < 0.05, **p < 0.01
To further confirm that the effect of LRP1 silencing on the decrease of MMP2 and MMP9 expression levels in U87 cells is specific, we restored LRP1 function by co-transfecting LRP1 minireceptor mLRP4. We observed that mLRP4 expression significantly restored mRNA levels of MMP2 and MMP9 in LRP1-silenced U87 cells (Fig. 5A). To further examine whether LRP1 modulates cell migration and invasion by regulating MMP expression and function, we tested whether ectopic expression of MMP2 or MMP9 can restore cell migration and invasion in LRP1-silenced U87 cells. We found that MMP2 or MMP9 overexpression successfully restored both migration and invasion (Fig. 5B and 5C), demonstrating that MMP2 and MMP9 are downstream targets of LRP1.
Figure 5.
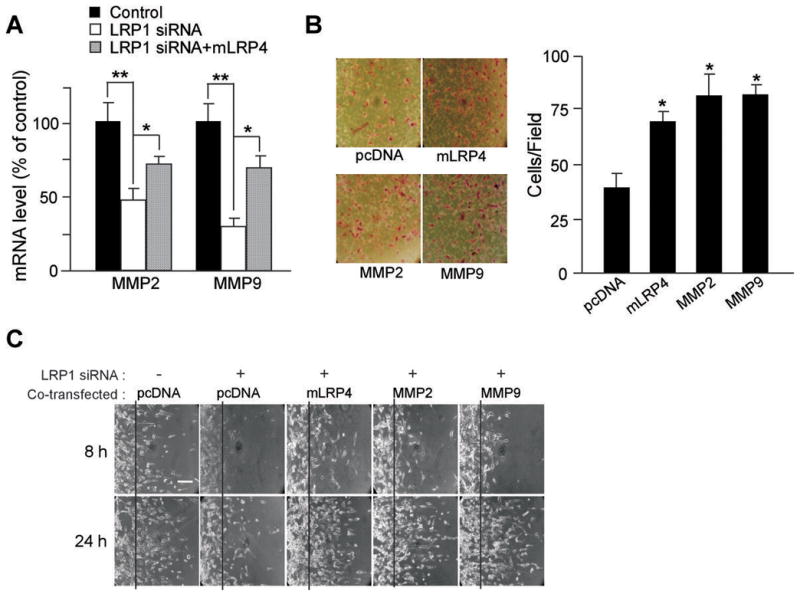
Decreased migration and invasion in LRP1 knockdown U87 cells were rescued by MMP2 and MMP9 overexpression. LRP1 minireceptor mLRP4, MMP2, or MMP9 expression vector was co-transfected with LRP1 siRNA into U87 cells and cellular migration and invasion were analyzed. A, Negative control or LRP1 siRNA, with or without mLRP4 were co-transfected into U87 cells. mRNA levels of MMP2 and MMP9 were quantified by real-time PCR. B, Invasion assay was conducted after pcDNA, mLRP4, MMP2, or MMP9 was co-transfected with LRP1 siRNA. C, Two-dimensional migration assay was performed after pcDNA, mLRP4, MMP2, or MMP9 was co-transfected with or without LRP1 siRNA. Bar indicates 100 μm. Values are the average of triple determinations with the S.D. indicated by error bars. *p < 0.05, **p < 0.01
ERK is a potential mediator of LRP1 regulation of MMP2 and MMP9 expression
The signal transduction pathways that modulate the activity of MMP transcription factors are highly diverse. MAPK signal transduction pathways, including p38, ERK, and JNK, are well known mediators, which stimulate or inhibit MMP expression depending on cell types (34–36). To understand the mechanism by which LRP1 regulates MMP2 and MMP9 expression, we analyzed the effects of LRP1-silencing on the activation of several potential signaling pathways. We found that the level of phosphorylated ERK was selectively decreased in LRP1-silenced U87 cells, which was restored upon ectopic expression of mLRP4 (Fig. 6A and 6B). Phosphorylated JNK and p38 were not significantly changed in LRP1-silenced U87 cells. The levels of phosphorylated Akt were also not changed in LRP1-silenced U87 cells. These results demonstrate that ERK is likely a downstream target of LRP1-mediated signaling that regulates MMP2 and MMP9 expression and their functions in cell migration and invasion in U87 cells.
Figure 6.
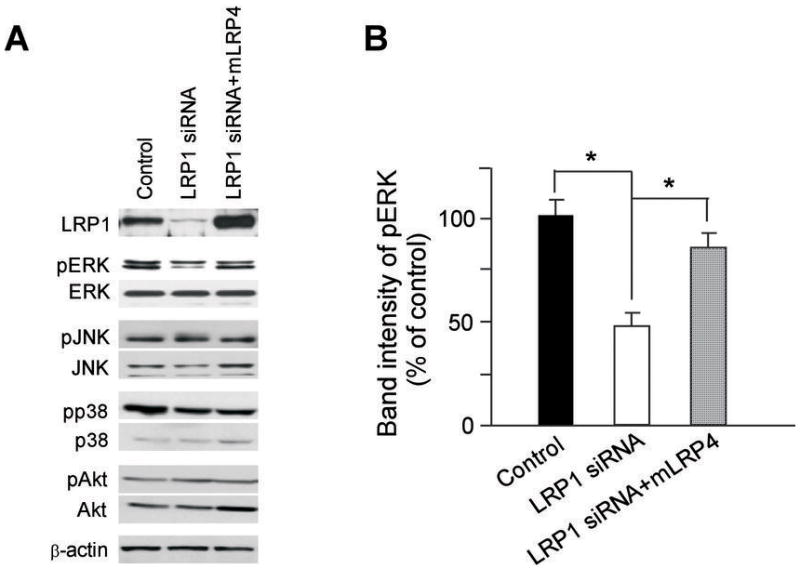
LRP1 silencing decreased phosphorylated ERK. Effects of LRP1 silencing on cellular signaling pathways were analyzed by Western blotting. A, Negative control or LRP1 siRNA, without or with mLRP4 were co-transfected into U87 cells. The same amounts of lysates were analyzed by Western blotting for LRP1 expression and the levels of indicated signaling molecules. Actin blot serves as a loading control and this blot is representative of three such experiments performed with similar data. B, Densitometric analysis of Western blots for pERK from triplicate samples was performed. Values are the average of triple determinations with the S.D. indicated by error bars. *p < 0.05
Discussion
In the present study, we show that LRP1 silencing leads to a significant decrease of glioblastoma cell migration and invasion and provide evidence that LRP1 regulates the transcriptional levels of MMP2 and MMP9 in U87 glioblastoma cells. We further show that LRP1 regulation of MMP2 and MMP9 expression is likely mediated by ERK signal pathways.
The finding that LRP1 silencing leads to a significant decrease of glioblastoma cell migration and invasion is consistent with Li and colleagues’ previous report, which showed that high levels of LRP1 expression promoted breast cancer cell invasiveness (17). We obtained similar results using two different gene silencing techniques, siRNA and shRNA. Moreover, functional blocking of LRP1 by RAP also led to decreased cell migration and invasion, which is also consistent with reports that RAP treatment decreased migration of breast cancer cells, myogenic cells (18, 37) as well as nontumorous smooth muscle cells (19, 38). In contrast, there are reports that knockdown or deletion of LRP1 increased cell migration, likely by decreasing the catabolic rate of the uPA:PAI-1:uPAR complex (14, 16). It has also been shown that low endogenous LRP1 levels are often related to the aggressive phenotype of tumor cells, a phenomenon seen in various tissues (11–13), and neutralization of LRP1 function has been commonly reported to increase cell migration and invasion (15, 16). The disparity of LRP1 function among these studies may reflect the diverse characteristics of the experimental systems including cell types, LRP1 expression levels, expression levels of other members of the LDLR family, and signaling pathways that participate in cellular migration. Nevertheless, our observations that ectopic expression of mLRP4 successfully rescued cell migration and invasion in LRP1-silenced glioblastoma cells strongly suggest that LRP1 expression levels are positively correlated with cancer cell migration and invasion rates. Indeed, we found that LRP1-silenced LN827 cells, which is known to be highly invasive in vivo (39), exhibit decreased migration and invasion rates. Although U87 cells showed migratory and invasive activity in the present in vitro results, previous report showed that the intracerebral xenograft U87 cells form well circumscribed tumors with no migration or invasion into normal brain tissue (40). This discrepancy of U87 cells between the in vitro and in vivo models may be due to their different microenvironment and/or host resistance in vivo. Therefore, in future studies it will be interesting to examine the invasiveness of alternative glioblastoma cell lines using xenograft mouse models following LRP1-silencing. We further observed that the number of invaded cells positively correlates with endogenous levels of LRP1 in different lung cancer cells, suggesting that LRP1 regulates cancer cell migration and invasion via a similar mechanism among different cancer cell types. In fact, the invasion rate of SK-LU-1 cells, which express the highest level of LRP1 among the lung cancer cell lines tested, was greatly decreased upon LRP1 silencing.
To determine how LRP1 promotes glioblastoma cell migration and invasion, we focused on delineating the relationship between LRP1 and MMPs, particularly LRP1 ligands, MMP2 and MMP9. It is well known that the mRNA and protein levels of MMP2 and MMP9 are highly increased upon glioblastoma progression (1–3). In this respect, it is interesting to note that several microarray studies have shown that the expression levels of LRP1 are highly up-regulated in human glioblastoma, suggesting that MMPa and LRP1 expression levels are likely coupled (4–6, 26). However, it is unknown how these genes regulate each other during tumorigenesis. Here, we found that LRP1 silencing significantly decreases the mRNA levels of both MMP2 and MMP9, but not the mRNA levels of the LDL receptor and MMP7, indicating that LRP1 specifically modulates the expression of MMP2 and MMP9. We confirmed that LRP1 regulates MMP2 and MMP9 expression at the transcriptional level by luciferase reporter assays using constructs containing the promoter regions of MMP2 and MMP9. Although ~90% of LRP1 expression was silenced by siRNA, the migration and invasion rates of U87 cells and the expression levels of MMP2 and MMP9 were reduced by only ~50–60%. A potential explanation for this discrepancy is the paradoxical function of some MMPs. Indeed, MMP2 has long been considered as a pro-tumorigenic factor, but MMP9 has a function of host resistance in cancer as well as pro-tumorigenic action (41). Additionally, other modulators may regulate MMP expression and U87 cell migration and invasion independent of LRP1. Further investigation will be needed to reveal the relationship of LRP1-dependent and LRP1-independent mechanisms in cancer cell migration and invasion. We further showed that the mRNA levels of MMP2 and MMP9 in both LRP1 deficient MEF2 and the brain of LRP1 forebrain knockout mice are significantly lower than those in the control samples. These results suggest that LRP1 is involved in transcriptional regulation of MMP2 and MMP9 in U87 cells. Hu and colleague’s previous report that tPA, an LRP1 ligand, regulates MMP9 expression in an LRP1-dependent manner (42) provides further support for our findings. We also demonstrated that the ectopic expression of MMP2 or MMP9 successfully restores both migration and invasion of LRP1-silenced U87 cells, indicating that MMP2 and MMP9 are indeed downstream targets of LRP1.
LRP1 is also a signaling receptor that regulates several signal transduction pathways in different cell types (42–44). It is known that ERK is a signal mediator of tPA-induced MMP9 transcription through LRP1 (42), and that LRP1 is a regulator of ERK phosphorylation in HT 1080 fibrosarcoma cells (16). Indeed, we found that the level of phosphorylated ERK is selectively decreased in LRP1 knockdown cells. Our results also show that LRP1 regulates the transcriptional activity of MMP9 to a greater extent than MMP2. Dedieu and colleagues have shown that LRP1 effectively controls cell invasiveness via inhibition of focal adhesion complex disassembly, a process mediated by ERK signaling (21). Thus, ERK signaling pathways are likely associated with several mechanisms of cell motility mediated by LRP1 including regulation of the transcriptional levels of MMP2 and MMP9.
As a therapeutic target, LRP1 has long been considered as a promising possibility for cancer treatment. Despite a number of contradicting reports, our results provide strong evidence that reducing LRP1 expression and function may well serve as a potential therapeutic strategy to treat cancer, particularly cancer metastasis. Further studies aimed at identifying the precise signal transduction pathways and transcription factors that mediate LRP1 regulation of MMP expression may provide a molecular understanding of the roles of LRP1 in cancer biology.
Supplementary Material
Acknowledgments
Grant support: NIH grant CA100520.
We thank Dr. Todd Zankel and Olivia Ying for critical reading of the article and Drs. Christopher C.W. Hughes and Jin-Moo Lee for kindly providing several plasmids.
References
- 1.Egebland M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2:161–74. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- 2.Vihinen P, Ala-aho R, Kahari VM. Matrix metalloproteinases as therapeutic targets in cancer. Curr Cancer Drug Targets. 2005;5:203–20. doi: 10.2174/1568009053765799. [DOI] [PubMed] [Google Scholar]
- 3.Forsyth PA, Wong H, Laing TD, et al. Gelatinase-A (MMP-2), gelatinase-B (MMP-9) and membrane type matrix metalloproteinase-1 (MT1-MMP) are involved in different aspects of the pathophysiology of malignant gliomas. B J Cancer. 1999;79:1828–35. doi: 10.1038/sj.bjc.6990291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sun L, Hui A, Su Q, et al. Neuronal and glioma-derived stem cell factor induces angiogenesis within the brain. Cancer Cell. 2006;9:287–300. doi: 10.1016/j.ccr.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 5.Bredel M, Bredel C, Juric D, et al. Tumor necrosis factor-a-induced protein 3 as a putative regulator of nuclear factor-kB-mediated resistance to O6-alkylating agents in human glioblastomas. J Clin Oncol. 2006;24:274–87. doi: 10.1200/JCO.2005.02.9405. [DOI] [PubMed] [Google Scholar]
- 6.Wang M, Wang T, Liu S, Yoshida D, Teramoto A. The expression of matrix metalloproteinase-2 and -9 in human gliomas of different pathological grades. Brain Tumor Pathol. 2003;20:65–72. doi: 10.1007/BF02483449. [DOI] [PubMed] [Google Scholar]
- 7.Chambers AF, Matrisian LM. Changing views of the role of matrix metalloproteinases in metastasis. J Natl Cancer Inst. 1997;89:1260–70. doi: 10.1093/jnci/89.17.1260. [DOI] [PubMed] [Google Scholar]
- 8.Komatsu K, Nakanishi Y, Nemoto N, Hori T, Sawada T, Kobayashi M. Expression and quantitative analysis of matrix metalloproteinase-2 and -9 in human gliomas. Brain Tumor Pathol. 2004;21:105–12. doi: 10.1007/BF02482184. [DOI] [PubMed] [Google Scholar]
- 9.Herz J, Strickland DK. LRP1: a multifunctional scavenger and signaling receptor. J Clin Invest. 2001;108:779–84. doi: 10.1172/JCI13992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herz J, Bock HH. Lipoprotein receptors in the nervous system. Annu Rev Biochem. 2002;71:405–34. doi: 10.1146/annurev.biochem.71.110601.135342. [DOI] [PubMed] [Google Scholar]
- 11.Foca C, Moses EK, Quinn MA, Rice GE. Differential expression of the alpha(2)-macroglobulin receptor and the receptor associated protein in normal human endometrium and endometrial carcinoma. Mol Hum Reprod. 2000;6:921–7. doi: 10.1093/molehr/6.10.921. [DOI] [PubMed] [Google Scholar]
- 12.Kancha RK, Stearns ME, Hussain MM. Decreased expression of the low density lipoprotein receptor-related protein/alpha 2-macroglobulin receptor in invasive cell clones derived from human prostate and breast tumor cells. Oncol Res. 1994;6:365–72. [PubMed] [Google Scholar]
- 13.Sid B, Dedieu S, Delorme N, et al. Human thyroid carcinoma cell invasion is controlled by the low density lipoprotein receptor-related protein-mediated clearance of urokinase plasminogen activator. Int J Biochem Cell Biol. 2006;38:1729–40. doi: 10.1016/j.biocel.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 14.Weaver AM, Hussaini IM, Mazar A, Henkin J, Gonias SL. Embryonic fibroblasts that are genetically deficient in low density lipoprotein receptor-related protein demonstrate increased activity of the urokinase receptor system and accelerated migration on vitronectin. J Biol Chem. 1997;272:14372–9. doi: 10.1074/jbc.272.22.14372. [DOI] [PubMed] [Google Scholar]
- 15.Desrosiers RR, Rivard ME, Grundy PE, Annabi B. Decrease in LDL receptor-related protein expression and function correlates with advanced stages of Wilms tumors. Pediatr Blood Cancer. 2006;46:40–9. doi: 10.1002/pbc.20566. [DOI] [PubMed] [Google Scholar]
- 16.Webb DJ, Nguyen DHD, Gonias SL. Extracellular signal-regulated kinase functions in the urokinase receptor dependent pathway by which neutralization of low density lipoprotein receptor-related protein promotes fibrosarcoma cell migration and matrigel invasion. J Cell Sci. 2000;113:123–34. doi: 10.1242/jcs.113.1.123. [DOI] [PubMed] [Google Scholar]
- 17.Li Y, Wood N, Grimsley P, Yellowlees D, Donnelly PK. In vitro invasiveness of human breast cancer cells is promoted by low density lipoprotein receptor-related protein. Invasion Metast. 1998;18:240–51. doi: 10.1159/000024517. [DOI] [PubMed] [Google Scholar]
- 18.Chazaud B, Ricoux R, Christov C, Plonquet A, Gherardi RK, Barlovatz-Meimon G. Promigratory effect of plasminogen activator inhibitor-1 on invasive breast cancer cell populations. Am J Pathol. 2002;160:237–46. doi: 10.1016/S0002-9440(10)64367-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wijnberg MJ, Quax PH, Nieuwenbroek NM, Verheijen JH. The migration of human smooth muscle cells in vitro is mediated by plasminogen activation and can be inhibited by alpha2-macroglobulin receptor associated protein. Thromb Haemost. 1997;78:880–6. [PubMed] [Google Scholar]
- 20.Li Y, Lu W, Bu G. Essential role of the low density lipoprotein receptor-related protein in vascular smooth muscle cell migration. FEBS Lett. 2003;555:346–50. doi: 10.1016/s0014-5793(03)01272-9. [DOI] [PubMed] [Google Scholar]
- 21.Dedieu S, Langlois B, Devy J, et al. LRP1-1 silencing prevents malignant cell invasion despite increased pericellular proteolytic activities. Mol Cell Biol. 2008;28:2980–95. doi: 10.1128/MCB.02238-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barmina OY, Walling HW, Fiacco GJ, et al. Collagenase-3 binds to a specific receptor and requires the low density lipoprotein receptor-related protein for internalization. J Biol Chem. 1999;274:30087–93. doi: 10.1074/jbc.274.42.30087. [DOI] [PubMed] [Google Scholar]
- 23.Hahn-Dantona E, Ruiz JF, Bornstein P, Strickland DK. The low density lipoprotein receptor-related protein modulates levels of matrix metalloproteinase 9 (MMP-9) by mediating its cellular catabolism. J Biol Chem. 2001;276:15498–503. doi: 10.1074/jbc.M100121200. [DOI] [PubMed] [Google Scholar]
- 24.Yang Z, Strickland DK, Bornstein P. Extracellular MMP2 levels are regulated by the LRP1 scavenger receptor and thrombospondin 2. J Biol Chem. 2001;276:8403–8. doi: 10.1074/jbc.M008925200. [DOI] [PubMed] [Google Scholar]
- 25.Emonard H, Bellon G, Troeberg L, et al. Low density lipoprotein receptor-related protein mediates endocytic clearance of pro-MMP-2. TIMP-2 complex through a thrombospondin-independent mechanism. J Biol Chem. 2004;279:54944–51. doi: 10.1074/jbc.M406792200. [DOI] [PubMed] [Google Scholar]
- 26.Kachra Z, Beaulieu E, Delbecchi L, et al. Expression of matrix metalloproteinases and their inhibitors in human brain tumors. Clin Exp Metastasis. 1999;17:555–66. doi: 10.1023/a:1006760632766. [DOI] [PubMed] [Google Scholar]
- 27.Williams SE, Ashcom JD, Argraves WS, Strickland DK. A novel mechanism for controlling the activity of alpha 2-macroglobulin receptor/low density lipoprotein receptor-related protein. Multiple regulatory sites for 39-kDa receptor-associated protein. J Biol Chem. 1992;267:9035–40. [PubMed] [Google Scholar]
- 28.Bu G, Maksymovitch EA, Schwartz AL. Receptor-mediated endocytosis of tissue-type plasminogen activator by low density lipoprotein receptor-related protein on human hepatoma HepG2 cells. J Biol Chem. 1993;268:13002–9. [PubMed] [Google Scholar]
- 29.Bu G, Geuze HJ, Strous GJ, Schwartz AL. 39 kDa receptor-associated protein is an ER resident protein and molecular chaperone for LDL receptor-related protein. EMBO J. 1995;14:2269–80. doi: 10.1002/j.1460-2075.1995.tb07221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li Y, Chen J, Lu W, McCormick LM, Wang J, Bu G. Mesd binds to mature LDL-receptor-related protein-6 and antagonizes ligand binding. J Cell Sci. 2005;118:5305–14. doi: 10.1242/jcs.02651. [DOI] [PubMed] [Google Scholar]
- 31.Bu G, Rennke S. Receptor-associated Protein Is a Folding Chaperone for Low Density Lipoprotein Receptor-related Protein. J Biol Chem. 1996;271:22218–24. doi: 10.1074/jbc.271.36.22218. [DOI] [PubMed] [Google Scholar]
- 32.Li Y, Marzolo MP, van Kerkhof P, Strous GJ, Bu G. The YXXL motif, but not the two NPXY motifs, serves as the dominant endocytosis signal for low density lipoprotein receptor-related protein. J Biol Chem. 2000;275:17187–94. doi: 10.1074/jbc.M000490200. [DOI] [PubMed] [Google Scholar]
- 33.Liu Q, Zerbinatti CV, Zhang J, et al. Amyloid precursor protein regulates brain apolipoprotein E and cholesterol metabolism through lipoprotein receptor LRP1. Neuron. 2008;56:66–78. doi: 10.1016/j.neuron.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Westermarck J, Kähäri VM. Regulation of matrix metalloproteinase expression in tumor invasion. FASEB J. 1999;13:781–92. [PubMed] [Google Scholar]
- 35.Simon C, Goepfert H, Boyd D. Inhibition of the p38 mitogen-activated protein kinase by SB 203580 blocks PMA-induced Mr 92,000 type IV collagenase secretion and in vitro invasion. Cancer Res. 1998;58:1135–9. [PubMed] [Google Scholar]
- 36.Johansson N, Ala-aho R, Uitto V, et al. Expression of collagenase-3 (MMP-13) and collagenase-1 (MMP-1) by transformed keratinocytes is dependent on the activity of p38 mitogen-activated protein kinase. J Cell Sci. 2000;113:227–35. doi: 10.1242/jcs.113.2.227. [DOI] [PubMed] [Google Scholar]
- 37.Chazaud B, Bonavaud S, Plonquet A, Pouchelet M, Gherardi RK, Barlovatz-Meimon G. Involvement of the [uPAR:uPA:PAI-1:LRP1] complex in human myogenic cell motility. Exp Cell Res. 2000;258:237–44. doi: 10.1006/excr.2000.4934. [DOI] [PubMed] [Google Scholar]
- 38.Okada SS, Grobmyer SR, Barnathan ES. Contrasting effects of plasminogen activators, urokinase receptor, and LDL receptor-related protein on smooth muscle cell migration and invasion. Arterioscler Thromb Vasc Biol. 1996;16:1269–76. doi: 10.1161/01.atv.16.10.1269. [DOI] [PubMed] [Google Scholar]
- 39.Zhang J, Sarker S, Yong VW. The chemokine stromal cell derived factor-1 (CXCL12) promotes glioma invasiveness through MT2-matrix metalloproteinase. Carcinogenesis. 2005;26:2069–77. doi: 10.1093/carcin/bgi183. [DOI] [PubMed] [Google Scholar]
- 40.Heimberger AB, Wang E, McGary EC, et al. Mechanisms of action of rapamycin in gliomas. Neuro-Oncology. 2005;7:1–11. doi: 10.1215/S1152851704000420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Overall CM, Kleifeld O. Towards third generation matrix metalloproteinase inhibitors for cancer therapy. Brit J Cancer. 2006;94:941–6. doi: 10.1038/sj.bjc.6603043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hu K, Yang J, Tanaka S, Gonias SL, Mars WM, Liu Y. Tissue-type plasminogen activator acts as a cytokine that triggers intracellular signal transduction and induces matrix metalloproteinase-9 gene expression. J Biol Chem. 2006;281:2120–7. doi: 10.1074/jbc.M504988200. [DOI] [PubMed] [Google Scholar]
- 43.Campana WM, Li X, Dragojlovic N, Janes J, Gaultier A, Gonias SL. The low-density lipoprotein receptor-related protein is a pro-survival receptor in Schwann cells: possible implications in peripheral nerve injury. J Neurosci. 2006;26:11197–207. doi: 10.1523/JNEUROSCI.2709-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang H, Lee JM, Wang Y, et al. Mutational analysis of the FXNPXY motif within LDL receptor-related protein 1 (LRP1) reveals the functional importance of the tyrosine residues in cell growth regulation and signal transduction. Biochem J. 2008;409:53–64. doi: 10.1042/BJ20071127. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


