Abstract
We have previously developed and tested a muscle model that predicts the effect of stimulation frequency on muscle force responses. The aim of this study was to enhance our isometric mathematical model to predict muscle forces in response to stimulation trains with a wide range of frequencies and intensities for the quadriceps femoris muscles of individuals with spinal cord injuries. Isometric forces were obtained experimentally from 10 individuals with spinal cord injuries (time after injury, 1.5-8 years) and then compared to forces predicted by the model. Our model predicted accurately the force-time integrals (FTI) and peak forces (PF) for stimulation trains of a wide range of frequencies (12.5-80 HZ) and intensities (150-600-μs pulse duration), and two different stimulation patterns (constant-frequency trains and doublet-frequency trains). The accurate predictions of our model indicate that our model, which now incorporates the effects of stimulation frequency, intensity, and pattern on muscle forces, can be used to design optimal customized stimulation strategies for spinal cord-injured patients.
Keywords: constant-frequency trains, doublet-frequency trains, force-time integral, isometric forces, mathematical muscle models, paralyzed muscle, peak force
Functional electrical stimulation (FES) is the application of electrical stimulation to generate functional movements such as standing, walking, and grasping in individuals with upper motor neuron lesions.2,25 However, in spite of the potential advantage of FES for improving the functional mobility of paralyzed individuals, it has not gained widespread application. Two of the limitations in current FES systems include rapid muscle fatigue and imprecise control of movements.25,27,31 During volitional activation, the central nervous system (CNS) modulates both the firing frequency and the number of activated motor units to control force output precisely for functional tasks and delay the onset of muscle fatigue.21 However, current FES systems typically only vary the stimulation intensity to control muscle force and maintain a constant stimulation frequency. In addition, current FES systems only use one stimulation pattern, namely, constant-frequency trains (CFTs) (see Fig. 1 for an example). Previous studies, however, showed that stimulation trains that contained two closely spaced pulses (doublet) throughout the train, doublet-frequency trains (DFTs), produced greater isometric forces from fatigued human skeletal muscles and greater power during dynamic contractions than the traditionally used CFTs.3-5,22,23 Modulating the stimulation frequency, pattern, and intensity during FES may help obtain more precise control of skeletal muscle force output and delay the onset of fatigue.
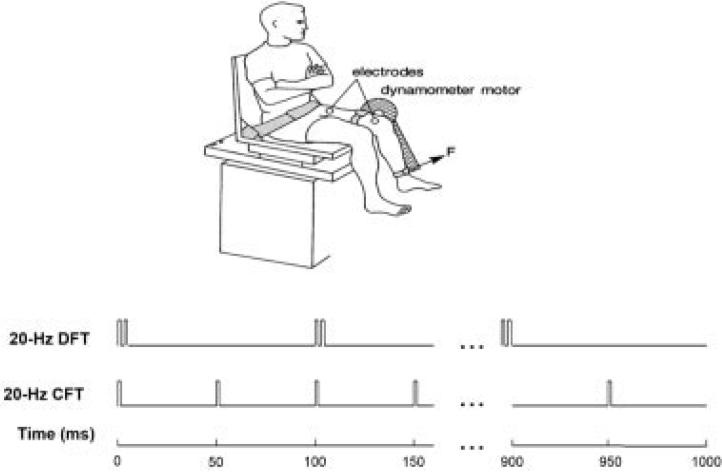
FIGURE 1.
Schematic representation of experimental arrangement for data collection and examples of stimulation trains used in the study. The top stimulation train is a 20-HZ doublet-frequency train (DFT) that contains pairs of doublets (two pulses separated by 5 ms) separated by 95-ms interdoublet intervals. The bottom stimulation train is a 20-HZ constant-frequency train (CFT) that contains pulses separated by 50-ms intervals.
To generate task-specific muscle forces during FES, it is important to determine the stimulation scheme that produces the targeted muscle force responses. The stimulation strategy that maximizes skeletal muscle performance, however, varies from person to person22 and, even for the same individual, varies with the physiological conditions of the muscle, such as the level of fatigue or muscle length.26 Thus, numerous tests would be needed to identify the best stimulation strategy for each individual and each task. Mathematical models that predict force responses to stimulation trains of different frequencies, intensities, and patterns can decrease the number of experimental sessions required to determine the best stimulation strategy for each subject. In addition, mathematical models can help to design subject-specific and task-specific stimulation trains as part of closed-loop feedback controllers in FES systems.15,31
Using data collected from soleus muscles of individuals with spinal cord injuries (SCIs), Frey Law and Shields18 evaluated the mathematical model that we developed using healthy subjects' muscles activated with 6-pulse stimulation trains.13 Their results showed that our model predicted the paralyzed muscle forces for all stimulation frequencies and pulse patterns reasonably well.18 Another recent study,8 which compared seven different muscle models, showed that our model14 and Bobet-Stein's7 model provided the best fits for ankle dorsiflexor forces over a range of joint angles in able-bodied individuals. Recently, we tested a newer version of our isometric model on quadriceps femoris muscles of SCI individuals and showed good predictions for stimulation trains containing up to 50 pulses.16 Thus, our previous model can predict the effect of stimulation frequency and pattern very well,16 but the effect of stimulation intensity on muscle force has not yet been incorporated into the model.
In the current study the effects of stimulation intensity, frequency, and pattern on muscle forces were incorporated into our isometric mathematical model. The purpose of this study was to test the model's ability to predict force responses of the quadriceps femoris muscles of individuals with SCI to stimulation trains with a wide range of frequencies, stimulation patterns, and physiologically relevant stimulation intensities.
MATERIALS AND METHODS
Isometric Force Model
Our previously developed mathematical model predicted isometric forces from human quadriceps femoris muscle in response to stimulation trains of different frequencies and patterns with the stimulation intensity kept constant throughout a testing session.12,14,16 The various physiological processes involved in the generation of skeletal muscle force were classified as two basic steps, muscle activation and force generation, modeled by two first-order ordinary differential equations.16
Muscle Activation Dynamics
Physiologically, the binding of Ca2+ to troponin is necessary to free the binding sites on actin for myosin. Similarly, the first step of our model captures the release and uptake of Ca2+ from the sarcoplasmic reticulum and the binding of Ca2+ to troponin. The rate-limiting steps leading to the formation of the Ca2+-troponin complex are modeled by equation 1,
| (1) |
with
where CN quantitatively describes the rate-limiting step before the myofilaments mechanically slide across each other and generate force, Ri accounts for the nonlinear summation of CN in response to two closely spaced pulses,12 and τC is the time constant controlling the transient shape of CN.
Force Generation
The force output of a muscle depends on the number and rate of formation of strongly bound actin-myosin cross bridges. In addition, visco-elastic components such as the connective tissue and tendon affect the muscle force. Similarly, in our model the instantaneous force (F) was driven by the strongly bound force-producing cross-bridges and mediated by a Michaelis-Menten term, CN/Km + CN. The rate of change of instantaneous force F was modeled by equation 2, which was derived from a linear spring, damper, and motor in series, representing the elastic, connective, and contractile component during muscle contraction, respectively.35
| (2) |
In this equation parameter Km represents the sensitivity of the force development to CN and parameter A represents a scaling factor for force. The second term in equation 2 accounts for the force decay due to two time constants, τ1 and τ2. Parameter τ1 models the force decay due to the visco-elastic components of the muscle following stimulation when CN is small, whereas parameter τ2 models the force decay due to these visco-elastic muscle components during stimulation.
Intensity Modulation
Two methods can be used to modulate stimulation intensity during electrical stimulation—varying the stimulation pulse duration or the stimulation pulse amplitude. We decided to vary the stimulation intensity by changing the stimulation pulse duration because it is easier to quantify and control than the stimulation pulse amplitude, provides a more consistent response across subjects, requires a smaller charge per stimulus pulse, and allows for greater selectivity of recruitment than amplitude modulation.10,20,33 In the present study the pulse amplitude was kept constant throughout the testing sessions while the pulse duration was varied to modulate the stimulation intensity. Preliminary studies on able-bodied subjects showed that most of the effects of stimulation intensity on force could be accounted for by modulating parameter A as a function of stimulation intensity (i.e., pulse duration).11 Thus, among all the parameters, we assumed that only parameter A is affected by stimulation intensity and that the intensity modulation is given by:
| (3) |
where a′ is a scaling factor; pd is the stimulation pulse duration; pd0 is the offset for stimulation pulse duration characterizing how sensitive the muscle is to the stimulation intensity; and pdt is the time constant controlling the steepness of the A-pd relationship.
Subjects
Ten subjects (six male, four female) with complete lower-extremity paralysis due to spinal cord injuries (American Spinal Injury Association, classification A or B) and with mean age of 14 years (range, 8-17 years) participated in this study. The mean time after injury was 4.8 years (range, 1.5-8 years).
At the beginning of each testing session the subject and parents were informed about the nature of the study, the testing procedures, how the force dynamometer, stimulator, and computer worked together for the testing, and the potential risks involved. Subjects were also told that participation was voluntary and that they could withdraw from the study at any time. Then, the subject (if not a minor) or parents signed an informed consent form approved by both the University of Delaware and the Temple University Human Subject Review Boards. Children 8 years of age and older signed an approved assent form.
Experimental Arrangement
Subjects were seated on a computerized force dynamometer (KinCom; Chat-tecx Corp., Chattanooga, Tennessee) with their hips flexed to ~75° and the tested knees flexed to 65° (Fig. 1). The force transducer pad was positioned against the anterior aspect of the leg to be tested about 5 cm proximal to the lateral malleolus. The axis of the dynamometer was aligned with the anatomical axis of the knee. The thigh, waist, and upper trunk were stabilized with straps. A Grass S8800 stimulator (Grass Instruments, West Warwick, Rhode Island) with a Grass Model SIU8T stimulus isolation unit was used to deliver the stimulation pulses. A custom-written LabView program was used to control the stimulator (National Instruments, Austin, Texas). A custom-made switch was connected in series with the stimulator to enable the control of stimulus pulse duration via the computer. Force data were collected directly from the force dynamometer, transferred to a personal computer via an analog-to-digital board at a sampling rate of 200 Hz, and analyzed using custom-written software (LabView; National Instruments).
After positioning the leg to be tested, two appropriately sized surface electrodes (Pals Platinum by Axelgaard Manufacturing Co., Fall Brook, California; 7.5 × 10 cm) were placed over the quadriceps femoris muscle. One electrode was placed over the proximal thigh and the other was placed distally, ~2 cm above the patella, over the vastus medialis. The electrode placement was checked using 770-ms, 14-Hz trains to ensure consistent and effective recruitment of the muscle. If inconsistent activation of the muscle was observed (i.e., lack of a clear response to each pulse within the train) the electrodes were repositioned and the placement was rechecked.
Next, the maximal twitch force of the subject's quadriceps muscle was recorded by stimulating the muscle with a series of single 600-μs pulses delivered at a rate of 1 pulse every 10 s. The pulse amplitude was gradually increased until the maximum twitch force was reached. The maximal twitch force ranged from 23 to 87N. The stimulation amplitude was then adjusted using 1-s, 100-Hz stimulation trains with pulse duration of 600 μs to produce a force equal to the subject's maximal twitch force. Because 100 Hz was the highest frequency and 600 μs was the longest pulse duration (i.e., the highest stimulation intensity) used during the testing session, subsequent testing trains produced lower forces. Previous work by others19,32 showed that the quadriceps femoris muscles of subjects with SCI have elevated twitch-to-tetanus ratios (0.25) compared to able-bodied subjects (0.13). Thus, we estimate that in response to the higher frequencies tested, ~25% of the force-generating ability of the muscles would be recruited. Once set, the stimulation amplitude was kept constant throughout the remainder of the testing session.
Experimental Testing
In this study, CFTs and DFTs with stimulation frequencies ranging from 12.5 to 80 Hz and stimulation pulse durations ranging from 150 to 600 μs were investigated (see Table 1 for the specific trains tested). The 12.5-Hz train with a 150-μs pulse duration was not tested because of the very low forces produced by this train. After the stimulation amplitude was set, a series of trains (40 trains) were delivered to the muscle at the rate of one train every 10 s to avoid fatigue (Table 1). The 50-Hz CFTs and 20-Hz DFTs at 150, 200, 300, and 500 μs were used to determine the model's parameter values for each subject (see the next section). The remaining trains were used to test the model's predictive ability for stimulation frequencies and intensities different from those used to calculate the model's parameter values for each subject. All 40 trains were either 1 s in duration or contained 50 pulses, whichever yielded the shorter train duration. Each testing train was delivered twice: first, trains were delivered in a random order, and then delivered again in reversed order.
Table 1.
Identification of stimulation trains used during testing.
| CFTs |
DFTs |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 12.5 HZ | 20 HZ | 33 HZ | 50 HZ | 80 HZ | 12.5 HZ | 20 HZ | 33 HZ | 50 HZ | |
| CFT, constant-frequency train; DFT, doublet-frequency train. | |||||||||
| o: the stimulation trains used to determine model parameter values. | |||||||||
| x: the stimulation trains used to test the model's predictive ability. | |||||||||
| Blank cells refer to trains that were not delivered. | |||||||||
| 150 μs | x | x | o | x | o | x | x | ||
| 200 μs | o | o | |||||||
| 250 μs | x | x | x | x | x | x | x | x | x |
| 300 μs | o | o | |||||||
| 350 μs | x | x | x | x | x | x | x | x | x |
| 500 μs | o | o | |||||||
| 600 μs | x | x | x | x | x | x | x | x | x |
Model Simulation
The modeling was carried out using a C++ program. The values for each free parameter in equations 1-3 were calculated for each subject using the force responses to the 50-Hz CFTs and 20-Hz DFTs collected at stimulation intensities of 150, 200, 300, and 500 μs using the following procedures. Once the parameter values were calculated, the model was used to predict the force responses to the remaining 32 trains tested. Because the simplest FES model is desired,1 we minimized the number of free parameters without affecting the model's predictive ability. Pilot testing of the model showed that parameter R0 could be fixed at 5 and parameter τC could be fixed at 11 ms for the quadriceps femoris muscles of individuals with SCI. Thus, the values of only seven free parameters, A as a function of (a′, pd0, pdt), Km, τ1, and τ2, needed to be calculated (Table 2). The following steps were involved to obtain predicted forces using the model.
Table 2.
Model parameter values determined for each subject.
| τ1 (ms) | τ2 (ms) | Km | a' (N/ms) | pdo (μs) | pdt (μs) | |
|---|---|---|---|---|---|---|
| For each subject, parameter Ro was fixed at 5 and τc was fixed at 11 ms. | ||||||
| Subject 1 | 53.645 | 1 | 0.159 | 0.421 | 118.357 | 89.827 |
| Subject 2 | 22.154 | 38.559 | 0.028 | 0.653 | 106.078 | 35.131 |
| Subject 3 | 51.684 | 1 | 0.109 | 1.034 | 76.986 | 355.973 |
| Subject 4 | 60.601 | 1 | 0.137 | 0.492 | 131.405 | 194.138 |
| Subject 5 | 28.163 | 1 | 0.189 | 1.359 | 96.285 | 184.054 |
| Subject 6 | 54.41 | 30.549 | 0.14 | 0.879 | 91.753 | 89.569 |
| Subject 7 | 76.472 | 1 | 0.546 | 0.200 | 60.963 | 64.378 |
| Subject 8 | 39.516 | 62.981 | 0.177 | 0.416 | 67.877 | 88.884 |
| Subject 9 | 19.62 | 12.462 | 0.092 | 0.620 | 47.752 | 162.760 |
| Subject 10 | 34.622 | 35.668 | 0.227 | 0.847 | 71.601 | 119.699 |
| Average | 44.099 | 18.522 | 0.180 | 0.692 | 86.906 | 138.441 |
Step 1: Calculation of parameter values for 300-μs pulse duration. Forces in response to 50-Hz CFT and 20-Hz DFT trains with pulse duration of 300 μs were collected. First, parameter τ1 was calculated by fitting the forces immediately following the tetanic stimulation while the muscle relaxed for forces between 50% to 25% of the peak force. This method has previously been described in detail.15 Then, the values of A, Km and μ2 were obtained by minimizing the objective function:
| (4) |
where Fpred were the forces predicted by the model (eqs. 1 and 2) and Fmeas were the measured forces.15,16
Steps 2 and 3: Identification of the A-pd relationship. All the aforementioned parameters, except parameter A, were fixed at the values obtained at the stimulation intensity of 300-μs pulse duration as described above (step 1). Next, the measured force responses to the 50-Hz CFTs and 20-Hz DFTs collected at the stimulation intensities of 150-, 200-, and 500-μs pulse durations were used to determine the values of parameter A by minimizing the objective function:
| (5) |
where Fpred were the forces predicted by the model (eqs. 1 and 2) and Fmeas were the measured forces. Next, using the A-values at these three pulse durations and the A-value calculated at the pulse duration of 300 μs in step 1, the A-pd relationship (eq. 3) was determined.
Step 4: Prediction of force responses to all stimulation trains. Finally, with all the parameter values calculated, the forces in response to two stimulation patterns (CFT and DFT) over a range of stimulation frequencies (12.5-80 Hz) and intensities (150-600 μs) were predicted and then compared to measured forces (see Figs. 2, 3).
FIGURE 2.
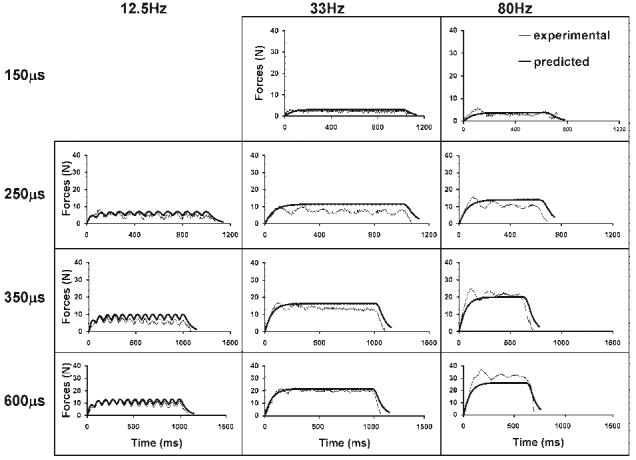
Force responses to constant-frequency trains (CFTs) of 12.5, 33, and 80 HZ at four intensities (150, 250, 350, and 600 μs) are shown for a typical subject.
FIGURE 3.
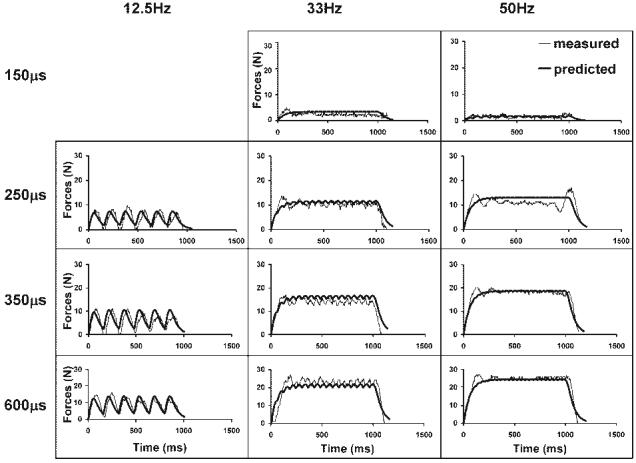
Force responses to doublet-frequency trains (DFTs) of 12.5, 33, and 50 HZ at four intensities (150, 250, 350, and 600 μs) are shown for a typical subject. Data were taken from the same subject shown in Figure 2.
Data Analysis
For each subject the two occurrences of each testing train were averaged and used as the measured force and then compared to the predicted force. Three statistical analyses were done to evaluate the predictions of the model.
First, to evaluate how well the model predicted the shape of the force response, R2 values (predicted R2) were calculated for each testing train between the predicted force and the averaged measured force. In addition, for each testing intensity the physiological R2 values were also calculated by comparing the first and second occurrence of each testing train. The R2 values for each testing train were then averaged across trains and then across subjects to determine the averaged physiological R2 for each intensity. The physiological R2 value between the first and second occurrence of each testing train showed an average value of 0.49, 0.58, 0.73, and 0.65 at a stimulation intensity of 150, 250, 350, and 600 μs, respectively. Then, for each intensity the percentage of testing trains with predicted R2 values above the calculated physiological R2 value was calculated.
Second, the peak force (PK) and force-time integral (FTI) were computed for both predicted and measured forces. Intraclass correlation coefficients (ICCs) between the predicted and measured data were calculated for FTIs and PKs. The predicted PKs and FTIs were plotted against the measured PKs and FTIs, respectively. Trendlines with intercepts of zero were used to evaluate agreement between the measured and predicted values. A slope of 1 and ICC value of 1 would represent a perfect prediction of the model.
Third, the force-frequency relationship was plotted for each testing intensity using measured and predicted FTIs and PKs data (averaged across subjects) plotted against the stimulation frequencies. To obtain a measure of physiological variability for both PKs and FTIs, the absolute percentage differences in PKs and FTIs between the two occurrences of each testing train were calculated using the formula:
| (6) |
and then averaged across all testing trains and subjects for each intensity (Table 3). For each testing train, the model error was calculated using the formula:
| (7) |
Table 3.
Physiological variability at each testing intensity.*
| Intensity tested | FTI | PK |
|---|---|---|
| FTI, force-time integral; PK, peak force. | ||
| 150 μs | 24% | 17% |
| 250 μs | 31% | 24% |
| 350 μs | 20% | 16% |
| 600 μs | 33% | 24% |
At each pulse duration, % variability was calculated for each train, then averaged across trains and subjects (see text for details).
If the percentage difference in FTI or PK between the measured and predicted forces (%model error) was larger than the % physiological variability, a paired t-test was used to determine whether there was a significant difference between the predicted and the measured forces for that testing train.
RESULTS
Complete data were collected from 10 subjects. Model parameter values that were used to obtain predicted forces are shown in Table 2. Inspection of measured and predicted force-time responses showed that the model was able to predict well the shape of the force-time responses (see Figs. 2 and 3 for examples from a representative subject). The model predicted well the shape of the force responses with the averaged predicted R2 values of 0.61, 0.71, 0.73, and 0.77 between the predicted and measured forces for stimulation intensity of 150, 250, 350, and 600 μs, respectively. About 77%, 79%, 61%, and 80% of the model's predictions produced R2 values above the physiological R2 values for the tested stimulation intensity 150, 250, 350, and 600 μs, respectively.
In addition, the model predicted well the FTI and PK. The trendline comparing the predicted to the measured FTI produced a slope of 0.99 and ICC values of 0.96 (Fig. 4). Similarly, the trendline comparing predicted to measured PK produced a slope of 0.88 and ICC value of 0.94 (Fig. 4). For both the CFTs and DFTs, the model predicted well the PK-frequency and FTI-frequency relationships for each of the four stimulation intensities tested (Figs. 5, 6). For FTI, significant differences on a paired t-test observed only for the 12.5-Hz DFT at 250-μs pulse duration (%model error = 33%, P < 0.05) and the 12.5-Hz CFT at 350-μs pulse duration (%model error = 59%, P < 0.05) (Fig. 5). For PK, significant differences were seen on the paired t-tests only for the 20-Hz CFT (%model error = 18%, P < 0.05) and the 20-Hz DFT (%model error = 22%, P < 0.05) at 150-μs pulse duration and the 20-Hz DFT with (%model error = 16%, P < 0.05) at 350-μs pulse duration (Fig. 6). Both the measured and predicted results showed that the 50-Hz trains produced the maximum FTI for all pulse durations tested and that the FTI declined at higher frequencies for CFTs (note that 50 Hz was the highest frequency tested for DFTs). Similarly, for PK the measured and predicted forces showed the maximum values at the same frequency, which was always the highest frequency tested. These data are consistent with previous reports.16
FIGURE 4.
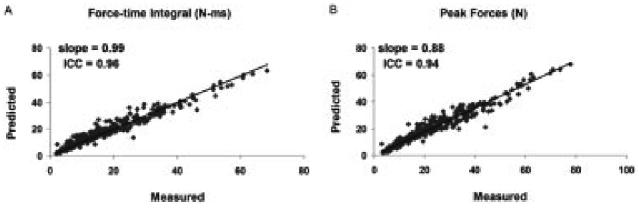
Plots of measured versus predicted force-time integrals (A) and peak forces (B) for all stimulation trains for 10 subjects. Trendlines were plotted using an intercept of zero and the slopes were reported.
FIGURE 5.
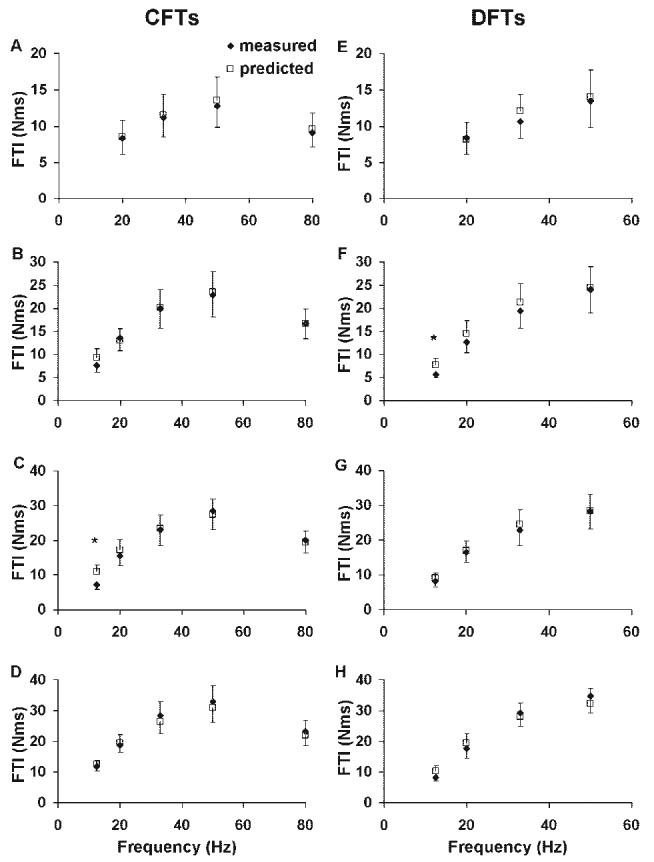
Comparisons between the measured (filled diamonds) and predicted (open squares) force-time integrals (FTIs) for CFTs (A-D) and DFTs (E-H) at each frequency tested. Data were collected at four different stimulation levels from the 10 subjects. Stimulus duration (intensity) was 150 μs, 250 μs, 350 μs, and 600 μs from the top panel down.
FIGURE 6.
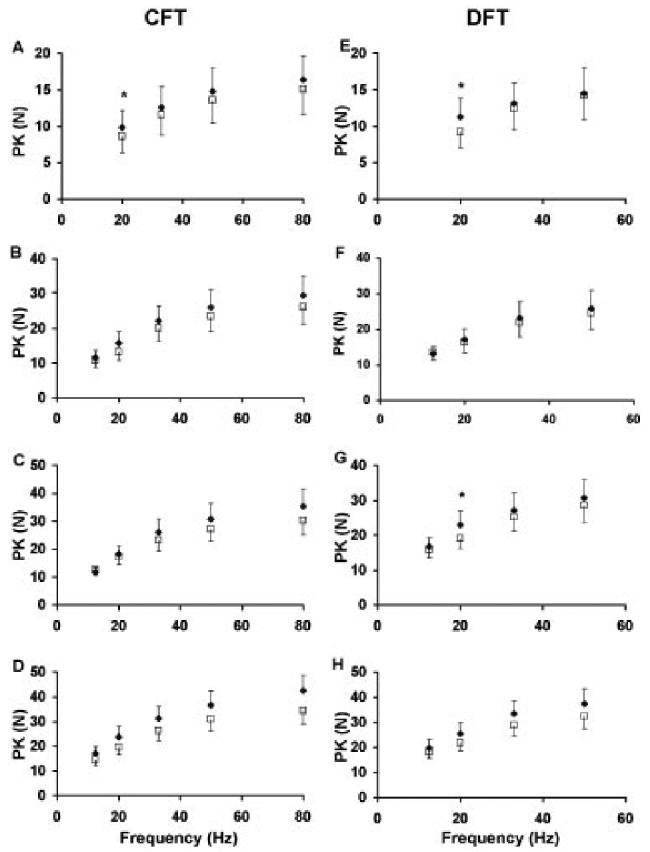
Comparisons between the measured (filled diamonds) and predicted (open squares) peak forces (PKs) for CFTs (A-D) and DFTs (E-H) at each frequency tested. Data were collected at four different stimulation levels from the 10 subjects. Stimulus duration (intensity) was 150 μs, 250 μs, 350 μs, and 600 μs from the top panel down.
DISCUSSION
Previously, we developed a model16 that successfully predicted the effects of stimulation frequency and pattern on force output of paralyzed skeletal muscle for patients with SCIs. In the current study the addition of an intensity component to our model, with only three additional parameters, enabled us to predict the effect of stimulation frequency, intensity (i.e., pulse duration), and pattern on muscle forces of SCI individuals. For each subject, after the values of the seven free model parameters (A, a′, pd0, pdt, Km, τ1, and τ2) were determined using measured forces in response to eight stimulation trains (50-Hz CFTs and 20-Hz DFTs at stimulation intensities of 150-, 200-, 300-, and 500-μs pulse durations), the model successfully predicted PKs and FTIs for trains of two different patterns (CFTs and DFTs) over a wide range of stimulation frequencies (10-80 Hz) and intensities (150-, 250-, 350-, and 600-μs pulse durations). It is notable that our model successfully predicted forces at intensities different from the ones that were used to determine the parameter values (see Table 1).
Accurate predictive mathematical models can help to enhance FES systems. An ideal FES system needs both a feedforward model, which designs subject-specific and task-specific stimulation patterns, and a feedback controller, which corrects errors by informing the feedforward model when changes in stimulation patterns are needed.1,6 When used in conjunction with a closed-loop controller, mathematical models can allow FES stimulators to deliver customized patient-specific stimulation patterns and perform FES tasks while continuously adapting stimulation parameters to the actual needs of the patient.31 The use of closed-loop control systems using customized stimulation patterns can reduce the energy expenditure and improve the speed at which functional tasks are performed during FES.31 Mathematical models have been developed to predict FES-induced standing movements,24 to derive and optimize open-loop and closed-loop controllers,30 and to provide insights into the internal dynamics of FES-induced muscle contractions.17
To our knowledge, only three previous modeling efforts9,29,34 have taken into account the effects of both frequency and intensity on muscle force output. A detailed musculoskeletal model of the human knee to describe shank motion was developed by Riener et al.29 and tested using measured data from quadriceps femoris muscles of paraplegic patients. The model was designed to take into account physiological conditions such as fatigue and the variation in the stimulation intensity at one stimulation frequency (20 Hz).29 The authors reported good matching between measured and simulated data. Two major shortcomings are noted regarding this model.29 First, the model is unable to predict the effects of varying stimulation frequency on force output. Second, the model is complex: it requires many parameters to describe the musculoskeletal system and would take longer to determine the parameter values for each subject. Watanabe et al.34 developed a model with both stimulation frequency and intensity as inputs. Their model was tested with force-frequency data obtained from the tibialis anterior and the triceps surae muscles of rabbits and the flexor carpi radialis and flexor carpi ulnaris muscles of one neurologically intact patient at one testing intensity. The results showed that the model fitted well the normalized isometric force-frequency relationship for all the muscles tested. However, the effect of varying recruitment order on force output (i.e., the effect of varying stimulation intensity on force output) was not evaluated.34 Chizeck et al.9 tested a muscle model on human subjects with lower-extremity paralysis due to SCI. Their model was able to predict the torque for stimulation trains up to 500 ms in duration; however, no predictive ability in stimulation pattern was demonstrated because the same stimulation patterns were used to determine model parameter values and to test the model.9 Once the stimulation pattern changed, the model parameter values needed to be recalculated, rendering their model incapable of identifying the optimal pattern for generating the targeted force.9
In 2005 we reported the ability of our isometric force model to predict the effects of stimulation frequency and pattern on quadriceps muscle forces of SCI individuals.16 The current version of our model has the advantage of also being able to predict the effects of stimulation intensity on paralyzed muscle forces. During previous testing of our model in SCI, τC was calculated by multiplying the subject's half-relaxation time by 0.22,16 resulting in an averaged τC value of 11 ms for both nonfatigued and fatigued muscles. In the present study, parameter τC was fixed at 11 ms because preliminary testing also showed that the model predicted well even with τC fixed at 11 ms. The scaling factor A from our previous model16 was modified with equation 3, which consists of three parameters—a, pd0, and pdt—to take into account the effect of stimulation intensity on muscle force output. Thus, the current model consists of seven free parameters, τ1, τ2, Km, and A as a function of (a, pd0, pdt) (Table 2) that need to be determined for each subject. Our present model, which is able to predict the effects of stimulation intensity and frequency on muscle force, showed similar predictive ability to our previous testing on SCI individuals16 with ICCs (measuring agreement between measured and predicted muscle forces) in our present study (≥0.94) comparable to the ICCs obtained in our previous study on SCI (>0.90).18
In conclusion, a simple modification to our previously developed model16 (eq. 3) enabled successful prediction of effects of stimulation intensity on muscle force for two different pulse patterns (CFTs and DFTs) over a wide range of frequencies (12.5-80 Hz) in SCI subjects. With this ability to predict forces in response to a wide range of physiologically relevant stimulation trains of different frequencies, intensities, and patterns, the model provides a multidimensional prediction of skeletal-muscle force responses. Our model can identify subject-specific optimal stimulation schemes and accurately predict muscle forces for feedforward control, making it ideal for use in FES systems. This study is another step toward our long-term goal of incorporating an intensity component into our recently developed nonisometric model28 to enable it to predict the effects of stimulation frequency, pattern, and intensity on muscle performance during FES for different muscle types in patient populations. We plan to incorporate this enhanced model into an FES control system to provide a more precise control of muscle output using both frequency and intensity modulation during FES tasks.
Acknowledgments
Supported by NIH grants HD36797 and HD38582. The authors thank Dr. R. Perumal and Mr. R. Maladen for the development of the software and hardware used during data acquisition.
Abbreviations
- CFT
constant-frequency trains
- CNS
central nervous system
- DFT
doublet-frequency train
- FES
functional electrical stimulation
- FTI
force-time integral
- ICC
intra-class correlation coefficient
- PK
peak force
- SCI
spinal cord injury
REFERENCES
- 1.Abbas JJ, Riener R. Using mathematical models and advanced control systems techniques to enhance neuroprosthesis function. Neuromodulation. 2001;4:187. doi: 10.1046/j.1525-1403.2001.00187.x. [DOI] [PubMed] [Google Scholar]
- 2.Andrews BJ, Baxendale RH, Barnett R, Phillips GF, Yamazaki T, Paul JP, et al. Hybrid FES orthosis incorporating closed loop control and sensory feedback. J Biomed Eng. 1988;10:189–195. doi: 10.1016/0141-5425(88)90099-4. [DOI] [PubMed] [Google Scholar]
- 3.Binder-Macleod S, Kesar T. Catchlike property of skeletal muscle: recent findings and clinical implications. Muscle Nerve. 2005;31:681–693. doi: 10.1002/mus.20290. [DOI] [PubMed] [Google Scholar]
- 4.Binder-Macleod SA, Lee SC, Baadte SA. Reduction of the fatigue-induced force decline in human skeletal muscle by optimized stimulation trains. Arch Phys Med Rehabil. 1997;78:1129–1137. doi: 10.1016/s0003-9993(97)90140-4. [DOI] [PubMed] [Google Scholar]
- 5.Binder-Macleod SA, Lee SC, Russ DW, Kucharski LJ. Effects of activation pattern on human skeletal muscle fatigue. Muscle Nerve. 1998;21:1145–1152. doi: 10.1002/(sici)1097-4598(199809)21:9<1145::aid-mus5>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 6.Bobet J. Can muscle models improve FES-assisted walking after spinal cord injury? J Electromyogr Kinesiol. 1998;8:125–132. doi: 10.1016/s1050-6411(97)00029-1. [DOI] [PubMed] [Google Scholar]
- 7.Bobet J, Stein RB. A simple model of force generation by skeletal muscle during dynamic isometric contractions. IEEE Trans Biomed Eng. 1998;45:1010–1016. doi: 10.1109/10.704869. [DOI] [PubMed] [Google Scholar]
- 8.Bobet J, Gossen ER, Stein RB. A comparison of models of force production during stimulated isometric ankle dorsiflexion in humans. IEEE Trans Neural Syst Rehabil Eng. 2005;13:444–451. doi: 10.1109/TNSRE.2005.858461. [DOI] [PubMed] [Google Scholar]
- 9.Chizeck HJ, Chang S, Stein RB, Scheiner A, Ferencz DC. Identification of electrically stimulated quadriceps muscles in paraplegic subjects. IEEE Trans Biomed Eng. 1999;46:51–61. doi: 10.1109/10.736755. [DOI] [PubMed] [Google Scholar]
- 10.Crago PE, Peckham PH, Thrope GB. Modulation of muscle force by recruitment during intramuscular stimulation. IEEE Trans Biomed Eng. 1980;27:679–684. doi: 10.1109/TBME.1980.326592. [DOI] [PubMed] [Google Scholar]
- 11.Ding J, Kesar T, Wexler AS, Binder-Macleod SA. A mathematical model that predicts the force-intensity relationship of human skeletal muscle. 10th International Conference of the International FES Society; Montreal, Canada. July, 2005.pp. 367–369. [Google Scholar]
- 12.Ding J, Binder-Macleod SA, Wexler AS. Two-step, predictive, isometric force model tested on data from human and rat muscles. J Appl Physiol. 1998;85:2176–2189. doi: 10.1152/jappl.1998.85.6.2176. [DOI] [PubMed] [Google Scholar]
- 13.Ding J, Wexler AS, Binder-Macleod SA. A predictive model of fatigue in human skeletal muscles. J Appl Physiol. 2000;89:1322–1332. doi: 10.1152/jappl.2000.89.4.1322. [DOI] [PubMed] [Google Scholar]
- 14.Ding J, Wexler AS, Binder-Macleod SA. A mathematical model that predicts the force-frequency relationship of human skeletal muscle. Muscle Nerve. 2002;26:477–485. doi: 10.1002/mus.10198. [DOI] [PubMed] [Google Scholar]
- 15.Ding J, Wexler AS, Binder-Macleod SA. Mathematical models for fatigue minimization during functional electrical stimulation. J Electromyogr Kinesiol. 2003;13:575–588. doi: 10.1016/s1050-6411(03)00102-0. [DOI] [PubMed] [Google Scholar]
- 16.Ding J, Lee SC, Johnston TE, Wexler AS, Scott WB, Binder-Macleod SA. Mathematical model that predicts isometric muscle forces for individuals with spinal cord injuries. Muscle Nerve. 2005;31:702–712. doi: 10.1002/mus.20303. [DOI] [PubMed] [Google Scholar]
- 17.Dorgan SJ, O'Malley MJ. A mathematical model for skeletal muscle activated by N-let pulse trains. IEEE Trans Rehabil Eng. 1998;6:286–299. doi: 10.1109/86.712226. [DOI] [PubMed] [Google Scholar]
- 18.Frey Law LA, Shields RK. Predicting human chronically paralyzed muscle force: a comparison of three mathematical models. J Appl Physiol. 2006;100:1027–1036. doi: 10.1152/japplphysiol.00935.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gerrits HL, De Haan A, Hopman MT, van Der Woude LH, Jones DA, Sargeant AJ. Contractile properties of the quadriceps muscle in individuals with spinal cord injury. Muscle Nerve. 1999;22:1249–1256. doi: 10.1002/(sici)1097-4598(199909)22:9<1249::aid-mus13>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 20.Grill WM, Jr, Mortimer JT. The effect of stimulus pulse duration on selectivity of neural stimulation. IEEE Trans Biomed Eng. 1996;43:161–166. doi: 10.1109/10.481985. [DOI] [PubMed] [Google Scholar]
- 21.Kanosue K, Yoshida M, Akazawa K, Fujii K. The number of active motor units and their firing rates in voluntary contraction of human brachialis muscle. Jpn J Physiol. 1979;29:427–443. doi: 10.2170/jjphysiol.29.427. [DOI] [PubMed] [Google Scholar]
- 22.Karu ZZ, Durfee WK, Barzilai AM. Reducing muscle fatigue in FES applications by stimulating with N-let pulse trains. IEEE Trans Biomed Eng. 1995;42:809–817. doi: 10.1109/10.398642. [DOI] [PubMed] [Google Scholar]
- 23.Kebaetse MB, Lee SC, Binder-Macleod SA. A novel stimulation pattern improves performance during repetitive dynamic contractions. Muscle Nerve. 2001;24:744–752. doi: 10.1002/mus.1065. [DOI] [PubMed] [Google Scholar]
- 24.Khang G, Zajac FE. Paraplegic standing controlled by functional neuromuscular stimulation. Part I. Computer model and control-system design. IEEE Trans Biomed Eng. 1989;36:873–884. doi: 10.1109/10.35296. [DOI] [PubMed] [Google Scholar]
- 25.Kralj A, Bajd T, Turk R. Enhancement of gait restoration in spinal injured patients by functional electrical stimulation. Clin Orthop. 1988:34–43. [PubMed] [Google Scholar]
- 26.Lee SC, Gerdom ML, Binder-Macleod SA. Effects of length on the catchlike property of human quadriceps femoris muscle. Phys Ther. 1999;79:738–748. [PubMed] [Google Scholar]
- 27.Marsolais EB, Edwards BG. Energy costs of walking and standing with functional neuromuscular stimulation and long leg braces. Arch Phys Med Rehabil. 1988;69:243–249. [PubMed] [Google Scholar]
- 28.Perumal R, Wexler AS, Binder-Macleod SA. Mathematical model that predicts lower leg motion in response to electrical stimulation. J Biomech. 2006;39:2826–2836. doi: 10.1016/j.jbiomech.2005.09.021. [DOI] [PubMed] [Google Scholar]
- 29.Riener R, Quintern J, Schmidt G. Biomechanical model of the human knee evaluated by neuromuscular stimulation. J Biomech. 1996;29:1157–1167. doi: 10.1016/0021-9290(96)00012-7. [DOI] [PubMed] [Google Scholar]
- 30.Riener R, Fuhr T. Patient-driven control of FES-supported standing up: a simulation study. IEEE Trans Rehabil Eng. 1998;6:113–124. doi: 10.1109/86.681177. [DOI] [PubMed] [Google Scholar]
- 31.Riener R. Model-based development of neuroprosthesis for paraplegic patients. Philos Trans R Soc Lond B Biol Sci. 1999;354:877–894. doi: 10.1098/rstb.1999.0440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scott WB, Lee SC, Johnston TE, Binkley J, Binder-Macleod SA. Contractile properties and the force-frequency relationship of the paralyzed human quadriceps femoris muscle. Phys Ther. 2006;86:788–799. [PubMed] [Google Scholar]
- 33.Szlavik RB, de Bruin H. The effect of stimulus current pulse width on nerve fiber size recruitment patterns. Med Eng Phys. 1999;21:507–515. doi: 10.1016/s1350-4533(99)00074-0. [DOI] [PubMed] [Google Scholar]
- 34.Watanabe T, Futami R, Hoshimiya N, Handa Y. An approach to a muscle model with a stimulus frequency-force relationship for FES applications. IEEE Trans Rehabil Eng. 1999;7:12–18. doi: 10.1109/86.750545. [DOI] [PubMed] [Google Scholar]
- 35.Wexler AS, Ding J, Binder-Macleod SA. A mathematical model that predicts skeletal muscle force. IEEE Trans Biomed Eng. 1997;44:337–348. doi: 10.1109/10.568909. [DOI] [PubMed] [Google Scholar]