Abstract
Anatomical and physiological studies conducted in the 1960s identified the periaqueductal gray (PAG) and its descending projections to the rostral ventromedial medulla (RVM) and spinal cord dorsal horn, as a primary anatomical pathway mediating opioid-based analgesia. Since these initial studies, the PAG-RVM-spinal cord pathway has been characterized anatomically and physiologically in a wide range of vertebrate species. Remarkably, the majority of these studies were conducted exclusively in males with the implicit assumption that the anatomy and physiology of this circuit were the same in females; however, this is not the case. It is well established that morphine administration produces greater antinociception in males compared to females. Recent studies indicate that the PAG-RVM pathway contributes to the sexually dimorphic actions of morphine. This manuscript will review our anatomical, physiological, and behavioral data identifying sex differences in the PAG-RVM pathway, focusing on its role in pain modulation and morphine analgesia.
1. Introduction
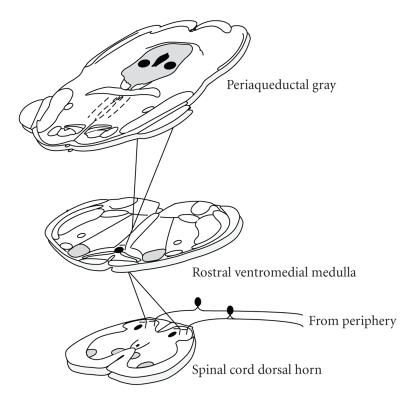
It was first reported that electrical stimulation of the midbrain periaqueductal gray (PAG) produced profound analgesia in the male rat in 1969 [1]. Since then, the anatomical and physiological organization of the PAG and its descending projections to the rostral ventromedial medulla (RVM) and dorsal horn of the spinal cord have been well characterized in a variety of species, including the rat [2–9], cat [10–18], primate [19, 20], and rabbit [21] (see Figure 1). The PAG-RVM-spinal cord pathway comprises an essential neural circuit for opioid-based antinociception [6, 18, 22]. Intra-PAG administration of the mu opioid receptor (MOR) agonist morphine, the most commonly prescribed opiate for persistent pain relief, produces naloxone-reversible analgesia [23] as well as naloxone-reversible excitation of RVM neurons [7, 24, 25]. Similarly, lesions of the PAG or intra-PAG administration of MOR antagonists [26–29] attenuate the antinociceptive effects of systemic morphine across a wide range of analgesiometric tests [30]. Studies utilizing autoradiography, immunohistochemistry, and in situ hybridization have shown that the PAG contains a high density of MOR [31–38], with approximately 27–50% of PAG neurons retrogradely labeled from the RVM expressing MOR [35, 37].
Figure 1.
A schematic of the descending inhibitory pathway for pain modulation illustrating the projections from the midbrain periaqueductal gray to the brainstem RVM and the spinal cord dorsal horn.
While it is well established that the PAG-RVM-spinal cord pathway is essential for the analgesic actions of both systemic and intra-PAG morphine, these early studies were conducted exclusively in male subjects. Only recently have studies begun including “sex” as an independent variable, and it is becoming increasingly clear that morphine does not produce the same degree of antinociception in males and females, especially following the induction of persistent pain. Sex differences in morphine potency were first reported in rodents in the late 1980s, when it was shown that systemic morphine administration produced a significantly greater degree of antinociception in males using acute pain assays [39–42]. This phenomenon has been repeated in multiple studies employing animal models of pain, including orofacial [43] and visceral [44, 45] pain models, as well as persistent somatic pain models [38, 46–52]. Although results on the contrary are also reported, generally these studies have shown that morphine produces a significantly greater degree of analgesia in males in comparison to females. Indeed, we have recently reported that male rats have a significantly higher MOR expression in the PAG, which is positively correlated with morphine analgesia in male but not female rats [38].
Recently, clinical studies in humans have also reported sex differences in morphine analgesia. Of the limited number of studies that examined “gender” or “sex” as an independent variable, it has been reported that males experience greater morphine analgesia compared to females [53–55]. In fact, one study reported that females required 30% more morphine to reach the same level of analgesia as males [55]. Similar to the rodent literature, the results in human studies are not unequivocal. Sarton et al. [56] reported greater morphine analgesia in females, while two studies reported no sex difference [57, 58]. Sex differences in morphine consumption also have been reported [59]; however, given that the majority of negative side effects associated with morphine consumption, including nausea, headache, and dysphoria [57, 60], are exacerbated in females compared to males, morphine consumption is not a reliable indicator of morphine analgesia.
Sex differences in opioid analgesia are not limited to mu opioid agonists. In both human and animal studies, sex differences in the analgesic effects of kappa or delta opioid agonists have also been reported, although again, not without controversy [61–65]. Several factors are likely to contribute to the disparate results between studies reporting the presence or absence of a sex difference in opioid analgesia, including differences in the type of pain being examined (e.g., experimental acute pain versus postoperative pain versus a chronic pain state), the route of drug administration (e.g., oral versus intravenous versus intrathecal), the strain differences in the rodents studies, and the efficacy of the opiate being administered. Sex differences in basal pain sensitivity, as well as estrous cycle effects, may also contribute [54, 55, 57, 66–79].
While it is clear that sex differences in opioid analgesia are not a simple and straightforward phenomenon, when sex differences are reported, they are not trivial in magnitude. In our persistent inflammatory [38, 46] and visceral pain [44, 45] studies, the ED50 for females is twice the ED50 of males. Similarly, morphine is approximately 5-fold more potent in producing antihyperalgesia in arthritic males compared to arthritic females [52]. Sex differences in morphine analgesia are not due to sex differences in the pharmacokinetics of morphine in humans [56] or rodents [50]. Rather, sex differences in morphine analgesia are likely related to the inherent differences in how the central nervous system of males and females respond to opiates. To date, the mechanism(s) underlying the sexually dimorphic actions of morphine remain unknown.
Given that the PAG and its descending projections to the RVM and dorsal horn of the spinal cord provide a primary pathway for the actions of opiates in pain modulation, inherent differences in this pathway could contribute to the sexually dimorphic actions of morphine. Thus, we tested three hypotheses: (1) are there sex differences in the anatomical organization of the PAG-RVM pathway? (2) is there a sexually dimorphic response of the PAG-RVM output neurons to persistent pain? (3) does the administration of morphine differentially engage the PAG-RVM pathway in male and female rats?
2. Sexually Dimorphic Organization of a Descending Pain Inhibitory Pathway
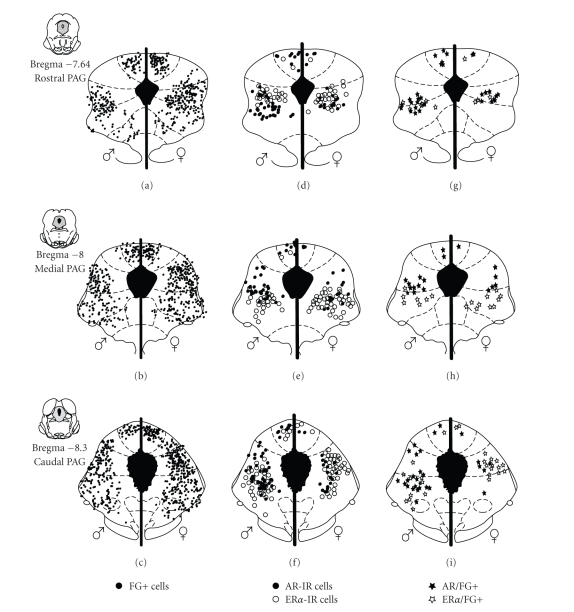
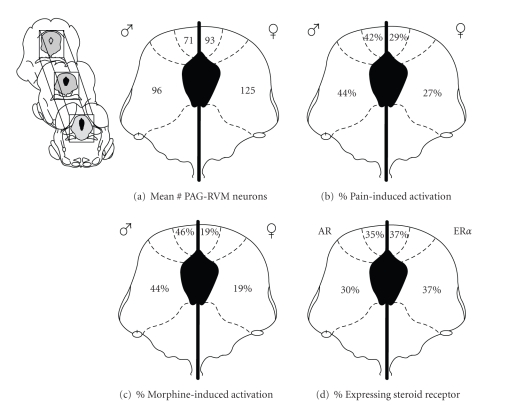
We used neuroanatomical tract-tracing techniques to examine whether there were qualitative and/or quantitative differences in the neural projection from the PAG to the RVM in male and female rats. Consistent with previous anatomical studies [2, 80, 81], we reported that the dorsomedial, lateral and ventrolateral PAG heavily project to the RVM in both male and female rats [82]. While no qualitative sex differences were noted in the overall distribution of PAG-RVM projection neurons, females had significantly more PAG-RVM output neurons across the rostrocaudal axis of the PAG compared to males [83, 84] (Figures 2(a)–2(c)). The average number of retrogradely labeled cells across the rostrocaudal extent of the PAG was greater by a third in female compared to male rats (Figure 3(a)). The most prominent sex difference in retrograde labeling was observed within the lateral and ventrolateral regions of the PAG, an area known to contain a dense distribution of MOR [34, 37].
Figure 2.
(a)–(c) Distribution of cells retrogradely labeled (FG+) from the RVM in males (left) and females (right) at three representative rostrocaudal levels of the periaqueductal gray. Each black circle represents one FG+ cell. (d)–(f) Distribution of PAG cells that were immunoreactive for AR (closed circles) or ERα (open circles). (g)–(i) Distribution of PAG cells retrogradely labeled from the RVM that were also immunoreactive for AR (closed stars) or ERα (open stars).
Figure 3.
(a) Mean number of PAG cells retrogradely labeled from the RVM across the rostrocaudal axis in males (left) and females (right). (b) Percentage of Fos-positive neurons that were retrogradely labeled from the RVM in males (left) and females (right) following twenty-four hours of inflammation. (c) Average of the percentage of AR (left) and ERα (right) receptor-expressing PAG cells retrogradely labeled from the RVM. (d) Percentage of Fos-positive neurons that were retrogradely labeled from the RVM in males (left) and females (right) following twenty-four hours of inflammation and one hour of morphine (5 mg/kg).
3. Sexually Dimorphic Response of the PAG-RVM Pathway to Persistent Inflammatory Pain
Inflammatory pain results in the activation of descending modulatory circuits [8, 85] and contributes to both hyperalgesia and antinociception [86–89]. We found that the persistent inflammatory pain induced by injection of complete Freund's adjuvant (CFA) into the rat hindpaw caused extensive activation of PAG neurons as measured by Fos labeling. Interestingly, this activation was comparable (both quantitatively and qualitatively) in male and female rats [82]. However, when the analysis was restricted to PAG neurons retrogradely labeled from the RVM, while females have almost twice the number of PAG-RVM output neurons in comparison to males, very few of these cells in female rats expressed inflammation-induced Fos, suggesting that this circuit is preferentially activated in males (Figure 3(b)). Indeed we found that, overall, persistent inflammatory pain activated approximately 43% of PAG-RVM neurons in the dorsomedial, lateral and ventrolateral PAG of males, but only half as many PAG-RVM output neurons were activated by inflammatory pain in females. Activation of the PAG and its descending outputs to the RVM results primarily in the inhibition of dorsal horn neuronal responses to acute noxious stimuli [90–95]; therefore, one would predict that given the greater activation of the circuit in males than females, males should have displayed reduced hyperalgesia following induction of plantar inflammation. However, in our behavioral studies, we found no sex differences in either baseline withdrawal latencies or in CFA-induced hyperalgesia. Therefore, our finding that the PAG-RVM descending circuit is not being engaged to the same degree by persistent inflammatory pain in males and females suggests that there is an alternative mechanism for endogenous pain modulation in female rats [96–99].
We have recently begun exploring this possibility using combinatorial anterograde and retrograde tract-tracing in combination with persistent pain-induced Fos labeling. The results of these studies suggest that there are indeed sex differences in both the efferent and afferent projections of the PAG. Specifically, the amygdala, ventromedial hypothalamus, and periventricular nucleus project more heavily to the PAG in females than males. In contrast, the medial preoptic area, parabrachial nucleus, and locus coeruleus project more heavily to the PAG in males than females [100]. In addition, our data indicate that the projections to the parabrachial nucleus, locus coeruleus, and the A5/A7 noradrenergic cell group appear to be greater in males (Loyd and Murphy, unpublished observations). Obviously, further research on the anatomy and physiology of pain modulatory circuits in females is warranted.
4. Sex Differences in the Activation of the Descending Inhibitory Pathway by Morphine
Although sex differences in PAG-RVM output neuron activation do not appear to contribute to sex differences in pain, they do appear to contribute to sex differences in morphine analgesia. Until recently, all studies examining the mechanisms of morphine action in the PAG were conducted exclusively in males; therefore it was unknown whether morphine administration has the same physiological effect on PAG neurons in females. Electrophysiological studies of PAG neurons are limited because they examine the response of a single neuron [7, 17, 101–109]. We have addressed this problem by using tract-tracing techniques and Fos labeling to measure the activity of populations of PAG-RVM neurons in the PAG of males and females.
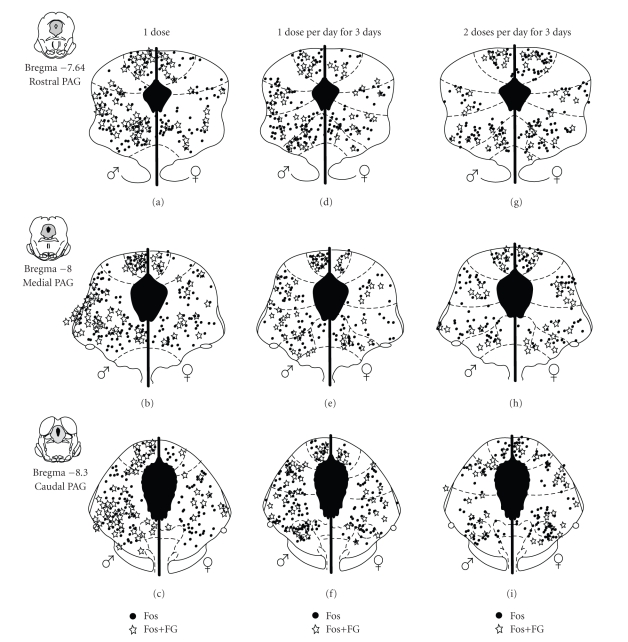
Systemic morphine administration attenuates the persistent pain-induced Fos expression within the PAG of male but not female rats [82] and is consistent with our data showing that the ED50 for systemic morphine is approximately twofold higher in females compared to males whether administered systemically [46] or directly into the PAG [38]. Interestingly, morphine administration, in the absence of pain, resulted in a twofold greater activation of PAG neurons compared to saline administration [84]. No sex difference was observed in the activation of PAG neurons by morphine (see the black circles in Figures 4(a)–4(c)), suggesting that in the absence of pain, morphine is equipotent in its ability to depolarize PAG neurons. However, when the analysis was limited to PAG neurons projecting to the RVM, the number of neurons activated by morphine was consistently and significantly higher in males compared to females (see the stars in Figures 4(a)–4(c)) [84]. Indeed, approximately half of PAG-RVM neurons in males were activated by morphine, whereas only 20% were activated in females (see Figure 3(c)). These results corroborate previous studies demonstrating that morphine results primarily in the net excitation of PAG-RVM neurons, most likely through the removal of tonic GABA inhibition [35, 104, 110, 111]. The finding that very few PAG-RVM neurons were activated by morphine in females suggests that morphine may be limited in effectiveness as a pain modulator.
Figure 4.
Distribution of PAG cells expressing Fos (black circles) and cells retrogradely labeled from the RVM expressing Fos (stars) following: (a)–(c) one 5 mg/kg dose of morphine; (d)–(f) one 5 mg/kg dose of morphine per day for three consecutive days; (g)–(i) or two 5 mg/kg doses of morphine per day for three consecutive days in males (left) and females (right) at three representative rostrocaudal levels of the PAG.
Given that more PAG neurons project to the RVM in female compared to male rats, it is possible that pain modulation in females is less dependent on opioids. If this is the case, then direct activation of PAG output neurons should produce greater antinociception in females, not males. Microinjection of the GABA antagonist bicuculline into the PAG produces antinociception [110, 112] by disinhibiting output neurons. Surprisingly, even though females have more output neurons, the antinociceptive effect of microinjecting bicuculline into the PAG is greater in males [113].
5. Sex Differences in the Development of Tolerance to Morphine
Repeated or continuous administration of morphine into the ventrolateral PAG of male rats has been shown to result in the development of tolerance [26, 114–118]. In addition, blocking opioid binding sites in the ventrolateral PAG attenuates the development of tolerance to systemically administered morphine [26]. Tolerance appears to be mediated by a reduction in MOR signaling efficacy in PAG neurons [119], an effect that is reversed when MOR coupling is enhanced via upregulated adenylate cyclase activity [120]. If the PAG-RVM pathway is essential for the development of tolerance, then activation of the PAG-RVM pathway by morphine should decline as tolerance develops, and changes in the activation of this pathway would correlate with sex differences in the development of tolerance to morphine. These hypotheses were tested in male and female rats using behavioral testing (hot plate) and immunohistochemistry to map the activation of the PAG-RVM pathway following repeated morphine administration.
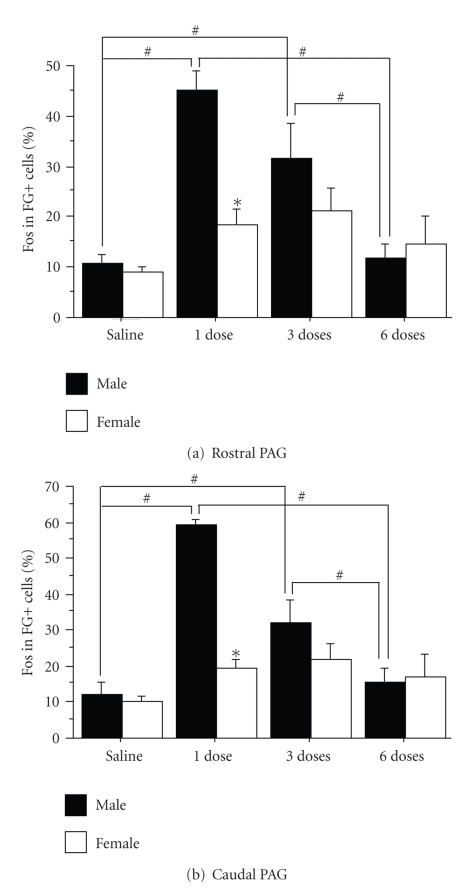
Morphine was administered once or twice a day for three days in rats that had previously received retrograde tracer injections into the RVM. To examine the activation of PAG-RVM neurons during the development of tolerance, males and females were both administered 5 mg/kg of morphine, the ED50 for males. Repeated administration of systemic morphine induced tolerance in males to a significantly greater extent than in females [83], consistent with previous research administering equipotent doses of morphine to examine sex differences in tolerance [47]. The half maximal antinociceptive effect of a single injection of morphine following the development of morphine tolerance was two times greater for female compared to male rats. In parallel, the activation of PAG-RVM neurons was significantly attenuated following repeated morphine administration in males [83]. While there was no sex difference in the activation of the PAG following three doses or six doses of morphine over three days (see the black circles in Figures 4(d)–4(i)), the activation of the PAG-RVM projection neurons steadily declined in males only (see the stars in Figures 4(d)–4(i)). Activation of the PAG-RVM pathway by morphine in female rats was minimal, and therefore did not decline significantly following repeated administration of morphine (Figure 5; previously published [83]).
Figure 5.
Percentage of Fos-positive neurons that were retrogradely labeled from the RVM (%Fos in FG+ cells) in male (solid bars) and female (open bars) rats injected with either morphine or saline once or twice daily for three days for the rostral ((a); Bregma −6.72, −7.04, −7.74) and caudal ((b); Bregma −8.00, −8.30, −8.80) PAG. A decrease in labeling is evident with an increase in the number of morphine injections for male rats. The # indicates a significant effect of treatment and the ∗ indicates a significant effect of sex. Saline: morphine naïve; 1 dose: saline pretreatment followed by one dose of morphine; 3 doses: one dose of morphine per day; 6 doses: two doses of morphine per day.
While together, these data provide compelling support for a central role of the PAG in the development of morphine tolerance; these studies administered the male ED50 dose of morphine. While a single administration of this dose of morphine resulted in comparable activation of the PAG in males and females, it was suboptimal in producing behaviorally defined antinociception in females and may account for why females did not develop tolerance to the same degree as males. Future studies employing sex-specific ED50 doses are clearly warranted.
6. Role of Gonadal Hormones in Sex Differences in Morphine Analgesia
Studies in rodents indicate that sex differences in the organizational and activational effects of the gonadal hormones estradiol and testosterone influence morphine analgesia. For example, male rats castrated at birth demonstrate decreased morphine potency in adulthood, while female rats masculinized at birth demonstrate greater morphine potency in adulthood [121, 122]. Similarly, morphine is less effective in gonadectomized adult males and is more effective in ovariectomized adult females [40, 123–128]; these effects can be reversed with hormone replacement [44, 123, 129]. Moreover, the antinociceptive potency of morphine has been reported to be greater during diestrus, when circulating estradiol levels are lowest [43, 124, 125, 127, 130], which is corroborated by our recent findings that MOR expression in female rats is the highest during diestrus compared to proestrus and estrus [38]. Recently, it was reported that microinjection of morphine directly into the PAG produces less antinociception during estrus (after estradiol peaks), while there was no sex difference in morphine potency between diestrus females and males [131]. We have recently reported similar findings in which the antihyperalgesic effects of intra-PAG morphine were significantly greater in females in diestrus in comparison to proestrus and estrus [38].
The anatomical substrate(s) whereby gonadal steroids influence pain and analgesia is unknown. Both androgen (AR) and estrogen receptorsα (ERα) have been localized in the PAG in the male rat [132]. Although it is not known if these receptors are present in the female rat, they have been localized in other species including the female cat [12], golden hamster [133], guinea pig [134], and the rhesus monkey [135, 136]. To date, however, the anatomical distribution of both types of steroid receptor within the PAG in reference to cells projecting to the RVM is not known.
We have combined neuroanatomical tract-tracing techniques and steroid receptor immunohistochemistry to characterize the expression of AR and ERα in the PAG-RVM pathway of male and female rats [137]. In these studies, we found that males had a significantly greater number of AR immunoreactive neurons localized within the dorsomedial, lateral and ventrolateral PAG compared to females. Interestingly, both the qualitative and quantitative expression of ERα in the PAG was comparable between the sexes (see Figures 2(d)–2(f)). Both receptor types were preferentially localized within the dorsomedial, lateral and ventrolateral subdivisions of the PAG and increased in density along the rostrocaudal axis of the PAG with the highest expression localized within the caudal PAG. In addition, 30–37% of PAG-RVM output neurons expressed AR or ERα (Figure 3(d)) with the highest density of colabeling in the lateral/ventrolateral region of PAG. ERa and AR colocalization in PAG neurons projecting to the RVM was comparable between the sexes [137] (Figures 2(g)–2(i)). The high density of steroid receptors localized on PAG-RVM output neurons may contribute to our observed sex differences in morphine analgesia. Although there was no sex difference in the anatomical localization of gonadal steroid receptors in the PAG despite the higher density of AR in males, 27–50% of PAG-RVM neurons contain MOR [37]. Given that morphine activates more of these neurons in male compared to female rats, the interaction between morphine and sex hormones is likely greater in the PAG of male compared to female rats.
There are several mechanisms whereby gonadal steroids may modulate opioid-sensitive PAG-RVM output neurons, thereby potentially resulting in a dimorphic response to morphine. First, estradiol has been shown to uncouple the MOR from G protein-gated inwardly rectifying potassium channels [138] resulting in an attenuation of morphine-induced hyperpolarization. Second, estradiol has also been shown to induce MOR internalization [139], thereby reducing available opioid binding sites on the cell membrane. Interestingly, ERα is required for estradiol-induced MOR internalization [140] supporting the hypothesis that colocalization of MOR and ERα in the PAG-RVM output neurons may provide a pain modulatory mechanism. Interestingly, administration of estradiol to gonadectomized males reinstates morphine analgesia while dihydrotestosterone does not [141], suggesting that estrogens affect morphine potency in both male and female rats [130].
7. Conclusions
Research spanning for four decades has shown that the PAG and its descending projections to the RVM and spinal cord dorsal horn constitute an essential neural circuit for opioid-based analgesia. During the last half of that period, numerous rodent and human studies have established sex differences in the antinociceptive and analgesic effects of morphine; however, the neural mechanisms underlying the sexually dimorphic actions of morphine remain poorly understood. It is now clear that the anatomical and physiological characteristics of the PAG and its descending projections to the RVM are sexually dimorphic, with clear biological consequences in terms of morphine potency. Our studies, as well as those of others, have shown that morphine is less potent in females compared to males in the alleviation of persistent pain. Future research efforts utilizing female subjects in both the investigation of persistent pain mechanisms and identification of both effective and potent pain therapeutics are clearly warranted.
Acknowledgments
This work was supported by the National Institutes of Health (NIH) Grants DA16272 and P50 AR49555 awarded to Anne Z. Murphy, and NIH Grant DA015498 awarded to Michael M. Morgan. The authors would like to thank Michael M. Morgan for his helpful comments on the earlier versions of this manuscript.
References
- 1.Reynolds DV. Surgery in the rat during electrical analgesia induced by focal brain stimulation. Science. 1969;164(3878):444–445. doi: 10.1126/science.164.3878.444. [DOI] [PubMed] [Google Scholar]
- 2.Beitz AJ, Shepard RD, Wells WE. The periaqueductal gray-raphe magnus projection contains somatostatin, neurotensin and serotonin but not cholecystokinin. Brain Research. 1983;261(1):132–137. doi: 10.1016/0006-8993(83)91292-1. [DOI] [PubMed] [Google Scholar]
- 3.Beitz AJ, Shepard RD. The midbrain periaqueductal gray in the rat. II. A Golgi analysis. The Journal of Comparative Neurology. 1985;237(4):460–475. doi: 10.1002/cne.902370404. [DOI] [PubMed] [Google Scholar]
- 4.Beitz AJ. The midbrain periaqueductal gray in the rat. I. Nuclear volume, cell number, density, orientation, and regional subdivisions. The Journal of Comparative Neurology. 1985;237(4):445–459. doi: 10.1002/cne.902370403. [DOI] [PubMed] [Google Scholar]
- 5.Beitz AJ. The organization of afferent projections to the midbrain periaqueductal gray of the rat. Neuroscience. 1982;7(1):133–159. doi: 10.1016/0306-4522(82)90157-9. [DOI] [PubMed] [Google Scholar]
- 6.Basbaum AI, Fields HL. Endogenous pain control mechanisms: review and hypothesis. Annals of Neurology. 1978;4(5):451–462. doi: 10.1002/ana.410040511. [DOI] [PubMed] [Google Scholar]
- 7.Behbehani MM, Fields HL. Evidence that an excitatory connection between the periaqueductal gray and nucleus raphe magnus mediates stimulation produced analgesia. Brain Research. 1979;170(1):85–93. doi: 10.1016/0006-8993(79)90942-9. [DOI] [PubMed] [Google Scholar]
- 8.Williams FG, Mullet MA, Beitz AJ. Basal release of Met-enkephalin and neurotensin in the ventrolateral periaqueductal gray matter of the rat: a microdialysis study of antinociceptive circuits. Brain Research. 1995;690(2):207–216. doi: 10.1016/0006-8993(95)00554-4. [DOI] [PubMed] [Google Scholar]
- 9.Jones SL, Light AR. Serotoninergic medullary raphespinal projection to the lumbar spinal cord in the rat: a retrograde immunohistochemical study. The Journal of Comparative Neurology. 1992;322(4):599–610. doi: 10.1002/cne.903220413. [DOI] [PubMed] [Google Scholar]
- 10.Mouton LJ, Holstege G. Segmental and laminar organization of the spinal neurons projecting to the periaqueductal gray (PAG) in the cat suggests the existence of at least five separate clusters of spino-PAG neurons. The Journal of Comparative Neurology. 2000;428(3):389–410. doi: 10.1002/1096-9861(20001218)428:3<389::aid-cne2>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 11.Vanderhorst VGJM, Mouton LJ, Blok BFM, Holstege G. Distinct cell groups in the lumbosacral cord of the cat project to different areas in the periaqueductal gray. The Journal of Comparative Neurology. 1996;376(3):361–385. doi: 10.1002/(SICI)1096-9861(19961216)376:3<361::AID-CNE2>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 12.Vanderhorst VGJM, Schasfoort FC, Meijer E, Van Leeuwen FW, Holstege G. Estrogen receptor-alpha-immunoreactive neurons in the periaqueductal gray of the adult ovariectomized female cat. Neuroscience Letters. 1998;240(1):13–16. doi: 10.1016/s0304-3940(97)00900-2. [DOI] [PubMed] [Google Scholar]
- 13.Holstege G, Cowie RJ. Projections from the rostral mesencephalic reticular formation to the spinal cord. An HRP and autoradiographical tracing study in the cat. Experimental Brain Research. 1989;75(2):265–279. doi: 10.1007/BF00247933. [DOI] [PubMed] [Google Scholar]
- 14.Abols IA, Basbaum AI. Afferent connections of the rostral medulla of the cat: a neural substrate for midbrain-medullary interactions in the modulation of pain. The Journal of Comparative Neurology. 1981;201(2):285–297. doi: 10.1002/cne.902010211. [DOI] [PubMed] [Google Scholar]
- 15.Sandkuhler J, Fu Q-G, Zimmermann M. Spinal pathways mediating tonic or stimulation-produced descending inhibition from the periaqueductal gray or nucleus raphe magnus are separate in the cat. Journal of Neurophysiology. 1987;58(2):327–341. doi: 10.1152/jn.1987.58.2.327. [DOI] [PubMed] [Google Scholar]
- 16.Sandkuhler J, Maisch B, Zimmermann M. Raphe magnus-induced descending inhibition of spinal nociceptive neurons is mediated through contralateral spinal pathways in the cat. Neuroscience Letters. 1987;76(2):168–172. doi: 10.1016/0304-3940(87)90710-5. [DOI] [PubMed] [Google Scholar]
- 17.Shah Y, Dostrovsky JO. Electrophysiological evidence for a projection of the periaqueductal gray matter to nucleus raphe magnus in cat and rat. Brain Research. 1980;193(2):534–538. doi: 10.1016/0006-8993(80)90183-3. [DOI] [PubMed] [Google Scholar]
- 18.Basbaum AI, Clanton CH, Fields HL. Three bulbospinal pathways from the rostral medulla of the cat: an autoradiographic study of pain modulating systems. The Journal of Comparative Neurology. 1978;178(2):209–224. doi: 10.1002/cne.901780203. [DOI] [PubMed] [Google Scholar]
- 19.Mantyh PW. Connections of midbrain periaqueductal gray in the monkey. II. Descending efferent projections. Journal of Neurophysiology. 1983;49(3):582–594. doi: 10.1152/jn.1983.49.3.582. [DOI] [PubMed] [Google Scholar]
- 20.Gerhart KD, Yezierski RP, Wilcox TK, Willis WD. Inhibition of primate spinothalamic tract neurons by stimulation in periaqueductal gray of adjacent midbrain reticular formation. Journal of Neurophysiology. 1984;51(3):450–466. doi: 10.1152/jn.1984.51.3.450. [DOI] [PubMed] [Google Scholar]
- 21.Haselton JR, Winters RW, Liskowsky DR, Haselton CL, McCabe PM, Schneiderman N. Anatomical and functional connections of neurons of the rostral medullary raphe of the rabbit. Brain Research. 1988;453(1-2):176–182. doi: 10.1016/0006-8993(88)90156-4. [DOI] [PubMed] [Google Scholar]
- 22.Basbaum AI, Fields HL. Endogenous pain control systems: brainstem spinal pathways and endorphin circuitry. Annual Review of Neuroscience. 1984;7:309–338. doi: 10.1146/annurev.ne.07.030184.001521. [DOI] [PubMed] [Google Scholar]
- 23.Yaksh TL, Rudy TA. Narcotic analgetics: CNS sites and mechanisms of action as revealed by intracerebral injection techniques. Pain. 1978;4(4):299–359. doi: 10.1016/0304-3959(77)90145-2. [DOI] [PubMed] [Google Scholar]
- 24.Cheng Z-F, Fields HL, Heinricher MM. Morphine microinjected into the periaqueductal gray has differential effects on 3 classes of medullary neurons. Brain Research. 1986;375(1):57–65. doi: 10.1016/0006-8993(86)90958-3. [DOI] [PubMed] [Google Scholar]
- 25.Morgan MM, Heinricher MM, Fields HL. Circuitry linking opioid-sensitive nociceptive modulatory systems in periaqueductal gray and spinal cord with rostral ventromedial medulla. Neuroscience. 1992;47(4):863–871. doi: 10.1016/0306-4522(92)90036-2. [DOI] [PubMed] [Google Scholar]
- 26.Lane DA, Patel PA, Morgan MM. Evidence for an intrinsic mechanism of antinociceptive tolerance within the ventrolateral periaqueductal gray of rats. Neuroscience. 2005;135(1):227–234. doi: 10.1016/j.neuroscience.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 27.Wilcox RE, Mikula JA, Levitt RA. Periaqueductal gray naloxone microinjections in morphine-dependent rats: hyperalgesia without “classical” withdrawal. Neuropharmacology. 1979;18(7):639–641. doi: 10.1016/0028-3908(79)90118-7. [DOI] [PubMed] [Google Scholar]
- 28.Ma QP, Han JS. Naloxone blocks the release of opioid peptides in periaqueductal gray and N. accumbens induced by intra-amygdaloid injection of morphine. Peptides. 1991;12(6):1235–1238. doi: 10.1016/0196-9781(91)90200-9. [DOI] [PubMed] [Google Scholar]
- 29.Zhang Y, Du L-N, Wu G-C, Cao X-D. Modulation of intrathecal morphine-induced immunosuppression by microinjection of naloxone into periaqueductal gray. Zhongguo Yao Li Xue Bao. 1998;19(6):519–522. [PubMed] [Google Scholar]
- 30.Lewis VA, Gebhart GF. Evaluation of the periaqueductal central gray (PAG) as a morphine specific locus of action and examination of morphine induced and stimulation produced analgesia at coincident PAG loci. Brain Research. 1977;124(2):283–303. doi: 10.1016/0006-8993(77)90886-1. [DOI] [PubMed] [Google Scholar]
- 31.Mansour A, Khachaturian H, Lewis ME, Akil H, Watson SJ. Autoradiographic differentiation of mu, delta, and kappa opioid receptors in the rat forebrain and midbrain. The Journal of Neuroscience. 1987;7(8):2445–2464. [PMC free article] [PubMed] [Google Scholar]
- 32.Mansour A, Lewis ME, Khachaturian H, Akil H, Watson SJ. Pharmacological and anatomical evidence of selective μ, δ, and χ opioid receptor binding in rat brain. Brain Research. 1986;399(1):69–79. doi: 10.1016/0006-8993(86)90601-3. [DOI] [PubMed] [Google Scholar]
- 33.Gutstein HB, Mansour A, Watson SJ, Akil H, Fields HL. Mu and kappa opioid receptors in periaqueductal gray and rostral ventromedial medulla. NeuroReport. 1998;9(8):1777–1781. doi: 10.1097/00001756-199806010-00019. [DOI] [PubMed] [Google Scholar]
- 34.Kalyuzhny AE, Arvidsson U, Wu W, Wessendorf MW. μ-opioid and δ-opioid receptors are expressed in brainstem antinociceptive circuits: studies using immunocytochemistry and retrograde tract-tracing. The Journal of Neuroscience. 1996;16(20):6490–6503. doi: 10.1523/JNEUROSCI.16-20-06490.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Commons KG, Aicher SA, Kow L-M, Pfaff DW. Presynaptic and postsynaptic relations of μ-opioid receptors to γ-aminobutyric acid-immunoreactive and medullary-projecting periaqueductal gray neurons. The Journal of Comparative Neurology. 2000;419(4):532–542. doi: 10.1002/(sici)1096-9861(20000417)419:4<532::aid-cne8>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 36.Commons KG, Van Bockstaele EJ, Pfaff DW. Frequent colocalization of mu opioid and NMDA-type glutamate receptors at postsynaptic sites in periaqueductal gray neurons. The Journal of Comparative Neurology. 1999;408(4):549–559. [PubMed] [Google Scholar]
- 37.Wang H, Wessendorf MW. μ- and δ-opioid receptor mRNAs are expressed in periaqueductal gray neurons projecting to the rostral ventromedial medulla. Neuroscience. 2002;109(3):619–634. doi: 10.1016/s0306-4522(01)00328-1. [DOI] [PubMed] [Google Scholar]
- 38.Loyd DR, Wang X, Murphy AZ. Sex differences in μ-opioid receptor expression in the rat midbrain periaqueductal gray are essential for eliciting sex differences in morphine analgesia. The Journal of Neuroscience. 2008;28(52):14007–14017. doi: 10.1523/JNEUROSCI.4123-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kepler KL, Standifer KM, Paul D, Kest B, Pasternak GW, Bodnar RJ. Gender effects and central opioid analgesia. Pain. 1991;45(1):87–94. doi: 10.1016/0304-3959(91)90168-W. [DOI] [PubMed] [Google Scholar]
- 40.Kepler KL, Kest B, Kiefel JM, Cooper ML, Bodnar RJ. Roles of gender, gonadectomy and estrous phase in the analgesic effects of intracerebroventricular morphine in rats. Pharmacology Biochemistry and Behavior. 1989;34(1):119–127. doi: 10.1016/0091-3057(89)90363-8. [DOI] [PubMed] [Google Scholar]
- 41.Baamonde AI, Hidalgo A, Andres-Trelles F. Sex-related differences in the effects of morphine and stress on visceral pain. Neuropharmacology. 1989;28(9):967–970. doi: 10.1016/0028-3908(89)90197-4. [DOI] [PubMed] [Google Scholar]
- 42.Kavaliers M, Innes DGL. Sex and day-night differences in opiate-induced responses of insular wild deer mice, Peromyscus maniculatus triangularis . Pharmacology Biochemistry and Behavior. 1987;27(3):477–482. doi: 10.1016/0091-3057(87)90351-0. [DOI] [PubMed] [Google Scholar]
- 43.Okamoto K, Tashiro A, Hirata H, Bereiter DA. Differential modulation of TMJ neurons in superficial laminae of trigeminal subnucleus caudalis/upper cervical cord junction region of male and cycling female rats by morphine. Pain. 2005;114(1-2):203–211. doi: 10.1016/j.pain.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 44.Ji Y, Murphy AZ, Traub RJ. Estrogen modulation of morphine analgesia of visceral pain in female rats is supraspinally and peripherally mediated. The Journal of Pain. 2007;8(6):494–502. doi: 10.1016/j.jpain.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 45.Ji Y, Murphy AZ, Traub RJ. Sex differences in morphine-induced analgesia of visceral pain are supraspinally and peripherally mediated. American Journal of Physiology. 2006;291(2):R307–R314. doi: 10.1152/ajpregu.00824.2005. [DOI] [PubMed] [Google Scholar]
- 46.Wang X, Traub RJ, Murphy AZ. Persistent pain model reveals sex difference in morphine potency. American Journal of Physiology. 2006;291(2):R300–R306. doi: 10.1152/ajpregu.00022.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barrett AC, Cook CD, Terner JM, Craft RM, Picker MJ. Importance of sex and relative efficacy at the μ opioid receptor in the development of tolerance and cross-tolerance to the antinociceptive effects of opioids. Psychopharmacology. 2001;158(2):154–164. doi: 10.1007/s002130100821. [DOI] [PubMed] [Google Scholar]
- 48.Bartok RE, Craft RM. Sex differences in opioid antinociception. Journal of Pharmacology and Experimental Therapeutics. 1997;282(2):769–778. [PubMed] [Google Scholar]
- 49.Boyer JS, Morgan MM, Craft RM. Microinjection of morphine into the rostral ventromedial medulla produces greater antinociception in male compared to female rats. Brain Research. 1998;796(1-2):315–318. doi: 10.1016/s0006-8993(98)00353-9. [DOI] [PubMed] [Google Scholar]
- 50.Cicero TJ, Nock B, Meyer ER. Sex-related differences in morphine's antinociceptive activity: relationship to serum and brain morphine concentrations. Journal of Pharmacology and Experimental Therapeutics. 1997;282(2):939–944. [PubMed] [Google Scholar]
- 51.Kest B, Wilson SG, Mogil JS. Sex differences in supraspinal morphine analgesia are dependent on genotype. Journal of Pharmacology and Experimental Therapeutics. 1999;289(3):1370–1375. [PubMed] [Google Scholar]
- 52.Cook CD, Nickerson MD. Nociceptive sensitivity and opioid antinociception and antihyperalgesia in Freund's adjuvant-induced arthritic male and female rats. Journal of Pharmacology and Experimental Therapeutics. 2005;313(1):449–459. doi: 10.1124/jpet.104.077792. [DOI] [PubMed] [Google Scholar]
- 53.Miller PL, Ernst AA. Sex differences in analgesia: a randomized trial of μ versus κ opioid agonists. Southern Medical Journal. 2004;97(1):35–41. doi: 10.1097/01.smj.0000085743.68121.a9. [DOI] [PubMed] [Google Scholar]
- 54.Cepeda MS, Africano JM, Manrique AM, Fragoso W, Carr DB. The combination of low dose of naloxone and morphine in PCA does not decrease opioid requirements in the postoperative period. Pain. 2002;96(1-2):73–79. doi: 10.1016/s0304-3959(01)00425-0. [DOI] [PubMed] [Google Scholar]
- 55.Cepeda MS, Carr DB. Women experience more pain and require more morphine than men to achieve a similar degree of analgesia. Anesthesia and Analgesia. 2003;97(5):1464–1468. doi: 10.1213/01.ANE.0000080153.36643.83. [DOI] [PubMed] [Google Scholar]
- 56.Sarton E, Olofsen E, Romberg R, et al. Sex differences in morphine analgesia: an experimental study in healthy volunteers. Anesthesiology. 2000;93(5):1245–1254. doi: 10.1097/00000542-200011000-00018. discussion 6A. [DOI] [PubMed] [Google Scholar]
- 57.Fillingim RB, Ness TJ, Glover TL, et al. Morphine responses and experimental pain: sex differences in side effects and cardiovascular responses but not analgesia. The Journal of Pain. 2005;6(2):116–124. doi: 10.1016/j.jpain.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 58.Gordon NC, Gear RW, Heller PH, Paul S, Miaskowski C, Levine JD. Enhancement of morphine analgesia by the GABA(B) agonist baclofen. Neuroscience. 1995;69(2):345–349. doi: 10.1016/0306-4522(95)00335-g. [DOI] [PubMed] [Google Scholar]
- 59.Miaskowski C, Gear RW, Levine JD. Sex related differences in analgesic responses. In: Fillingim RB, editor. Sex, Gender, and Pain. Seattle, Wash, USA: IASP Press; 2000. pp. 209–232. [Google Scholar]
- 60.Cepeda MS, Farrar JT, Baumgarten M, Boston R, Carr DB, Strom BL. Side effects of opioids during short-term administration: effect of age, gender, and race. Clinical Pharmacology and Therapeutics. 2003;74(2):102–112. doi: 10.1016/S0009-9236(03)00152-8. [DOI] [PubMed] [Google Scholar]
- 61.Kest B, Sarton E, Dahan A. Gender differences in opioid-mediated analgesia: animal and human studies. Anesthesiology. 2000;93(2):539–547. doi: 10.1097/00000542-200008000-00034. [DOI] [PubMed] [Google Scholar]
- 62.Craft RM. Sex differences in opioid analgesia: “from mouse to man”. Clinical Journal of Pain. 2003;19(3):175–186. doi: 10.1097/00002508-200305000-00005. [DOI] [PubMed] [Google Scholar]
- 63.Fillingim RB, Gear RW. Sex differences in opioid analgesia: clinical and experimental findings. European Journal of Pain. 2004;8(5):413–425. doi: 10.1016/j.ejpain.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 64.Dahan A, Kest B, Waxman AR, Sarton E. Sex-specific responses to opiates: animal and human studies. Anesthesia & Analgesia. 2008;107(1):83–95. doi: 10.1213/ane.0b013e31816a66a4. [DOI] [PubMed] [Google Scholar]
- 65.Barrett AC, Smith ES, Picker MJ. Capsaicin-induced hyperalgesia and μ-opioid-induced antihyperalgesia in male and female Fischer 344 rats. Journal of Pharmacology and Experimental Therapeutics. 2003;307(1):237–245. doi: 10.1124/jpet.103.054478. [DOI] [PubMed] [Google Scholar]
- 66.Rosseland LA, Stubhaug A. Gender is a confounding factor in pain trials: women report more pain than men after arthroscopic surgery. Pain. 2004;112(3):248–253. doi: 10.1016/j.pain.2004.08.028. [DOI] [PubMed] [Google Scholar]
- 67.Aubrun F, Salvi N, Coriat P, Riou B. Sex- and age-related differences in morphine requirements for postoperative pain relief. Anesthesiology. 2005;103(1):156–160. doi: 10.1097/00000542-200507000-00023. [DOI] [PubMed] [Google Scholar]
- 68.Cogan R, Spinnato JA. Pain and discomfort thresholds in late pregnancy. Pain. 1986;27(1):63–68. doi: 10.1016/0304-3959(86)90223-X. [DOI] [PubMed] [Google Scholar]
- 69.Hellström B, Anderberg UM. Pain perception across the menstrual cycle phases in women with chronic pain. Perceptual and Motor Skills. 2003;96(1):201–211. doi: 10.2466/pms.2003.96.1.201. [DOI] [PubMed] [Google Scholar]
- 70.LaCroix-Fralish ML, Tawfik VL, DeLeo JA. The organizational and activational effects of sex hormones on tactile and thermal hypersensitivity following lumbar nerve root injury in male and female rats. Pain. 2005;114(1-2):71–80. doi: 10.1016/j.pain.2004.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gaumond I, Arsenault P, Marchand S. The role of sex hormones on formalin-induced nociceptive responses. Brain Research. 2002;958(1):139–145. doi: 10.1016/s0006-8993(02)03661-2. [DOI] [PubMed] [Google Scholar]
- 72.Aloisi AM, Ceccarelli I, Fiorenzani P, De Padova AM, Massafra C. Testosterone affects formalin-induced responses differently in male and female rats. Neuroscience Letters. 2004;361(1–3):262–264. doi: 10.1016/j.neulet.2003.12.023. [DOI] [PubMed] [Google Scholar]
- 73.Mogil JS, Chesler EJ, Wilson SG, Juraska JM, Sternberg WF. Sex differences in thermal nociception and morphine antinociception in rodents depend on genotype. Neuroscience and Biobehavioral Reviews. 2000;24(3):375–389. doi: 10.1016/s0149-7634(00)00015-4. [DOI] [PubMed] [Google Scholar]
- 74.Tall JM, Crisp T. Effects of gender and gonadal hormones on nociceptive responses to intraplantar carrageenan in the rat. Neuroscience Letters. 2004;354(3):239–241. doi: 10.1016/j.neulet.2003.09.081. [DOI] [PubMed] [Google Scholar]
- 75.Tall JM, Stuesse SL, Cruce WLR, Crisp T. Gender and the behavioral manifestations of neuropathic pain. Pharmacology Biochemistry and Behavior. 2001;68(1):99–104. doi: 10.1016/s0091-3057(00)00461-5. [DOI] [PubMed] [Google Scholar]
- 76.Gintzler AR. Endorphin-mediated increases in pain threshold during pregnancy. Science. 1980;210(4466):193–195. doi: 10.1126/science.7414330. [DOI] [PubMed] [Google Scholar]
- 77.Aloisi AM, Ceccarelli I. Role of gonadal hormones in formalin-induced pain responses of male rats: modulation by estradiol and naloxone administration. Neuroscience. 1999;95(2):559–566. doi: 10.1016/s0306-4522(99)00445-5. [DOI] [PubMed] [Google Scholar]
- 78.LeResche L, Mancl L, Sherman JJ, Gandara B, Dworkin SF. Changes in temporomandibular pain and other symptoms across the menstrual cycle. Pain. 2003;106(3):253–261. doi: 10.1016/j.pain.2003.06.001. [DOI] [PubMed] [Google Scholar]
- 79.Korszun A, Young EA, Engleberg NC, et al. Follicular phase hypothalamic-pituitary-gonadal axis function in women with fibromyalgia and chronic fatigue syndrome. Journal of Rheumatology. 2000;27(6):1526–1530. [PubMed] [Google Scholar]
- 80.Beitz AJ. The sites of origin brain stem neurotensin and serotonin projections to the rodent nucleus raphe magnus. The Journal of Neuroscience. 1982;2(7):829–842. doi: 10.1523/JNEUROSCI.02-07-00829.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.van Bockstaele EJ, Aston-Jones G, Pieribone VA, Ennis M, Shipley MT. Subregions of the periaqueductal gray topographically innervate the rostral ventral medulla in the rat. The Journal of Comparative Neurology. 1991;309(3):305–327. doi: 10.1002/cne.903090303. [DOI] [PubMed] [Google Scholar]
- 82.Loyd DR, Murphy AZ. Sex differences in the anatomical and functional organization of the periaqueductal gray-rostral ventromedial medullary pathway in the rat: a potential circuit mediating the sexually dimorphic actions of morphine. The Journal of Comparative Neurology. 2006;496(5):723–738. doi: 10.1002/cne.20962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Loyd DR, Morgan MM, Murphy AZ. Sexually dimorphic activation of the periaqueductal gray-rostral ventromedial medullary circuit during the development of tolerance to morphine in the rat. European Journal of Neuroscience. 2008;27(6):1517–1524. doi: 10.1111/j.1460-9568.2008.06100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Loyd DR, Morgan MM, Murphy AZ. Morphine preferentially activates the periaqueductal gray-rostral ventromedial medullary pathway in the male rat: a potential mechanism for sex differences in antinociception. Neuroscience. 2007;147(2):456–468. doi: 10.1016/j.neuroscience.2007.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Morgan MM, Gold MS, Liebeskind JC, Stein C. Periaqueductal gray stimulation produces a spinally mediated, opioid antinociception for the inflamed hindpaw of the rat. Brain Research. 1991;545(1-2):17–23. doi: 10.1016/0006-8993(91)91264-2. [DOI] [PubMed] [Google Scholar]
- 86.Guan Y, Guo W, Zou S-P, Dubner R, Ren K. Inflammation-induced upregulation of AMPA receptor subunit expression in brain stem pain modulatory circuitry. Pain. 2003;104(1-2):401–413. doi: 10.1016/s0304-3959(03)00048-4. [DOI] [PubMed] [Google Scholar]
- 87.Miki K, Zhou Q-Q, Guo W, et al. Changes in gene expression and neuronal phenotype in brain stem pain modulatory circuitry after inflammation. Journal of Neurophysiology. 2002;87(2):750–760. doi: 10.1152/jn.00534.2001. [DOI] [PubMed] [Google Scholar]
- 88.Guan Y, Terayama R, Dubner R, Ren K. Plasticity in excitatory amino acid receptor-mediated descending pain modulation after inflammation. Journal of Pharmacology and Experimental Therapeutics. 2002;300(2):513–520. doi: 10.1124/jpet.300.2.513. [DOI] [PubMed] [Google Scholar]
- 89.Ren K, Dubner R. Descending modulation in persistent pain: an update. Pain. 2002;100(1-2):1–6. doi: 10.1016/s0304-3959(02)00368-8. [DOI] [PubMed] [Google Scholar]
- 90.Jones SL, Gebhart GF. Inhibition of spinal nociceptive transmission from the midbrain, pons and medulla in the rat: activation of descending inhibition by morphine, glutamate and electrical stimulation. Brain Research. 1988;460(2):281–296. doi: 10.1016/0006-8993(88)90373-3. [DOI] [PubMed] [Google Scholar]
- 91.Aimone LD, Jones SL, Gebhart GF. Stimulation-produced descending inhibition from the periaqueductal gray and nucleus raphe magnus in the rat: mediation by spinal monoamines but not opioids. Pain. 1987;31(1):123–136. doi: 10.1016/0304-3959(87)90012-1. [DOI] [PubMed] [Google Scholar]
- 92.Waters AJ, Lumb BM. Inhibitory effects evoked from both the lateral and ventrolateral periaqueductal grey are selective for the nociceptive responses of rat dorsal horn neurones. Brain Research. 1997;752(1-2):239–249. doi: 10.1016/s0006-8993(96)01462-x. [DOI] [PubMed] [Google Scholar]
- 93.Budai D, Fields HL. Endogenous opioid peptides acting at μ-opioid receptors in the dorsal horn contribute to midbrain modulation of spinal nociceptive neurons. Journal of Neurophysiology. 1998;79(2):677–687. doi: 10.1152/jn.1998.79.2.677. [DOI] [PubMed] [Google Scholar]
- 94.Gray BG, Dostrovsky JO. Descending inhibitory influences from periaqueductal gray, nucleus raphe magnus, and adjacent reticular formation. I. Effects on lumbar spinal cord nociceptive and nonnociceptive neurons. Journal of Neurophysiology. 1983;49(4):932–947. doi: 10.1152/jn.1983.49.4.932. [DOI] [PubMed] [Google Scholar]
- 95.Carstens E, Yokota T, Zimmermann M. Inhibition of spinal neuronal responses to noxious skin heating by stimulation of mesencephalic periaqueductal gray in the cat. Journal of Neurophysiology. 1979;42(2):558–568. doi: 10.1152/jn.1979.42.2.558. [DOI] [PubMed] [Google Scholar]
- 96.Barrett AC, Cook CD, Terner JM, Roach EL, Syvanthong C, Picker MJ. Sex and rat strain determine sensitivity to κ opioid-induced antinociception. Psychopharmacology. 2002;160(2):170–181. doi: 10.1007/s00213-001-0949-2. [DOI] [PubMed] [Google Scholar]
- 97.Berkley KJ. Sex differences in pain. Behavioral and Brain Sciences. 1997;20(3):371–380. doi: 10.1017/s0140525x97221485. [DOI] [PubMed] [Google Scholar]
- 98.Fillingim RB. Sex, gender, and pain: women and men really are different. Current Review of Pain. 2000;4(1):24–30. doi: 10.1007/s11916-000-0006-6. [DOI] [PubMed] [Google Scholar]
- 99.Fillingim RB, Ness TJ. Sex-related hormonal influences on pain and analgesic responses. Neuroscience and Biobehavioral Reviews. 2000;24(4):485–501. doi: 10.1016/s0149-7634(00)00017-8. [DOI] [PubMed] [Google Scholar]
- 100.Loyd DR, Murphy AZ. Anatomical and physiological characterization of the midbrain periaqueductal gray in the female rat: identification of novel pain pathways. Society for Neuroscience, abstract control number 921.3, San Diego, Calif, USA, 2007.
- 101.Vaughan CW, Ingram SL, Connor MA, Christie MJ. How opioids inhibit GABA-mediated neurotransmission. Nature. 1997;390(6660):611–614. doi: 10.1038/37610. [DOI] [PubMed] [Google Scholar]
- 102.Chieng B, Christie MJ. Inhibition by opioids acting on μ-receptors of GABAergic and glutamatergic postsynaptic potentials in single rat periaqueductal gray neurones in vitro. British Journal of Pharmacology. 1994;113(1):303–309. doi: 10.1111/j.1476-5381.1994.tb16209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chieng B, Christie MJ. Hyperpolarization by opioids acting on μ-receptors of a sub-population of rat periaqueductal gray neurones in vitro. British Journal of Pharmacology. 1994;113(1):121–128. doi: 10.1111/j.1476-5381.1994.tb16183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Vaughan CW, Christie MJ. Presynaptic inhibitory action of opioids on synaptic transmission in the rat periaqueductal grey in vitro. The Journal of Physiology. 1997;498, part 2:463–472. doi: 10.1113/jphysiol.1997.sp021872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Osborne PB, Vaughan CW, Wilson HI, Christie MJ. Opioid inhibition of rat periaqueductal grey neurones with identified projections to rostral ventromedial medulla in vitro. The Journal of Physiology. 1996;490, part 2:383–389. doi: 10.1113/jphysiol.1996.sp021152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Behbehani MM. The role of acetylcholine in the function of the nucleus raphe magnus and in the interaction of this nucleus with the periaqueductal gray. Brain Research. 1982;252(2):299–307. doi: 10.1016/0006-8993(82)90397-3. [DOI] [PubMed] [Google Scholar]
- 107.Behbehani MM, Pomeroy SL, Mack CE. Interaction between central gray and nucleus raphe magnus: role of norepinephrine. Brain Research Bulletin. 1981;6(5):361–364. doi: 10.1016/s0361-9230(81)80004-4. [DOI] [PubMed] [Google Scholar]
- 108.Behbehani MM. Effect of chronic morphine treatment on the interaction between the periaqueductal grey and the nucleus raphe magnus of the rat. Neuropharmacology. 1981;20(6):581–586. doi: 10.1016/0028-3908(81)90211-2. [DOI] [PubMed] [Google Scholar]
- 109.Basbaum AI, Fields HL. The origin of descending pathways in the dorsolateral funiculus of the spinal cord of the cat and rat: further studies on the anatomy of pain modulation. The Journal of Comparative Neurology. 1979;187(3):513–531. doi: 10.1002/cne.901870304. [DOI] [PubMed] [Google Scholar]
- 110.Moreau J-L, Fields HL. Evidence for GABA involvement in midbrain control of medullary neurons that modulate nociceptive transmission. Brain Research. 1986;397(1):37–46. doi: 10.1016/0006-8993(86)91367-3. [DOI] [PubMed] [Google Scholar]
- 111.Behbehani MM, Jiang MR, Chandler SD, Ennis M. The effect of GABA and its antagonists on midbrain periaqueductal gray neurons in the rat. Pain. 1990;40(2):195–204. doi: 10.1016/0304-3959(90)90070-T. [DOI] [PubMed] [Google Scholar]
- 112.Morgan MM, Clayton CC, Lane DA. Behavioral evidence linking opioid-sensitive GABAergic neurons in the ventrolateral periaqueductal gray to morphine tolerance. Neuroscience. 2003;118(1):227–232. doi: 10.1016/s0306-4522(02)00822-9. [DOI] [PubMed] [Google Scholar]
- 113.Fossum EN, McNeal AL, Ingram SL, Morgan MM. Sex differences in antinociception following opioid and non-opioid activation of the periaqueductal gray of the rat. Society for Neuroscience, abstract control number 71.17, Washington, DC, USA, 2008.
- 114.Tortorici V, Morgan MM. Comparison of morphine and kainic acid microinjections into identical PAG sites on the activity of RVM neurons. Journal of Neurophysiology. 2002;88(4):1707–1715. doi: 10.1152/jn.2002.88.4.1707. [DOI] [PubMed] [Google Scholar]
- 115.Morgan MM, Fossum EN, Levine CS, Ingram SL. Antinociceptive tolerance revealed by cumulative intracranial microinjections of morphine into the periaqueductal gray in the rat. Pharmacology Biochemistry and Behavior. 2006;85(1):214–219. doi: 10.1016/j.pbb.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 116.Siuciak JA, Advokat C. Tolerance to morphine microinjections in the periaqueductal gray (PAG) induces tolerance to systemic, but not intrathecal morphine. Brain Research. 1987;424(2):311–319. doi: 10.1016/0006-8993(87)91476-4. [DOI] [PubMed] [Google Scholar]
- 117.Jacquet YF, Lajtha A. The periaqueductal gray: site of morphine analgesia and tolerance as shown by 2-way cross tolerance between systemic and intracerebral injections. Brain Research. 1976;103(3):501–513. doi: 10.1016/0006-8993(76)90448-0. [DOI] [PubMed] [Google Scholar]
- 118.Tortorici V, Robbins CS, Morgan MM. Tolerance to the antinociceptive effect of morphine microinjections into the ventral but not lateral-dorsal periaqueductal gray of the rat. Behavioral Neuroscience. 1999;113(4):833–839. doi: 10.1037//0735-7044.113.4.833. [DOI] [PubMed] [Google Scholar]
- 119.Bagley EE, Chieng BCH, Christie MJ, Connor M. Opioid tolerance in periaqueductal gray neurons isolated from mice chronically treated with morphine. British Journal of Pharmacology. 2005;146(1):68–76. doi: 10.1038/sj.bjp.0706315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hack SP, Vaughan CW, Christie MJ. Modulation of GABA release during morphine withdrawal in midbrain neurons in vitro. Neuropharmacology. 2003;45(5):575–584. doi: 10.1016/s0028-3908(03)00205-3. [DOI] [PubMed] [Google Scholar]
- 121.Krzanowska EK, Ogawa S, Pfaff DW, Bodnar RJ. Reversal of sex differences in morphine analgesia elicited from the ventrolateral periaqueductal gray in rats by neonatal hormone manipulations. Brain Research. 2002;929(1):1–9. doi: 10.1016/s0006-8993(01)03350-9. [DOI] [PubMed] [Google Scholar]
- 122.Cicero TJ, Nock B, O'Connor L, Meyer ER. Role of steroids in sex differences in morphine-induced analgesia: activational and organizational effects. Journal of Pharmacology and Experimental Therapeutics. 2002;300(2):695–701. doi: 10.1124/jpet.300.2.695. [DOI] [PubMed] [Google Scholar]
- 123.Ratka A, Simpkins JW. Effects of estradiol and progesterone on the sensitivity to pain and on morphine-induced antinociception in female rats. Hormones and Behavior. 1991;25(2):217–228. doi: 10.1016/0018-506x(91)90052-j. [DOI] [PubMed] [Google Scholar]
- 124.Stoffel EC, Ulibarri CM, Craft RM. Gonadal steroid hormone modulation of nociception, morphine antinociception and reproductive indices in male and female rats. Pain. 2003;103(3):285–302. doi: 10.1016/s0304-3959(02)00457-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Stoffel EC, Ulibarri CM, Folk JE, Rice KC, Craft RM. Gonadal hormone modulation of mu, kappa, and delta opioid antinociception in male and female rats. The Journal of Pain. 2005;6(4):261–274. doi: 10.1016/j.jpain.2004.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Terner JM, Barrett AC, Grossell E, Picker MJ. Influence of gonadectomy on the antinociceptive effects of opioids in male and female rats. Psychopharmacology. 2002;163(2):183–193. doi: 10.1007/s00213-002-1143-x. [DOI] [PubMed] [Google Scholar]
- 127.Terner JM, Lomas LM, Picker MJ. Influence of estrous cycle and gonadal hormone depletion on nociception and opioid antinociception in female rats of four strains. The Journal of Pain. 2005;6(6):372–383. doi: 10.1016/j.jpain.2005.01.354. [DOI] [PubMed] [Google Scholar]
- 128.Krzanowska EK, Bodnar RJ. Morphine antinociception elicited from the ventrolateral periaqueductal gray is sensitive to sex and gonadectomy differences in rats. Brain Research. 1999;821(1):224–230. doi: 10.1016/s0006-8993(98)01364-x. [DOI] [PubMed] [Google Scholar]
- 129.Kiefel JM, Bodnar RJ. Roles of gender and gonadectomy in pilocarpine and clonidine analgesia in rats. Pharmacology Biochemistry and Behavior. 1992;41(1):153–158. doi: 10.1016/0091-3057(92)90075-q. [DOI] [PubMed] [Google Scholar]
- 130.Craft RM, Mogil JS, Aloisi AM. Sex differences in pain and analgesia: the role of gonadal hormones. European Journal of Pain. 2004;8(5):397–411. doi: 10.1016/j.ejpain.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 131.Bernal SA, Morgan MM, Craft RM. PAG mu opioid receptor activation underlies sex differences in morphine antinociception. Behavioural Brain Research. 2007;177(1):126–133. doi: 10.1016/j.bbr.2006.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Murphy AZ, Hoffman GE. Distribution of gonadal steroid receptor-containing neurons in the preoptic-periaqueductal gray-brainstem pathway: a potential circuit for the initiation of male sexual behavior. The Journal of Comparative Neurology. 2001;438(2):191–212. doi: 10.1002/cne.1309. [DOI] [PubMed] [Google Scholar]
- 133.Boers J, Gerrits PO, Meijer E, Holstege G. Estrogen receptor-alpha-immunoreactive neurons in the mesencephalon, pons and medulla oblongata of the female golden hamster. Neuroscience Letters. 1999;267(1):17–20. doi: 10.1016/s0304-3940(99)00318-3. [DOI] [PubMed] [Google Scholar]
- 134.Turcotte JC, Blaustein JD. Immunocytochemical localization of midbrain estrogen receptor- and progestin receptor-containing cells in female guinea pigs. The Journal of Comparative Neurology. 1993;328(1):76–87. doi: 10.1002/cne.903280106. [DOI] [PubMed] [Google Scholar]
- 135.Vanderhorst VGJM, Terasawa E, Ralston HJ., III Estrogen receptor-alpha immunoreactive neurons in the ventrolateral periaqueductal gray receive monosynaptic input from the lumbosacral cord in the rhesus monkey. The Journal of Comparative Neurology. 2002;443(1):27–42. doi: 10.1002/cne.10098. [DOI] [PubMed] [Google Scholar]
- 136.Vanderhorst VGJM, Terasawa E, Ralston HJ., III Projections from estrogen receptor-α immunoreactive neurons in the periaqueductal gray to the lateral medulla oblongata in the rhesus monkey. Neuroscience. 2004;125(1):243–253. doi: 10.1016/j.neuroscience.2003.12.044. [DOI] [PubMed] [Google Scholar]
- 137.Loyd DR, Murphy AZ. Androgen and estrogen (α) receptor localization on periaqueductal gray neurons projecting to the rostral ventromedial medulla in the male and female rat. Journal of Chemical Neuroanatomy. 2008;36(3-4):216–226. doi: 10.1016/j.jchemneu.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kelly MJ, Qiu J, Rønnekleiv OK. Estrogen modulation of G-protein-coupled receptor activation of potassium channels in the central nervous system. Annals of the New York Academy of Sciences. 2003;1007:6–16. doi: 10.1196/annals.1286.001. [DOI] [PubMed] [Google Scholar]
- 139.Eckersell CB, Popper P, Micevych PE. Estrogen-induced alteration of μ-opioid receptor immunoreactivity in the medial preoptic nucleus and medial amygdala. The Journal of Neuroscience. 1998;18(10):3967–3976. doi: 10.1523/JNEUROSCI.18-10-03967.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Micevych PE, Rissman EF, Gustafsson J-Å, Sinchak K. Estrogen receptor-α is required for estrogen-induced μ-opioid receptor internalization. Journal of Neuroscience Research. 2003;71(6):802–810. doi: 10.1002/jnr.10526. [DOI] [PubMed] [Google Scholar]
- 141.Stoffel EC, Ulibarri CM, Craft RM. Gonadal steroid hormone modulation of nociception, morphine antinociception and reproductive indices in male and female rats. Pain. 2003;103(3):285–302. doi: 10.1016/s0304-3959(02)00457-8. [DOI] [PMC free article] [PubMed] [Google Scholar]