Abstract
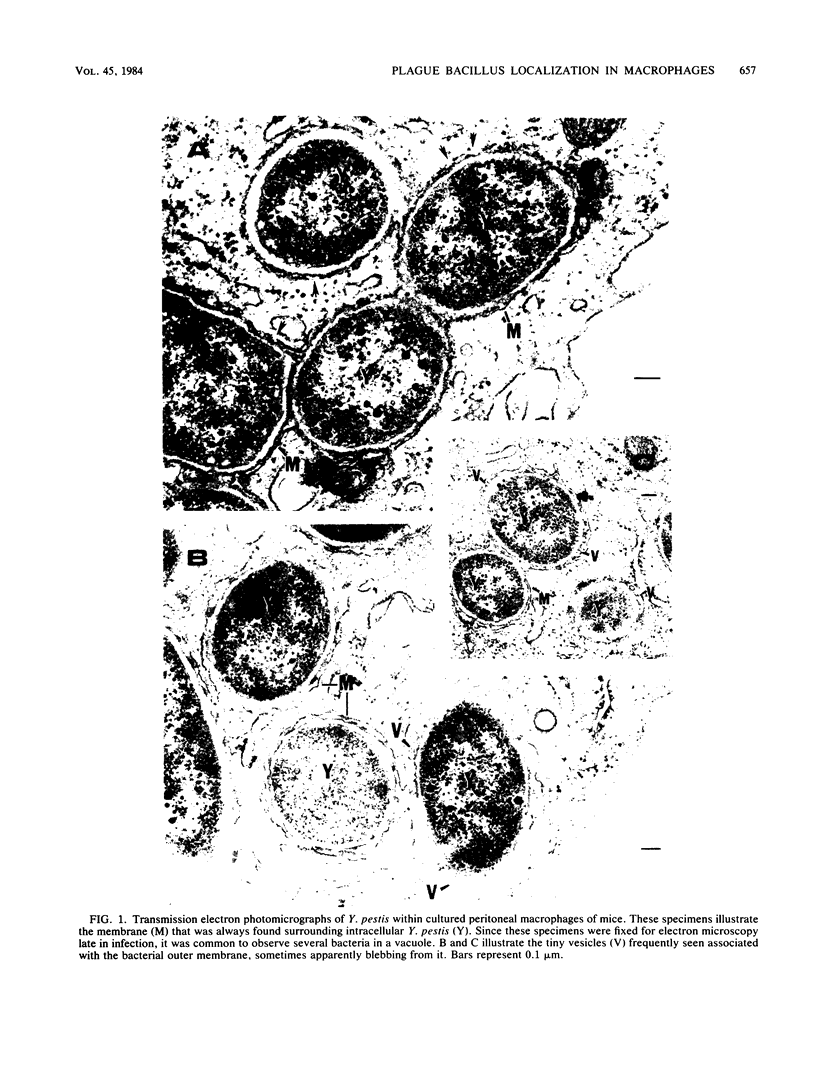
Transmission electron microscopy was used to determine the intracellular localization of Yersinia pestis growing within cultured resident peritoneal macrophages of mice. Monolayers fixed immediately, or as long as 4.5 h, after infection contained yersiniae closely surrounded by a membrane. The vesicles containing the yersiniae were determined to be phagolysosomes by labeling secondary lysosomes of macrophages with electron-dense particles of thorium dioxide. The presence of the label within vesicles containing yersiniae indicates that this pathogen grows within the lysosomal compartment of its target host cell.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong J. A., Hart P. D. Phagosome-lysosome interactions in cultured macrophages infected with virulent tubercle bacilli. Reversal of the usual nonfusion pattern and observations on bacterial survival. J Exp Med. 1975 Jul 1;142(1):1–16. doi: 10.1084/jem.142.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BACON G. A., BURROWS T. W. The basis of virulence in Pasteurella pestis: an antigen determining virulence. Br J Exp Pathol. 1956 Oct;37(5):481–493. [PMC free article] [PubMed] [Google Scholar]
- BRUBAKER R. R., SURGALLA M. J. THE EFFECT OF CA++ AND MG++ ON LYSIS, GROWTH, AND PRODUCTION OF VIRULENCE ANTIGENS BY PASTEURELLA PESTIS. J Infect Dis. 1964 Feb;114:13–25. doi: 10.1093/infdis/114.1.13. [DOI] [PubMed] [Google Scholar]
- Brubaker R. R. Mutation rate to nonpigmentation in Pasteurella pestis. J Bacteriol. 1969 Jun;98(3):1404–1406. doi: 10.1128/jb.98.3.1404-1406.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brubaker R. R. The genus Yersinia: biochemistry and genetics of virulence. Curr Top Microbiol Immunol. 1972;57:111–158. doi: 10.1007/978-3-642-65297-4_4. [DOI] [PubMed] [Google Scholar]
- Burton P. R., Kordová N., Paretsky D. Electron microscopic studies of the rickettsia Coxiella burneti: entry, lysosomal response, and fate of rickettsial DNA in L-cells. Can J Microbiol. 1971 Feb;17(2):143–150. doi: 10.1139/m71-025. [DOI] [PubMed] [Google Scholar]
- Burton P. R., Stueckemann J., Welsh R. M., Paretsky D. Some ultrastructural effects of persistent infections by the rickettsia Coxiella burnetii in mouse L cells and green monkey kidney (Vero) cells. Infect Immun. 1978 Aug;21(2):556–566. doi: 10.1128/iai.21.2.556-566.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAVANAUGH D. C., RANDALL R. The role of multiplication of Pasteurella pestis in mononuclear phagocytes in the pathogenesis of flea-borne plague. J Immunol. 1959 Oct;83:348–363. [PubMed] [Google Scholar]
- Carrol M. E., Jackett P. S., Aber V. R., Lowrie D. B. Phagolysosome formation, cyclic adenosine 3':5'-monophosphate and the fate of Salmonella typhimurium within mouse peritoneal macrophages. J Gen Microbiol. 1979 Feb;110(2):421–429. doi: 10.1099/00221287-110-2-421. [DOI] [PubMed] [Google Scholar]
- Charnetzky W. T., Brubaker R. R. RNA synthesis in Yersinia pestis during growth restriction in calcium-deficient medium. J Bacteriol. 1982 Mar;149(3):1089–1095. doi: 10.1128/jb.149.3.1089-1095.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DULBECCO R., VOGT M. Plaque formation and isolation of pure lines with poliomyelitis viruses. J Exp Med. 1954 Feb;99(2):167–182. doi: 10.1084/jem.99.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOX E. N., HIGUCHI K. Synthesis of the fraction I antigenic protein by Pasteurella pestis. J Bacteriol. 1958 Feb;75(2):209–216. doi: 10.1128/jb.75.2.209-216.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferber D. M., Fowler J. M., Brubaker R. R. Mutations to tolerance and resistance to pesticin and colicins in Escherichia coli phi. J Bacteriol. 1981 May;146(2):506–511. doi: 10.1128/jb.146.2.506-511.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finegold M. J. Pneumonic plague in monkeys. An electron microscopic study. Am J Pathol. 1969 Feb;54(2):167–185. [PMC free article] [PubMed] [Google Scholar]
- HIGUCHI K., KUPFERBERG L. L., SMITH J. L. Studies on the nutrition and physiology of Pasteurella pestis. III. Effects of calcium ions on the growth of virulent and avirulent strains of Pasteurella pestis. J Bacteriol. 1959 Mar;77(3):317–321. doi: 10.1128/jb.77.3.317-321.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones T. C., Hirsch J. G. The interaction between Toxoplasma gondii and mammalian cells. II. The absence of lysosomal fusion with phagocytic vacuoles containing living parasites. J Exp Med. 1972 Nov 1;136(5):1173–1194. doi: 10.1084/jem.136.5.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAWTON W. D., ERDMAN R. L., SURGALLA M. J. BIOSYNTHESIS AND PURIFICATION OF V AND W ANTIGEN IN PASTEURELLA PESTIS. J Immunol. 1963 Aug;91:179–184. doi: 10.21236/ad0299868. [DOI] [PubMed] [Google Scholar]
- Perry R. D., Brubaker R. R. Vwa+ phenotype of Yersinia enterocolitica. Infect Immun. 1983 Apr;40(1):166–171. doi: 10.1128/iai.40.1.166-171.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth M. G., Compans R. W. Delayed appearance of pseudotypes between vesicular stomatitis virus influenza virus during mixed infection of MDCK cells. J Virol. 1981 Dec;40(3):848–860. doi: 10.1128/jvi.40.3.848-860.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein S. C., Steinman R. M., Cohn Z. A. Endocytosis. Annu Rev Biochem. 1977;46:669–722. doi: 10.1146/annurev.bi.46.070177.003321. [DOI] [PubMed] [Google Scholar]
- Straley S. C., Harmon P. A. Growth in mouse peritoneal macrophages of Yersinia pestis lacking established virulence determinants. Infect Immun. 1984 Sep;45(3):649–654. doi: 10.1128/iai.45.3.649-654.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Une T. Studies on the pathogenicity of Yersinia enterocolitica. III. Comparative studies between Y. enterocolitica and Y. pseudotuberculosis. Microbiol Immunol. 1977;21(9):505–516. doi: 10.1111/j.1348-0421.1977.tb00316.x. [DOI] [PubMed] [Google Scholar]
- Zahorchak R. J., Brubaker R. R. Effect of exogenous nucleotides on Ca2+ dependence and V antigen synthesis in Yersinia pestis. Infect Immun. 1982 Dec;38(3):953–959. doi: 10.1128/iai.38.3.953-959.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahorchak R. J., Charnetzky W. T., Little R. V., Brubaker R. R. Consequences of Ca2+ deficiency on macromolecular synthesis and adenylate energy charge in Yersinia pestis. J Bacteriol. 1979 Sep;139(3):792–799. doi: 10.1128/jb.139.3.792-799.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]





