Abstract
Background
Cytochrome P450 (CYP) 2J2 is expressed in the vascular endothelium and metabolizes arachidonic acid to biologically active epoxyeicosatrienoic acids (EETs). The EETs are potent endogenous vasodilators and inhibitors of vascular inflammation. However, it is not known whether genetic polymorphisms of CYP2J2 are associated with increased cardiovascular risks.
Methods and Results
All 9 exons of the CYP2J2 gene and its proximal promoter were sequenced in 132 patients to identify potential variants. Functional consequence of a single nucleotide polymorphism (SNP) in the promoter of CYP2J2 was further evaluated by use of transcription factor–binding and reporter assays. A total of 17 polymorphisms were identified. One of the most relevant polymorphisms in terms of frequency and functional importance is located at −50 (G-50T) in the proximal promoter of CYP2J2. Screening of 289 patients with coronary artery disease and 255 control subjects revealed 77 individuals with the G-50T SNP (17.3% of coronary artery disease patients, 10.6% of control subjects; P=0.026). The association of the G-50T polymorphism remained significant after adjustment for age, gender, and conventional cardiovascular risk factors (OR, 2.23; 95% CI, 1.04 to 4.79). The G-50T mutation resulted in the loss of binding of the Sp1 transcription factor to the CYP2J2 promoter and resulted in a 48.1±2.4% decrease in CYP2J2 promoter activity (P<0.01). Plasma concentrations of stable EET metabolites were significantly lower in individuals with the G-50T SNP.
Conclusions
A functionally relevant polymorphism of the CYP2J2 gene is independently associated with an increased risk of coronary artery disease.
Keywords: atherosclerosis, coronary disease, genetics, inflammation, molecular biology
Cytochrome P450 epoxygenases metabolize arachidonic acid to epoxyeicosatrienoic acids (EETs). The human cytochrome P450 enzyme, CYP2J2, is abundantly expressed in coronary artery endothelial and smooth muscle cells and in cardiac myocytes.1,2 One of the primary products of the NADPH-dependent epoxidation of arachidonic acid by CYP2J2 is the production of 11,12-EET. This eicosanoid exerts antiinflammatory effects by inhibiting endothelial nuclear factor-κB, a transcription factor that plays a key role in the induction of many proinflammatory gene products in the vascular wall.2,3 Additionally, 5,6-, 8,9-, 11,12-, and 14,15-EETs have important vasodilator properties via hyperpolarization and relaxation of vascular smooth muscle cells.4–6 Recently, additional vascular protective effects of EETs, including antithrombotic, antimigratory, antioxidant, and antiapoptotic effects, have been observed.7,8
A large degree of interindividual variation in CYP2J2 expression has been observed.1 Among the factors known to be associated with differential human P450 gene expression are genetic polymorphisms.9,10 Given the potential importance of CYP2J2 in vascular function, we investigated whether genetic variants of this novel gene are associated with cardiovascular disease and, if so, to determine the potential mechanism involved.
Methods
Study Population
We studied 544 unrelated male and female white subjects, including 289 cases of coronary artery disease (CAD), as defined by angiographically documented coronary artery stenosis of >50% severity. Control patients (n=255) had no coronary vessels with >50% stenosis. The studied subjects were consecutive patients with an age limit of 65 years encountered in the cardiac catheterization laboratory from August 1998 to July 2000 and from October 2001 to August 2002. All participants came from central Germany and had comparable socioeconomic backgrounds.
All patients gave informed consent that explicitly provided permission for DNA analysis and collection of relevant clinical data. Whole blood (10 mL) was collected in EDTA-anticoagulant tubes by phlebotomy. At the same time, a complete clinical history, including cardiovascular risk factors, was obtained from all study subjects. Criteria for the diagnosis of myocardial infarction were chest pain lasting >30 minutes, elevated cardiac enzymes, and ECG changes suggestive of myocardial infarction. Diagnosis of stroke or transient ischemic attack was based on the findings of physical examination by a neurologist and confirmed by cranial CT. The Institutional Review Board approved the study protocol.
Polymorphism Analysis
Patient DNA was isolated from whole-blood samples by the phenol-chloroform extraction method. In the first 132 patients (78 with CAD, 54 without CAD), all 9 exons, intron-exon boundaries, and the proximal promoter of CYP2J2 were analyzed for genetic variants by polymerase chain reaction (PCR) and direct sequencing of PCR products. A total of 4613 base pairs (bp) were screened. Primers, size of amplification products, and PCR conditions used for amplification of CYP2J2 exons have been described previously by King et al.11 A 273-bp promoter region proximal to the transcriptional start site was amplified with the exon 1 primers described by King et al11 The sequence reaction used dye terminator chemistry (Applied Biosystems), and the sequence products were resolved on an ABI 377 automated sequencer. The promoter polymorphism G-50T was further verified by direct sequencing in 154 patients with angiographically documented CAD and in 145 patients without CAD. The numbering of the polymorphisms refers to the GenBank sequence AF272142 (accession number).
Subsequently, genotype identification by a restriction endonuclease digestion system was established and used for further genotype analysis in the remaining 245 patients. The G-50T polymorphism creates a novel restriction site for the AluI endonuclease (recognition sequence AGCT). PCR (using the following primers: sense, 5′-TTTTCTGAGACCGGTGCGTG-3′; antisense, 5′-TAGGAGAGTCCGAGGATGGA-3′) yielded a 242-bp product. Incubation with AluI resulted in 2 fragments (99 and 143 bp) in PCR products with the G-50T single nucleotide polymorphism (SNP) but not in wild-type (WT) PCR products. Results of restriction analysis were confirmed by Light-Cycler PCR with specific sensor and anchor primers.
Functional Promoter Analysis
Electrophoretic mobility shift assays were performed as described.12 Oligonucleotides (22-mer) corresponding to portions of the WT and G-50T variant CYP2J2 promoter region were labeled with digoxigenin and incubated with nuclear extracts (5 to 10 μg) from quiescent human umbilical vein endothelial cells. Equal loading of oligonucleotides was ensured by test blots with dilution series of each pair of labeled oligonucleotides (WT and G-50T variant). Densitometry was performed with multiple replicates using the NIH Image 1.63 program. Mean area density was measured, and background signal was subtracted.
For transfection experiments, CYP2J2 promoter constructs were obtained by PCR using DNA from patients with either the WT or the G-50T variant sequence as a template and primers containing KpnI and XhoI restriction sites (sense, 5′-CATAGGAGAGACGGTGATTGAACC -3′; antisense, 5′-GGCGTCTTCTCGTCCTCCTGCA -3′). PCR yielded 269 bp products: 253 bp of the promoter and 16 bp of the 5′-end noncoding region. These promoter constructs were subcloned into the luciferase reporter plasmid pGL2 enhancer (Promega). Reporter gene constructs were confirmed by direct DNA sequencing. The empty pGL2 enhancer vector was used as control. Transient transfection of bovine aortic endothelial cells and luciferase measurement were performed as described.12 Five independent experiments were performed in duplicate.
Eicosanoids were extracted from plasma samples 3 times with ethyl acetate after acidification with acetic acid. After evaporation, saponification with 0.4N KOH in methanol, and re-extraction, concentrations of the stable EET metabolite 14,15-dihydroxyeicosatrienoic acid (DHET) were determined by an ELISA kit (Detroit R&D).
Statistical Analysis
The OR between frequencies of the G-50T polymorphism and cardiovascular events was analyzed. For quantitative covariables, mean and SD were calculated. Qualitative variables are expressed in percent of the respective population. Differences in the covariables over genotypes were tested with the t test and χ2 test for quantitative and qualitative variables, respectively. The effect of age, gender, and classic CAD risk factors on the association of CAD with the G-50T polymorphism was studied with multiple logistic regression analysis for each covariable separately and in 1 complete model. Adjusted ORs with 95% CIs were computed. Experimental data from transfection and gel shift studies are expressed as mean±SEM.
Results
Sequencing of the CYP2J2 gene in 132 white patients revealed several genetic variants in the promoter region, in exons 1, 2, 4, 6, and 9; in intronic regions; and in the 3′-untranslated region (Table 1). Among them, 3 rare (frequency <0.01) polymorphisms in exons 2, 4, and 9 are predictive of amino acid substitutions in the CYP2J2 protein. More frequent polymorphisms in exons 1 and 6 were silent and therefore not considered to be of clinical importance. An SNP affecting the promoter region at position −50 (relative to the transcriptional start site), representing a substitution of a guanidine by a thymidine nucleotide (G-50T), was identified. The TT genotype was found in 1.5% and the GT genotype in 11.4% of patients. Given the prominent frequency of this G-50T SNP compared with the 3 rare coding region polymorphisms, functional studies were performed.
TABLE 1.
Frequency of CYP2J2 Polymorphisms
| Genotypes |
||||
|---|---|---|---|---|
| Nucleotide Polymorphism | Location | Heterozygous | Homozygous | Effect |
| G-50T | Promoter | 0.114 (15) | 0.015 (2) | |
| C173T | Exon 1 | 0.227 (30) | 0.023 (3) | Leu-Leu |
| G10812A | Exon 2 | 0.008 (1) | 0 | Arg→Gln |
| G15079C | Exon 4 | 0.008 (1) | 0 | Arg→His |
| C18944A | Exon 6 | 0.03 (4) | 0 | Arg-Arg |
| T33112A | Exon 9 | 0.008 (1) | 0 | Cys→Ser |
| G10860A | Intron 2 | 0.076 (10) | 0.008 (1) | |
| G14192A | Intron 3 | 0.038 (5) | 0 | |
| T18778G | Intron 5 | 0.189 (25) | 0.023 (3) | |
| T19206G | Intron 6 | 0.008 (1) | 0 | |
| C21708T | Intron 6 | 0.008 (1) | 0 | |
| T25865C | Intron 8 | 0.008 (1) | 0 | |
| C33291T | 3′-UTR | 0.212 (28) | 0 | |
| T33370A | 3′-UTR | 0.166 (22) | 0.008 (1) | |
| T33411A | 3′-UTR | 0.008 (1) | 0 | |
| A33465G | 3′-UTR | 0.166 (22) | 0.008 (1) | |
| A33497G | 3′-UTR | 0.038 (5) | 0 | |
Numbers in parentheses are numbers of patients.
Polymorphisms are numbered relative to transcription start site.
Analysis of the CYP2J2 promoter with G-50T polymorphism revealed the loss of binding site for the transcription factor Sp1 (Figure 1). We performed DNA binding studies to assess the functional relevance. Using a G→T mutated oligonucleotide in electrophoretic mobility shift assay studies, we reduced Sp1 binding significantly compared with WT oligonucleotide (Figure 2A). Addition of unlabeled oligonucleotide abolished the Sp1 binding, and preincubation with an Sp1 antibody supershifted the Sp1 band, confirming the specificity of the Sp1-DNA binding (data not shown). To determine the functional consequences of reduced Sp1-DNA binding on transcriptional activation of the CYP2J2 gene, transfection studies with promoter-reporter gene constructs were performed. In nonstimulated endothelial cells, the construct containing the WT promoter induced a 42-fold increase in promoter activity compared with the empty pGL2 vector (Figure 2B). The basal activity of the G-50T mutant promoter, however, was reduced by 48±2% (P<0.01) compared with the WT construct. Preincubation of transfected cells with tumor necrosis factor-α, interleukin-1, nifedipine, cortisol, and diclofenac had no effect on basal WT or mutant CYP2J2 promoter activity (data not shown).
Figure 1.
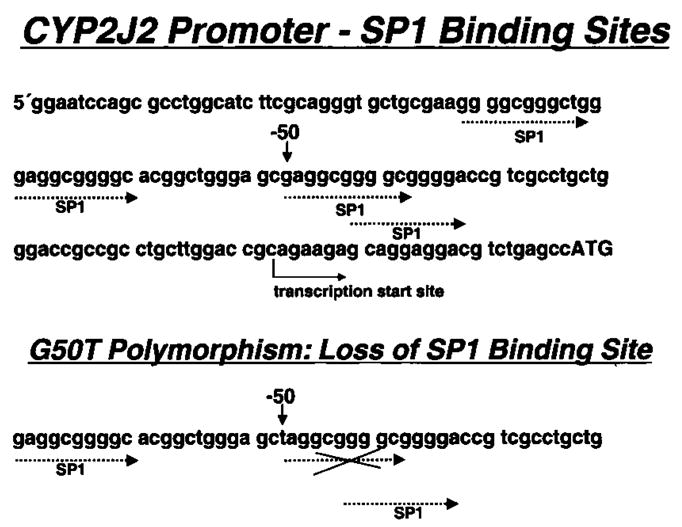
Sense strand sequence of proximal CYP2J2 promoter with Sp1 binding sites. Position −50 corresponds to promoter polymorphism site for G-50T.
Figure 2.
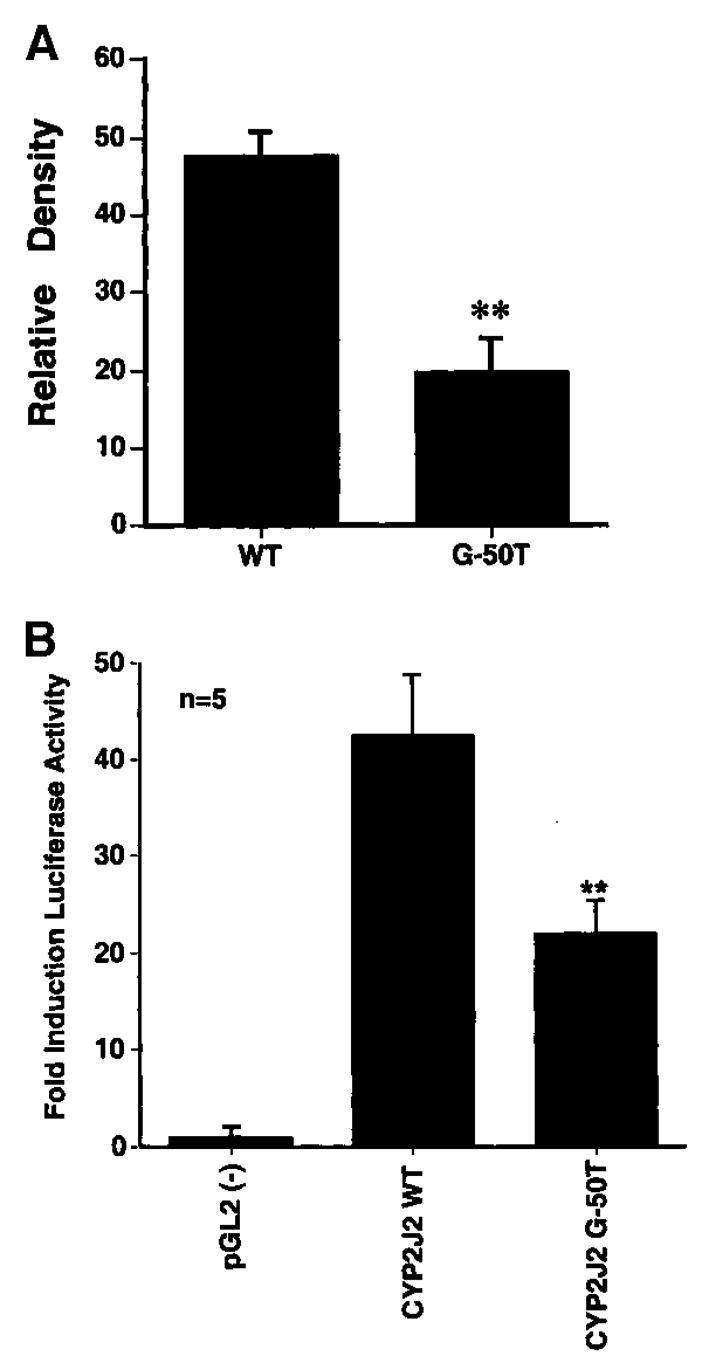
A, Electrophoretic mobility shift assay, using oligonucleotides with WT sequence at −50 Sp1 binding site or G-50T variant. Densitometry data are from 5 experiments. **P<0.001. B, Transient transfection of endothelial cells with CYP2J2-promoter-luciferase plasmid (pGL2 enhancer). CYP2J2 WT indicates reporter gene plasmid with WT promoter; CYP2J2 G-50T, same construct with G-50T variant. **P<0.01 vs CYP2J2 WT.
To further investigate the functional role of the G-50T polymorphism, we measured the plasma concentrations of the major CYP2J2-dependent epoxidation product from arachidonic acid. Given the instability of the primary products, EETs, concentration of the stable metabolite 14,15-DHET was determined after extraction from plasma samples (Figure 3). Median DHET plasma concentrations were significantly lower in samples from individuals with the G-50T polymorphism (5.25 ng/mL) compared with WT individuals (7.4 ng/mL; P=0.028)
Figure 3.

Plasma concentrations of stable EET metabolite DHET in individuals with (MT, n=8) or without (WT, n=7) G-50T polymorphism. Results are given for minimum, 25% quartile, median, 75% quartile, and maximum for each group. *P=0.028.
Given these functional findings, we analyzed the frequency of GT and TT genotypes in 289 patients with angiographically documented CAD and 255 control subjects with angiographic exclusion of CAD. The G-50T (GT and TT genotype) SNP was found in 10.6% of control subjects and 17.3% of CAD patients. The GT genotype was present in 10.2% of control subjects and 14.9% of CAD patients; the TT genotype was found in only 0.4% of control subjects and 2.4% of CAD patients. Considering the low frequency of the TT genotype, the G-50T variant with TT and GT genotype was compared with the GG genotype. The G-50T variant was significantly more frequent in CAD patients (Table 2). To determine whether the association of the mutant genotypes with CAD frequency is independent of age, gender, and conventional cardiovascular risk factors, ORs (CAD with the G-50T variant) were calculated with adjustment for covariables (Table 2). CAD was still significantly more frequent in patients with the G-50T promoter polymorphism after adjustment for these factors (Table 2). A potential confounding effect of specific covariables was excluded by calculating probability values for the differences in means or proportion between WT and mutant categories.
TABLE 2.
Adjusted and Unadjusted Association of G-50T SNP with CAD
| Wild Type (GG) (n=467) |
G-50T (GT, TT) (n=77) |
Difference in Covariables, Pd | Adjusted Odds Ratio for CAD With G-50T (95% CI) |
P | |
|---|---|---|---|---|---|
| CAD, % | 51.2 | 64.9 | 1.77 (1.07–2.92) | 0.026 | |
| Age, y | 50.8±9.3 | 51.8±8.5 | 0.390 | 1.73 (1.03–2.92) | 0.037 |
| Male gender, % | 76.6 | 70.1 | 0.216 | 2.06 (1.21–3.52) | 0.008 |
| Body mass index, kg/m2 | 27.9±4.1 | 27.5±4.4 | 0.422 | 2.08 (1.21–3.58) | 0.008 |
| Serum cholesterol, mg/dL | |||||
| Total | 211±48 | 216±52 | 0.483 | 1.68 (1.01–2.78) | 0.046 |
| LDL | 136±42 | 142±50 | 0.353 | 1.63 (0.89–2.97) | 0.113 |
| HDL | 40±15 | 43±17 | 0.228 | 1.64 (0.90–2.97) | 0.105 |
| Serum triglycerides, mg/dL | 183±136 | 191±133 | 0.628 | 1.68 (1.01–2.79) | 0.045 |
| Smoking, % | 55.8 | 52.0 | 0.608 | 1.68 (1.00–2.81) | 0.049 |
| Hypertension, % | 63.6 | 65.3 | 0.775 | 1.69 (1.00–2.85) | 0.049 |
| Diabetes mellitus, % | 15.3 | 13.3 | 0.660 | 1.74 (1.03–2.93) | 0.038 |
| All covariables | … | … | … | 2.23 (1.04–4.79) | 0.040 |
Values are expressed as mean±SD unless otherwise noted.
To test, whether the higher frequency of the promoter polymorphism in CAD patients is associated with clinical events, we retrospectively examined the prevalence of adverse cardiovascular events between the mutant and WT genotypes. The frequency of cerebral ischemia and acute coronary syndromes was slightly higher in the G-50T SNP subjects compared with the GG genotype subjects without reaching statistical significance (Table 3). The OR for total adverse cardiovascular events (acute coronary syndromes and cerebral ischemia) in the G-50T group compared with the GG genotype group was 1.53 (95% CI, 0.94 to 2.48).
TABLE 3.
Cardiovascular Events
| Wild Type (n=467) n (%) |
G-50T (GT, TT) (n=77) n (%) |
P | OR (95 % CI) | |
|---|---|---|---|---|
| Acute coronary syndromes | 170 (36.4) | 34 (44.2) | 0.19 | 1.38 (0.85–2.25) |
| Cerebral ischemia | 17 (3.6) | 6 (8.5) | 0.1 | 2.24 (0.85–5.87) |
| Total cardiovascular events | 176 (37.7) | 37 (48.1) | 0.09 | 1.53 (0.94–2.48) |
CAD is defined in most studies as angiographic coronary artery stenosis of >50%. This cutoff point is an arbitrary definition. Therefore, we additionally analyzed the frequency of the G-50T variant in individuals with any form of angiographically visible atherosclerosis compared with patients with absence of stenosis or visible coronary sclerosis. Again, the G-50T variant was significantly more frequent in the coronary atherosclerosis group (55 of 315) compared with control subjects (22 of 229) (OR, 1.99; 95% CI, 1.18 to 3.37; P=0.012).
Discussion
We analyzed the CYP2J2 gene for genetic variants in patients with and without angiographically documented CAD. The frequency of coding SNPs was <1%. Functional importance of these relatively rare variants is not known. In contrast to the coding SNPs, the relatively frequent promoter polymorphism G-50T is much more likely to influence the cardiovascular risk of a population.
The G-50T variant has previously been described by King et al11 with variable frequency in different racial groups. They also described 5 relatively rare (frequency <2%) coding region variants that previously were observed primarily in individuals of African descent. Thus, as a result of 2 independent sequencing studies of the entire CYP2J2 gene-coding region, there does not appear to be any frequent polymorphisms with amino acid substitutions in the CYP2J2 gene.
There are 4 putative Sp1 binding sites in the proximal CYP2J2 promoter, with their 5-ends in positions −84, −72, −50, and −45. The loss of an SP1 binding site in G-50T variants significantly reduces binding of Sp1. This finding is in accordance with our transfection studies demonstrating strong basal transcriptional activity from the WT but not G-50T CYP2J2 promoter, although no inducibility by cytokines and other substances able to induce CYP2C8/9 was observed. Constitutive expression of CYP2J2 mRNA and protein has been found in human heart tissue.1 These findings are consistent with the CYP2J2 promoter being TATA-less and that its basal activity is regulated predominantly by Sp1.11 To the best of our knowledge, inducibility of CYP2J2 has not been reported so far.
The relevance of our experimental findings with reduced gene activity of the G-50T mutant is confirmed by reduced concentrations of the stable CYP2J2-dependent arachidonic acid metabolite 14,15-DHET in individuals with the polymorphism. Endothelial cells do contain an active soluble epoxide hydrolase; therefore, most of the EET that is formed will be rapidly hydrated to the corresponding diol (DHET), which is the stable metabolite.13 After transfection of endothelial cells with CYP2J2, most epoxygenase metabolites observed are DHET, indicating that even high amounts of epoxygenase metabolites are predominantly hydrated to DHET.2 Therefore, we believe that 14,15-DHET levels are more reflective of CYP2J2 activity than soluble epoxide hydrolase activity. Also 11,12-EET is 1 of the primary products of CYP2J2 epoxidation; the major CYP2J2 product is 14,15-EET.1,11 For the reasons mentioned above, 14,15-DHET can be considered a relevant marker of CYP2J2 activity.
We demonstrated a higher frequency of the G-50T polymorphism in subjects with angiographically documented CAD. Individuals with angiographic exclusion of CAD instead of a random population sample were chosen as a matched control group, because the morphological diagnosis of CAD cannot be excluded in some “healthy individuals.” Our findings indicate a close association between variation in the CYP2J2 gene and human disease. Although our data do not prove a causal relationship, they are certainly consistent with a vascular protective role of products of this gene in humans. Strong constitutive expression of CYP2J2 would contribute to the biosynthesis of EETs, leading to protective vascular effects. Individuals with the G-50T polymorphism would have considerably less basal transcriptional activity of CYP2J2, which might result in reduced vascular protection. Given the multifactorial nature of atherosclerosis, it is unlikely that a polymorphism in a single gene will have a profound effect on the risk of atherosclerotic diseases. On the other hand, given a polymorphism frequency of 8% to 11% in whites, the G-50T SNP might contribute significantly to the risk of cardiovascular disease by allowing other proinflammatory and proatherogenic risk factors to go unchecked. The level of CYP2J2 protein expression in WT and variant individuals would be interesting to know. However, given the organ-specific expression of CYP2J2, protein expression cannot be simply measured in blood samples but requires organ biopsies (ie, endomyocardial biopsy).
Interestingly, lower CYP2J2 gene transcription might contribute to hypertension via reduced levels of the vasodilator eicosanoid 11,12-EET. Indeed, gene deletion of soluble epoxide hydrolase, which degrades EETs, in mice results in systemic hypotension.14 However, many other known and unknown factors contribute to systemic hypertension. In our study population, systemic hypertension as a single factor was not significantly associated with the G-50T polymorphism, and after adjustment for systemic hypertension, the association between G-50T SNP and CAD remains significant.
The incidence of adverse cardiovascular events is based on a retrospective analysis. Therefore, only nonfatal events were recorded. Additionally, a retrospective analysis is limited to what has been clinically documented. The incidence of acute coronary syndromes and cerebral ischemia needs further prospective evaluation, because a higher incidence of cardiovascular fatalities within 1 group could bias our findings. Nevertheless, our findings provide the first evidence for disease relevance of a polymorphism of a novel gene, CYP2J2. The association between the G-50T promoter polymorphism in younger patients and the prevalence of CAD further supports the role of P450-derived eicosanoids in limiting vascular inflammatory diseases such as atherosclerosis.
Acknowledgments
This work was supported by grants from the National Institutes of Health (HL-52233) to Dr Liao and from Alexander von Humboldt-Stiftung, FoRUM and Deutsche Forschungsgemeinschaft (DFG SP 537/3-1) to Dr Spiecker.
References
- 1.Wu S, Moomaw CR, Tomer KB, et al. Molecular cloning and expression of CYP2J2, a human cytochrome P450 arachidonic acid epoxygenase highly expressed in heart. J Biol Chem. 1996;271:3460–3468. doi: 10.1074/jbc.271.7.3460. [DOI] [PubMed] [Google Scholar]
- 2.Node K, Huo Y, Ruan X, et al. Anti-inflammatory properties of cytochrome P450 epoxygenase– derived eicosanoids. Science. 1999;285:1276–1279. doi: 10.1126/science.285.5431.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Collins T, Cybulsky MI. NF-κB: pivotal mediator or innocent bystander in atherogenesis? J Clin Invest. 2001;107:255–264. doi: 10.1172/JCI10373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oltman CL, Weintraub NL, VanRollins M, et al. Epoxyeicosatrienoic acids and dihydroxyeicosatrienoic acids are potent vasodilators in the canine coronary microcirculation. Circ Res. 1998;83:932–939. doi: 10.1161/01.res.83.9.932. [DOI] [PubMed] [Google Scholar]
- 5.Pinto A, Abraham NG, Mullane KM. Arachidonic acid–induced endothelial-dependent relaxations of canine coronary arteries: contribution of a cytochrome P-450–dependent pathway. J Pharmacol Exp Ther. 1987;240:856–863. [PubMed] [Google Scholar]
- 6.Fisslthaler B, Popp R, Kiss L, et al. Cytochrome P450 2C is an EDHF synthase in coronary arteries. Nature. 1999;401:493–497. doi: 10.1038/46816. [DOI] [PubMed] [Google Scholar]
- 7.Node K, Ruan XL, Dai J, et al. Activation of Gαs mediates induction of tissue-type plasminogen activator gene transcription by epoxyeicosatrienoic acids. J Biol Chem. 2001;276:15983–15989. doi: 10.1074/jbc.M100439200. [DOI] [PubMed] [Google Scholar]
- 8.Sun J, Sui X, Bradbury JA, et al. Inhibition of vascular smooth muscle cell migration by cytochrome p450 epoxygenase–derived eicosanoids. Circ Res. 2002;90:1020–1027. doi: 10.1161/01.res.0000017727.35930.33. [DOI] [PubMed] [Google Scholar]
- 9.Guengerich FP. Human cytochrome P-450 enzymes. Life Sci. 1992;50:1471–1478. doi: 10.1016/0024-3205(92)90136-d. [DOI] [PubMed] [Google Scholar]
- 10.Wolf CR, Miles JS, Gough A, et al. Molecular genetics of the human cytochrome P-450 system. Biochem Soc Trans. 1990;18:21–24. doi: 10.1042/bst0180021. [DOI] [PubMed] [Google Scholar]
- 11.King LM, Ma J, Srettabunjong S, et al. Cloning of CYP2J2 gene and identification of functional polymorphisms. Mol Pharmacol. 2002;61:840–852. doi: 10.1124/mol.61.4.840. [DOI] [PubMed] [Google Scholar]
- 12.Spiecker M, Lorenz I, Marx N, et al. Tranilast inhibits cytokine-induced nuclear factor kB activation in vascular endothelial cells. Mol Pharmacol. 2002;62:856–863. doi: 10.1124/mol.62.4.856. [DOI] [PubMed] [Google Scholar]
- 13.VanRollins M, Kaduce TL, Knapp HR, et al. 14,15-Epoxyeicosatrienoic acid metabolism in endothelial cells. J Lipid Res. 1993;34:1931–1942. [PubMed] [Google Scholar]
- 14.Sinal CJ, Miyata M, Tohkin M, et al. Targeted disruption of soluble epoxide hydrolase reveals a role in blood pressure regulation. J Biol Chem. 2000;275:40504–40510. doi: 10.1074/jbc.M008106200. [DOI] [PubMed] [Google Scholar]


