Abstract
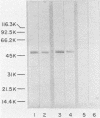
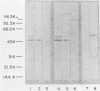
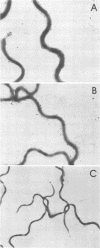
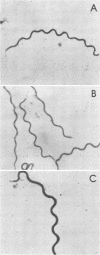
Murine monoclonal antibodies directed against a 47,000-dalton immunodominant surface-exposed antigen of Treponema pallidum subsp. pallidum (Nichols) were isolated. These monoclonal antibodies cross-reacted with analogous 47,000-dalton antigens of two other virulent treponemes, T. pallidum subsp. pertenue and T. pallidum subsp. endemicum (Bosnia A), as determined by radioimmunoassay and immunoblot analyses. Immunoelectron microscopy confirmed that the 47,000-dalton antigen of T. pallidum subsp. pallidum was a surface-associated cellular component. Surface binding assays and immunoelectron microscopic studies also suggested that the analogous 47,000-dalton antigenic component of T. pallidum subsp. pertenue may not have been oriented toward the bacterial surface in the same way as the T. pallidum subsp. pallidum antigen or that the relevant antigenic determinant(s) may not have been exposed to the outer surface in the same way. The significance of this antigen relative to its apparent conservation among pathogenic treponemes and its possible diagnostic and vaccinogenic potentials are discussed.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alderete J. F., Baseman J. B. Surface characterization of virulent Treponema pallidum. Infect Immun. 1980 Dec;30(3):814–823. doi: 10.1128/iai.30.3.814-823.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderete J. F., Baseman J. B. Surface-associated host proteins on virulent Treponema pallidum. Infect Immun. 1979 Dec;26(3):1048–1056. doi: 10.1128/iai.26.3.1048-1056.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker-Zander S. A., Lukehart S. A. Molecular basis of immunological cross-reactivity between Treponema pallidum and Treponema pertenue. Infect Immun. 1983 Nov;42(2):634–638. doi: 10.1128/iai.42.2.634-638.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baseman J. B., Hayes E. C. Molecular characterization of receptor binding proteins and immunogens of virulent Treponema pallidum. J Exp Med. 1980 Mar 1;151(3):573–586. doi: 10.1084/jem.151.3.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- COCKBURN T. A. The origin of the treponematoses. Bull World Health Organ. 1961;24:221–228. [PMC free article] [PubMed] [Google Scholar]
- HACKETT C. J. ON THE ORIGIN OF THE HUMAN TREPONEMATOSES (PINTA, YAWS, ENDEMIC SYPHILIS AND VENEREAL SYPHILIS). Bull World Health Organ. 1963;29:7–41. [PMC free article] [PubMed] [Google Scholar]
- HILL K. R. Non-specific factors in the epidemiology of yaws. Bull World Health Organ. 1953;8(1-3):17–51. [PMC free article] [PubMed] [Google Scholar]
- HUDSON E. H. TREPONEMATOSIS AND AFRICAN SLAVERY. Br J Vener Dis. 1964 Mar;40:43–52. doi: 10.1136/sti.40.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Hanff P. A., Bishop N. H., Miller J. N., Lovett M. A. Humoral immune response in experimental syphilis to polypeptides of Treponema pallidum. J Immunol. 1983 Oct;131(4):1973–1977. [PubMed] [Google Scholar]
- KHAN A. S., NELSON R. A., Jr, TURNER T. B. Immunological relationships among species and strains of virulent treponemes as determined with the treponemal immobilization test. Am J Hyg. 1951 May;53(3):296–316. doi: 10.1093/oxfordjournals.aje.a119455. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lukehart S. A., Baker-Zander S. A., Gubish E. R., Jr Identification of Treponema pallidum antigens: comparison with a nonpathogenic treponeme. J Immunol. 1982 Aug;129(2):833–838. [PubMed] [Google Scholar]
- MAGNUSON H. J., THOMAS E. W., OLANSKY S., KAPLAN B. I., DE MELLO L., CUTLER J. C. Inoculation syphilis in human volunteers. Medicine (Baltimore) 1956 Feb;35(1):33–82. doi: 10.1097/00005792-195602000-00002. [DOI] [PubMed] [Google Scholar]
- McLEOD C. P., MAGNUSON H. J. A study of cross immunity between syphilis and yaws in treated rabbits. J Vener Dis Inf. 1951 Nov;32(11):305–309. [PubMed] [Google Scholar]
- Metzger M., Michalska E., Podwińska J., Smogór W. Immunogenic properties of the protein component of Treponema pallidum. Br J Vener Dis. 1969 Dec;45(4):299–304. doi: 10.1136/sti.45.4.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao R. M., Fieldsteel A. H. Genetic relationship between Treponema pallidum and Treponema pertenue, two noncultivable human pathogens. J Bacteriol. 1980 Jan;141(1):427–429. doi: 10.1128/jb.141.1.427-429.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. N. Immunity in experimental syphilis. VI. Successful vaccination of rabbits with Treponema pallidum, Nichols strain, attenuated by -irradiation. J Immunol. 1973 May;110(5):1206–1215. [PubMed] [Google Scholar]
- Robertson S. M., Kettman J. R., Miller J. N., Norgard M. V. Murine monoclonal antibodies specific for virulent Treponema pallidum (Nichols). Infect Immun. 1982 Jun;36(3):1076–1085. doi: 10.1128/iai.36.3.1076-1085.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schell R. F., Azadegan A. A., Nitskansky S. G., LeFrock J. L. Acquired resistance of hamsters to challenge with homologous and heterologous virulent treponemes. Infect Immun. 1982 Aug;37(2):617–621. doi: 10.1128/iai.37.2.617-621.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schell R. F., Le Frock J. L., Babu J. P., Chan J. K. Use of CB hamsters in the study of Treponema pertenue. Br J Vener Dis. 1979 Oct;55(5):316–319. doi: 10.1136/sti.55.5.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schell R. F., LeFrock J. L., Chan J. K., Bagasra O. LSH hamster model of syphilitic infection. Infect Immun. 1980 Jun;28(3):909–913. doi: 10.1128/iai.28.3.909-913.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TURNER T. B., KLUTH F. C. Protective antibodies in the serum of syphilitic patients. Am J Hyg. 1948 Sep;48(2):173–181. doi: 10.1093/oxfordjournals.aje.a119233. [DOI] [PubMed] [Google Scholar]
- TURNER T. B., NELSON R. A., Jr The relationship of treponemal immobilizing antibody to immunity in syphilis. Trans Assoc Am Physicians. 1950;63:112–117. [PubMed] [Google Scholar]
- Thornburg R. W., Baseman J. B. Comparison of major protein antigens and protein profiles of Treponema pallidum and Treponema pertenue. Infect Immun. 1983 Nov;42(2):623–627. doi: 10.1128/iai.42.2.623-627.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tight R. R., White A. C. Quantitative microhaemagglutination assay for Treponema pallidum antibodies in experimental syphilis. Br J Vener Dis. 1980 Oct;56(5):291–296. doi: 10.1136/sti.56.5.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]