Preface
Cervical cancer is the leading cause of cancer death for women in developing countries1. Optical technologies can improve the accuracy and availability of cervical cancer screening. For example, battery-powered digital cameras can obtain multi-spectral images of the entire cervix highlighting suspicious areas, and high-resolution optical technologies can further interrogate suspicious areas, providing in vivo diagnosis with high sensitivity and specificity. In addition, targeted contrast agents can highlight changes in biomarkers of cervical neoplasia. Such advances should provide a much needed global approach to cervical cancer prevention.
Cervical Cancer: A Global Challenge
Cervical cancer is the second most common cancer in women worldwide1; more than 80% of cervical cancers occur in the developing world where the least resources exist for management1. Most cases of cervical cancer can be prevented through screening programs, such as the Papanicolaou (Pap) smear aimed at detecting precancerous lesions for treatment. In countries in which organized programs have been established, the incidence and mortality of cervical cancer have dramatically reduced2. Yet, the necessary resources and infrastructure for screening are not available in many countries; as a result, 274,000 women die each year as a result of this preventable disease1.
Cervical cancer is caused by chronic infection with high risk types of the human papilloma virus (HPV)3. The recent development of vaccines to prevent HPV infection promises to further reduce the incidence of cervical cancer in countries where vaccines are available4. However, the significant cost of vaccines and, in some cases, political or logistical barriers, could delay implementation of universal mass vaccination in many developing countries5. Furthermore, current vaccines are effective only against high-risk HPV types 16 and 18, which together account for approximately 70% of cervical cancers worldwide5. Because the vaccine does not cover all high-risk HPV subtypes, routine cervical cancer screening is still necessary, even for women who have been vaccinated, so cervical cancer screening remains necessary for the foreseeable future.
Clinical Approaches to Cervical Cancer Screening
Current screening and diagnosis of cervical precancer is based on optical techniques developed in the early 1900s2. As shown in the top row of Fig. 1, an abnormal Pap smear is followed by examination of the cervix using a low-power light microscope (colposcope) to visualize changes in tissue reflectance, which might indicate precancer. For decades, clinical investigators have searched for ways to improve the optical contrast between normal cervix and precancerous lesions. The use of simple agents such as acetic acid and Lugol's iodine, together with the use of a green illumination filter, can highlight suspicious regions. However, because the specificity of visual examination is low, colposcopically abnormal areas are routinely biopsied to confirm the presence of disease6. Implementing this approach requires extensive infrastructure, personnel and economic resources; as a result, the vast majority of women in the world do not have access to life-saving screening programs7.
Figure 1.
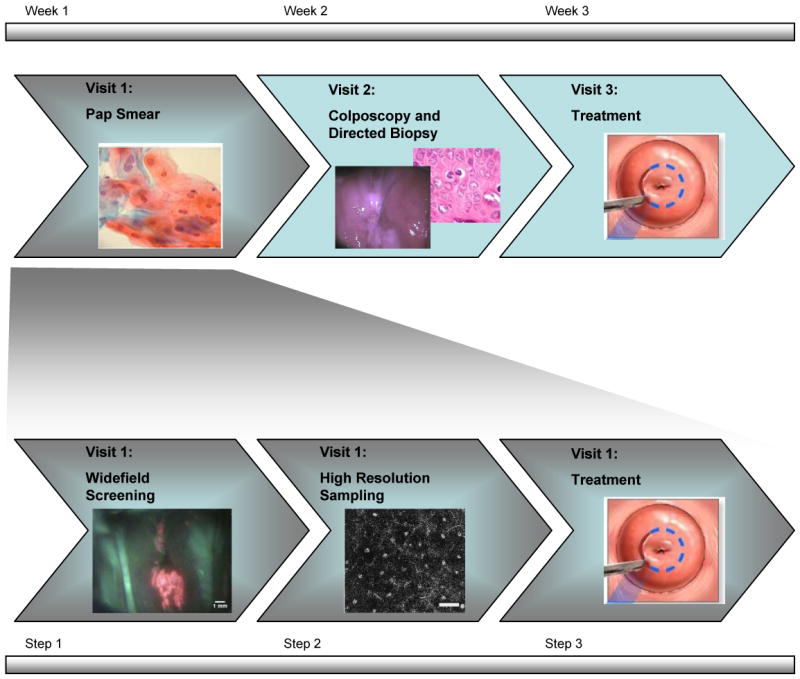
Simple visual approaches have been explored to enable cervical cancer screening in resource-poor settings. For example, the use of visual inspection with acetic acid (VIA) is being explored as an alternative to Pap smear screening and colposcopy in many developing countries7-10. In VIA, a trained health care provider examines the cervix with the naked eye before and after application of acetic acid. Large clinical trials have been conducted to evaluate the performance of VIA for screening; Table 1 summarizes the findings of several of these trials. A recent review of the performance of VIA in six studies involving more than 65,000 women in South Africa, India, Zimbabwe, China, Burkina Faso, Congo, Guinea, Mali and Niger found that the sensitivity of VIA varied from 67-79% while specificity ranged from 49%-86%. The sensitivity of VIA is similar to that reported for Pap smear screening while specificity is lower, although some studies suffered from verification bias11. The use of low level magnification does not improve the performance of VIA appreciably12, 13.
Table 1.
Recent clinical trials (top) and pilot studies (bottom) testing in vivo optical imaging techniques for early detection of cervical cancer and its precursors
| Type of Detection | N = # of Patients in Analysis | Sensitivity (%) / Specificity (%) | Type of Study | |
|---|---|---|---|---|
| Major Trials | Colposcopy6 | 5378 | 85/69 | Prospective |
| VIA90 | 2148 | 77/64 | Prospective | |
| VIA91 | 2817 | 67/83* | Prospective | |
| VIA92 | 1997 | 71/74* | Prospective | |
| VIA13 | 2575 | 70/79 | Prospective | |
| VIA93 | 1093 | 79/49 | Prospective | |
| VIA94 | 54981 | 79/86 | Prospective | |
| Widefield44 | 604 | 92/50 | Prospective | |
| Widefield46 | 111 | 97/70* | Prospective | |
| Widefield47 | 572 | 95/55 | Prospective | |
| Pilot Studies | Spectroscopy51 | 161 | 83/80 | Cross-Validation |
| Spectroscopy52 | 44 | 92/90 | Cross-Validation | |
| High Resolution29 | 28‡ | 100/100 | Prospective | |
| High Resolution66 | 38‡ | 100/91 | Retrospective | |
Visual Inspection with Acetic Acid (VIA)
The threshold for positive test result is LGD. For all others the threshold is HGD.
N = # of biopsies and not the number of patients.
VIA has many advantages — it is inexpensive, requires minimal infrastructure and if abnormal areas are observed the patient can be referred for immediate treatment, circumventing the need for the expense and infrastructure of histology. However, because VIA relies on subjective visual interpretation, it is crucial to define consistent criteria for suspicious lesions and to train providers to correctly implement these criteria. Denny noted that restricting the definition of a positive VIA test to a well-defined acetowhite lesion significantly improved specificity, but reduced sensitivity13. In a series of 1,921 women screened in Peru, Jeronimo found that the VIA positivity rate dropped from 13.5% in the first few months to 4% during subsequent months of a two-year study; the drop was hypothesized to be due to a learning curve for the evaluator10.
Recent advances in consumer electronics have led to inexpensive, high dynamic range charge-coupled device (CCD) cameras with excellent low light sensitivity. These technologies have been used to acquire digital images of the cervix in a relatively inexpensive way, with or without magnification14. Moreover, automated image diagnosis algorithms based on modern image processing techniques can assist and complement subjective visual interpretation15-17. These approaches, which we refer to as Digital Inspection with Acetic Acid (DIA), can potentially improve the performance of VIA.
Changes in Optical Properties
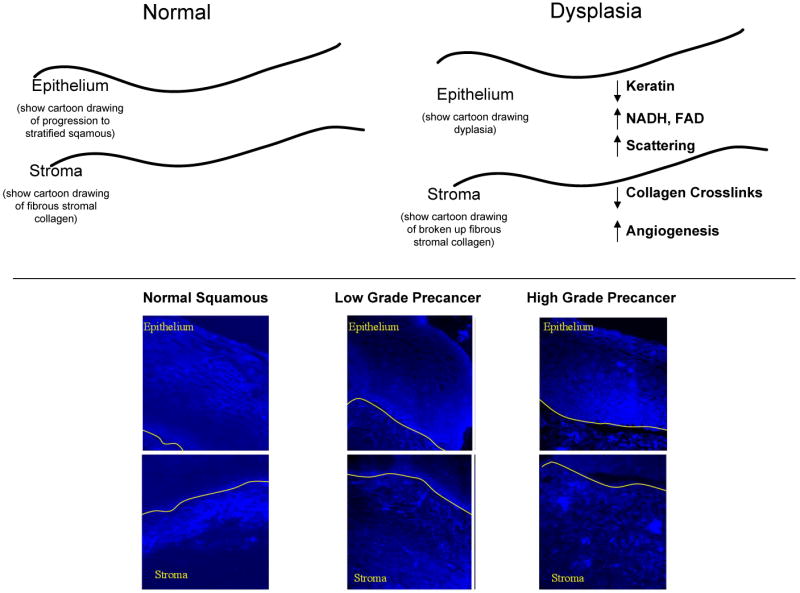
Both VIA and colposcopy rely on changes in the optical properties of neoplastic tissue to detect precancerous lesions. Image contrast between normal tissue and precancerous areas can be enhanced in a number of ways. Illuminating tissue with green light during colposcopy highlights the contrast associated with atypical blood vessels because hemoglobin absorbs green light. The application of acetic acid differentially increases the light scattering of neoplastic lesions18, making them easier to visualize. While these simple approaches help clinicians recognize suspicious lesions, they exploit only a few of the changes in optical properties associated with the development of neoplasia. Recent studies have characterized a broad range of changes in tissue optical properties that occur with precancer19-21. Results of these studies are summarized in Fig. 2, and indicate that optical methods can be used to probe changes associated with known hallmarks of cancerous changes in tissue such as epithelial cell morphology, metabolic activity and differentiation22, stromal angiogenesis23, 24, and epithelial-stromal communication25.
Figure 2.
Light Scattering and Absorption
Optical technologies can interrogate changes in tissue architecture, cell morphology and biochemical composition. Most high grade precancers present with vascular changes due to the development of new blood vessels26. This angiogenesis can be seen visually, and quantified using image analysis approaches17. Hemoglobin has a characteristic absorption spectrum with peaks at 420 nm, 542 nm and 577 nm. Changing the wavelength of illumination can enhance vascular contrast and can probe vessels at different depths below the visual surface of the cervix. Acetic acid increases light scattering from cervical cell nuclei. Following application of acetic acid18, 27, the mean scattering coefficient of precancerous tissue is approximately three times higher than that of normal epithelium28, 29. The difference in scattering between normal and precancerous epithelium is attributed to increased nuclear size, increased optical density of the nucleus and changes in chromatin texture30, 31 that have been documented in cancerous cells. Finally, cervical precancer is associated with decreased stromal scattering, attributed to a degradation in collagen fibers possibly due to proteases secreted by pre-neoplastic epithelial cells32.
Autofluorescence
The use of fluorescence interrogation can extend the range of biochemical changes which can be probed optically. In normal cervical tissue, collagen crosslinks give rise to bright fluorescence in the stroma over a broad range of excitation wavelengths 20, 33. In normal women, this stromal fluorescence increases with age and with menopause21, however, stromal fluorescence is greatly diminished in cervical precancers20, 33 and cancers34. Cervical epithelial cells show cytoplasmic autofluorescence attributed to mitochondrial NADH using UV excitation wavelengths (∼330-370 nm) and mitochondrial FAD using green excitation wavelengths (∼510-550 nm) 20, 33. In addition, cervical epithelial cells show autofluorescence at the periphery of the cells, often attributed to cytokeratins28. In normal epithelium, basal epithelial cells show strong cytoplasmic fluorescence; parabasal, intermediate and superficial cells showed fluorescence only at the periphery of the cell20, 33. Figure 2 compares confocal fluorescence images of organ cultures of normal human cervical tissue and precancer. In low-grade precancers, cytoplasmic fluorescence is visible in the bottom 1/3 of the epithelium and in high grade precancers, cytoplasmic fluorescence is visible throughout the lower two-thirds of the epithelium, with reduced fluorescence attributed to keratin20, 33. This is consistent with recent studies which show that HPV immortalized keratinocytes show increased NADH and FAD fluorescence relative to normal keratinocytes35.
In Vivo Optical Imaging Technologies
In the last decade, advances in high-performance, low-cost electronics, have enabled development of sensitive systems for optical imaging and interrogation of cervical precancer in vivo36-38. Table 2 summarizes the capabilities of a variety of different optical interrogation methods under investigation. As illustrated in the bottom row of Fig. 1, these optical tools can be used to monitor biologically predictive features of cervical cancer, providing a global approach to detect cervical cancer which bridges the molecular, cellular, and tissue scales.
Table 2.
Overview of optical technologies being used for the detection of cervical cancer.
| Technique | Spatial Resolution | Field of View | Depth | Sources of contrast | Cost | In clinical use? |
|---|---|---|---|---|---|---|
| Visual Inspection | 100u-200u | Entire cervix | Surface | Induced change in scattering | $ | Yes |
| Widefield Imaging | 50-100μ | Entire cervix | Surface | Fuorescence: Collagen Reflectance: Hemaglobin absorption, Acetic acid induced change in scattering |
$$-$$$ | Yes |
| Spectroscopy | 1mm | 1mm | .3-1mm | Fuorescence: Collagen, NADH, FAD Reflectance: Hemaglobin absorption, morphologic changes, DNA content, chromatin texture |
$$ | No |
| High Resolution Imaging* | 1-2μm | <1mm | <1mm | Fluorescence: Fluorescently labeled probes Reflectance: Acetic acid induced change in scattering, morphologic changes, scattering coefficient |
$$$ | No |
$, <$100; $$, $5,000-$30,000; $$$, >$30,000 (in U.S. Dollars)
Laser scanning confocal microscopy
Multispectral Widefield Imaging
Widefield imaging relies on cameras to image changes in reflectance and autofluorescence at multiple wavelengths across the entire cervical epithelium. Typically, widefield imaging can achieve 50-100 micron spatial resolution, and can highlight suspicious regions of tissue. Point-probe techniques, which use a small fiber optic probe placed in contact with the tissue surface, can then be used to interrogate suspicious areas with higher spatial or spectral resolution.
A number of pilot studies have investigated features of multi-spectral reflectance imaging to assess which give rise to greatest image contrast. Reflectance imaging with green wavelength illumination consistently gives best contrast because of hemoglobin absorption39. Alternatively, color reflectance images obtained with white light illumination can be separated into red, green, and blue channels and analyzed to enhance image contrast15, 40. A pilot study of digital reflectance images of the cervix acquired from 29 women showed that an automated image analysis algorithm could identify the presence and spatial extent of high grade precancers with 79% sensitivity and 88% specificity compared to histopathologic analysis 15. Illuminating tissue with light which has been passed through a linear polarizer while imaging reflectance through an orthogonally oriented linear polarizer can reduce specular reflection, bright areas caused by light reflected from the tissue surface, and improve visualization of subepithelial vascular patterns39.
Imaging the time course of acetowhitening can also improve the ability to discriminate high grade precancer39, 40; a multispectral reflectance imaging study of 123 women found that the increase in light scattering after application of acetic acid was greater and persisted for a longer time in high grade precancers41.
Similarly, tissue autofluorescence can be imaged in widefield mode42. Exciting autofluorescence in the UV and blue wavelengths (∼440-470 nm) has been shown to give greatest contrast between normal tissue and precancer42, 43. Results of several large clinical studies investigating hyperspectral autofluorescence and reflectance imaging have recently been reported and are summarized in Table 144-47. In these studies, autofluorescence and reflectance spectral data were collected with relatively high spectral (∼5 nm) and spatial (∼ 1 mm) resolution. Pattern recognition approaches were used to classify tissue and results were compared to the gold standard of histology to assess performance. In a study of 111 women, Ferris found a sensitivity of 97% and a specificity of 70% for hyperspectral widefield imaging compared to colposcopic directed biopsy or loop electro-surgical excision46. A larger series of 572 women were assessed with this device47, yielding a sensitivity of 95.1% and a specificity of 55.2%. Huh and colleagues found similar results using a different hyperspectral imaging approach to detect UV induced fluorescence at 337 nm and reflectance in 604 women44. With a sensitivity of 92% and a specificity of 50%, they found that hyperspectral imaging could detect 1/3 more high grade precancers than colposcopy alone, with a relatively small increase in the false positive rate44. In a multi-center trial testing the device as an adjunct to colposcopy in 193 women, researchers found that the use of hyperspectral imaging resulted in a 22% relative gain in the true positive rate of colposcopy with an 18.1% incremental gain in the false positive rate48. A multi-center trial of this device involving 2,299 women randomized to receive colposcopy alone or colposcopy plus hyperspectral imaging showed similar results45. In addition, for women with a Pap smear showing atypical cells of uncertain significance or low grade precancer, hyperspectral imaging increased the true positive rate by 26.8% compared to colposcopy alone with a minimal increase in the false positive rate.
This device was approved by the US Food and Drug Administration in March of 2006 to enhance the sensitivity of colposcopic detection of high grade cervical precancers49. Thus, while widefield imaging can objectively detect cervical precancers with high sensitivity, specificity is limited. The use of other optical approaches to probe suspicious areas identified with widefield imaging may increase specificity. Two approaches which have been considered include point optical spectroscopy and high resolution optical imaging.
Optical Spectroscopy
Fiber optic probes can be used to record fluorescence and reflectance spectra of small areas of tissue with 1-5 nm spectral resolution, providing detailed, quantitative information about the distributions of optically active molecules within a tissue (Figure 3).
Figure 3.
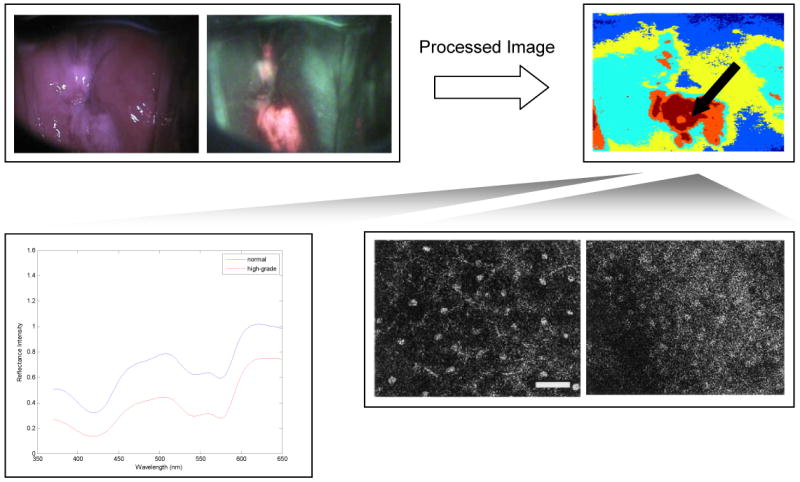
Top row: Widefield reflectance and autofluorescence imaging can interrogate the entire field of the cervix, indicating suspicious areas; digital image analysis approaches can help to objectify and automate the recognition of abnormal areas with high sensitivity. High spectral or spatial resolution techniques can be used to probe suspicious areas to confirm diagnosis of precancerous areas. Spectroscopy (lower left) can probe changes in the concentration of tissue chromophores, while confocal microscopy (lower right) can directly images changes in cell morphology and nuclear to cytoplasmic ratio without the need to biopsy, section and stain tissue. Scale bars measure 1mm in the widefield images, and 50 um in the confocal images.
A number of studies have been carried out to assess whether optical spectroscopy can provide accurate in vivo diagnosis of cervical precancer; Table 1 summarizes results of key studies. Reflectance spectroscopy measures the intensity of light reflected as a function of illumination wavelength, providing information about changes in epithelial cell scattering, stromal scattering and stromal angiogenesis. Using empirical algorithms to classify tissue based on reflectance alone achieved sensitivity of 72% and specificity of 81% in one series of 161 patients50, but approaches which use fluorescence spectroscopy alone or the combination of reflectance and fluorescence generally yield better classification accuracy51, 52. Much current effort is focused on the design of fiber optic probes53, 54 and analysis strategies to separate reflectance signals from the epithelium and the stroma32, 55 in an attempt to increase accuracy.
In vivo fluorescence spectroscopy can detect high grade precancer with good accuracy; early pilot studies focused on UV and blue excitation56, 57. More recently, a study of 146 patients comparing 18 different excitation wavelengths found that three broad ranges of excitation - 330-340 nm (UV), 350-380 nm (UV) and 400-450 nm (blue) excitation – gave best sensitivity and specificity for detection of high grade precancer58. Across all studies, fluorescence intensity of precancerous lesions is lower than that of normal squamous tissue, and the peak emission wavelength of precancers is shifted to longer emission wavelengths relative to that of normal tissue56-59. The decreased fluorescence intensity has been attributed to the decreased stromal fluorescence and increased stromal absorption of cervical precancers60-62, while the spectral shift is attributed to both increased hemoglobin absorption and increased mitochondrial fluorescence in precancers60-62. Drezek showed that at 380 nm excitation, approximately 20% of detected fluorescence of squamous normal tissue is due to NADH, while 40-50% of detected fluorescence in high grade precancer is due to NADH60. Brookner found that the fluorescence of columnar normal tissue and metaplasia are lower than that of squamous normal tissue63. Since cervical precancers frequently develop at the junction between squamous and columnar epithelium, the performance of optical algorithms are often limited by the challenge of discriminating precancers at the squamo-columnar junction.
Several small studies have compared the performance of reflectance and fluorescence spectroscopy alone and in combination with reflectance spectroscopy for cervical precancer detection51, 52, 64, with combined methods giving best results. Georgakoudi discovered that combining three modes of spectroscopy - fluorescence, reflectance, and light scattering - yielded better results than any individual mode52. Mirabal noted that reflectance spectroscopy could distinguish columnar normal from high grade dysplasia with higher specificity than fluorescence alone50.
Importantly, Weingandt noted that inflammatory lesions may give rise to false positive fluorescence measurements59, and recent studies in other organ sites indicate that inflammation and precancer both exhibit a similar loss in stromal autofluorescence65. Techniques which better probe changes in epithelial signatures, such as depth-resolved spectroscopy54 or high resolution imaging29, 66, may give rise to better specificity.
High Resolution Imaging
Small, flexible confocal microscopes have been developed to image cervical tissue and microfabrication techniques can be used to manufacture confocal microscopes with minimal power requirements. High resolution techniques can image tissue with sub-cellular resolution to probe changes in epithelial cell morphology and epithelial architecture without the need for biopsy, sectioning and staining67 (Figure 3). Video-rate reflectance confocal microscopy yields images of intact epithelial tissue with 1-2 micron spatial resolution68 and with the use of acetic acid, which increases nuclear scattering, determination of image parameters such as nuclear to cytoplasmic (N/C) ratio are possible69. Collier showed that the N/C ratio measured via confocal microscopy could separate high grade cervical precancers with a sensitivity and specificity greater than 90%29, 66. Automated image analysis routines can be used to segment nuclei in confocal images of cervical tissue and objectively calculate N/C ratio70. More recently, fiber optic confocal microscopes have become available to acquire confocal images of cervical tissue in vivo at near video rate in both reflectance71 and fluorescence modes72. While it is difficult to image weak autofluorescence in vivo using confocal fluorescence microscopy due to photobleaching limits, advances in optically active, targeted contrast agents can be used to tag biomarkers of interest with an optical signal which can be measured and quantified in vivo.
Contrast Agents for Molecular Imaging
Recent developments in confocal fluorescence imaging have shown the utility of new vital stains, such as IV administered fluorescein to highlight vascular changes and topically applied acriflavine to visualize cell nuclei73. In the last decade, enormous progress has been made to understand the molecular events that accompany carcinogenesis. Optically active, molecular-targeted contrast agents can be used to image these biomarkers in vivo74, 75.
In general, targeted optical contrast agents consist of a probe molecule for molecular specific recognition of biomarkers conjugated to an optically interrogatable label74. Optically active contrast agents have been developed using antibodies76 or peptides77 to target biomarkers and using a number of different types of optically active labels, including metal nanoparticles78, 79, quantum dots80 and organic fluorescent dyes76. Fluorescent dyes conjugated to monoclonal antibodies provide a mechanism to target multiple cell surface receptors overexpressed on tumor cells, such as the epidermal growth factor receptor76. Alternatively, peptides such as the epidermal growth factor, can target receptors77, yielding smaller molecular weight agents which provide an advantage for topical application. The broad excitation range and narrow emission spectra of quantum dots provides the ability to simultaneously image expression of multiple biomarkers81, 82, although concerns exist about the cytotoxicity of these materials83.
As an alternative, some contrast agents incorporate optically active metal nanoparticles74, 78, 79, 82. Gold and silver nanoparticles provide a strong source of backscattered light for contrast in widefield and high resolution imaging84; the scattering signal from a single nanoparticle has been shown to be equivalent to approximately 1 million fluorophores85. Unlike fluorescent dyes, metal nanoparticles are not susceptible to photobleaching, and gold is non-toxic and biocompatible79. Gold nanoparticles conjugated to anti-EGFR antibodies have been used to image cervical precancer in vitro with high contrast78, 79. EGFR overexpressing cells induce aggregation of gold nanoparticles, leading to non-linear enhancements in scattering which can magnify signal differences resulting from moderate levels of overexpression78. The aggregation-induced increase in signal yielded an image contrast ratio of 10-20 fold between images of normal and high grade cervical precancer labeled with anti-EGFR gold nanoparticles in one study, dramatically increasing contrast beyond values reported for antibody targeted fluorescent dyes78.
Conclusions and Perspectives
New screening technologies should work for developed and developing countries. The decreasing incidence of cervical cancer in many developed countries is a testament to the impact of comprehensive screening programs86. Since current HPV vaccines do not prevent all cervical cancers, and since women in low resource areas may not have access to new vaccines for decades, we have incentive to continue developing low-cost, high-impact screening technologies that can continue to reduce the incidence of cervical cancer.
Optical imaging and spectroscopy can non-invasively assess the morphologic and biochemical changes associated with the development of precancer at the point-of-care. Driven by advances in consumer electronics, high quality optical images can now be obtained with low cost devices; tandem advances in digital signal processing provide the ability to automate image analysis. Thus, optical imaging is ideally suited for use as a screening technology. Results of large, multi-center trials of widefield hyperspectral imaging show that this approach has high sensitivity, but lower specificity. In addition, currently available imaging instrumentation is expensive and bulky, making it difficult to use in low-resource settings87; efforts to engineer lower cost, battery powered, portable devices are essential to support global translation. Further work is needed to improve specificity; efforts should focus on improving the ability to discriminate precancer from normal columnar tissue, metaplasia and inflammation.
Alternatively, depth-resolved spectroscopy or high resolution optical imaging may provide complementary information about optical changes in the epithelium, which can improve specificity. In particular, the sensitivity and specificity of high resolution in vivo imaging in pilot studies, coupled with recent developments in low cost, high resolution fluorescence imaging systems88, 89 makes this approach especially appealing. However, large scale clinical trials are needed to confirm and optimize diagnostic performance of high resolution approaches. Harnessing the benefits of optically active targeted contrast agents to image cancer-related biomarkers may further aid performance.
As shown in Fig. 3, optical technologies provide a flexible approach to sample the full range of biochemical and morphologic changes which accompany precancer development. This multi-modal optical approach has the potential to improve the performance of precancer screening, and once appropriately validated, also has the potential to expand access to screening.
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA: a cancer journal for clinicians. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Koss LG. The Papanicolaou test for cervical cancer detection. A triumph and a tragedy. JAMA. 1989;261:737–43. [PubMed] [Google Scholar]
- 3.Walboomers JM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189:12–9. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 4.Cohen J. Public health. High hopes and dilemmas for a cervical cancer vaccine. Science. 2005;308:618–21. doi: 10.1126/science.308.5722.618. [DOI] [PubMed] [Google Scholar]
- 5.Agosti JM, Goldie SJ. Introducing HPV vaccine in developing countries--key challenges and issues. N Engl J Med. 2007;356:1908–10. doi: 10.1056/NEJMp078053. [DOI] [PubMed] [Google Scholar]
- 6.Mitchell MF, Schottenfeld D, Tortolero-Luna G, Cantor SB, Richards-Kortum R. Colposcopy for the diagnosis of squamous intraepithelial lesions: a meta-analysis. Obstet Gynecol. 1998;91:626–31. doi: 10.1016/s0029-7844(98)00006-4. [DOI] [PubMed] [Google Scholar]
- 7.Goldie SJ, et al. Cost-effectiveness of cervical-cancer screening in five developing countries. N Engl J Med. 2005;353:2158–68. doi: 10.1056/NEJMsa044278. [DOI] [PubMed] [Google Scholar]
- 8.Sankaranarayanan R, et al. Visual inspection of the uterine cervix after the application of acetic acid in the detection of cervical carcinoma and its precursors. Cancer. 1998;83:2150–6. [PubMed] [Google Scholar]
- 9.Sankaranarayanan R, et al. Effect of visual screening on cervical cancer incidence and mortality in Tamil Nadu, India: a cluster-randomised trial. Lancet. 2007;370:398–406. doi: 10.1016/S0140-6736(07)61195-7. [DOI] [PubMed] [Google Scholar]
- 10.Jeronimo J, et al. Visual inspection with acetic acid for cervical cancer screening outside of low-resource settings. Rev Panam Salud Publica. 2005;17:1–5. doi: 10.1590/s1020-49892005000100001. [DOI] [PubMed] [Google Scholar]
- 11.Sankaranarayanan R, Gaffikin L, Jacob M, Sellors J, Robles S. A critical assessment of screening methods for cervical neoplasia. Int J Gynaecol Obstet. 2005;89 2:S4–S12. doi: 10.1016/j.ijgo.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 12.Sankaranarayanan R, et al. The role of low-level magnification in visual inspection with acetic acid for the early detection of cervical neoplasia. Cancer Detect Prev. 2004;28:345–51. doi: 10.1016/j.cdp.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 13.Denny L, Kuhn L, Pollack A, Wright TC., Jr Direct visual inspection for cervical cancer screening: an analysis of factors influencing test performance. Cancer. 2002;94:1699–707. doi: 10.1002/cncr.10381. [DOI] [PubMed] [Google Scholar]
- 14.Thekkek N, Martinez J, Follen M, Richards-Kortum R. Biomedical Engineering Society 2007 Annual Fall Meeting; Los Angeles, California. 2007. [Google Scholar]
- 15.Park SY, et al. Automated Image Analysis of Digital Colposcopy for the Detection of Cervical Intraepithelial Neoplasia. J Biomed Opt. 2008 doi: 10.1117/1.2830654. in press. [DOI] [PubMed] [Google Scholar]
- 16.Cristoforoni PM, et al. Computerized colposcopy: results of a pilot study and analysis of its clinical relevance. Obstet Gynecol. 1995;85:1011–6. doi: 10.1016/0029-7844(95)00051-R. [DOI] [PubMed] [Google Scholar]
- 17.Mikhail MS, Palan PR, Basu J, Romney SL. Computerized measurement of intercapillary distance using image analysis in women with cervical intraepithelial neoplasia: correlation with severity. Acta Obstet Gynecol Scand. 2004;83:308–10. doi: 10.1111/j.0001-6349.2004.0429.x. [DOI] [PubMed] [Google Scholar]
- 18.Drezek RA, et al. Laser scanning confocal microscopy of cervical tissue before and after application of acetic acid. Amer J Obstet Gynecol. 2000;182:1135–9. doi: 10.1067/mob.2000.104844. [DOI] [PubMed] [Google Scholar]
- 19.Skala MC, et al. Multiphoton microscopy of endogenous fluorescence differentiates normal, precancerous, and cancerous squamous epithelial tissues. Cancer Res. 2005;65:1180–6. doi: 10.1158/0008-5472.CAN-04-3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pavlova I, et al. Microanatomical and biochemical origins of normal and precancerous cervical autofluorescence using laser-scanning fluorescence confocal microscopy. Photochem Photobiol. 2003;77:550–5. doi: 10.1562/0031-8655(2003)077<0550:maboon>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 21.Brookner CK, et al. Autofluorescence patterns in short-term cultures of normal cervical tissue. Photochem Photobiol. 2000;71:730–6. doi: 10.1562/0031-8655(2000)071<0730:apistc>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 22.Gulledge CJ, Dewhirst MW. Tumor oxygenation: a matter of supply and demand. Anticancer Res. 1996;16:741–9. [PubMed] [Google Scholar]
- 23.Smith-McCune KK, Weidner N. Demonstration and characterization of the angiogenic properties of cervical dysplasia. Cancer Res. 1994;54:800–4. [PubMed] [Google Scholar]
- 24.Guidi AJ, et al. Vascular permeability factor (vascular endothelial growth factor) expression and angiogenesis in cervical neoplasia. J Natl Cancer Inst. 1995;87:1237–45. doi: 10.1093/jnci/87.16.1237. [DOI] [PubMed] [Google Scholar]
- 25.Davidson B, et al. Expression of matrix metalloproteinase-9 in squamous cell carcinoma of the uterine cervix-clinicopathologic study using immunohistochemistry and mRNA in situ hybridization. Gynecol Oncol. 1999;72:380–6. doi: 10.1006/gyno.1998.5285. [DOI] [PubMed] [Google Scholar]
- 26.Triratanachat S, Niruthisard S, Trivijitsilp P, Tresukosol D, Jarurak N. Angiogenesis in cervical intraepithelial neoplasia and early-staged uterine cervical squamous cell carcinoma: clinical significance. Int J Gynecol Cancer. 2006;16:575–80. doi: 10.1111/j.1525-1438.2006.00568.x. [DOI] [PubMed] [Google Scholar]
- 27.Zuluaga AF, et al. Contrast agents for confocal microscopy: how simple chemicals affect confocal images of normal and cancer cells in suspension. J Biomed Opt. 2002;7:398–403. doi: 10.1117/1.1481047. [DOI] [PubMed] [Google Scholar]
- 28.Collier T, Follen M, Malpica A, Richards-Kortum R. Sources of scattering in cervical tissue: determination of the scattering coefficient by confocal microscopy. Appl Opt. 2005;44:2072–81. doi: 10.1364/ao.44.002072. [DOI] [PubMed] [Google Scholar]
- 29.Collier T, Guillaud M, Follen M, Malpica A, Richards-Kortum R. Real-time reflectance confocal microscopy: comparison of two-dimensional images and three-dimensional image stacks for detection of cervical precancer. J Biomed Opt. 2007;12:024021. doi: 10.1117/1.2717899. [DOI] [PubMed] [Google Scholar]
- 30.Drezek R, et al. Light scattering from cervical cells throughout neoplastic progression: influence of nuclear morphology, DNA content, and chromatin texture. J Biomed Opt. 2003;8:7–16. doi: 10.1117/1.1528950. [DOI] [PubMed] [Google Scholar]
- 31.Arifler D, et al. Light scattering from normal and dysplastic cervical cells at different epithelial depths: finite-difference time-domain modeling with a perfectly matched layer boundary condition. J Biomed Opt. 2003;8:484–94. doi: 10.1117/1.1578640. [DOI] [PubMed] [Google Scholar]
- 32.Arifler D, MacAulay C, Follen M, Richards-Kortum R. Spatially Resolved Reflectance Spectroscopy for Diagnosis of Cervical Pre-Cancer: Monte Carlo Modeling and Comparison to Clinical Measurements. J Biomed Opt. 2006;11:064027. doi: 10.1117/1.2398932. [DOI] [PubMed] [Google Scholar]
- 33.Drezek R, et al. Autofluorescence microscopy of fresh cervical-tissue sections reveals alterations in tissue biochemistry with dysplasia. Photochem Photobiol. 2001;73:636–41. doi: 10.1562/0031-8655(2001)073<0636:AMOFCT>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 34.Lohmann W, Mussmann J, Lohmann C, Kunzel W. Native fluorescence of unstained cryo-sections of the cervix uteri compared with histological observations. Naturwissenschaften. 1989;76:125–7. doi: 10.1007/BF00366606. [DOI] [PubMed] [Google Scholar]
- 35.Mujat C, et al. Endogenous optical biomarkers of normal and human papillomavirus immortalized epithelial cells. Int J Cancer. 2008;122:363–71. doi: 10.1002/ijc.23120. [DOI] [PubMed] [Google Scholar]
- 36.DaCosta RS, Wilson BC, Marcon NE. Fluorescence and spectral imaging. ScientificWorldJournal. 2007;7:2046–71. doi: 10.1100/tsw.2007.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Perelman LT. Optical diagnostic technology based on light scattering spectroscopy for early cancer detection. Expert Rev Med Devices. 2006;3:787–803. doi: 10.1586/17434440.3.6.787. [DOI] [PubMed] [Google Scholar]
- 38.Ramanujam N. Fluorescence spectroscopy of neoplastic and non-neoplastic tissues. Neoplasia. 2000;2:89–117. doi: 10.1038/sj.neo.7900077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Balas C. A novel optical imaging method for the early detection, quantitative grading, and mapping of cancerous and precancerous lesions of cervix. IEEE Trans Biomed Eng. 2001;48:96–104. doi: 10.1109/10.900259. [DOI] [PubMed] [Google Scholar]
- 40.Pogue BW, et al. Analysis of acetic acid-induced whitening of high-grade squamous intraepithelial lesions. J Biomed Opt. 2001;6:397–403. doi: 10.1117/1.1412850. [DOI] [PubMed] [Google Scholar]
- 41.Orfanoudaki IM, et al. A clinical study of optical biopsy of the uterine cervix using a multispectral imaging system. Gynecol Oncol. 2005;96:119–31. doi: 10.1016/j.ygyno.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 42.Benavides JM, C S, Park SY, MacKinnon N, MacAulay C, Milbourne A, Malpica A, Follen M, Richards-Kortum R. Multispectral Digital Colposcopy for in vivo Detection of Cervical Cancer. Opt Express. 2003;11:1223–1236. doi: 10.1364/oe.11.001223. [DOI] [PubMed] [Google Scholar]
- 43.Milbourne A, et al. Results of a pilot study of multispectral digital colposcopy for the in vivo detection of cervical intraepithelial neoplasia. Gynecol Oncol. 2005;99:S67–75. doi: 10.1016/j.ygyno.2005.07.047. [DOI] [PubMed] [Google Scholar]
- 44.Huh WK, et al. Optical detection of high-grade cervical intraepithelial neoplasia in vivo: results of a 604-patient study. Am J Obstet Gynecol. 2004;190:1249–57. doi: 10.1016/j.ajog.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 45.Alvarez RD, Wright TC. Effective cervical neoplasia detection with a novel optical detection system: a randomized trial. Gynecol Oncol. 2007;104:281–9. doi: 10.1016/j.ygyno.2006.08.056. [DOI] [PubMed] [Google Scholar]
- 46.Ferris DG, et al. Multimodal Hyperspectral Imaging for the Noninvasive Diagnosis of Cervical Neoplasia. J Low Genit Tract Dis. 2001;5:65–72. doi: 10.1046/j.1526-0976.2001.005002065.x. [DOI] [PubMed] [Google Scholar]
- 47.DeSantis T, et al. Spectroscopic imaging as a triage test for cervical disease: a prospective multicenter clinical trial. J Low Genit Tract Dis. 2007;11:18–24. doi: 10.1097/01.lgt.0000230207.50495.05. [DOI] [PubMed] [Google Scholar]
- 48.Alvarez RD, Wright TC., Jr Increased detection of high-grade cervical intraepithelial neoplasia utilizing an optical detection system as an adjunct to colposcopy. Gynecol Oncol. 2007;106:23–8. doi: 10.1016/j.ygyno.2007.02.028. [DOI] [PubMed] [Google Scholar]
- 49.Kendrick JE, Huh WK, Alvarez RD. LUMA cervical imaging system. Expert Rev Med Devices. 2007;4:121–9. doi: 10.1586/17434440.4.2.121. [DOI] [PubMed] [Google Scholar]
- 50.Mirabal YN, et al. Reflectance spectroscopy for in vivo detection of cervical precancer. J Biomed Opt. 2002;7:587–94. doi: 10.1117/1.1502675. [DOI] [PubMed] [Google Scholar]
- 51.Chang SK, et al. Combined reflectance and fluorescence spectroscopy for in vivo detection of cervical pre-cancer. J Biomed Opt. 2005;10:024031. doi: 10.1117/1.1899686. [DOI] [PubMed] [Google Scholar]
- 52.Georgakoudi I, et al. Trimodal spectroscopy for the detection and characterization of cervical precancers in vivo. Am J Obstet Gynecol. 2002;186:374–82. doi: 10.1067/mob.2002.121075. [DOI] [PubMed] [Google Scholar]
- 53.Mourant JR, et al. In vivo light scattering measurements for detection of precancerous conditions of the cervix. Gynecol Oncol. 2007;105:439–45. doi: 10.1016/j.ygyno.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 54.Wang A, Nammalavar V, Drezek R. Targeting spectral signatures of progressively dysplastic stratified epithelia using angularly variable fiber geometry in reflectance Monte Carlo simulations. J Biomed Opt. 2007;12:044012. doi: 10.1117/1.2769328. [DOI] [PubMed] [Google Scholar]
- 55.Arifler D, Schwarz RA, Chang SK, Richards-Kortum R. Reflectance spectroscopy for diagnosis of epithelial precancer: model-based analysis of fiber-optic probe designs to resolve spectral information from epithelium and stroma. Appl Opt. 2005;44:4291–305. doi: 10.1364/ao.44.004291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ramanujam N, et al. Fluorescence spectroscopy: a diagnostic tool for cervical intraepithelial neoplasia (CIN) Gynecol Oncol. 1994;52:31–8. doi: 10.1006/gyno.1994.1007. [DOI] [PubMed] [Google Scholar]
- 57.Ramanujam N, et al. Cervical precancer detection using a multivariate statistical algorithm based on laser-induced fluorescence spectra at multiple excitation wavelengths. Photochem Photobiol. 1996;64:720–35. doi: 10.1111/j.1751-1097.1996.tb03130.x. [DOI] [PubMed] [Google Scholar]
- 58.Chang SK, et al. Optimal excitation wavelengths for discrimination of cervical neoplasia. IEEE Trans Biomed Eng. 2002;49:1102–11. doi: 10.1109/TBME.2002.803597. [DOI] [PubMed] [Google Scholar]
- 59.Weingandt H, et al. Autofluorescence spectroscopy for the diagnosis of cervical intraepithelial neoplasia. Br J Obstet Gynecol. 2002;109:947–51. doi: 10.1111/j.1471-0528.2002.01311.x. [DOI] [PubMed] [Google Scholar]
- 60.Drezek R, et al. Understanding the contributions of NADH and collagen to cervical tissue fluorescence spectra: modeling, measurements, and implications. J Biomed Opt. 2001;6:385–96. doi: 10.1117/1.1413209. [DOI] [PubMed] [Google Scholar]
- 61.Chang SK, Arifler D, Drezek R, Follen M, Richards-Kortum R. Analytical model to describe fluorescence spectra of normal and preneoplastic epithelial tissue: comparison with Monte Carlo simulations and clinical measurements. J Biomed Opt. 2004;9:511–22. doi: 10.1117/1.1695559. [DOI] [PubMed] [Google Scholar]
- 62.Georgakoudi I, et al. NAD(P)H and collagen as in vivo quantitative fluorescent biomarkers of epithelial precancerous changes. Cancer Res. 2002;62:682–7. [PubMed] [Google Scholar]
- 63.Brookner CK, Utzinger U, Staerkel G, Richards-Kortum R, Mitchell MF. Cervical fluorescence of normal women. Lasers Surg Med. 1999;24:29–37. doi: 10.1002/(sici)1096-9101(1999)24:1<29::aid-lsm6>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 64.Nordstrom RJ, Burke L, Niloff JM, Myrtle JF. Identification of cervical intraepithelial neoplasia (CIN) using UV-excited fluorescence and diffuse-reflectance tissue spectroscopy. Lasers Surg Med. 2001;29:118–27. doi: 10.1002/lsm.1097. [DOI] [PubMed] [Google Scholar]
- 65.Pavlova I, Williams M, El-Naggar A, Richards-Kortum R, Gillenwater A. Understanding the biological basis of autofluorescence imaging for oral cancer detection: High resolution fluorescence microscopy in viable tissue. Clin Cancer Res. 2008 doi: 10.1158/1078-0432.CCR-07-1609. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Collier T, Lacy A, Richards-Kortum R, Malpica A, Follen M. Near real-time confocal microscopy of amelanotic tissue: detection of dysplasia in ex vivo cervical tissue. Acad Radiol. 2002;9:504–12. doi: 10.1016/s1076-6332(03)80326-4. [DOI] [PubMed] [Google Scholar]
- 67.Evans JA, Nishioka NS. Endoscopic confocal microscopy. Curr Opin Gastroenterol. 2005;21:578–84. doi: 10.1097/01.mog.0000174217.62214.10. [DOI] [PubMed] [Google Scholar]
- 68.MacAulay C, Lane P, Richards-Kortum R. In vivo pathology: microendoscopy as a new endoscopic imaging modality. Gastrointest Endosc Clin N Am. 2004;14:595–620. xi. doi: 10.1016/j.giec.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 69.Collier T, Shen P, de Pradier B, Richards-Kortum R. Near Real Time Confocal Microscopy of Amelanotic Tissue: Dynamics of Aceto-Whitening Enable Nuclear Segmentation. Opt Express. 2000;6:40–48. doi: 10.1364/oe.6.000040. [DOI] [PubMed] [Google Scholar]
- 70.Luck BL, Carlson KD, Bovik AC, Richards-Kortum RR. An image model and segmentation algorithm for reflectance confocal images of in vivo cervical tissue. IEEE Trans Image Process. 2005;14:1265–76. doi: 10.1109/tip.2005.852460. [DOI] [PubMed] [Google Scholar]
- 71.Sung KB, et al. Near real time in vivo fibre optic confocal microscopy: sub-cellular structure resolved. J Microsc. 2002;207(Pt 2):137–45. doi: 10.1046/j.1365-2818.2002.01049.x. [DOI] [PubMed] [Google Scholar]
- 72.Tan J, Delaney P, McLaren WJ. Confocal endomicroscopy: a novel imaging technique for in vivo histology of cervical intraepithelial neoplasia. Expert Rev Med Devices. 2007;4:863–71. doi: 10.1586/17434440.4.6.863. [DOI] [PubMed] [Google Scholar]
- 73.Kiesslich R, et al. Confocal laser endoscopy for diagnosing intraepithelial neoplasias and colorectal cancer in vivo. Gastroenterology. 2004;127:706–13. doi: 10.1053/j.gastro.2004.06.050. [DOI] [PubMed] [Google Scholar]
- 74.Sokolov K, et al. Optical systems for in vivo molecular imaging of cancer. Technol Cancer Res Treat. 2003;2:491–504. doi: 10.1177/153303460300200602. [DOI] [PubMed] [Google Scholar]
- 75.Hsiung PL, et al. Detection of colonic dysplasia in vivo using a targeted heptapeptide and confocal microendoscopy. Nat Med. 2008;14:454–8. doi: 10.1038/nm1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Koyama Y, et al. In vivo molecular imaging to diagnose and subtype tumors through receptor-targeted optically labeled monoclonal antibodies. Neoplasia. 2007;9:1021–9. doi: 10.1593/neo.07787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Adams KE, et al. Comparison of visible and near-infrared wavelength-excitable fluorescent dyes for molecular imaging of cancer. J Biomed Opt. 2007;12:024017. doi: 10.1117/1.2717137. [DOI] [PubMed] [Google Scholar]
- 78.Aaron J, et al. Plasmon Resonance Coupling of Metal Nanoparticles for Molecular Imaging of Carcinogenesis In Vivo. J Biomed Opt. 2007;12 doi: 10.1117/1.2737351. [DOI] [PubMed] [Google Scholar]
- 79.Sokolov K, et al. Real-time vital optical imaging of precancer using anti-epidermal growth factor receptor antibodies conjugated to gold nanoparticles. Cancer Res. 2003;63:1999–2004. [PubMed] [Google Scholar]
- 80.Wu X, et al. Immunofluorescent labeling of cancer marker Her2 and other cellular targets with semiconductor quantum dots. Nat Biotechnol. 2003;21:41–6. doi: 10.1038/nbt764. [DOI] [PubMed] [Google Scholar]
- 81.Nida DL, Rahman MS, Carlson KD, Richards-Kortum R, Follen M. Fluorescent nanocrystals for use in early cervical cancer detection. Gynecol Oncol. 2005;99:S89–94. doi: 10.1016/j.ygyno.2005.07.050. [DOI] [PubMed] [Google Scholar]
- 82.Cuenca AG, et al. Emerging implications of nanotechnology on cancer diagnostics and therapeutics. Cancer. 2006;107:459–66. doi: 10.1002/cncr.22035. [DOI] [PubMed] [Google Scholar]
- 83.Chang E, Thekkek N, Yu WW, Colvin VL, Drezek R. Evaluation of quantum dot cytotoxicity based on intracellular uptake. Small. 2006;2:1412–7. doi: 10.1002/smll.200600218. [DOI] [PubMed] [Google Scholar]
- 84.Nitin N, Javier DJ, Roblyer DM, Richards-Kortum R. Widefield and high-resolution reflectance imaging of gold and silver nanospheres. J Biomed Opt. 2007;12:051505. doi: 10.1117/1.2800314. [DOI] [PubMed] [Google Scholar]
- 85.Aslan K, Zhang J, Lakowicz JR, Geddes CD. Saccharide sensing using gold and silver nanoparticles--a review. J Fluoresc. 2004;14:391–400. doi: 10.1023/b:jofl.0000031820.17358.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vizcaino AP, et al. International trends in incidence of cervical cancer: II. Squamous-cell carcinoma. Int J Cancer. 2000;86:429–35. doi: 10.1002/(sici)1097-0215(20000501)86:3<429::aid-ijc20>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 87.Roblyer D, Richards-Kortum R, Park SY, Adewole I, Follen M. Objective screening for cervical cancer in developing nations: lessons from Nigeria. Gynecol Oncol. 2007;107:S94–7. doi: 10.1016/j.ygyno.2007.07.042. [DOI] [PubMed] [Google Scholar]
- 88.Muldoon T, et al. Subcellular resolution molecular imaging within living tissue by fiber microendoscopy. Opt Express. 2007 doi: 10.1364/oe.15.016413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Muldoon TJ, Anandasabapathy S, Maru D, Richards-Kortum R. High-Resolution Imaging in Barrett's Esophagus: A Novel, Low- Cost Endoscopic Microscope. Gastrointestinal Endoscopy. 2008 doi: 10.1016/j.gie.2008.05.018. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Visual inspection with acetic acid for cervical-cancer screening: test qualities in a primary-care setting. University of Zimbabwe/JHPIEGO Cervical Cancer Project. Lancet. 1999;353:869–73. [PubMed] [Google Scholar]
- 91.Denny L, Kuhn L, Pollack A, Wainwright H, Wright TC., Jr Evaluation of alternative methods of cervical cancer screening for resource-poor settings. Cancer. 2000;89:826–33. doi: 10.1002/1097-0142(20000815)89:4<826::aid-cncr15>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 92.Belinson JL, et al. Cervical cancer screening by simple visual inspection after acetic acid. Obstet Gynecol. 2001;98:441–4. doi: 10.1016/s0029-7844(01)01454-5. [DOI] [PubMed] [Google Scholar]
- 93.Cronje HS, et al. A comparison of four screening methods for cervical neoplasia in a developing country. Am J Obstet Gynecol. 2003;188:395–400. doi: 10.1067/mob.2003.153. [DOI] [PubMed] [Google Scholar]
- 94.Sankaranarayanan R, et al. Accuracy of visual screening for cervical neoplasia: Results from an IARC multicentre study in India and Africa. Int J Cancer. 2004;110:907–13. doi: 10.1002/ijc.20190. [DOI] [PubMed] [Google Scholar]


