Abstract
Melanin-concentrating hormone (MCH) is a neuropeptide that exhibits potent orexigenic activity. In rodents, it exerts its actions by interacting with one receptor, MCH1 receptor which is expressed in many parts of the central nervous system (CNS). To study the physiological implications of the MCH system, we need to be able to block it locally and acutely. This necessitates the use of MCH1 receptor antagonists. While MCH1 receptor antagonists have been previously reported, they are mainly not accessible to academic research. We apply here a strategy that leads to the isolation of a high affinity and selective MCH1 receptor antagonist amenable to in vivo analyses without further chemical modifications. This antagonist, TPI 1361-17, was identified through the screening of multiple non-peptide positional scanning synthetic combinatorial libraries (PS-SCL) totaling more than eight hundred thousand compounds in conditions that allow for the identification of only high-affinity compounds. TPI 1361-17 exhibited an IC50 value of 6.1 nM for inhibition of 1 nM MCH-induced Ca2+ mobilization and completely displaced the binding of [125I] MCH to rat MCH1 receptor. TPI 1361-17 was found specific, having no affinity for a variety of other G-protein coupled receptors and channels. TPI 1361-17 was found active in vivo since it blocked MCH-induced food intake by 75 %. Our results indicate that TPI 1361-17 is a novel and selective MCH1 receptor antagonist and is an effective tool to study the physiological functions of the MCH system. These results also illustrate the successful application of combinatorial library screening to identify specific surrogate antagonists in an academic setting.
Index words: MCH1 receptor, combinatorial libraries, antagonist, G-protein coupled receptors, TPI 1361-17
1. Introduction
Melanin-concentrating hormone (MCH) is a cyclic, 19-amino-acid peptide isolated from salmon pituitary as a melanophore concentrating factor (Kawauchi et al., 1983), that is also present in rat and human (Mouri et al., 1993; Vaughan et al., 1989). In the mammalian brain, MCH is predominantly expressed in the perikarya of the lateral hypothalamic area (LHA), and the zona incerta (ZI) and projects widely throughout the central nervous system (Bittencourt et al., 1992; Vaughan et al., 1989). MCH is known to interact with two receptors, MCH1 receptor (Saito et al., 1999) and MCH2 receptor (Mori et al., 2001; Sailer et al., 2001). MCH1 receptor is expressed in the brain regions where MCH fibers were identified, in particular in centers modulating feeding behavior (Bittencourt et al., 1992; Saito et al., 2001a; Saito et al., 1999), and in some peripheral tissues (Bradley et al., 2000; Pissios et al., 2006; Saito et al., 2000; Saito et al., 2001b; Tadayyon et al., 2000; Verlaet et al., 2002). MCH2 receptor, identified on the basis of its sequence similarity to MCH1 receptor, is species-specific, most notably it is absent in rodents (Tan et al., 2002), a fact that has impaired study of its function.
In mammals, MCH has been mostly implicated in the regulation of food consumption and energy metabolism. Central administration of MCH has been shown to promote feeding (Qu et al., 1996), while MCH1 receptor mRNA levels rise as a result of starvation and leptin deficiency (Kokkotou et al., 2005) and MCH circulatory levels are high in obese Zucker rats (Stricker-Krongrad et al., 2001). MCH also stimulates insulin and leptin release in insulinoma cell lines and 3T3-L1 adipose cells, respectively (Bradley et al., 2000; Tadayyon et al., 2000) and regulates pituitary hormones (Kennedy et al., 2003; Kennedy et al., 2001; Murray et al., 2000; Tsukamura et al., 2000). The importance of MCH system in energy metabolism was confirmed by genetic interventions. Mice devoid of MCH are lean and hypophagic (Shimada et al., 1998), while mice over-expressing MCH are obese and hyperphagic (Ludwig et al., 2001). Genetic disruption of MCH1 receptor on the other hand results in mice that are surprisingly hyperphagic, but also hypermetabolic, and obesity-resistant (Chen et al., 2002; Marsh et al., 2002). The reasons for this discrepancy are unknown.
MCH1 receptor antagonists have been shown to be effective at modulating feeding and diet induced obesity (Borowsky et al., 2002; Kowalski et al., 2004; Kowalski et al., 2006; Mashiko et al., 2005; Shearman et al., 2003); reviewed in Handlon and Zhou, 2006). Consistent with the wide distribution of MCH1 receptor in the brain, the MCH system is expected to be involved in various physiological functions and indeed a MCH1 receptor antagonist has been shown to have antidepressant and anxiolytic activity (Borowsky et al., 2002; Chaki et al., 2005; David et al., 2007; Georgescu et al., 2005; Shimazaki et al., 2006; Smith et al., 2006). MCH1 receptor antagonists have also been reported to improve social memory in a social recognition test (Millan et al., 2008). On the other hand, MCH1 receptor knockout mice exhibit cognitive deficits in inhibitory passive avoidance test (Adamantidis et al., 2005).
To fully understand physiological roles of the MCH system, we need to be able to study the behavioral effects resulting from acute blockade of the MCH system. This necessitates the isolation of an antagonist. A MCH antagonist presents the following advantages: 1) its effects can be monitored after both acute or chronic administration; 2) it is not plagued by the developmental complications that may be associated with genetic manipulations; 3) it can be injected locally allowing to differentiate peripheral and central effects for example; 4) it is not restricted to one animal species; 5) it allows to set up parameters for testing the system in humans. In order to isolate a specific MCH1 receptor antagonist, we chose to screen mixture based combinatorial libraries. Combinatorial chemistry has emerged as a powerful tool for the discovery and optimization of new leads in the pharmaceutical industry (Pinilla et al., 2003). This has been largely due to advances in high-throughput screening as well as more efficient parallel synthesis and purification techniques. The most commonly used mixture libraries and deconvolution methods include iterative, positional scanning and the sequencing of resin-bound peptides or tags from one-bead, one compound library. Furthermore, provided that access to a semi high-throughput technology is available, screening for a specific antagonist in the academic setting is possible. In this study, we screen heterocyclic positional scanning synthetic combinatorial libraries (PS-SCL) to isolate a specific MCH1 receptor antagonist which will be useful to study physiological functions of the MCH system.
2. Materials and Methods
2.1. Drugs
Rat / human MCH, rat / human NPY, rat urotensin II, Dynorphin and angiotensin II were purchased from Bachem California (Torrance, CA). Human Orexin B was purchased from Anaspec (San Jose, CA). [125I]-MCH was purchased from AP Biotech (Piscataway, NJ). Phosphoramidon and phenylmethylsulfonylfuloride (PMSF) were purchased from Sigma (St. Louis, MO).
2.2. Non-peptide positional scanning combinatorial library
We used several heterocyclic and peptidomimetic positional scanning combinatorial libraries (PS-SCL) for screening. Each library was prepared by using simultaneous multiple synthesis technology (Houghten, 1985) as described elsewhere (Ostresh, 1998). List of the libraries and a representation of an N-benzyl aminocyclic thiourea are presented (Table 1). The identity and purity of each individual compound were analyzed by mass spectral analysis interfaced with a liquid-chromatography system (Finnigan LCQ) and/or analytical reverse-phase high performance liquid chromatography (RP-HPLC) using a Vydac C18 column and a Beckman system Gold HPLC. The compounds were purified by using a Waters Milliprep 3400 preparative HPLC with a preparative Foxy fraction collector.
Table 1. PS-SCLs tested for MCH1 receptor antagonism.
| Templates | Diversity (# of total cpds.) |
# of active mixture / # of total mixture |
|---|---|---|
| Heterocycles | ||
| N-benzyl aminocyclic thioureas | 118,400 | 26 / 80 |
| N-methyl amynocyclic thioureas | 118,400 | 1 / 80 |
| N-methyl diketopiperazines | 31,320 | 0 / 40 |
| N-benzyl diketopiperazines | 31,320 | 0 / 40 |
| N-methyl piperazines | 31,320 | 0 / 40 |
| N-benzyl piperadines | 31,320 | 0 / 40 |
| C-6-acylamino bicyclic guanidines | 72,283 | 0 / 40 |
| bicyclic guanidines | 102,459 | 0 / 88 |
| bis-diketopiperazines | 45,864 | 0 / 40 |
| Peptidemimetics | ||
| triphenylureas | 85,248 | 0 / 40 |
| N-acyl triamines | 125,000 | 0 / 50 |
| N-methyltriamines | 31,320 | 0 / 40 |
2.3. Cell culture and transfection
MCH1 receptor was mixed with LipofectAMINE transfection reagents (Life Technologies, MD), and the mixture was diluted with Opti-MEM and added to 60–70 % confluent HEK 293T cells plated on 100-mm dishes. The transfected cells were cultured in DMEM containing 10 % FBS. For Calcium mobilization assay and radioligand binding assay, CHO cells or HEK-293T cells stably expressing rat MCH1 receptor were used (Saito et al., 1999).
2.4. Measurement of calcium influx
Transfected HEK293T cells seeded on black-walled 96-well plates (Sigma, St Louis USA) were loaded for 1 h at 37°C with Ca2+-sensitive fluorescent dye, fluo-4 (Molecular Device, CA, USA) in Hanks’ balanced salt solution containing 20 mM HEPES (pH 7.4). The level of [Ca2+]i was then monitored using a FLIPR system (Fluorometric Imaging Plate Reader; Molecular Devices, San Francisco, CA). For antagonists screening, each mixture from the compound library was first incubated with the cell for 10 minutes, before the addition of MCH. Data were expressed as fluorescence (arbitrary units) versus time.
2.5. MCH1 receptor-binding experiments
HEK293T cells expressing the rat MCH1 receptor were scraped with ice-cold PBS and centrifuged at 1000 × g for 5 min. Cell pellet was homogenized with ice-cold 50 mM Tris-HCl buffer (pH 7.4) containing 5 mM EDTA, and ultra-centrifuged twice at 48,000 × g for 20 min at 4°C. The pellets were then suspended in 50 mM Tris-HCl (pH 7.4) buffer containing 5 mM EDTA and used as membrane fractions. The membrane fraction (30 μg protein for each assay) dissolved in 500 μl assay buffer containing 50 mM Tris-HCl buffer (pH 7.4), 1 μM phosphoramidon, 0.5 mM phenylmethylsulfonylfluoride and 0.2 % BSA with 0.1 nM [125I] (Phe13, Tyr19) MCH and various concentrations of cold MCH and TPI1361-17 at room temperature for 2 h. Nonspecific binding was determined by including 1 μM MCH in the binding reaction. The binding reaction was terminated by rapid filtration through GF/C filter plates pre-soaked in 0.2 % polyethylenimine, followed by washing three times with 3 ml of PBS. The radioactivity retained in the filters was determined with a γ-counter. IC50 values calculated as described above are expressed as the mean ± S.E.M. for three independent determinations.
2.6. Selectivity screening of TPI 1361-17
Binding analyses were carried out by MDS Pharma services (Taipei, Taiwan) and assay details and literature references are available online at http://discovery.mdsps.com/catalog/. hERG channel assay was carried out by AVIVA biosciences (San Diego, CA). Other activity analyses were carried out by using the FLIPR assay format as described above. Activity implies that activation of the listed receptors was tested in the presence of TPI 1361-17 by changes in intracellular calcium levels as described above.
2.7. Animals
Adult male Sprague-Dawley rats weighing 200 – 300 g were obtained from Charles River Laboratories (Wilmington, MA), bearing a 23-gauge stainless steel cannula placed into the right lateral ventricle. Rats were housed individually and maintained on a 12 h light-dark cycle (6:00 am–6:00 pm light) with free access to tap water and rat chaw (PROLAB RMH 2500, containing 23.0% protein, 4.5% fat, gross energy 4.5 kcal/g. PMI nutrition international, LLC. Brentwood, MO). Prior to all studies, rats were handled and habituated for 7 days, and 10 μl of artificial cerebrospinal fluid (aCSF: NaCl 124, KCl 5, CaCl2 2.4 MgSO4 2, KH2PO4 1.25, NaHCO3 26 and glucose 10, in mM) was injected through the cannula to minimize stress effects at the time of the experiment. Cannula placement was confirmed in all animals by evaluating the response to 50 ng of angiotensin II. Only animals drinking more than 5 ml of water in 1h were used in feeding studies. This study design was approved by the Institutional Animal Care and Use Committee, University of California, Irvine.
2.8. Pharmacokinetics
Pharmacokinetic studies in rats were carried out by Ricerca Biosciences (Concord, OH). Assay details are available at http://www.ricerca.com/pages/RC3/metavivo2.html.
2.9. Spontaneous locomotor activity
Ambulatory movements were recorded using a Hamilton-Kinder Activity Monitor (with the infrared beams in a 4 × 8 configured frame). Locomotion was quantified in transparent polycarbonate cages (32 × 26 × 20 cm) placed within the activity frames. Animal position is detected by two dimensional beam breaks. The ambulation is tabulated as a movement that results in the change of location. All other beam breaks are categorized as non-ambulatory, fine movement. Animals were acclimatized to the novel home cage-environment for 60 minutes before injection, then were removed, injected and immediately replaced in the same cage, and monitoring began. Locomotion was measured for 60 minutes after the i.c.v. administration of either vehicle or 5 nmole TPI 1361-17.
2.10. Conditioned taste aversion test
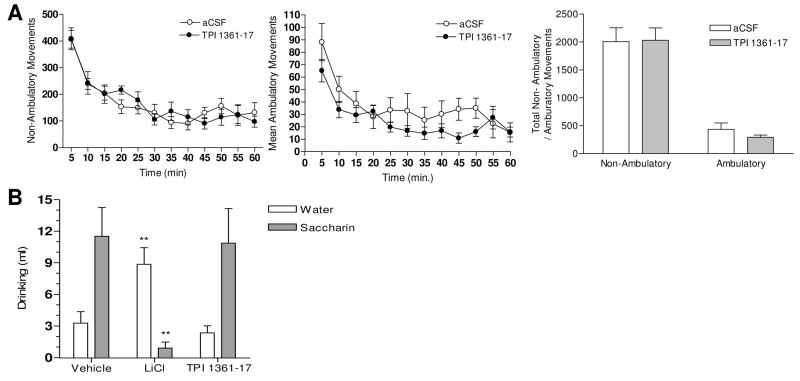
Rats had free access to food in their home cage, but water was restricted to 20 ml per day. Rats were accustomed to a 1hr tap water drinking session (per day) for 5 days in a separate cage where the amount of liquid consumed is monitored in an automated feeding/drinking recording system (TSE, Germany). On day 6, the conditioning day, rats were exposed to 0.1% saccharin solution instead of water and the volume consumed was recorded. 15 min after saccharin bottle removal, rats were injected either with vehicle, LiCl (300 mg/kg, i.p.) or TPI 1361-17 (10 nmole, i.c.v.). On day 7, rats were offered both water and saccharin in separate bottles and the drinking volume was monitored for 1 hr. The volume of water and saccharin solution consumed was used to quantify the conditioned taste aversion (CTA). Lithium chloride (LiCl), a classic reagent to induce conditioned taste aversion was used as a positive control to induce aversion after saccharin consumption on conditioning day (day 6), and therefore influence rats to drink water instead of saccharin on the test day (day 7).
2.11. Measurement of MCH - induced food intake
To determine the effects of TPI 1361-17 on MCH-induced food intake, rats were randomly divided into three groups; 1) TPI 1361-17 and 2 nmole of MCH; 2) MCH 2 nmole, 3) aCSF. Each mixture contained a DMSO equivalent concentration to that of the antagonist solution (up to 5 % (v/v)). Ten μl of each solution was administered into lateral ventricle for 2 minutes. The injections were done between 7:00 – 8:00 a.m., and food intake was monitored for 4 h after the injection.
2.12. Data analysis
Prism software (GraphPad, San Diego, CA) was used for statistical analysis. Results were analyzed by t test or ANOVA followed by the appropriate post hoc comparisons, and P<0.05 was considered statistically significant.
3. Results
3.1. Screening of the positional scanning synthetic combinatorial libraries (PS-SCL)
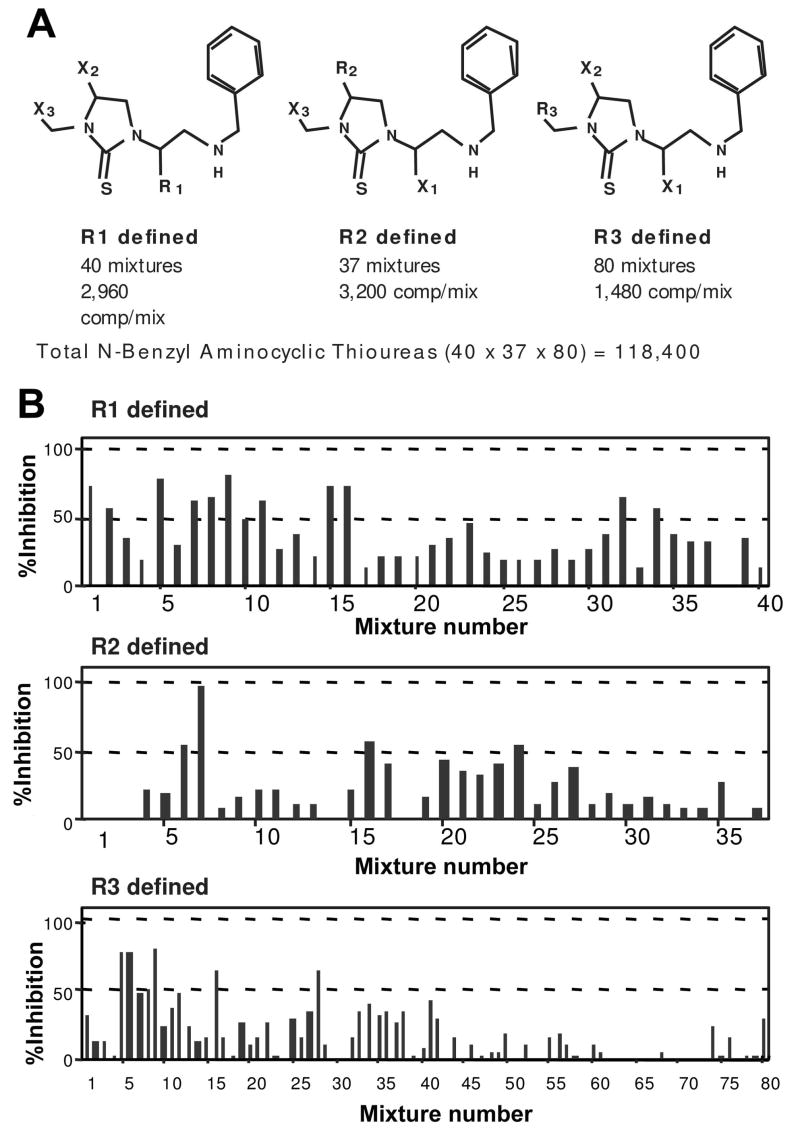
A total of twelve heterocyclic or peptidomimetics PS-SCLs were tested (Table 1). Most libraries were generated in a PS format by using 40 ~ 50 amino acids at each of the R1 and R2 positions, and 40 ~ 80 carboxylic acids at the R3 position. A sample set of PS-SCLs was screened initially, sub-libraries with defined R3 positions and a mixture of building blocks at each of the other two positions were prepared (all compounds in the library are represented). Each sub-library was composed of 40 ~ 80 mixtures containing an average of 1300 individual compounds. A total of 618 separate mixtures were screened for inhibition of 30 nM MCH-induced Ca2+ mobilization in CHO cells expressing MCH1 receptor. Mixtures showing IC50 values lower than 2.5 μg/ml were considered bioactive. At this concentration, an individual compound should have a nanomolar affinity for MCH1 receptor. The N-benzyl aminocyclic thiourea PS-SCL exhibited 26 hits out of 80 sub-libraries, while the N-methyl aminocyclic thiourea PS-SCL had one out of 80. There were no agonistic or MCH1 receptor antagonistic activities in the other libraries.
3.2. Deconvolution of the N-benzyl aminocyclic thiourea PS-SCL
The N-benzyl aminocyclic thiourea PS-SCL had been generated in a PS format by using 40 amino acids at R1 Position, 37 amino acids at the R2 position, and 80 carboxylic acids at the R3 position for a total of 118,400 (40 × 37 × 80) individual compounds (Fig. 1A). The building blocks are described in Table 2. The complete PS-SCL was composed of three sub-libraries, each of which had a single defined building block at one position and a mixture of building blocks at each of the other two positions (Fig. 1B). Each sub-library contained the same number of compounds (118,400). Pooling of each sub-library varied based on the number of building blocks included at the defined position of that sub-library. The PS-SCL was composed of 157 separate mixtures (i.e., 40 + 37 + 80 = 157 samples to be assayed), each mixture contained 1,480 (40 × 37) to 3,200 (40 × 80) individual compounds, depending on the location of the defined position. The structure(s) of the active individual compound(s) present in the library can be directly determined from the screening of these 157 mixtures, since each individual compound is present in a single mixture in each of the three sub-libraries.
Figure 1.
A, Representation of the N-benzyl aminocyclic thiourea PS-SCL. R is defined as a single block; X is a mixture of building blocks. B, Profile of the inhibitory activity of the PS-SCL on 30 nM MCH-induced Ca2+ mobilization on MCH1 receptor expressing CHO cells. Each graph represents a building block with one-position defined mixtures. The bars show the % inhibition by a single mixture at 0.5 μg / ml. A representative data set from three individual assays is shown. See Table 2 for the details of each mixture.
Table 2. Building blocks used in the N-benzyl thiourea PS-SCL.
| No. | Building block | ||
|---|---|---|---|
| R1 | R2 | R3 | |
| 1 | L-Ala | L-Ala | 1-Phenyl-1-cyclopropanecarboxylic acid |
| 2 | L-Phe | L-Phe | 2-Phenylbutyric acid |
| 3 | L-Gly | Gly | 3-Phenylbutyric acid |
| 4 | L-Ile | L-Ile | m-Tolylacetic acid |
| 5 | L-Lys(Boc) | L-Leu | 3-Fluorophenylacetic acid |
| 6 | L-Leu | L-Met(O) | 3-Bromophenylacetic acid |
| 7 | L-Met(O) | L-Arg(Pmc) | (α-α-α-Trifluoro-m-tolyl)acetic acid |
| 8 | L-Asn | L-Ser(tBu) | p-Tolylacetic acid |
| 9 | L-Gln | L-Thr(tBu) | 4-Fluorophenylacetic acid |
| 10 | L-Arg(Pmc) | L-Val | 3-Methoxyphenylacetic acid |
| 11 | L-Ser(tBu) | L-Trp(Boc) | 4-Bromophenylacetic acid |
| 12 | L-Thr(tBu) | L-Tyr(BrZ) | 4-Methoxyphenylacetic acid |
| 13 | L-Val | L-Tyr(tBu) | 4-Ethoxyphenylacetic acid |
| 14 | L-Trp | D-Ala | 4-Isobutyl-α-methylphenylacetic acid |
| 15 | L-Tyr(BrZ) | D-Phe | 3,4-Dichlorophenylacetic acid |
| 16 | L-Tyr(tBu) | D-Ile | 3,5-bis-(Trifluoromethyl)phenylacetic acid |
| 17 | D-Ala | D-Leu | 3-(3,4-Dimethoxyphenyl)propionic acid |
| 18 | D-Phe | D-Ser | 4-Biphenylacetic acid |
| 19 | D-Ile | D-Thr(tBu) | α-Methylcinnamic acid |
| 20 | D-Lys(Boc) | D-Val | 2-(Trifluoromethyl)cinnamic acid |
| 21 | D-Leu | D-Trp(Boc) | (3,4-Dimethoxyphenyl)acetic acid |
| 22 | D-Asn | D-Tyr(tBu) | 3,4-(Methylenedioxy)phenylacetic acid |
| 23 | D-Gln | D-Arg(Pmc) | 2-Methoxycinnamic acid |
| 24 | D-Ser | L-Nle | Benzoic acid |
| 25 | D-Thr(tBu) | D-Nle | 4-Chlorocinnamic acid |
| 26 | D-Val | L-Nva | trans-Cinnamic acid |
| 27 | D-Trp | D-Nva | m-Toluic acid |
| 28 | D-Tyr(tBu) | L-NapAla | Phenylacetic acid |
| 29 | D-Arg(Pmc) | D-NapAla | Hydrocinnamic acid |
| 30 | L-Nle | L-Phg | 4-Phenylbutyric acid |
| 31 | D-Nle | L-Glu(tBu) | 3,5-bis-(Trifluoromethyl)benzoic acid |
| 32 | L-Nva | D-Glu(tBu) | Butyric acid |
| 33 | D-Nva | β–Ala | Heptanoic acid |
| 34 | L-NapAla | L-ChAla | Isobutyric acid |
| 35 | D-NapAla | D-ChAla | (+/−)-2-Methylbutyric acid |
| 36 | L-Phg | L-His(Trt) | Isovaleric acid |
| 37 | L-ChAla | D-His(Trt) | 3-Methylvaleric acid |
| 38 | D-ChAla | 4-Methylvaleric acid | |
| 39 | L-His(Trt) | Crotonic acid | |
| 40 | D-His(Trt) | Vinylacetic acid | |
| 41 | p-Toluic acid | ||
| 42 | Trimethylacetic acid | ||
| 43 | tert-Butylacetic acid | ||
| 44 | Cyclohexanecarboxylic acid | ||
| 45 | Cyclohexylacetic acid | ||
| 46 | Cyclohexanebutyric acid | ||
| 47 | Cycloheptanecarboxylic acid | ||
| 48 | Acetic acid | ||
| 49 | 2-Methylcyclopropanecarboxylic acid | ||
| 50 | Cyclobutanecarboxylic acid | ||
| 51 | Cyclopentanecarboxylic acid | ||
| 52 | 3-Cyclopentylpropionic acid | ||
| 53 | Cyclohexanepropionic acid | ||
| 54 | 4-Methyl-1-cyclohexanecarboxylic acid | ||
| 55 | 4-tert-Butyl-cyclohexanecarboxylic acid | ||
| 56 | 4-Methylcyclohexaneacetic acid | ||
| 57 | Tiglic acid | ||
| 58 | 1-Adamantaneacetic acid | ||
| 59 | Niflumic acid | ||
| 60 | 4-Nitrophenylacetic acid | ||
| 61 | 4-(Nitrophenyl)-butyric acid | ||
| 62 | 4-Nitrocinnamic acid | ||
| 63 | 2-Nitrobenzoic acid | ||
| 64 | 2,4-Dinitrophenyl acetic acid | ||
| 65 | 4-Biphenylacetic acid | ||
| 66 | 2-Chloro-5-nitrobenzoic acid | ||
| 67 | (4-Pyridylthio)acetic acid | ||
| 68 | 3-3 Diphenylpropionic acid | ||
| 69 | 2-Chloro-4-nitrobenzoic acid | ||
| 70 | 4-Dimethylaminobenzoic acid | ||
| 71 | 4-Nitrobenzoic acid | ||
| 72 | 3-Dimethylaminobenzoic acid | ||
| 73 | Abietic acid | ||
| 74 | 2-Methyl-4-nitro-1-imidizole-propionic acid | ||
| 75 | trans-Styrylacetic acid |
3.3. Screening of the N-benzyl aminocyclic thiourea PS-SCL
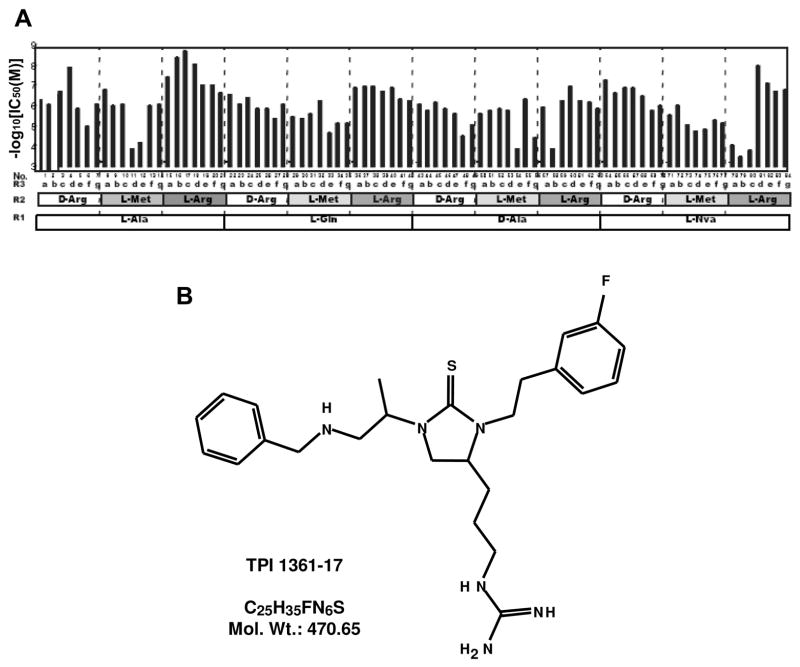
Each mixture was assayed in duplicate in three separate assays at three concentrations (1, 0.1 and 0.01 μg/ml) (Fig. 1B). Inhibition rates were calculated as the percent decrease in intracellular Ca2+ mobilization in MCH1 receptor expressing CHO cells induced by 30 nM MCH in the presence or absence of the mixture. None of the 157 mixtures elicited increases in Ca2+ mobilization alone. Mixtures showing highest reproducible antagonism and dose-dependency were selected for deconvolution. A set of 84 individual N- benzyl aminocyclic thioureas was then generated to confirm the connectivity between the selected building blocks (i.e., if the activities of the selected mixtures are due to the same individual compounds), as well as to determine the relative activities of the individual compounds. These 84 selected compounds were generated containing 4, 3 and 7 different building blocks at position R1, R2 and R3 respectively (Fig. 2A). Each compound was assayed at five different concentrations derived from serial ten-fold dilutions starting at 10 μM (Fig. 2A). The lowest IC50 values were in the nM range, found with the combination of L-Alanine at R1 position, L-Arginine at R2, and phenylacetic acids at R3. The compound with the highest affinity, TPI 1361-17 (N-(3-{(4S)-1-[(1S)-2-(benzylamino)-1-methylethyl]-3-[2-(3-fluorophenyl)ethyl]-2-thioxoimidazolidin-4-yl}propyl)guanidine) (Fig. 2B) was selected for further pharmacological analyses. By mass spectrometry, the molecular weight of purified TPI 1361-17 was 470.26, which matched exactly the one expected.
Figure 2.
A, Inhibitory activity of a series of 84 compounds individually synthesized based on the blocking profile of the library screening. IC50 values of each compound inhibiting 30 nM MCH-induced Ca2+ mobilization in MCH1 receptor expressing CHO cells. Details of the each compound are shown in Table 2. Functionalities at the three diversity positions (R1-R3) for each compound is illustrated. For R3 position, each letter represents organic acids as follows; a; 3-Bromophenylacetic Acid, b; Phenylacetic Acid, c; 3-Fluorophenylacetic Acid, d; 4-Fluorophenylacetic Acid, e; 3,5 Bis (Trifluoromethyl) Phenylacetic acid, f; 4-Methylacetic Acid, and g; p-Tolylacetic acid. B, Chemical structure of TPI 1361-17.
3.4. TPI 1361-17 antagonism at MCH1 receptor
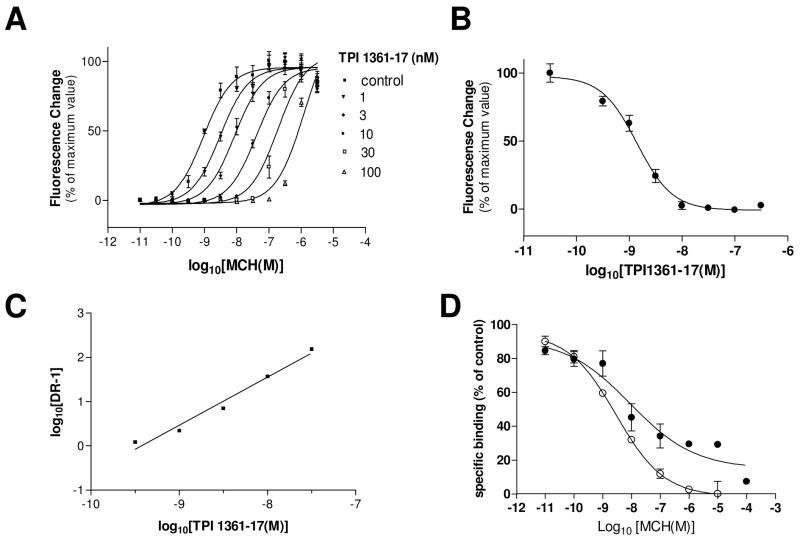
MCH stimulated the Ca2+ mobilization in HEK 293T cell expressing rat MCH1 receptor with an EC50 value of 0.9 nM. TPI 1361-17 showed IC50 value of 6.1 nM on 1 nM MCH-induced Ca2+ mobilization (Fig. 3B). TPI 1361-17 did not change the basal Ca2+ level by itself, but significantly inhibited the MCH-induced Ca2+ mobilization. The addition of increasing concentrations of TPI 1361-17 caused a progressive shift of the curve to the right (Fig. 3A). The Schild regression estimated a pA2 of 9.43 with a slope of 1.085 ± 0.010 (r2 = 0.975), which predicts a Kb of 0.37 nM (Fig 3C). This compound was more than 1,000 fold selective for MCH1 receptor when compared to the human MCH2 receptor, as well as other G-protein coupled receptors including the neuropeptide Y2, orexin 1R, prolactin releasing peptide receptor, and kappa opioid receptors. TPI 1361-17 did also not show activity in a hERG channel assay. Its binding was also tested on a battery of receptors, channels and enzymes and showed no activity (Table 3). TPI 1361-17 exhibited a high affinity for rat MCH1 receptor in binding assays as well. TPI 1361-17 completely displaced the binding of [125I] MCH to rat MCH1 receptor, with an IC50 value of 31.8 ± 8.66 nM (mean ± S.E.M.) (Fig. 3D).
Figure 3.
In vitro pharmacology of TPI 1361-17. A, Dose response curves obtained for inhibition of MCH-induced fluorescence in MCH1 receptor expressing HEK-293T cells by increasing concentrations of TPI 1361-17. B, IC50 value for TPI 1361-17 on 1 nM MCH-induced Ca2+ mobilization. C, Schild plot of the TPI 1361-17 antagonism. Data are mean ± S.E.M., n = 3. D, Displacement of [125I] MCH binding to membrane fractions of MCH1 receptor expressing HEK-293T, by MCH (●) and TPI 1361-17 (○). The data representative of three separate experiments with similar results are shown. Data are mean ± S.E.M.
Table 3. Selectivity of TPI 1361-17.
Activity implies that activations of the listed receptors were tested in the FLIPR assay in the presence of increasing concentrations of TPI 1361-17 as described in Methods. hERG channel assay was carried out by using in vitro hERG electrophysiology assay at AVIVA biosciences. Binding implies that the following receptors and channels were tested for displacement of specific radioactive ligands by TPI 1361-17 (done by MDS Pharma Services).
| ACTIVITY | BINDING | ||
|---|---|---|---|
| MCH2R | >10uM | Adenosine A1 | >1uM |
| NPY1R | >10uM | Adenosine A2A | >1uM |
| NPY2R | >10uM | Adrenergic α1A/α1B/α2A | >1uM |
| NPY5R | >10uM | Adrenergic β1/β2 | >1uM |
| GPR7 | >10uM | Dopamine D2s | >1uM |
| GPR10 | >10uM | Dopamine D1 | >1uM |
| GPH14 | >10uM | GPR103 | >1uM |
| Dopannine D1 | >10uM | Histamine H1 | >1uM |
| DopannineD2 | >10uM | Imidazoline I2, Central | >1uM |
| Opiate κ (KOR) | >10uM | Muscarinic M2 | >1uM |
| Orexin 1R | >10uM | Muscarinic M3 | >1uM |
| hERG | >10uM | Prostanoid EP4 | >1uM |
| Opiate μ (OP3, MOP) | >1uM | ||
| Phorobol Ester | >1uM | ||
| Potassium Channel [KATP] | >1uM | ||
| Rolipram | >1uM | ||
| Nicotinic Acetylcholine | >1uM | ||
| Nicotinic Acetylcholine α1, Bungarotoxin | >1uM | ||
| GABAA, Agonist site | >1uM | ||
| GABAA, Benzodiazepine, Central, Flunitrazepam | >1uM | ||
| Glutarmate, NMDA, Phencyclidine | >1uM | ||
| Calcium channel L-type | >1uM | ||
| Transporter, Norepinephrine (NET) | >1uM |
3.5. Pharmacokinetics of TPI 1361-17
After intravenous administration of TPI 1361-17 (5 mg/kg), peak plasma concentrations were attained at 0.083 hr, the first sampling point postdose. TPI 1361-17 concentrations then declined, and values for plasma elimination half-life ranged from 1.7 to 4.2 hr (mean value of 2.7 hr). AUC0-∞ values ranged from 1964.6 to 2684.1 hr*ng/mL (mean value of 2438.6 hr*ng/mL).
3.6. Inhibitory effects of TPI 1361-17 on MCH-induced food intake
Rats were habituated to a wire bottom cage for more than 3 weeks prior to the experiment. Experiments were carried out from 7 a.m. to 8 a.m. when rats were satiated. Intracerebroventricular injection of MCH (2 nmole) induced rapid and robust feeding in satiated rats. Addition of TPI 1361-17 (1 or 5 nmole) significantly suppressed MCH-induced food consumption in a dose dependent manner. One nmole TPI 1361-17 decreased MCH-induced food intake for 1h and 2h but this decrease was reversed over the next two hours. On the other hand, five nmole of TPI 1361-17 suppressed cumulative food intake by 75 % even at 4h (Fig. 4).
Figure 4.
Effect of i.c.v. administration of TPI 1361-17 on MCH-induced food intake. Rats were administered vehicle (□, n = 10), or 2 nmole MCH (○, n = 9), or 2 nmole MCH and TPI 1361-17 (●, n= 9). TPI 1361-17 was tested at two doses: 1 nmole and 5 nmole. Experiments were performed one to two hours into the light cycle. Ten minutes after the administration, rats were given access to regular low fat-diet, food intake was monitored and cumulative food intake was calculated (Each data point expresses mean value with S.E.M. * P< 0.05; ANOVA/Bonferoni multiple comparison test for the effect of TPI 1361-17 + MCH versus MCH alone).
3.7. Effects of TPI 1361-17 on locomotor activity and taste aversion test
Intracerebroventricular cannulated rats were acclimatized to the testing situation. Five or one nmole of TPI 1361-17 administered i.c.v. did not exhibit any detectable changes in movement. Ambulatory movements and non-ambulatory movements were monitored for 60 minutes upon administration of five nmole TPI 1361-17 or vehicle (Fig. 5A). Both animal groups showed highest movements just after the injection, which gradually decreased and stabilizes. There were no significant differences at any time points, as there was no difference in the total amounts of non-ambulatory or ambulatory movements (Fig. 5A). TPI 1361-17 also did not result in any aversive effect in taste aversion test. Neither TPI 1361-17 (10 nmole, i.c.v.) nor vehicle changed the preference to drink a saccharin solution, whereas LiCl elicited a robust taste aversion effect (** P<0.01 vs. vehicle group) (Fig. 5B).
Figure 5.
A, Effect of i.c.v. administration of TPI 1361-17 on non-ambulatory and ambulatory movements. Both movements were monitored for one hour after the administration of vehicle (○) or 5 nmole TPI 1361-17 (●); Sums of non-ambulatory and ambulatory movements (Each data point represents the value with the S.E.M.; n=10). B, Effect of TPI 1361-17 (10 nmole, i.c.v.) on conditioned taste aversion (□ water; ■ saccharin) (** P<0.01 vs. vehicle group, ANOVA/Dunnett’s multiple comparison test, n=6-8). Data expressed as mean ± SEM.
4. Discussion
The use of orphan G-protein coupled receptors as targets to identify novel transmitters has led to the discoveries of several new neuropeptides as well as to the matching of known peptides to their specific G-protein coupled receptors. Discovery of a new neuropeptide system ultimately leads to the question of its function. This question can be addressed either by genetic or chemical approaches. The development of specific antagonists allows evaluation of blockade of a system and of this effect on the organism in a timely fashion. It also provides putative candidates for therapeutic research. Our approach was to screen large numbers of randomly synthesized compounds in pools of an average 1300 compounds. By choosing appropriate pool concentrations, we calculated that any positive pool would include a compound that should inhibit MCH-induced intracellular calcium release with a low nanomolar affinity that reflected high specificity and could be of use in vivo without further chemical modification.
We describe the identification and characterization of a specific MCH1 receptor antagonist, TPI 1361-17, isolated from a N-benzyl aminocyclic thiourea PS-SCL. The technology of mixture-based combinatorial library enables us to assay thousands of compounds at a time, thus decreasing the time and costs of screening and as shown here is suitable for an academic environment. So far, this strategy has proven useful for analysis of T-cell specificity (Zhao et al., 2001), to characterize enzymes (Nazif and Bogyo, 2001) and to discover ion channel blockers (Tai et al., 2001) and both agonists and antagonists of G-protein coupled receptors (Dooley et al., 1998). Since we had little information about structure-function relationship of MCH1 receptor (Macdonald et al., 2000), we chose to test as many PS-SCL libraries as possible. Among the twelve non-peptide libraries tested, the N-benzyl aminocyclic thiourea library was found to have high affinity to the MCH1 receptor. Since our high-throughput receptor assay system is able to detect both agonistic and antagonistic activities, we have also identified a few mixtures showing agonistic activities (data not shown). Upon deconvolution of the libraries, we identified one compound, TPI 1361-17, which most effectively inhibited MCH-induced Ca2+ mobilization in CHO or HEK 293T cells expressing the rat MCH1 receptor. TPI 1361-17 exhibited an IC50 value of 6.1 nM at 1 nM MCH and completely displaced [125I] MCH binding to rat MCH1 receptor. TPI 1361-17 was found specific for MCH1 receptor since it displayed no affinity to an array of G-protein coupled receptors and channels. In particular, no activity was detected on hERG channel assay which has been shown to plague many MCH1 receptor antagonists (Mendez-Andino and Wos, 2007).
To prove its efficacy in vivo, TPI 1361-17 was tested for its ability to block MCH-induced food intake. TPI 1361-17 inhibited MCH-induced food intake up to 75% in dose dependent manner at the beginning of the light phase, when MCH shows maximal effect (Rossi et al., 1997). This effect lasted for over 4h, in agreement with the half-life that we found for the compound in plasma. TPI 1361-17 did not affect locomotion or fine movements and did not induce any taste aversion, indicating that its administration does not induce aversive effects.
Numerous MCH1 receptor antagonists have been reported (Luthin, 2007). They have been developed by the pharmaceutical industry and show different pharmacological properties. TPI 1361-17 differs in that it has been isolated using an approach that aimed at identifying high affinity antagonists that did not require further chemical modifications for activity. TPI 1361-17 exhibits pharmacological characteristics that allow it to be used as a tool for studying the responses associated with the activation of the MCH system. MCH has been implicated in a variety of physiological functions, most notably the regulation of energy homeostasis. Centrally administered MCH stimulates feeding (Qu et al., 1996), while MCH-deficient mice are hypophagic, lean, and hypermetabolic (Shimada et al., 1998). Targeted disruption of MCH1 receptor causes hyperphagia, hypermetabolism, and diet-resistant obesity (Chen et al., 2002; Marsh et al., 2002). This accumulating evidence suggests that MCH1 receptor regulates energy metabolism. While there exist two MCH receptors in human, only MCH1 receptor is expressed in rodents, blockade of MCH1 receptor should therefore equal total blockade of the MCH system in rodents. Also, MCH1 receptor antagonists have been reported to exhibit antidepressant effects when administered orally or centrally (Borowsky et al., 2002; Georgescu et al., 2005). Finally, a MCH1 receptor antagonist has been shown to improve social memory suggesting that the blockade of the MCH system using an antagonist affects cognition (Millan et al., 2008).
In conclusion, we have screened PS-SCL libraries under conditions that would allow to identify high affinity antagonists. We have succeeded in isolating a MCH1 receptor antagonist, TPI 1361-17 that exhibits high-affinity and is selective for the MCH1 receptor. It does not induce aversive effects when administered centrally and thus can be used to explore the functions of the MCH1 receptor system.
Acknowledgments
We would like to thank Kaman Chung, Tim Wong, Jessica Lin, and Armen Chivichyan for excellent technical assistance. We thank our colleagues and Rainer Reinscheid for discussions about the studies. This work was supported by grants MH 060231, DK 063001 and BIO 05-10485 and by an award from the Stanley Research Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adamantidis A, Thomas E, Foidart A, Tyhon A, Coumans B, Minet A, Tirelli E, Seutin V, Grisar T, Lakaye B. Disrupting the melanin-concentrating hormone receptor 1 in mice leads to cognitive deficits and alterations of NMDA receptor function. Eur J Neurosci. 2005;21:2837–2844. doi: 10.1111/j.1460-9568.2005.04100.x. [DOI] [PubMed] [Google Scholar]
- Bittencourt JC, Presse F, Arias C, Peto C, Vaughan J, Nahon JL, Vale W, Sawchenko PE. The melanin-concentrating hormone system of the rat brain: an immuno- and hybridization histochemical characterization. J Comp Neurol. 1992;319:218–245. doi: 10.1002/cne.903190204. [DOI] [PubMed] [Google Scholar]
- Borowsky B, Durkin MM, Ogozalek K, Marzabadi MR, DeLeon J, Lagu B, Heurich R, Lichtblau H, Shaposhnik Z, Daniewska I, Blackburn TP, Branchek TA, Gerald C, Vaysse PJ, Forray C. Antidepressant, anxiolytic and anorectic effects of a melanin-concentrating hormone-1 receptor antagonist. Nat Med. 2002;8:825–830. doi: 10.1038/nm741. [DOI] [PubMed] [Google Scholar]
- Bradley RL, Kokkotou EG, Maratos-Flier E, Cheatham B. Melanin-concentrating hormone regulates leptin synthesis and secretion in rat adipocytes. Diabetes. 2000;49:1073–1077. doi: 10.2337/diabetes.49.7.1073. [DOI] [PubMed] [Google Scholar]
- Chaki S, Yamaguchi J, Yamada H, Thomsen W, Tran TA, Semple G, Sekiguchi Y. ATC0175: an orally active melanin-concentrating hormone receptor 1 antagonist for the potential treatment of depression and anxiety. CNS Drug Rev. 2005;11:341–352. doi: 10.1111/j.1527-3458.2005.tb00052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Hu C, Hsu CK, Zhang Q, Bi C, Asnicar M, Hsiung HM, Fox N, Slieker LJ, Yang DD, Heiman ML, Shi Y. Targeted disruption of the melanin-concentrating hormone receptor-1 results in hyperphagia and resistance to diet-induced obesity. Endocrinology. 2002;143:2469–2477. doi: 10.1210/endo.143.7.8903. [DOI] [PubMed] [Google Scholar]
- David DJ, Klemenhagen KC, Holick KA, Saxe MD, Mendez I, Santarelli L, Craig DA, Zhong H, Swanson CJ, Hegde LG, Ping XI, Dong D, Marzabadi MR, Gerald CP, Hen R. Efficacy of the MCHR1 antagonist N-[3-(1-{[4-(3,4-difluorophenoxy)phenyl]methyl}(4-piperidyl))-4-methylphen yl]-2-methylpropanamide (SNAP 94847) in mouse models of anxiety and depression following acute and chronic administration is independent of hippocampal neurogenesis. J Pharmacol Exp Ther. 2007;321:237–248. doi: 10.1124/jpet.106.109678. [DOI] [PubMed] [Google Scholar]
- Dooley CT, Ny P, Bidlack JM, Houghten RA. Selective ligands for the mu, delta, and kappa opioid receptors identified from a single mixture based tetrapeptide positional scanning combinatorial library. J Biol Chem. 1998;273:18848–18856. doi: 10.1074/jbc.273.30.18848. [DOI] [PubMed] [Google Scholar]
- Georgescu D, Sears RM, Hommel JD, Barrot M, Bolanos CA, Marsh DJ, Bednarek MA, Bibb JA, Maratos-Flier E, Nestler EJ, DiLeone RJ. The hypothalamic neuropeptide melanin-concentrating hormone acts in the nucleus accumbens to modulate feeding behavior and forced-swim performance. J Neurosci. 2005;25:2933–2940. doi: 10.1523/JNEUROSCI.1714-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handlon AL, Zhou H. Melanin-concentrating hormone-1 receptor antagonists for the treatment of obesity. J Med Chem. 2006;49:4017–4022. doi: 10.1021/jm058239j. [DOI] [PubMed] [Google Scholar]
- Houghten RA. General method for the rapid solid-phase synthesis of large numbers of peptides: specificity of antigen-antibody interaction at the level of individual amino acids. Proceedings of the National Academy of Sciences of the United States of America. 1985;82:5131–5135. doi: 10.1073/pnas.82.15.5131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawauchi H, Kawazoe I, Tsubokawa M, Kishida M, Baker BI. Characterization of melanin-concentrating hormone in chum salmon pituitaries. Nature. 1983;305:321–323. doi: 10.1038/305321a0. [DOI] [PubMed] [Google Scholar]
- Kennedy AR, Todd JF, Dhillo WS, Seal LJ, Ghatei MA, O’Toole CP, Jones M, Witty D, Winborne K, Riley G, Hervieu G, Wilson S, Bloom SR. Effect of direct injection of melanin-concentrating hormone into the paraventricular nucleus: further evidence for a stimulatory role in the adrenal axis via SLC-1. J Neuroendocrinol. 2003;15:268–272. doi: 10.1046/j.1365-2826.2003.00997.x. [DOI] [PubMed] [Google Scholar]
- Kennedy AR, Todd JF, Stanley SA, Abbott CR, Small CJ, Ghatei MA, Bloom SR. Melanin-concentrating hormone (MCH) suppresses thyroid stimulating hormone (TSH) release, in vivo and in vitro, via the hypothalamus and the pituitary. Endocrinology. 2001;142:3265–3268. doi: 10.1210/endo.142.7.8374. [DOI] [PubMed] [Google Scholar]
- Kokkotou E, Jeon JY, Wang X, Marino FE, Carlson M, Trombly DJ, Maratos-Flier E. Mice with MCH ablation resist diet-induced obesity through strain-specific mechanisms. Am J Physiol Regul Integr Comp Physiol. 2005;289:R117–124. doi: 10.1152/ajpregu.00861.2004. [DOI] [PubMed] [Google Scholar]
- Kowalski TJ, Farley C, Cohen-Williams ME, Varty G, Spar BD. Melanin-concentrating hormone-1 receptor antagonism decreases feeding by reducing meal size. Eur J Pharmacol. 2004;497:41–47. doi: 10.1016/j.ejphar.2004.06.027. [DOI] [PubMed] [Google Scholar]
- Kowalski TJ, Spar BD, Weig B, Farley C, Cook J, Ghibaudi L, Fried S, O’Neill K, Del Vecchio RA, McBriar M, Guzik H, Clader J, Hawes BE, Hwa J. Effects of a selective melanin-concentrating hormone 1 receptor antagonist on food intake and energy homeostasis in diet-induced obese mice. Eur J Pharmacol. 2006;535:182–191. doi: 10.1016/j.ejphar.2006.01.062. [DOI] [PubMed] [Google Scholar]
- Ludwig DS, Tritos NA, Mastaitis JW, Kulkarni R, Kokkotou E, Elmquist J, Lowell B, Flier JS, Maratos-Flier E. Melanin-concentrating hormone overexpression in transgenic mice leads to obesity and insulin resistance. J Clin Invest. 2001;107:379–386. doi: 10.1172/JCI10660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luthin DR. Anti-obesity effects of small molecule melanin-concentrating hormone receptor 1 (MCHR1) antagonists. Life Sci. 2007;81:423–440. doi: 10.1016/j.lfs.2007.05.029. [DOI] [PubMed] [Google Scholar]
- Macdonald D, Murgolo N, Zhang R, Durkin JP, Yao X, Strader CD, Graziano MP. Molecular characterization of the melanin-concentrating hormone/receptor complex: identification of critical residues involved in binding and activation. Mol Pharmacol. 2000;58:217–225. doi: 10.1124/mol.58.1.217. [DOI] [PubMed] [Google Scholar]
- Marsh DJ, Weingarth DT, Novi DE, Chen HY, Trumbauer ME, Chen AS, Guan XM, Jiang MM, Feng Y, Camacho RE, Shen Z, Frazier EG, Yu H, Metzger JM, Kuca SJ, Shearman LP, Gopal-Truter S, MacNeil DJ, Strack AM, MacIntyre DE, Van der Ploeg LH, Qian S. Melanin-concentrating hormone 1 receptor-deficient mice are lean, hyperactive, and hyperphagic and have altered metabolism. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:3240–3245. doi: 10.1073/pnas.052706899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashiko S, Ishihara A, Gomori A, Moriya R, Ito M, Iwaasa H, Matsuda M, Feng Y, Shen Z, Marsh DJ, Bednarek MA, MacNeil DJ, Kanatani A. Antiobesity effect of a melanin-concentrating hormone 1 receptor antagonist in diet-induced obese mice. Endocrinology. 2005;146:3080–3086. doi: 10.1210/en.2004-1150. [DOI] [PubMed] [Google Scholar]
- Mendez-Andino JL, Wos JA. MCH-R1 antagonists: what is keeping most research programs away from the clinic? Drug discovery today. 2007;12:972–979. doi: 10.1016/j.drudis.2007.08.010. [DOI] [PubMed] [Google Scholar]
- Millan MJ, Gobert A, Panayi F, Rivet JM, Dekeyne A, Brocco M, Ortuno JC, Di Cara B. The melanin-concentrating hormone1 receptor antagonists, SNAP-7941 and GW3430, enhance social recognition and dialysate levels of acetylcholine in the frontal cortex of rats. Int J Neuropsychopharmacol. 2008:1–18. doi: 10.1017/S1461145708008894. [DOI] [PubMed] [Google Scholar]
- Mori M, Harada M, Terao Y, Sugo T, Watanabe T, Shimomura Y, Abe M, Shintani Y, Onda H, Nishimura O, Fujino M. Cloning of a novel G protein-coupled receptor, SLT, a subtype of the melanin-concentrating hormone receptor. Biochem Biophys Res Commun. 2001;283:1013–1018. doi: 10.1006/bbrc.2001.4893. [DOI] [PubMed] [Google Scholar]
- Mouri T, Takahashi K, Kawauchi H, Sone M, Totsune K, Murakami O, Itoi K, Ohneda M, Sasano H, Sasano N. Melanin-concentrating hormone in the human brain. Peptides. 1993;14:643–646. doi: 10.1016/0196-9781(93)90158-d. [DOI] [PubMed] [Google Scholar]
- Murray JF, Mercer JG, Adan RA, Datta JJ, Aldairy C, Moar KM, Baker BI, Stock MJ, Wilson CA. The effect of leptin on luteinizing hormone release is exerted in the zona incerta and mediated by melanin-concentrating hormone. J Neuroendocrinol. 2000;12:1133–1139. doi: 10.1046/j.1365-2826.2000.00577.x. [DOI] [PubMed] [Google Scholar]
- Nazif T, Bogyo M. Global analysis of proteasomal substrate specificity using positional-scanning libraries of covalent inhibitors. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:2967–2972. doi: 10.1073/pnas.061028898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostresh JM, Schoner CC, Hamashin VT, Nefzi A, Meyer J, Houghten RA. The solid phase synthesis of tri-substituted bicyclic guanidines via cyclization of reduced N-acylated dipeptides. J Org Chem. 1998;63:8622–8623. [Google Scholar]
- Pinilla C, Appel JR, Borras E, Houghten RA. Advances in the use of synthetic combinatorial chemistry: mixture-based libraries. Nat Med. 2003;9:118–122. doi: 10.1038/nm0103-118. [DOI] [PubMed] [Google Scholar]
- Pissios P, Bradley RL, Maratos-Flier E. Expanding the scales: The multiple roles of MCH in regulating energy balance and other biological functions. Endocr Rev. 2006;27:606–620. doi: 10.1210/er.2006-0021. [DOI] [PubMed] [Google Scholar]
- Qu D, Ludwig DS, Gammeltoft S, Piper M, Pelleymounter MA, Cullen MJ, Mathes WF, Przypek R, Kanarek R, Maratos-Flier E. A role for melanin-concentrating hormone in the central regulation of feeding behaviour. Nature. 1996;380:243–247. doi: 10.1038/380243a0. [DOI] [PubMed] [Google Scholar]
- Rossi M, Choi SJ, O’Shea D, Miyoshi T, Ghatei MA, Bloom SR. Melanin-concentrating hormone acutely stimulates feeding, but chronic administration has no effect on body weight. Endocrinology. 1997;138:351–355. doi: 10.1210/endo.138.1.4887. [DOI] [PubMed] [Google Scholar]
- Sailer AW, Sano H, Zeng Z, McDonald TP, Pan J, Pong SS, Feighner SD, Tan CP, Fukami T, Iwaasa H, Hreniuk DL, Morin NR, Sadowski SJ, Ito M, Bansal A, Ky B, Figueroa DJ, Jiang Q, Austin CP, MacNeil DJ, Ishihara A, Ihara M, Kanatani A, Van der Ploeg LH, Howard AD, Liu Q. Identification and characterization of a second melanin-concentrating hormone receptor, MCH-2R. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:7564–7569. doi: 10.1073/pnas.121170598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito Y, Cheng M, Leslie FM, Civelli O. Expression of the melanin-concentrating hormone (MCH) receptor mRNA in the rat brain. J Comp Neurol. 2001a;435:26–40. doi: 10.1002/cne.1191. [DOI] [PubMed] [Google Scholar]
- Saito Y, Nothacker HP, Civelli O. Melanin-concentrating hormone receptor: an orphan receptor fits the key. Trends Endocrinol Metab. 2000;11:299–303. doi: 10.1016/s1043-2760(00)00290-3. [DOI] [PubMed] [Google Scholar]
- Saito Y, Nothacker HP, Wang Z, Lin SH, Leslie F, Civelli O. Molecular characterization of the melanin-concentrating-hormone receptor. Nature. 1999;400:265–269. doi: 10.1038/22321. [DOI] [PubMed] [Google Scholar]
- Saito Y, Wang Z, Hagino-Yamagishi K, Civelli O, Kawashima S, Maruyama K. Endogenous melanin-concentrating hormone receptor SLC-1 in human melanoma SK-MEL-37 cells. Biochem Biophys Res Commun. 2001b;289:44–50. doi: 10.1006/bbrc.2001.5926. [DOI] [PubMed] [Google Scholar]
- Shearman LP, Camacho RE, Sloan Stribling D, Zhou D, Bednarek MA, Hreniuk DL, Feighner SD, Tan CP, Howard AD, Van der Ploeg LH, MacIntyre DE, Hickey GJ, Strack AM. Chronic MCH-1 receptor modulation alters appetite, body weight and adiposity in rats. Eur J Pharmacol. 2003;475:37–47. doi: 10.1016/s0014-2999(03)02146-0. [DOI] [PubMed] [Google Scholar]
- Shimada M, Tritos NA, Lowell BB, Flier JS, Maratos-Flier E. Mice lacking melanin-concentrating hormone are hypophagic and lean. Nature. 1998;396:670–674. doi: 10.1038/25341. [DOI] [PubMed] [Google Scholar]
- Shimazaki T, Yoshimizu T, Chaki S. Melanin-concentrating hormone MCH1 receptor antagonists: a potential new approach to the treatment of depression and anxiety disorders. CNS drugs. 2006;20:801–811. doi: 10.2165/00023210-200620100-00002. [DOI] [PubMed] [Google Scholar]
- Smith DG, Davis RJ, Rorick-Kehn L, Morin M, Witkin JM, McKinzie DL, Nomikos GG, Gehlert DR. Melanin-concentrating hormone-1 receptor modulates neuroendocrine, behavioral, and corticolimbic neurochemical stress responses in mice. Neuropsychopharmacology. 2006;31:1135–1145. doi: 10.1038/sj.npp.1300913. [DOI] [PubMed] [Google Scholar]
- Stricker-Krongrad A, Dimitrov T, Beck B. Central and peripheral dysregulation of melanin-concentrating hormone in obese Zucker rats. Brain Res Mol Brain Res. 2001;92:43–48. doi: 10.1016/s0169-328x(01)00130-9. [DOI] [PubMed] [Google Scholar]
- Tadayyon M, Welters HJ, Haynes AC, Cluderay JE, Hervieu G. Expression of melanin-concentrating hormone receptors in insulin-producing cells: MCH stimulates insulin release in RINm5F and CRI-G1 cell-lines. Biochem Biophys Res Commun. 2000;275:709–712. doi: 10.1006/bbrc.2000.3357. [DOI] [PubMed] [Google Scholar]
- Tai KK, Blondelle SE, Ostresh JM, Houghten RA, Montal M. An N-methyl-D-aspartate receptor channel blocker with neuroprotective activity. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:3519–3524. doi: 10.1073/pnas.061449498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan CP, Sano H, Iwaasa H, Pan J, Sailer AW, Hreniuk DL, Feighner SD, Palyha OC, Pong SS, Figueroa DJ, Austin CP, Jiang MM, Yu H, Ito J, Ito M, Ito M, Guan XM, MacNeil DJ, Kanatani A, Van der Ploeg LH, Howard AD. Melanin-concentrating hormone receptor subtypes 1 and 2: species-specific gene expression. Genomics. 2002;79:785–792. doi: 10.1006/geno.2002.6771. [DOI] [PubMed] [Google Scholar]
- Tsukamura H, Thompson RC, Tsukahara S, Ohkura S, Maekawa F, Moriyama R, Niwa Y, Foster DL, Maeda K. Intracerebroventricular administration of melanin-concentrating hormone suppresses pulsatile luteinizing hormone release in the female rat. J Neuroendocrinol. 2000;12:529–534. doi: 10.1046/j.1365-2826.2000.00482.x. [DOI] [PubMed] [Google Scholar]
- Vaughan JM, Fischer WH, Hoeger C, Rivier J, Vale W. Characterization of melanin-concentrating hormone from rat hypothalamus. Endocrinology. 1989;125:1660–1665. doi: 10.1210/endo-125-3-1660. [DOI] [PubMed] [Google Scholar]
- Verlaet M, Adamantidis A, Coumans B, Chanas G, Zorzi W, Heinen E, Grisar T, Lakaye B. Human immune cells express ppMCH mRNA and functional MCHR1 receptor. FEBS Lett. 2002;527:205–210. doi: 10.1016/s0014-5793(02)03232-5. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Gran B, Pinilla C, Markovic-Plese S, Hemmer B, Tzou A, Whitney LW, Biddison WE, Martin R, Simon R. Combinatorial peptide libraries and biometric score matrices permit the quantitative analysis of specific and degenerate interactions between clonotypic TCR and MHC peptide ligands. J Immunol. 2001;167:2130–2141. doi: 10.4049/jimmunol.167.4.2130. [DOI] [PubMed] [Google Scholar]