Abstract
Pro- and mature neurotrophins often elicit opposing biological effects. For example, mature brain-derived neurotrophic factor (mBDNF) is critical for long-term potentiation induced by high-frequency stimulation, whereas proBDNF facilitate long-term depression induced by low-frequency stimulation. Because mBDNF is derived from proBDNF by endoproteolytic cleavage, mechanisms regulating the cleavage of proBDNF may control the direction of BDNF regulation. Using methods that selectively detect proBDNF or mBDNF, we show that low-frequency stimulation induced predominant proBDNF secretion in cultured hippocampal neurons. In contrast, high-frequency stimulation preferentially increased extracellular mBDNF. Inhibition of extracellular, but not intracellular cleavage of proBDNF greatly reduced high-frequency stimulation-induced extracellular mBDNF. Moreover, high-frequency, but not low-frequency stimulation selectively induced the secretion of tissue plasminogen activator, a key protease involved in extracellular proBDNF to mBDNF conversion. Thus, high-frequency neuronal activity controls the ratio of extracellular proBDNF/mBDNF by regulating the secretion of extracellular proteases. Our study demonstrates activity-dependent control of extracellular proteolytic cleavage of a secretory protein, and reveals an important mechanism that controls diametrically opposed functions of BDNF isoforms.
Keywords: BDNF, hippocampus, LTP, proteases, tPA
Brain-derived neurotrophic factor (BDNF) plays a critical role in activity-dependent processes, such as synapse development and plasticity (1–3). Synthesized as a precursor, proBDNF is then sorted into one of the two secretory pathways: constitutive (passive) or regulated (induced or active) (4–6). Until recently, only mature BDNF (mBDNF) was considered biologically active. This view was challenged by findings that exogenous proBDNF induces apoptosis in peripheral neurons (7) and facilitates long-term depression (LTD) in the hippocampus (8). ProBDNF, is believed to be cleaved intracellularly by furin in trans-Golgi or by pro-protein convertase 1/3 (PC1/3) in secretory granules (9, 10) to generate mBDNF. Recently, Lee et al. demonstrated that proBDNF could be converted to mBDNF by extracellular proteases, such as plasmin and matrixmetalloprotease-7 (11). The physiological significance of extracellular proBDNF cleavage became evident when proBDNF → mBDNF conversion through the tissue plasminogen activator (tPA)/plasminogen cascade was shown to be essential for late phase long-term potentiation (L-LTP) in the hippocampus (12). Given the opposing biological effects of proBDNF and mBDNF, mechanisms controlling the cleavage of proBDNF have emerged as important mechanisms in controlling the direction of BDNF regulation (13).
Neuronal release of endogenous proBDNF remains controversial. While studies using neuronal cultures in absence of glial cells and in the presence of cell impermeant α2 antiplasmin inhibitors successfully detected proBDNF in the extracellular culture medium (7), a recent study by Matsumoto et al. (14) failed to detect proBDNF secretion in neurons derived from BDNF-myc knock-in mice. We successfully detected both exogenous and endogenous proBDNF and mBDNF from the culture medium in our experimental system. Because BDNF is secreted by 2 different pathways, it is unclear which of the 2 secretory pathways is responsible for proBDNF and mBDNF secretion, and how the extracellular levels of these 2 isoforms are regulated. Most importantly, how neuronal activity controls the extracellular levels of proBDNF and mBDNF is a question of significant importance in understanding activity-dependent synaptic potentiation and depression. These questions were difficult to address because of the lack of sensitive biochemical tools to distinguish proBDNF from mature BDNF. To circumvent this problem, we have developed an antibody that specifically recognizes mBDNF, without any cross-reactivity to uncleaved proBDNF. Moreover, we have used field electrical stimulation to measure regulated secretion of BDNF and tPA under more physiologically relevant conditions. Our results demonstrate that both low- and high- frequency neuronal activities increased proBDNF in the extracellular milieu, and only high-frequency neuronal activity induced tPA secretion, resulting in extracellular conversion of proBDNF → mBDNF. These results demonstrate how high-frequency neuronal activity controls diametrically opposite functions of BDNF isoforms.
Results
Constitutive and Regulated Secretion of proBDNF and mBDNF.
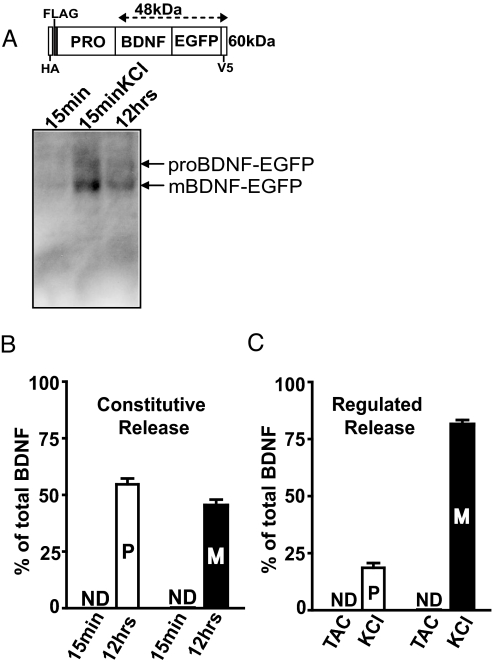
To effectively detect proBDNF and mBDNF secretion, we expressed epitope tagged human BDNF cDNA (HA and FLAG tags following the signal peptide at the amino terminal, EGFP and V5 at the carboxyl terminal) in rat/mouse hippocampal neurons (Fig. 1A). Two issues are of potential concern. First, epitope tags may mask the cleavage site, preventing proBDNF cleavage. Western blot analysis detected substantial amounts of intracellular mBDNF following exogenous BDNF expression [Fig. S1A, mBDNF-EGFP, lane 4], suggesting that the epitope tags did not block intracellular processing of exogenously expressed proBDNF. However, it should be noted that the proBDNF-EGFP/mBDNF-EGFP ratio was higher than that of endogenous proBDNF/mBDNF (see Fig. S1A). While more proBDNF-EGFP secretion is expected upon stimulation, it was not the case (compare high KCl in Fig. 1C and Fig. 5A, see below). Second, over-expression may affect neurotrophin sorting into constitutive or regulated secretory pathway. A lentiviral vector with a minimal CMV promoter was used to express epitope-tagged BDNF at moderate levels (30 ± 8% of the endogenous BDNF) (see Fig. S1A) to alleviate this concern. All BDNF secretion experiments were performed in mature neurons (≥14 days in culture). To determine the levels of BDNF secreted under constitutive conditions, spontaneous action potentials and synaptic transmission were completely blocked by the Na+-channel blocker tetrodotoxin, the NMDA receptor antagonist APV, and the AMPA receptor antagonist CNQX (this medium is called TAC). To measure regulated secretion, the cultures were depolarized with high concentration of KCl (50 mM). Secretion of both proBDNF and mBDNF was measured by immunoprecipitation of culture media using monoclonal anti-GFP antibody to pull down total BDNF through the C-terminal GFP tag, followed by Western blotting using either anti-GFP polyclonal antibody to detect both proBDNF and mBDNF (see Fig. 1A, Bottom), or anti-HA antibody (data not shown) to specifically detect proBDNF. For both constitutive and regulated release, we set the total amount of BDNF (the sum of signals from proBDNF and mBDNF bands) detected by the GFP antibody as 100%, and then calculated the percentages of proBDNF and mBDNF. Constitutive secretion of total BDNF was extremely low, detectable from the culture medium only after 10 to 12 h, but not within 15 min of accumulation in TAC (Fig. 1B and Fig. S1C). Quantitative analysis revealed that roughly equal amounts of proBDNF and mBDNF was secreted (54.6 ± 2.6% vs. 45.4 ± 2.6%). Depolarization with 15-min KCl in the culture media, resulted in ≈6- to 8-fold increase in BDNF compared to 12 h of constitutive secretion. Interestingly, more mBDNF (81.3 ± 2%) than proBDNF (18.7 ± 2.0%) was detected in the medium (see Fig. 1C). Thus, unlike in the resting conditions, membrane depolarization preferentially increased extracellular levels of mBDNF.
Fig. 1.
Constitutive vs. regulated secretion of proBDNF and mBDNF. (A) Western blot detection of proBDNF and mBDNF secreted from cultured hippocampal neurons. Hippocampal neurons transfected with epitope-tagged BDNF construct (Top) were treated with either TAC for 15 min or 12 h, or KCl (50 mM) for 15 min. Culture media was immunoprecipitated using monoclonal anti-GFP antibody followed by Western blotting using polyclonal anti-GFP antibody. (B) Steady-state levels of proBDNF and mBDNF secreted under constitutive conditions in 15 min or 12 h. ProBDNF- and mBDNF-specific signals were normalized to total BDNF (sum of the two) in 12 h TAC, and presented as % of total BDNF. (C) Quantification of proBDNF and mBDNF secreted from neurons treated with TAC or KCl for 15 min. proBDNF and mBDNF specific signals were presented as % of total BDNF. n = 4 independent experiments. Data are presented as mean ± SEM. P, proBDNF; M, mBDNF; ND, not detectable.
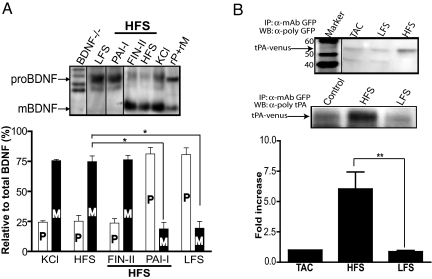
Fig. 5.
(A) Intracellular versus extracellular cleavage of endogenous proBDNF detected by Western blot. Neurons were stimulated with KCl, HFS, or LFS in the absence or presence of FIN-II (13 μM) or PAI-I (1 μg/ml) as indicated. Endogenous BDNF secretion was measured by immunoprecipitation using chicken polyclonal “pro/m” antibody, which recognizes both proBDNF and mBDNF with same affinity and chicken IgY precipitating agarose, followed by Western blot using rabbit polyclonal “pro/m” antibody. The bands were quantified and represented as % of total BDNF (n = 4 independent experiments). *, P < 0.001, 2-way ANOVA followed by Bonferroni posthoc analysis; RP, recombinant proBDNF; rM, recombinant mBDNF. (B) Frequency-dependent secretion of tPA. Cultured hippocampal neurons transfected with tPA-Venus was stimulated for tPA secretion exactly as described in Fig. 4. Representative Western blots showing tPA-Venus detected by a polyclonal anti-GFP antibody and a anti-tPA antibody. The relative values for tPA-Venus release in HFS and LFS normalized to tPA signals in TAC condition are presented as fold increase. The data for the same condition in multiple experiments (n = 3) were averaged and presented as mean± SEM. *, P < 0.001, 2-way ANOVA followed by Bonferroni posthoc analysis.
Specific Detection of mBDNF.
To specifically detect mBDNF, we devised a strategy based on the premise that cleavage of proBDNF would generate a previously unexposed epitope, which could be used to generate a specific antibody as illustrated in Fig. S2A (B-peptide). In a Western blot with purified recombinant proteins, the B-peptide antibody detected only mBDNF but not proBDNF or the pro-fragment (GST-pro) (Fig. S2B), which was visualized with the anti-proBDNF antibody (Fig. S2C). Moreover, the B-antibody specifically detected mBDNF, but not proBDNF, in wild-type hippocampal lysates (Fig. S2D). mBDNF band was validated with an alternate antibody raised against a completely different epitope in the mature domain of BDNF (N-20) (Fig. S1D). A semiquantitative analysis of mBDNF band detected by the B-antibody in wild-type hippocampal lysates in comparison with known amounts of recombinant mBDNF revealed the presence of ≈0.6 to 0.8 pg of mBDNF per μg of total soluble hippocampal protein (or 600–800 ng/g tissue wet weight) (see Fig. S1D), an estimate comparable to previous reports. Neither mBDNF nor proBDNF was detectable in lysates prepared from BDNF–/– animals (see Fig. S2 D and E). The presence of both proBDNF and mature BDNF in the wild-type hippocampal lysates were also confirmed using a rabbit polyclonal antibody (Santa Cruz N-20) (Fig. S6A, lane 2). Finally, immunocytochemistry using the B-antibody detected specific immunoreactive signals under cell permeabilizing conditions in hippocampal neurons derived from wild type, but not in BDNF–/– animals (Fig. S3A). Henceforth, we will refer to B-antibody as mBDNF-specific antibody.
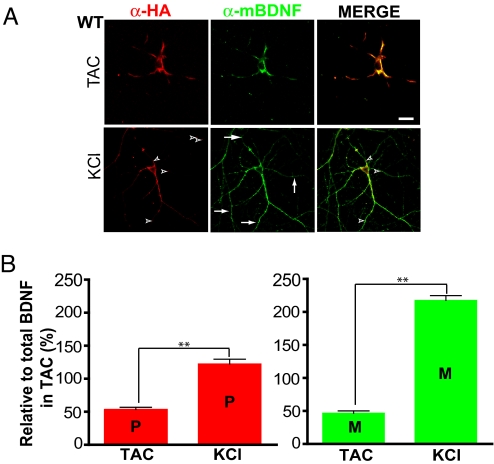
mBDNF and proBDNF are positively charged under physiological conditions (pI = 9.59 and 8.98, respectively) in the extracellular space, and therefore likely to electrostatically interact with the negatively charged cell membranes after their secretion, in a manner similar to that of nerve growth factor (NGF) (15). We took advantage of this property to measure the secretion of proBDNF and mBDNF by surface immunostaining. Neurons transfected with HA-tagged BDNF were treated with either TAC or KCl, fixed briefly under nonpermeabilizing conditions, and double-stained with the mouse anti-HA antibody and the rabbit mBDNF-specific antibody. Similar techniques have been used previously to measure the secretion of NGF in hippocampal neurons (15). This method detected only cell surface proteins, because staining for intracellular proteins [MAP2 (Fig. S3B) or Tau (not shown)] was negative. In TAC, similar levels of proBDNF (red) and mBDNF (green) were observed on the cell surface (Fig. 2A). Depolarization with high KCl increased the surface levels of mBDNF, particularly in distal dendrites (see Fig. 2A). While few punctae stained only for proBDNF (see Fig. 2A, arrowheads, red), many branches in the distal dendrites stained exclusively for mBDNF (see Fig. 2A, arrows, green-only processes). When the fluorescence intensities of proBDNF and mBDNF along the dendrites were quantified and normalized to that of total BDNF in TAC, KCl treatment showed a significant increase in proBDNF, as well as mBDNF on the dendritic surface (Fig. 2B). Therefore, mBDNF-specific antibody can be used to study the cellular distribution of mBDNF, which is difficult with any other method.
Fig. 2.
Detection of constitutive and regulated secretion of proBDNF and mBDNF by cell surface staining. BDNF transfected neurons (>14-days old) treated with either TAC for 12 h or KCl (50 mM) for 15 min, fixed under nonpermeabilizing conditions, and processed for surface immunolabeling using the monoclonal anti-HA antibody for proBDNF (red) or the mBDNF-specific antibody (green). (A) Representative micrographs of cell surface staining. Superimposed images are shown on the right. Arrows indicate mBDNF-only signals and arrowhead indicate proBDNF-only signals. In this and all micrographs, scale bar is 10 μm. (B) Quantification of surface-bound proBDNF and mBDNF in dendrites by immunostaining. The fluorescence intensity for total BDNF (proBDNF + mBDNF) in the entire dendritic areas of a neuron in TAC was set as 100%, and values for proBDNF and mBDNF in various conditions were normalized to total BDNF. A total of 26 dendrites from 7 neurons (n = 7) in TAC and 33 dendrites from 8 neurons (n = 8) in high KCl conditions from n = 3 independent experiments. In this and all other figures the data were analyzed using InStat3. **, P < 0.001, paired two-tailed Student t test.
Different Forms of Extracellular BDNF Induced by High- and Low-Frequency Stimulation.
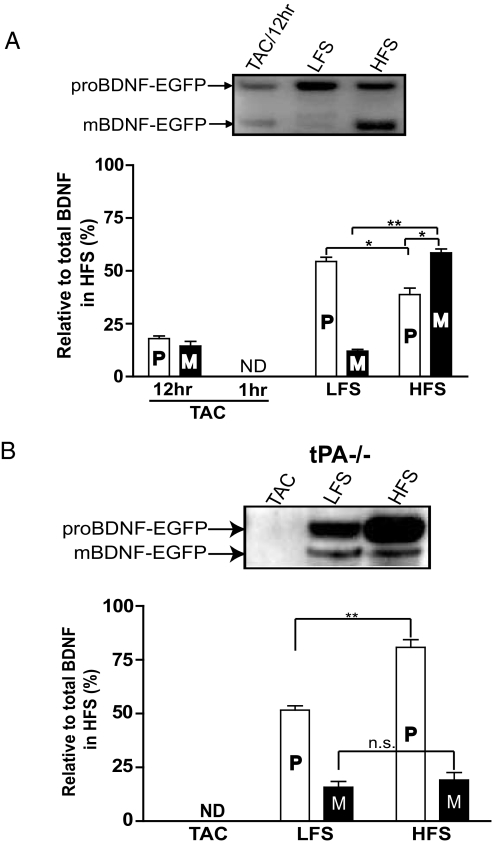
To measure the ratio of proBDNF and mBDNF under more physiologically relevant conditions, we applied field electrical stimulation to cultured hippocampal neurons transfected with the BDNF construct (see Fig. 1A), using a protocol previously shown to reliably induce action potentials throughout the culture dishes (16). For high-frequency stimulation (HFS), we used theta burst stimulation (14,400 pulses, 60 min), and for low-frequency stimulation (LFS), we delivered a 5-Hz train with the same number of pulses (14,400 pulses, 48 min). Longer HFS and LFS stimulation protocols were used compared with those used in slice electrophysiology for the following reasons. First, HFS and LFS mimic continuous theta-burst firing during learning and the low-frequency stochastic firings recorded in the hippocampus in vivo, respectively. Thus, these protocols may be more relevant to neuronal firings in vivo. Second, and more importantly, a longer stimulation time is needed to accumulate enough secreted BDNF for immunoprecipitation and Western analysis. Last, but not the least, the efficiency of stimulating neurons in dissociated cultures is not as high as that of stimulating the well-organized CA3-CA1 Schaffer collateral circuits. Following stimulation, the culture media was immunoprecipitated using a monoclonal anti-GFP antibody followed by Western blotting with a polyclonal anti-GFP antibody (Fig. 3A, Upper). Because the antibodies recognize the C-terminal GFP tag, they detect both proBDNF and mBDNF with same affinity. Constitutive secretion of BDNF in TAC was undetectable in 1 h, but discernible after a 12-h period. While both LFS and HFS induced a marked increase in BDNF secretion, HFS was more effective (see Fig. 3A). Neither the electrical stimulation nor high K+ resulted in cell death as measured by lactate dehydrogenase (LDH) activity (Fig. S4). Two major differences were observed when neurons in culture were stimulated with HFS and LFS. First, LFS conditioned medium contained only ≈66% of total BDNF compared to HFS. Second, LFS induced predominant secretion of proBDNF (pro-, 54.5 ± 1.9%; mBDNF, 12.08 ± 0.78%), compared to mBDNF in HFS (pro-, 38.77 ± 3.1%; mBDNF, 58.5 ± 1.8%) (see Fig. 3A, Lower). Similar results were obtained when neurons were stimulated with LFS or HFS for the same amount of time (60 min, data not shown). Therefore, our results suggest that the ratio of mBDNF to proBDNF (M/P) in the extracellular space may differ depending on the frequency of neuronal activity.
Fig. 3.
Western blot analyses of proBDNF and mBDNF secretion induced by electrical stimulation at different frequencies. Rat hippocampal neurons (A) or tPA–/– neurons (B) transfected with BDNF construct shown in Fig. 1A were stimulated with the same number of electrical pulses (14,400) of HFS or LFS. Secretion of proBDNF and mBDNF was detected from culture medium (1 ml per well × 3 wells in a 12-well plate, with a plating density of 0.12 × 106 cells/well) by the method described in Fig. 1, using antibodies against the GFP tag and quantified by normalizing all data to average total BDNF signal under HFS condition (n = 3 independent experiments). ND, not detectable. Data are presented as mean ± SEM and analyzed by 2-way ANOVA followed by Bonferroni posthoc analysis. *, P < 0.01; **, P < 0.001.
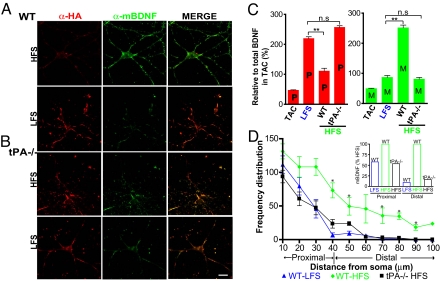
To examine the release of BDNF isoforms from different neuronal compartments (soma vs. dendrites), we performed surface immunolabeling using the anti-HA antibody for proBDNF (red) and the mBDNF-specific antibody (green) following different frequencies of electrical stimulation. Negligible staining was detected in control (TAC) condition (see Fig. 2A Upper) for both proBDNF and mBDNF. Consistent with biochemical analysis, LFS primarily increased proBDNF on neuronal surface, whereas HFS increased mBDNF staining on neuronal surface (Fig. 4A). The ratio of proBDNF in LFS vs. HFS was ≈2, whereas the ratio of mBDNF was ≈0.35 (Fig. 4C). These results were further confirmed by switching the primary-secondary antibody pairs (data not shown). Interestingly, mBDNF staining was intense and more frequent (≈10-fold) in the distal dendritic regions (>40 μm from soma) (see Fig. 4D and inset) following HFS, compared to only a 2-fold increase in the proximal dendritic region (<40 μm). The biochemical (see Fig. 3A) and immunostaining results together suggest that HFS is more effective than LFS in converting proBDNF to mBDNF.
Fig. 4.
HFS- and LFS-induced secretion of proBDNF and mBDNF in wild-type and tPA–/– neurons revealed by cell surface staining. Images of surface stained wild-type (A) and tPA–/– neurons (B) following 15 min stimulation. (C) Quantification of the surface bound proBDNF and mBDNF on the dendrites. Wild type, TAC: 30 dendrites from 10 neurons; Wild type, HFS: 55 dendrites from 12 neurons; Wild type, LFS, 37 dendrites from 9 neurons; tPA–/–, HFS, 31 dendrites from 5 neurons. n.s., non-significant; **, P < 0.001, 1-way ANOVA. (D) Distribution of the number of mBDNF fluorescent puncta in distal dendrites. Number of fluorescent spots corresponding to mBDNF on distal dendrites, binned at intervals of 10 μm, were plotted against the distance from the cell body (10 to 40 μm from soma as proximal and >40 μm as distal). The same neurons in (C) were used for this analysis. Inset: Distribution of mBDNF puncta in WT-LFS and tPA(–/–)-HFS normalized to WT-HFS in proximal and distal regions. *, P < 0.05, Mann-Whitney test. Results are derived from 3 independent preparations.
Activity-Dependent Control of Extracellular proBDNF → mBDNF Conversion.
An increase in mBDNF staining on the dendritic surface in response to HFS could be because of either increased dendritic secretion of mBDNF or an increase in extracellular cleavage of proBDNF by the tPA/plasmin system (12). To distinguish these possibilities, we repeated the surface staining in neurons derived from tPA knockout mice (tPA–/–). Similar to the wild-type neurons, LFS predominantly induced the secretion of proBDNF in both cell body and dendritic areas (Fig. 4B Lower). However, in tPA–/– neurons, HFS no longer induced an increase in dendritic surface mBDNF (see Fig. 4B and Fig. S5). Staining for proBDNF and mBDNF along the dendritic surface was very similar to that seen in wild-type neurons stimulated with LFS (see Fig. 4C). Further analysis indicated that mBDNF was uniformly distributed along the dendrites at barely detectable levels (see Fig. 4D), and almost identical to that of wild-type LFS (see Fig. 4D inset). These results support a model in which secretion of proBDNF is induced by either HFS or LFS, but only HFS is capable of converting proBDNF to mBDNF, possibly because of dendritic secretion of tPA. The surface immunostaining results were further confirmed by immunoprecipitation experiments using cultured tPA–/– neurons transfected with epitope-tagged BDNF (as in Fig. 1A). In TAC, neither proBDNF nor mBDNF was detectable. Again, LFS predominantly induced the secretion of proBDNF in tPA–/– neurons (Fig. 3B). Unlike the substantial increase in mBDNF induced by HFS seen in wild-type neurons (see Fig. 3A), proBDNF was the major form detected after HFS in tPA–/– neurons, although trace amounts of mBDNF were detectable (see Fig. 3B). Failure to detect large amount of mBDNF in the medium of tPA–/– cultures after HFS suggests that extracellular conversion of proBDNF to mBDNF by tPA is a major contributor of extracellular mBDNF in response to high-frequency neuronal activity.
To demonstrate that the frequency-dependent extracellular conversion of proBDNF to mBDNF is not limited to exogenously expressed epitope-tagged BDNF but applicable to the endogenously synthesized BDNF as well, we measured endogenous BDNF secreted from wild-type neurons in response to different stimulation paradigms in the absence or presence of protease inhibitors that selectively inhibit either intracellular or extracellular proteolytic processing of proBDNF. To inhibit furin and prohormone convertase 1/3, the two major intracellular prohormone convertases expressed in hippocampal neurons (17), we incubated the wild-type neuronal cultures with furin inhibitor II (FIN-II, 13 μM) (4, 9). To inhibit extracellular proteolytic processing of secreted proBDNF, neurons were incubated with the membrane impermeable inhibitor for tPA (18), plasminogen activator inhibitor-I (PAI-I). Neurons were stimulated by high KCl, LFS, or HFS, and the secreted proBDNF and mBDNF were measured by immunoprecipitation and Western blotting. While both KCl and HFS predominantly contained mBDNF in the extracellular milieu (75.6 ± 1.1% and 74.75 ± 4.75%, respectively), LFS primarily induced proBDNF secretion (80.626 ± 5.67%) (see Fig. 5A). Inhibition of intracellular proteases by FIN-II did not affect extracellular proBDNF to mBDNF conversion (mBDNF − 76.4 ± 3.67%), whereas PAI-I significantly reduced its conversion (pro, 81.21 ± 5.53%) (see Fig. 5A). These results, together with the finding that tPA–/– neurons predominantly secreted proBDNF into the extracellular medium under HFS (see Fig. 3B), strongly suggest that proBDNF is the major form secreted during HFS in the absence of extracellular tPA or when its activity was blocked. There was still ≈15 to 20% mBDNF present in the medium of wild-type neurons containing PAI-I (see Fig. 5A, column 8) as well as in tPA–/– neurons (see Fig. 3B), suggesting a minor contribution of mBDNF from intracellular conversion. Consistently, analysis of whole-cell lysates indicated that a significant proportion of proBDNF may be cleaved inside neurons (Fig. S6B, lane 1), and the absolute levels of intracellular mBDNF were significantly reduced when the cells were treated with FIN-II but not with PAI-I (see Fig. S6B, lane 3 and lane 2, respectively). Thus, while proBDNF constitutes a small proportion (≈20%) of total BDNF intracellularly, it is the predominant isoform secreted in response to neuronal activity, which is subsequently converted to mBDNF by extracellular cleavage. Together, these results support the notion that majority of extracellular mBDNF during HFS is derived from the conversion of proBDNF to mBDNF by the extracellular tPA/plasmin system, rather than from direct secretion of mBDNF from the intracellular pool.
Secretion of tPA Induced by HFS but not LFS.
The most likely explanation for the frequency-dependent cleavage of proBDNF by tPA is the selective secretion of tPA by HFS. To test this possibility, rat hippocampal neurons transfected with Venus-tagged rat tPA cDNA (19) was subjected to field electrical stimulation with different frequencies. tPA in the media was measured by immunoprecipitation using a monoclonal anti-GFP antibody followed by blotting with a polyclonal anti-GFP antibody. Indeed, HFS, but not LFS, induced the secretion of tPA (Fig. 5B Upper). Quantification of the results showed that HFS increased tPA secretion by 6-fold (see Fig. 5B Lower). Identity of the immunoprecipitated band was confirmed using an anti-tPA antibody (see Fig. 5B Middle). Consistent with studies by Lochner et al. (20), double immunostaining under cell permeable conditions using antibodies against rat tPA-Venus (anti-GFP), dendritic and synaptic marker proteins (MAP2 and Synapsin I, respectively) revealed that after HFS, intracellular tPA localized to dendritic spines (Fig. S7), suggesting that tPA-containing vesicles are mobilized into spines of active synapses in response to HFS. Taken together, these results support the hypothesis that the secretion of tPA in response to high-frequency neuronal firing is responsible for the extracellular conversion of proBDNF to mBDNF.
Discussion
BDNF, a neurotrophin that plays a key role in synapse development and plasticity, is secreted in response to neuronal activity (4). Using newly developed tools, we showed that when neurons are completely silent, very small amounts of proBDNF and mBDNF were constitutively secreted. When neurons were depolarized with high KCl to induce regulated secretion, there was a dramatic increase in the amount of extracellular BDNF, suggesting that BDNF is primarily secreted through the regulated pathway. Given that many of our findings were based on exogenous epitope-tagged BDNF expression, caution must be exercised during interpretation. However, the following results suggest that it did not significantly affect our conclusion that most of the proBDNF → BDNF conversion occurs outside, and is dependent on the extracellular protease tPA/plasmin cascade. First, high KCl induced similar percent of exogenous (see Fig. 1C) and endogenous (see Fig. 5A, far Left) proBDNF. Second, inhibition of intracellular proBDNF → mBDNF conversion by FIN-II did not significantly affect proBDNF/mBDNF ratio following HFS stimulation (see Fig. 5A). Together, these results suggest that irrespective of the intracellular levels of proBDNF and mBDNF, the majority of BDNF secreted by the regulatory secretory pathway is proBDNF and is extracellularly converted to mBDNF.
Given that mBDNF enhances neuronal survival (21) and promotes LTP (12, 22, 23), while proBDNF induces apoptosis (7) and facilitates LTD (8), it is essential to determine the relative extracellular levels of the 2 isoforms under various conditions. The most striking finding of the present study is that LFS predominantly induced proBDNF secretion, whereas substantial amount of mBDNF was detected in the extracellular milieu in HFS. Thus, the ratio of mBDNF and proBDNF, and hence the direction of BDNF regulation, appears to be controlled by the frequency of neuronal activity. HFS-induced increase in extracellular mBDNF occurred in dendrites of wild-type neurons, but not in tPA–/– neurons, suggesting that tPA is secreted only in response to high frequency, but not low-frequency neuronal activity. These results support a model in which high frequency neuronal activity triggers simultaneous secretion of proBDNF and tPA to generate mBDNF in situ, whereas low frequency stimulation induces the secretion of only proBDNF (Fig. S8).
A recent study by Matsumoto et al. reported that no proBDNF was secreted from the cultured hippocampal neurons even when neuronal activity was elevated (14). This result is at odd with the present finding that BDNF is secreted primarily as proBDNF. The following reasons may have contributed to this discrepancy: (i) We used neuronal cultures free of glial cells to measure BDNF secretion, whereas the Matsumoto study did not. Glial cells are a major source of proteases such as uPA and tPA, and therefore could have readily converted secreted proBDNF to mature BDNF in response to stimuli. (ii) Including BSA as a competitor in the culture medium allowed us to recover all of the BDNF released by immunoprecipitation by preventing its binding to membrane or receptors. (iii) Inclusion of protease inhibitors in our study during the experimental procedure may have effectively prevented the conversion of secreted proBDNF to mBDNF. Consistent with these reasons, Yang et al. (24), using a BDNF-HA knock-in mice (HA-tag vs. myc-tag at the C terminus of BDNF by Matsumoto et al, ref. 14), showed that hippocampal neurons primarily secreted proBDNF in response to high KCl when α2 anti-plasmin inhibitor was included in the culture medium. Therefore, failure to detect proBDNF secretion in the Matsumoto study may not necessarily reflect its release from the neurons.
An important finding is that LFS predominantly triggered secretion of proBDNF, but HFS resulted in substantial amounts of extracellular mBDNF. Using conventional ELISA, previous studies showed that while tetanic stimulation or TBS evoked the secretion of BDNF, no extracellular BDNF could be detected after LFS (5, 25–27). In contrast, we show that LFS reliably triggered the secretion of proBDNF, as measured by both Western blot and by cell surface staining. Several differences between the present and previous studies may explain this discrepancy. Most obviously, the previous studies used an ELISA that could not distinguish between proBDNF and mBDNF. Although this antibody should be able to detect both proBDNF and mBDNF, the experimental conditions used in the earlier study may not have allowed effective measurement of proBDNF. The experimental conditions, such as brain regions [perirhinal cortex (27) vs. hippocampus present study], maturity of the neurons [3-d (5) vs. >14-d present study], magnitude of over-expression [10- to 20-fold using adenovirus (26) vs. 30% increase present study], and stimulation time [5 min (25, 26) vs. 48 min present study] may also have contributed to this variation.
The present study may provide important insights into how different and sometime opposite forms of synaptic plasticity can be regulated by BDNF. Our previous studies demonstrate that proBDNF to mBDNF conversion by the tPA/plasmin system is important for hippocampal L-LTP (12). More recently, we have shown that proBDNF, through activation of p75NTR, facilitate LTD at the same hippocampal synapses (8). Is proBDNF secreted in response to an LTD stimulus? If so, why not tPA/plasmin or other extracellular enzymes convert proBDNF to mBDNF? We now show that proBDNF is the predominant form secreted in the extracellular milieu in response to LTD-inducing LFS, and that HFS-induced secretion of tPA is critical for the elevation of extracellular mBDNF. Taken together, we propose that proBDNF, secreted in response to LFS during LTD, activates p75NTR, leading to NMDA-dependent LTD. In addition to proBDNF secretion, HFS also induces the secretion of tPA, which converts the extracellular proBDNF to mBDNF by activating the plasminogen at the synapses in situ. Through activation of TrkB, mBDNF facilitates L-LTP. Thus, activity-dependent secretion of proBDNF could be involved in either LTD or LTP, depending on whether there is extracellular cleavage. In addition to its temporal specificity, the extracellular conversion of proBDNF to mBDNF at active synapses offers an explanation for synapse specificity of L-LTP. In addition to our scientific findings, we have developed new tools to selectively detect mBDNF. Because recent studies have shown alterations in BDNF levels in the brains of Alzheimer's patients (28, 29), in cerebrospinal fluid after brain injuries (30), and in various neurodegenerative disorders (31–34), detecting the proBDNF/mBDNF ratio may serve as a biomarker in neuropsychiatric and neuropathological conditions.
Materials and Methods
Neuronal Cultures, Nucleofection, and Electrical Stimulation.
Neuronal cultures were prepared and transfected with cDNA constructs by nucleofection as described in SI Methods. Bipolar field stimulation was applied through a custom-made lid with platinum wires immersed in the medium (1.3 ml). Each stimulation pulse (1 msec, 2–8 V) was sufficient to elicit action potentials in these cultured neurons (16). The whole procedure was done at 37 °C in a 5% CO2 incubator. Following are the 2 stimulation paradigms that we have used in this study: (i) HFS: theta-burst stimulus consisting of four bursts, each with 5 biphasic pulses at 100 Hz (10-msec interval), separated by an interburst interval of 200 msec. One episode was given every 5 sec, and stimulation continued for 60 min. (ii) LFS: 5 Hz applied continuously for 48 min to match the number of pulses used in HFS.
Surface Immunofluorescence Staining.
All staining procedures were performed on ice. Neurons grown on cover slips were washed with PBS (PBS), fixed with 4% paraformaldehyde in PBS containing 120 mM sucrose for 3 to 5 min, and then incubated with 100-mM glycine for 5 min. Blocking was done using 3% BSA (Pierce) in PBS for 60 min followed by primary antibody for 2 h, 3 × 5 min PBS washes and secondary antibody (Alexa Fluor antibodies from Molecular Probes) for 1 h. Following PBS washes, the cover slips were mounted on medium containing Mowiol 4–88 and the anti-fade DABCO, and left overnight in dark for curing. Images were acquired using a 63X oil plan Apochromat lens with the numeric aperture of 1.4 and multitrack option in the Zeiss confocal LSM 510 Meta for all samples under identical conditions (laser power, pinhole, gain and offset for 2 different colors). Neurons that stained positive for both mBDNF and proBDNF (HA-positive) were acquired. Fluorescence intensity values (in pixel, 0 minimum–255 maximum) for all of the stained spots along the entire length of the dendrites were obtained using the region of interest tool in IP lab software and subtracted for background before normalizing to total BDNF.
Supplementary Material
Acknowledgments.
We thank Drs. Newton Woo and Keri Martinowich for their thoughtful comments and suggestions. The rat tPA-Venus in the pEGFP-N1 vector was kindly provided by Dr. Wolfhard Almers. Microscopy imaging was performed at the Microscopy & Imaging Core (National Institute of Child Health and Development, National Institutes of Health) with the assistance of Dr. Vincent Schram. This work is supported by the Intramural Research Programs of National Institute of Child Health and National Institue of Mental Health.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0807322106/DCSupplemental.
References
- 1.McAllister AM, Katz LC, Lo DC. Neurotrophins and synaptic plasticity. Annu Rev Neurosci. 1999;22:295–318. doi: 10.1146/annurev.neuro.22.1.295. [DOI] [PubMed] [Google Scholar]
- 2.Huang EJ, Reichardt LF. Neurotrophins: roles in neuronal development and function. Ann Rev Neurosci. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lu B, Figurov A. Role of neurotrophins in synapse development and plasticity. Rev Neurosci. 1997;8:1–12. doi: 10.1515/revneuro.1997.8.1.1. [DOI] [PubMed] [Google Scholar]
- 4.Mowla SJ, et al. Differential sorting of nerve growth factor and brain-derived neurotrophic factor in hippocampal neurons. J Neurosci. 1999;19:2069–2080. doi: 10.1523/JNEUROSCI.19-06-02069.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balkowiec A, Katz DM. Cellular mechanisms regulating activity-dependent release of native brain-derived neurotrophic factor from hippocampal neurons. J Neurosci. 2002;22:10399–10407. doi: 10.1523/JNEUROSCI.22-23-10399.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heymach JV, Jr, Kruttgen A, Suter U, Shooter EM. The regulated secretion and vectorial targeting of neurotrophins in neuroendocrine and epithelial cells. J Biol Chem. 1996;271:25430–25437. doi: 10.1074/jbc.271.41.25430. [DOI] [PubMed] [Google Scholar]
- 7.Teng HK, et al. ProBDNF induces neuronal apoptosis via activation of a receptor complex of p75NTR and sortilin. J Neurosci. 2005;25:5455–5463. doi: 10.1523/JNEUROSCI.5123-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Woo NH, et al. Activation of p75NTR by proBDNF facilitates hippocampal long-term depression. Nat Neurosci. 2005;8:1069–1077. doi: 10.1038/nn1510. [DOI] [PubMed] [Google Scholar]
- 9.Seidah NG, et al. Cellular processing of the neurotrophin precursors of NT3 and BDNF by the mammalian proprotein convertases. FEBS Lett. 1996;379:247–250. doi: 10.1016/0014-5793(95)01520-5. [DOI] [PubMed] [Google Scholar]
- 10.Mowla SJ, et al. Biosynthesis and post-translational processing of the precursor to brain-derived neurotrophic factor. J Biol Chem. 2001;276:12660–12666. doi: 10.1074/jbc.M008104200. [DOI] [PubMed] [Google Scholar]
- 11.Lee R, Kermani P, Teng KK, Hempstead BL. Regulation of cell survival by secreted proneurotrophins. Science. 2001;294:1945–1948. doi: 10.1126/science.1065057. [DOI] [PubMed] [Google Scholar]
- 12.Pang PT, et al. Cleavage of proBDNF by tPA/plasmin is essential for long-term hippocampal plasticity. Science. 2004;306:487–491. doi: 10.1126/science.1100135. [DOI] [PubMed] [Google Scholar]
- 13.Lu B, Pang PT, Woo NH. The yin and yang of neurotrophin action. Nat Rev Neurosci. 2005;6:603–614. doi: 10.1038/nrn1726. [DOI] [PubMed] [Google Scholar]
- 14.Matsumoto T, et al. Biosynthesis and processing of endogenous BDNF: CNS neurons store and secrete BDNF, not pro-BDNF. Nat Neurosci. 2008;11:131–133. doi: 10.1038/nn2038. [DOI] [PubMed] [Google Scholar]
- 15.Blochl A, Thoenen H. Localization of cellular storage compartments and sites of constitutive and activity-dependent release of nerve growth factor (NGF) in primary cultures of hippocampal neurons. Mol Cell Neurosci. 1996;7:173–190. doi: 10.1006/mcne.1996.0014. [DOI] [PubMed] [Google Scholar]
- 16.Du J, Feng L, Yang F, Lu B. Activity- and Ca(2+)-dependent modulation of surface expression of brain-derived neurotrophic factor receptors in hippocampal neurons. J Cell Biol. 2000;150:1423–1434. doi: 10.1083/jcb.150.6.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meyer A, et al. Kainic acid increases the expression of the prohormone convertases furin and PC1 in the mouse hippocampus. Brain Res. 1996;732:121–132. doi: 10.1016/0006-8993(96)00502-1. [DOI] [PubMed] [Google Scholar]
- 18.Baranes D, et al. Tissue plasminogen activator contributes to the late phase of LTP and to synaptic growth in the hippocampal mossy fiber pathway. Neuron. 1998;21:813–825. doi: 10.1016/s0896-6273(00)80597-8. [DOI] [PubMed] [Google Scholar]
- 19.Taraska JW, et al. Secretory granules are recaptured largely intact after stimulated exocytosis in cultured endocrine cells. Proc Natl Acad Sci USA. 2003;100:2070–2075. doi: 10.1073/pnas.0337526100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lochner JE, et al. Activity-dependent release of tissue plasminogen activator from the dendritic spines of hippocampal neurons revealed by live-cell imaging. J Neurobiol. 2006;66:564–577. doi: 10.1002/neu.20250. [DOI] [PubMed] [Google Scholar]
- 21.Ghosh A, Carnahan J, Greenberg ME. Requirement for BDNF in activity-dependent survival of cortical neurons. Science. 1994;263:1618–1623. doi: 10.1126/science.7907431. [DOI] [PubMed] [Google Scholar]
- 22.Figurov A, et al. Regulation of synaptic responses to high-frequency stimulation and LTP by neurotrophins in the hippocampus. Nature. 1996;381:706–709. doi: 10.1038/381706a0. [DOI] [PubMed] [Google Scholar]
- 23.Patterson SL, et al. Recombinant BDNF rescues deficits in basal synaptic transmission and hippocampal LTP in BDNF knockout mice. Neuron. 1996;16:1137–1145. doi: 10.1016/s0896-6273(00)80140-3. [DOI] [PubMed] [Google Scholar]
- 24.Yang, et al. Neuronal release of proBDNF. Nat Neurosci. 2009 doi: 10.1038/nn.2244. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Balkowiec A, Katz DM. Activity-dependent release of endogenous brain-derived neurotrophic factor from primary sensory neurons detected by ELISA in situ. J Neurosci. 2000;20:7417–7423. doi: 10.1523/JNEUROSCI.20-19-07417.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gartner A, Staiger V. Neurotrophin secretion from hippocampal neurons evoked by long-term-potentiation-inducing electrical stimulation patterns. Proc Natl Acad Sci USA. 2002;99:6386–6391. doi: 10.1073/pnas.092129699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aicardi G, et al. Induction of long-term potentiation and depression is reflected by corresponding changes in secretion of endogenous brain-derived neurotrophic factor. Proc Natl Acad Sci USA. 2004;101:15788–15792. doi: 10.1073/pnas.0406960101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peng S, Wuu J, Mufson EJ, Fahnestock M. Precursor form of brain-derived neurotrophic factor and mature brain-derived neurotrophic factor are decreased in the pre-clinical stages of Alzheimer's disease. J Neurochem. 2005;93:1412–1421. doi: 10.1111/j.1471-4159.2005.03135.x. [DOI] [PubMed] [Google Scholar]
- 29.Lee J, et al. Decreased levels of BDNF protein in Alzheimer temporal cortex are independent of BDNF polymorphisms. Exp Neurol. 2005;194:91–96. doi: 10.1016/j.expneurol.2005.01.026. [DOI] [PubMed] [Google Scholar]
- 30.Korhonen L, Riikonen R, Nawa H, Lindholm D. Brain derived neurotrophic factor is increased in cerebrospinal fluid of children suffering from asphyxia. Neurosci Lett. 1998;240:151–154. doi: 10.1016/s0304-3940(97)00937-3. [DOI] [PubMed] [Google Scholar]
- 31.Azoulay D, et al. Lower brain-derived neurotrophic factor in serum of relapsing remitting MS: reversal by glatiramer acetate. J Neuroimmunol. 2005;167:215–218. doi: 10.1016/j.jneuroim.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 32.Tan YL, et al. Decreased BDNF in serum of patients with chronic schizophrenia on long-term treatment with antipsychotics. Neurosci Lett. 2005;382:27–32. doi: 10.1016/j.neulet.2005.02.054. [DOI] [PubMed] [Google Scholar]
- 33.Tan YL, Zhou DF, Zhang XY. Decreased plasma brain-derived neurotrophic factor levels in schizophrenic patients with tardive dyskinesia: association with dyskinetic movements. Schizophr Res. 2005;74:263–270. doi: 10.1016/j.schres.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 34.Manni L, et al. Reduced plasma levels of NGF and BDNF in patients with acute coronary syndromes. Int J Cardiol. 2005;102:169–171. doi: 10.1016/j.ijcard.2004.10.041. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.