Abstract
Ticks are among the most important vectors of a wide range of human and animal diseases. During blood feeding, ticks are exposed to an enormous amount of free iron that must be appropriately used and detoxified. However, the mechanism of iron metabolism in ticks is poorly understood. Here, we show that ticks possess a complex system that efficiently utilizes, stores and transports non-heme iron within the tick body. We have characterized a new secreted ferritin (FER2) and an iron regulatory protein (IRP1) from the sheep tick, Ixodes ricinus, and have demonstrated their relationship to a previously described tick intracellular ferritin (FER1). By using RNA interference-mediated gene silencing in the tick, we show that synthesis of FER1, but not of FER2, is subject to IRP1-mediated translational control. Further, we find that depletion of FER2 from the tick plasma leads to a loss of FER1 expression in the salivary glands and ovaries that normally follows blood ingestion. We therefore suggest that secreted FER2 functions as the primary transporter of non-heme iron between the tick gut and the peripheral tissues. Silencing of the fer1, fer2, and irp1 genes by RNAi has an adverse impact on hatching rate and decreases postbloodmeal weight in tick females. Importantly, knockdown of fer2 dramatically impairs the ability of ticks to feed, thus making FER2 a promising candidate for development of an efficient anti-tick vaccine.
Keywords: cytosolic aconitase, IRP, ferritin, RNAi
Ticks are bloodfeeding ectoparasite disease vectors. They transmit some of most devastating viral, bacterial and protozoal diseases known to humans and animals (1). Ixodes ricinus (subphylum Chelicerata, class Arachnida), an European species closely related to the American deer tick I. scapularis, is a tick with 3-host cycle. Its larvae and nymphs feed once on blood before they molt; the adults feed once for up to 1 week and when fully engorged drop from the host and lay thousands of eggs.
Ticks ingest up to several hundred times their unfed weight during a single blood meal (2). Although other blood feeding vectors, such as mosquitoes, digest the blood meal in the gut lumen, tick digestion occurs inside digestive cells in the gut epithelium that employ a lysosomal machinery of acidic cysteine and aspartic peptidases (3). Similar to most blood feeding arthropods, ticks must have strategies to manage the excess iron originating from the host blood.
The fate of heme (a prosthetic group containing iron) in ticks has been well described in the cattle tick, Boophilus microplus (4, 5). Heme released during the digestion of hemoglobin is detoxified by storage in organelles called hemosomes (4). A small portion of heme is bound to the heme-binding lipoprotein, HeLp, and transported in hemolymph to the peripheral tissues, primarily the ovaries (6). The heme prosthetic group is apparently used as such because B. microplus lacks the heme synthetic pathway (7). Whether ticks can catabolize and recover the iron from heme remains unknown. In contrast to heme, very little is known about the fate of non-heme iron from the blood meal.
Iron is essential for most organisms because it serves as an electron donor and acceptor in various metabolic processes. However, these chemical properties also allow iron to participate in the formation of toxic free radicals that cause substantial damage to proteins, lipids and DNA. For this reason, iron homeostasis has to be maintained by an orchestrated set of proteins that conduct iron uptake, utilization, transport, and storage (8).
The identity and character of the proteins involved in vertebrate iron metabolism has grown rapidly during the last decade (9). Three of the primary proteins involved in this process are ferritin (FER) and the iron regulatory proteins, IRP1 and IRP2. Ferritin is the main protein for intracellular iron storage and consists of 2 types of subunits - a heavy (ferroxidase sites) and a light chain (nucleation sites). Translational control of ferritin in response to iron levels is mediated by the binding of IRP to an iron responsive element (IRE) in the 5′ untranslated region (UTR) of the subunit mRNA (10).
Similar systems to those for iron control in vertebrates exist in other organisms (for review, see ref. 11). In flies and mosquitoes, ferritin is predominately a secreted hemolymph protein that consists of heavy and light chains (12, 13). IRP also exists as 2 similar variants in Drosophila and both are cytosolic aconitases. However, only 1 binds the IRE (14). The only iron metabolism protein characterized in ticks is the intracellular ferritin, FER1, a homo-oligomer. FER1 was first described in I. ricinus and Ornithodoros moubata (15). It shows greatest similarity to the heavy chains and conserved ferroxidase sites. The fer1 mRNA contains an IRE indicating that IRP control could be active in these animals.
Here, we further characterize ferritin and IRP in ticks. We show that up-regulation of fer1 translation that occurs after a bloodmeal is controlled by IRP1 and we introduce a tick ferritin, FER2, that is expressed in the gut tissue and is required for iron transport to the ovaries and salivary glands. Functional analysis by silencing fer1, irp1, or fer2 indicates that each protein serves unique roles in blood feeding and egg development. These studies support that interference with proteins involved in iron metabolism has great potential as a control strategy to reduce tick numbers or for tick vaccine development and thereby reduction of tick-transmitted diseases.
Results
Transcript for fer1 Is Abundant in All Tissues.
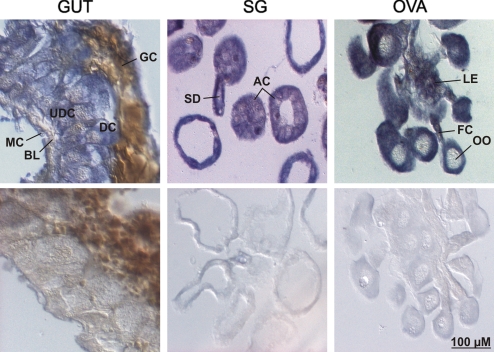
We reported that fer1 mRNA expression in tick tissues is not influenced by the bloodmeal (15). To further establish which tissues and cellsexpress fer1, we performed in situ hybridization with a fer1 specific probe. Positive labeling (blue color) was found in all tissues examined from partially fed (day 5) ticks (Fig. 1). In the gut, fer1 mRNA was evenly distributed in both undifferentiated (basal) and mature digestive cells (16) indicating that fer1 expression is not altered by bloodmeal processing. Muscle cells under the basal lamina also stained positively. In the ovary, luminal epithelium and funicle cells, and oocytes showed positive staining. In the salivary glands, fer1 mRNA was detected in all acini types and cells surrounding saliva ducts. The sense probe (controls) showed no signal above background in any tissue. These data demonstrate that fer1 expression is ubiquitous in cells of many types and all tissues, and support a role for FER1 as a primary intracellular iron storage protein in ticks.
Fig. 1.
Localization of fer1 message in tick tissues by in situ hybridization. Fer1 mRNA is ubiquitously expressed in the gut diverticulum (GUT), salivary glands (SG), and ovaries (OVA) of partially engorged (fed for 5 days) ticks. Antisense probe (Upper); Sense probe control (Lower). MC, muscle cells; BL, basal lamina; UDC, undifferentiated digestive cells; DC, differentiated digestive cells; GC, gut content; SD, salivary duct; AC, acini; OO, oocyte; FC, folicular cells; LE, luminal epithelium.
Blood Feeding Stimulates fer1 Translation.
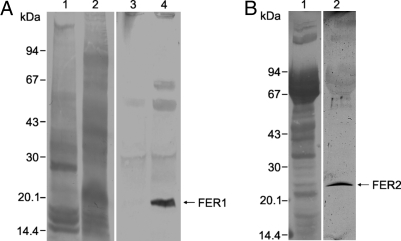
Although there is an abundance of fer1 mRNA within the cells, FER1 protein levels in unfed ticks are very low as seen by Western blot analysis (Fig. 2A). However, levels of FER1 dramatically increase during blood feeding as shown for salivary glands (Fig. 2A). These results support our previous hypothesis that fer1 mRNA translation is posttranscriptionally regulated by the IRE in the 5′ UTR (15).
Fig. 2.
Immunodetection of intracellular ferritin (FER1) and secreted ferritin (FER2) by Western blot analysis. (A) The amount of FER1 markedly increases in salivary glands (SG) during blood meal. (Lanes 1 and 3) SG from unfed ticks. (Lanes 2 and 4) SG from partially engorged (fed for 5 days) ticks. (Lanes 1 and 2) Amidoblack-stained SDS/PAGE profile of SG. (Lanes 3 and 4) Immunoblotting using Ra×FER1 antiserum. (B) Detection of secreted FER2 in plasma of partially fed ticks. (Lane 1) Amidoblack-stained SDS/PAGE profile of tick hemolymph. (Lane 2) Immunoblotting using Ra×FER2 antiserum.
Molecular Cloning of irp1.
To identify tick IRP, and other components of the iron metabolism pathway, the robust EST database of a closely-related tick, I. scapularis, was analyzed for homologues of sequences of human or insect genes coding proteins involved in iron metabolism (Table S1). We identified a homolog of mammalian cytosolic aconitase 1 (irp1), a new sequence for a ferritin (referred to herein as fer2) and a homolog of mammalian and insect divalent metal transporter1 (dmt1) (17, 18). Interestingly, sequences related to the vertebrate serum transferrin1, its receptor (TFR) and heme oxygenase (catalyses degradation of heme) were not found. The only transferrin related sequence found in tick databases was a homolog of insect transferrin2, a molecule likely not involved in iron transport (19). We subsequently sequenced irp1 from I. ricinus (GenBank accession no. EU885952). The full length cDNA is 2,835 bp. The ORF encodes a protein of 890 aa lacking a signal sequence. The deduced amino acid sequence indicates that the tick protein is an IRP1 (Fig. S1) that has the 4 domains typical of aconitase structure (20) and contains all of the amino acids involved in aconitase activity (21), Fe-S cluster binding and IRE interaction (10).
FER2 Is a Secreted Heavy Chain Ferritin.
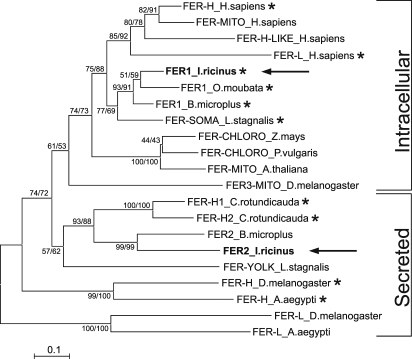
We also cloned and sequenced fer2 (GenBank accession no. EU885951). The fer2 sequence lacks a canonical 5′ UTR IRE. The ORF encodes a protein of 196 aa and the first 16 aa of FER2 constitute a secretory signal peptide (Fig. S2). Calculated mass of the mature peptide is 20,707 Da. Multiple comparative alignments with other ferritins (Fig. S3) show that FER2 is a heavy chain subunit with all of the residues important for ferroxidase activity conserved (22). The sequence of fer2 is closely related to that of the plasma heavy chain ferritin subunit from the horseshoe crab Carcinoscorpius rotundicauda (23), and to a yolk ferritin from the snail, Lymnea stagnalis (24) (Fig. 3). To determine whether FER2 is a secreted protein, we prepared antiserum against recombinant FER2 (Ra×FER2). The antiserum detected a specific band of ≈25 kDa in tick hemolymph (Fig. 2B). This is in contrast to FER1 that is never found in hemolymph (15).
Fig. 3.
Phylogenetic tree of selected vertebrate and invertebrate ferritins. Unrooted tree of ferritin amino acid sequences reconstructed using the Neighbor Joining (NJ) method based on alignment using ClustalX. Alignment and sequence descriptions are provided as supporting information. The Ixodes ricinus FER1 clusters together with intracellular ferritins of different origin. The tick FER2 is closely related to plasma ferritins from the horseshoe crab and form a separate branch with other secreted ferritins from invertebrates. Arrows indicate ferritins of I. ricnius. Asterisk shows a presence of the iron responsive element (IRE) in the 5′- untranslated region of mRNA. Numbers at branches represent bootstrap support using NJ/minimal evolution criterion with 1,000 replicates each. (Bar: 0.1 substitutions per site.)
Expression Profiles of fer1, irp1, and fer2.
To obtain a more complete picture of the expression of fer1, irp1, and fer2 in tick developmental stages and tissues, all 3 genes were studied in the same samples. As shown in Fig. S4, each gene is transcribed in all developmental stages and expression in tissues is independent of blood feeding. In keeping with the in situ hybridization results, fer1 is expressed in all tissues and stages, as is irp1. In contrast, fer2 shows primary expression in gut tissues with limited transcription in ovary and salivary glands.
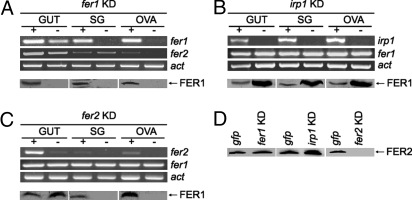
Silencing of irp1 Increases FER1 Synthesis.
As shown in the Fig. 4, expression of each gene was efficiently silenced by RNAi in all tissues of partially engorged ticks. Western blot analysis for FER1 showed that the protein is synthesized in all tissues we analyzed and that silencing of fer1 reduces the protein levels (Fig. 4A). The presence of fer1 transcript in the absence of FER1 protein in salivary glands in unfed ticks (Fig. 2A and Fig. S4) and the increase in FER1 protein levels after blood feeding (Fig. 2A) indicates to us that FER1 synthesis is repressed at the translational level most likely by IRP1 in unfed ticks. We reasoned that if this were the case, silencing of irp1 would result in increased synthesis of FER1. Western blots for FER1 in tissues taken at day 5 of blood feeding clearly show that when irp1 is silenced, FER1 synthesis is markedly increased (Fig. 4B). These data confirm that FER1 synthesis is subject to translational control by IRP1 in vivo in ticks.
Fig. 4.
RNAi silencing of fer1, irp1 and fer2 in partially engorged (fed for 5 days) ticks. (A) Silencing of fer1 specifically silenced fer1 mRNA levels (row fer1) and translation (Western blot in the row FER1) whereas fer2 and actin mRNA levels were unchanged (rows fer2 and act, respectively). (B) Silencing of irp1 specifically silenced irp1 message (row irp1). When silenced, protein levels of FER1 in various tissues significantly increased (Western blot in the row FER1) indicating that fer1 translation is blocked by IRP1. (C) Silencing of fer2 specifically silenced fer2 message (row fer2). When silenced, the amount of FER1 slightly increased in the gut, but no FER1 could be detected in salivary glands and ovaries (Western blot in the row FER1). (Upper) Separation of PCR products on agarose gels. (Lower) Western blots. (D) Western blot of the tick plasma shows that silencing of fer2 specifically abolishes proteosynthesis of FER2, which was not influenced by silencing of fer1 or irp1. +, gfp dsRNA control; −, gene specific silencing. GUT, gut tissue; SG, salivary glands; OVA, ovaries.
Silencing of fer2 Prevents Synthesis of FER1 in Peripheral Tissues.
Because fer2 is expressed predominantly in the gut and FER2 is secreted into the hemolymph, we wanted to test whether FER2 might be required for dietary iron transport from the gut to the ovaries and salivary glands. Because we were unable to measure the iron levels of hemolymph directly in fer2 silencing, we used FER1 expression in ovaries and salivary glands as sensors for meal iron delivery. We reasoned that after blood feeding, the iron released from the bloodmeal inside the gut cells would up-regulate local FER1 expression in gut tissues regardless of FER2 knockdown. In contrast, if FER2 was required to move meal iron from the gut to the ovaries and salivary gland, then when FER2 was knocked down, FER1 in these two non-gut tissues would not increase. In keeping with this hypothesis, Western blot analyses of tissues at 5 days of blood feeding shows that indeed despite silencing of fer2 (Fig. 4C), gut FER1 modestly increased indicating an iron excess inside the gut cells. However, up-regulation of FER1 in salivary glands and ovaries is virtually absent relative to controls. From these data we conclude that meal iron up-regulates FER1 locally in gut tissues regardless of fer2 silencing. In contrast, silencing of fer2 results in reduced iron delivery to salivary glands and ovaries and this is reflected in an absence of FER1 up-regulation in these tissues. These data strongly support that FER2 functions in the tick as the primary transport protein for meal iron. Importantly, silencing of fer1 or irp1 does not affect the amount of FER2 in the hemolymph (Fig. 4D) showing that FER2 synthesis is not regulated by these 2 proteins.
Tick Feeding, Survival, and Development Are Dependent on Ferritin and IRP1.
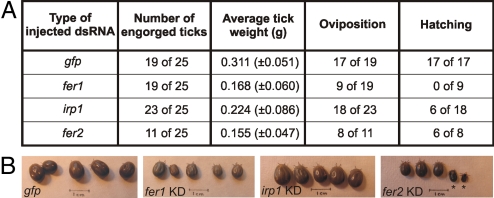
Our goal is to determine whether we can interfere with proteins involved in iron metabolism and thereby, reduce tick numbers and disease transmission. We evaluated the effects of knockdown of each gene, fer1, irp1, and fer2, on tick feeding, oviposition and egg hatching. Ticks injected with gfp were used as negative controls. We observed that control ticks fed for ≈10 days until replete (full engorgement) with the bloodmeal. Two weeks after feeding, the females began to lay eggs and 1 month later the eggs hatched. Silencing of fer1 reduced post bloodmeal weight by half indicating this protein is required for full repletion with blood. Silencing fer1 also significantly decreased oviposition and prevented hatching of those eggs that were laid (Fig. 5A). When irp1 was silenced, although repletion was only modestly reduced and oviposition was not altered, hatching of eggs was reduced by ≈66%. The results of silencing fer2 show that like FER1, this protein is crucial for blood ingestion. In the absence of FER2, more than half of the females dried and fell from the host soon after attachment. Of those that survived blood feeding, most laid eggs. From our data taken together, we conclude that all 3 proteins, FER1, FER2, and IRP1, serve unique roles in female tick iron metabolism. FER1 is required for iron storage after the bloodmeal and without this storage, reproduction is significantly reduced. IRP1 appears to serve important roles in the developing egg and interference with this protein can significantly reduce tick numbers primarily by reducing hatching of eggs. Finally, FER2 is required for repletion and iron distribution. Without this protein, many animals will die during blood feeding.
Fig. 5.
Effect of fer1, irp1 and fer2 silencing on tick feeding, survival, and development. (A) Summarized effect of each silencing on tick feeding, weight after repletion, oviposition, and hatching. (B) Appearance of fully engorged ticks after individual gene knockdown. gfp dsRNA was used as a negative control. Significant reduction in size was observed in ticks with fer1 and fer2 knockdown (KD). Ticks marked with an asterisk in fer2 KD group dried during feeding. Five representative members of each group are shown.
Discussion
In this report, we present the first overview of iron metabolism in ticks and we show that interference with the proteins involved limits tick reproduction. Silencing of fer1, irp1, and fer2 revealed the importance of each gene in tick development during and after feeding (Fig. 5). Knockout of fer or irp in mouse (25, 26) or flies (27) results in embryonic lethality. In ticks, silencing of fer1 reduced oviposition and prevented egg hatching. This implies that storage of iron is required for the developing embryo and that in the absence of adequate iron storage, hatching fails. However, we found that silencing of irp1 allows overexpression of FER1, and yet this also reduces egg hatching. The increased FER1 expression could have reduced intracellular iron availability by enhancing iron storage and limiting iron availability for the developing egg. Alternatively, we speculate that IRP1 is involved in controlling the levels of some yet unidentified proteins involved in organism development. Interestingly, although silencing of each of the genes influenced blood feeding behavior, only the knockdown of FER2 reduces the capacity of females to fully engorge. It seems likely that this occurs because meal iron could not be adequately processed and removed from gut cells into hemolymph in these animals. We suggest that FER2 serves a role in supplying peripheral tissues with iron needed for their normal function. If this is the case, then this protein has important potential for vaccine development, because FER2 sequence shows a relatively low homology to vertebrate ferritins. Moreover, it has been shown that host antibodies can freely pass the tick gut and target proteins in the hemolymph (28).
The biomedical and economical importance of ticks has led to detailed studies by others on many aspects of tick biology (29). Now, the use of reverse genetic approaches and the feasibility of RNA interference (30) has markedly increased their potential as a valuable invertebrate model (31). We are unaware of another study in which RNA interference is used to reveal the functions of proteins involved in a complex metabolic pathway in ticks. For such studies, RNAi silencing is the method of choice as other genetic manipulations in ticks are not feasible.
IRP-IRE interaction is a well characterized translational regulatory system of vertebrate iron metabolism that allows timely and appropriate responses to changes in intracellular iron status. In mammals, the IRP/IRE system controls the translation of ferritins, mitochondrial aconitase, aminolevulinate synthase, ferroportin, transferrin receptor 1, and DMT1 (8). In insects, IRP1 drives translation of ferritins and succinate dehydrogenase (32, 33, 34). The only transcript in ticks known to contain an IRE is fer1 (15) that is expressed in all tissues in both fed and unfed ticks. Although abundant message is present, the protein is found only in fed animals indicating repression of translation in the unfed state. Silencing of tick irp1 escalates FER1 synthesis and demonstrates IRP1 regulation of fer1 translation in vivo. These results imply that IRP1 is responsible for repression of fer1 we observed in unfed ticks, and that the release of IRP1 from fer1 mRNA contributes to up-regulation of FER1 for iron storage in tissues in response to the bloodmeal.
Unlike fer1, fer2 has no canonical IRE in the mRNA suggesting translation of this message is not under IRP1 control. Further, fer2 is expressed mainly in the gut with limited expression in other tissues such as ovaries and salivary glands in either unfed or fed ticks. FER1 clusters phylogenetically with other intracellular ferritins with plant chloroplast and mitochondrial ferritins. This branch of intracellular ferritins is well separated from invertebrate secreted ferritins, including tick FER2. To date, snails and ticks are the only two species that show conserved intracellular and secreted ferritins. Several functions have been suggested for secreted ferritins. Plasma ferritin from the horseshoe crab C. rotundicauda is suggested to play a role in immunity by sequestering iron from invading bacteria during an infection (35). In vertebrates, Fisher et al. (36) demonstrated that iron delivery to the brain is mediated by serum ferritin via a clathrin-dependent transport mechanism. In the mosquito, Aedes aegypti, hemolymph ferritin transports iron to the ovaries (13). Our data support that the gut is the primary site of fer2 expression, and that FER2 is secreted into hemolymph. Further, we show that silencing of fer2 abolishes FER1 synthesis in peripheral tissues, but not in the gut. These data imply that FER1 translation occurs in gut tissues when meal iron is available regardless of fer2 silencing, but that fer2 silencing results in insufficient iron delivery to the peripheral tissues to up-regulate FER1 synthesis in ovaries and salivary glands during blood ingestion. Taken together, our data strongly support that FER2 is primarily responsible for iron transport from the gut to the peripheral tissues in these animals.
Ticks feed exclusively on vertebrate blood that contains minimum free iron, iron as ferric transferrin, and iron as heme in hemoglobin. Work by others and our database search reported herein show that ticks do not have the heme synthetic (7) or catabolic pathway (lack of heme oxygenase). Thus, the primary source of iron for ticks is likely the host ferric-transferrin found in the blood meal. Our calculation that is based on the situation in mosquitoes (13) indicates that a fully engorged tick female would receive almost 900 ng of Fe from the host Fe-transferrin in 730 μL of blood meal (2). These levels of iron are likely sufficient for egg development in these animals. A system that delivers the iron to the tick peripheral tissues is required and our data indicate that the tick FER2 is used here as the main iron transporter.
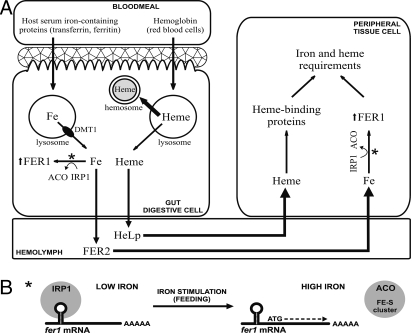
From the available evidence in ticks and from work in insects and mammals, we propose a model for tick iron metabolism (Fig. 6). Although incomplete, such a model can provide a working guide for post genomic research in these animals. We suggest that host Fe-transferrin taken in the bloodmeal enters and is degraded in the endosomes of the digestive cells. The iron is released by protein degradation and/or lowered pH, and could be transported from the endosomes via the putative DMT1. Intracellular iron is then stored in intracellular ferritin (FER1) or concentrated into FER2 that is subsequently secreted into hemolymph. FER1 likely serves a role as an antioxidant by sequestering excess intracellular iron, whereas FER2 removes iron from the gut cells and delivers it to peripheral tissues to meet iron requirements. Heme released from the host hemoglobin during digestion is partially used by the tick, but is not the primary source of iron that is used to meet tissue iron requirements. We do not know the role of the putative tick transferrin2. However, our preliminary data indicate that a significant role in meal iron delivery via hemolymph is unlikely (tf2 KD). We also do not know how iron from ferritin2 is delivered to the peripheral tissues. We are pursuing studies on tick FER2 and transferrin2 in our group at this time, but the answers to these and many other questions must await further research in these important disease vectors.
Fig. 6.
A model of iron metabolism in ticks. (A) Based on our data, we assume an independent pathway for both heme and iron. Heme released from hemoglobin is detoxified in hemosomes or transported into peripheral tissues by the HeLp protein to meet cell heme requirements. Iron released from host serum proteins could be transported from endosome via the putative DMT1 into cytoplasm, where it is scavenged by FER1 or loaded into FER2 and transported via hemolymph into peripheral tissues to meet cell iron requirements. Asterisks depict a process shown in detail in B. (B) Increasing concentrations of iron in the tick cell allow insertion of newly synthesized Fe-S clusters into IRP1, which becomes an active aconitase (ACO) and detaches from fer1 mRNA IRE loop. This triggers fer1 mRNA translation and newly synthesized FER1 sequester the free iron and protect the cell against oxidative stress.
Materials and Methods
Biological Materials.
Nymphs and adult males and females of Ixodes ricinus were collected in Ceske Budejovice in the Czech Republic. Adult females were fed on laboratory guinea pigs and engorged ticks were kept separately in glass vials in wet chambers at 26 °C until oviposition and hatching. Laboratory animals were treated in accordance with the Animal Protection Law of the Czech Republic no. 246/1992 Sb.
Database Search and Phylogenetic Analysis.
The search for tick genes related to iron metabolism (ferritin, cytosolic aconitase, transferrin, transferrin receptor, divalent metal transporter1, duodenal cytochrome b reductase1, and heme oxygenase) was performed using BLASTN and the National Center for Biotechnology Information I. scapularis EST database (www.ncbi.nlm.nih.gov). Full length cDNA sequences were analyzed using the programs SignalP 3.0 (www.cbs.dtu.dk/services/SignalP) for the presence of signal sequence. Phylogenetic analysis was done as described in ref. 37.
RT-PCR Profiling and Western Blot Analysis.
Adult I. ricinus females were allowed to feed on laboratory guinea pigs for 5 days. Tissues (gut, salivary glands, and ovaries) were dissected into TRI Reagent solution (Sigma) for RNA isolation, PBS for protein analysis, SDS/PAGE sample buffer for Western blot analysis. Whole body homogenates were prepared by freezing ticks in liquid nitrogen and grinding using a mortar and pestle. Total RNA (0.5 μg) was reversibly transcribed into cDNA using Enhanced Avian First Strand synthesis kit (Sigma). The pairs of gene-specific primers for PCR were designed from sequences of I. ricinus fer1 (AF068224), actin (AJ889837), irp1 and fer2 and are listed in Table S2. The PCR products obtained were cloned and the sequences verified. The Western blot was processed as described earlier (38). The detection of FER1 was performed using rabbit anti-Ornithodoros moubata ferritin antibody (Ra×FER1, 1:100) (15). For FER2, a newly prepared rabbit antibody against recombinant FER2 (Ra×FER2, 1:100) was used (see below). Recombinant FER2 was obtained using E. coli strain BL21 using Champion pET Directional TOPO expression kit (Invitrogen, Carlsbad, CA); fer2 was cloned into pET100/D-TOPO vector using primers FER2-Exp-F/FER2-Exp-R (Table S2). Protein purification, refolding and rabbit immunization was carried out as described in ref. 37.
Molecular Cloning of irp1 and fer2 Full-Length cDNAs.
The cDNA sequences of irp1 and fer2 were determined from overlapping PCR, 3′ RACE and 5′ RACE PCR fragments. Middle segments of irp1 and fer2 were amplified using IRP1-F2/IRP1-R2 and FER2-F/FER2-R primers (Table S2). For 5′ and 3′ RACE PCR, the common cDNA was prepared from salivary glands total RNA by reverse transcription, using a 5′ RACE system (Invitrogen) and a mix of oligo dT23 and random nonamer primers (Sigma). The 3′ ends of irp1 and fer2 were amplified using dT23 primer and IRP1-F1 and FER2-F, respectively (Table S2). The 5′ ends of irp1 and fer2 were amplified using AAP anchor primer (Invitrogen) in combination with IRP1-R3 and FER2-R primers, respectively (Table S2). Products obtained were sequenced and sequences assembled in SeqMan program (DNASTAR).
In Situ Hybridization.
Plasmid pll10 with a cloned fer1 fragment (see below) was used as template to synthesize sense and antisense DIG-labeled ssDNA probes using Dig Probe Synthesis kit (Roche). Probe yield was checked by dot blot. For in situ hybridization, dissected tissues were fixed in Bouin–Hollande sublimate (BHS), embedded in paraplast and sectioned (39). In situ hybridization was done using the mRNA locator kit (Ambion) with a hybridization temperature of 50 °C. The detection of hybridized probes was performed using alkaline phosphatase-conjugated anti-digoxigenin antibody 1:500 (Roche Molecular Biochemicals) and BCIP/NBT substrate (PerkinElmer). Dig-labeled sense probes were used in control experiments.
RNA Silencing.
A 368-bp fragment of I. ricinus fer1 (position 32–399 of AF068224), a 243-bp fragment of fer2 (position 203–445 of EU885951) and a 376-bp fragment of irp1 (position 1957–2333 of EU885952) were amplified from tick salivary gland cDNA and cloned into pll10 vector with two T7 promoters in reverse orientation (40), using the primer pairs FER1-F/FER1-R, FER2-F/FER2-R, and IRP1-F/IRP1-R (Table S2) containing the additional restriction sites ApaI and XbaI for fer1 and fer2 and KpnI and XbaI for irp1. Purified linear plasmids served as templates for RNA syntheses using MEGAscript T7 transcription kit (Ambion). The dsRNA (0.5 μL, 3 μg/μL) was injected into the hemolymph of unfed female ticks using a micromanipulator (Narishige). Control ticks were injected with the same volume of GFP dsRNA synthesized under the same conditions from linearized plasmid pll6 (40). After 24 h of rest in a humid chamber at room temperature, ticks were fed on guinea pigs (25 females and 25 males per animal). The gene silencing was checked by RT-PCR and/or Western blot analysis on tissues dissected from half-engorged (5 days of feeding) females. For survival studies, ticks were allowed to feed until repletion, photographed, weighed and put into separate vials to evaluate ensuing oviposition and hatching.
Supplementary Material
Acknowledgments.
We thank Dr. Elena Levashina, Centre National de la Recherche Scientifique, Strasbourgh, France for her help with set-up the RNAi experiments in our laboratory and Dr. Marek Jindra, Biology Centre, Academy of Sciences of the Czech Republic for his help with the manuscript revision. This work was supported by Research Centres LC06009 and LC07032 and Grant Agency of the Academy of Sciences of the Czech Republic Grant IAA60022603. Research at the Faculty of Science of the University of South Bohemia and Institute of Parasitology; the Biology Centre of the Academy of Sciences of the Czech Republic is covered by Research Plans MSM 6007665801 and Z60220518, respectively.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The sequences reported in this paper have been deposited in the GenBank database {accession nos. EU885951 [Tick ferritin2 (FER2)] and EU885952 [tick iron regulatory protein1 (IRP1)]}.
This article contains supporting information online at www.pnas.org/cgi/content/full/0807961106/DCSupplemental.
References
- 1.Jongejan F, Uilenberg G. The global importance of ticks. Parasitology. 2004;129:S3–14. doi: 10.1017/s0031182004005967. [DOI] [PubMed] [Google Scholar]
- 2.Balashov YS. Bloodsucking ticks (Ixodoidea)—vectors of disease of man and animals (English translation) Misc Publ Entomol Soc Amer. 1972;8:163–376. [Google Scholar]
- 3.Sojka D, et al. Profiling of proteolytic enzymes in the gut of the tick Ixodes ricinus reveals an evolutionarily conserved network of aspartic and cysteine peptidases. Parasit Vectors. 2008;1:7. doi: 10.1186/1756-3305-1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lara FA, et al. A new intracellular pathway of haem detoxification in the midgut of the cattle tick Boophilus microplus: Aggregation inside a specialized organelle, the hemosome. J Exp Biol. 2003;206:1707–1715. doi: 10.1242/jeb.00334. [DOI] [PubMed] [Google Scholar]
- 5.Lara FA, Lins U, Bechara GH, Oliveira PL. Tracing heme in a living cell: Hemoglobin degradation and heme traffic in digest cells of the cattle tick Boophilus microplus. J Exp Biol. 2005;208:3093–3101. doi: 10.1242/jeb.01749. [DOI] [PubMed] [Google Scholar]
- 6.Maya-Monteiro CM, et al. HeLp, a heme lipoprotein from the hemolymph of the cattle tick, Boophilus microplus. J Biol Chem. 2000;275:36584–36589. doi: 10.1074/jbc.M007344200. [DOI] [PubMed] [Google Scholar]
- 7.Braz GR, Coelho HS, Masuda H, Oliveira PL. A missing metabolic pathway in the cattle tick Boophilus microplus. Curr Biol. 1999;9:703–706. doi: 10.1016/s0960-9822(99)80312-1. [DOI] [PubMed] [Google Scholar]
- 8.Hentze MW, Muckenthaler MU, Andrews NC. Balancing acts: Molecular control of mammalian iron metabolism. Cell. 2004;117:285–297. doi: 10.1016/s0092-8674(04)00343-5. [DOI] [PubMed] [Google Scholar]
- 9.Dunn LL, Rahmanto YS, Richardson DR. Iron uptake and metabolism in the new millennium. Trends Cell Biol. 2007;17:93–100. doi: 10.1016/j.tcb.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 10.Walden WE, et al. Structure of dual function iron regulatory protein 1 complexed with ferritin IRE-RNA. Science. 2006;314:1903–1908. doi: 10.1126/science.1133116. [DOI] [PubMed] [Google Scholar]
- 11.Nichol H, Law JH, Winzerling JJ. Iron metabolism in insects. Annu Rev Entomol. 2002;47:535–559. doi: 10.1146/annurev.ento.47.091201.145237. [DOI] [PubMed] [Google Scholar]
- 12.Winzerling JJ, D-Pham DQ. In: Comprehensive Molecular Insect Science. Gilbert LI, Iatrou K, Gill S, editors. Oxford: Elsevier Pergamon; 2004. pp. 341–356. [Google Scholar]
- 13.Zhou G, et al. Fate of blood meal iron in mosquitoes. J Insect Physiol. 2007;53:1169–1178. doi: 10.1016/j.jinsphys.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lind MI, et al. Of two cytosolic aconitases expressed in Drosophila, only one functions as an iron-regulatory protein. J Biol Chem. 2006;281:18707–18714. doi: 10.1074/jbc.M603354200. [DOI] [PubMed] [Google Scholar]
- 15.Kopacek P, et al. Molecular cloning, expression and isolation of ferritins from two tick species–Ornithodoros moubata and Ixodes ricinus. Insect Biochem Mol Biol. 2003;33:103–113. doi: 10.1016/s0965-1748(02)00181-9. [DOI] [PubMed] [Google Scholar]
- 16.Coons LB, Alberti G. In: Microscopic Anatomy of Invertebrates. Harrison FW, Foelix R, editors. New York: Wiley-Liss; 1999. pp. 267–514. [Google Scholar]
- 17.Andrews NC. The iron transporter DMT1. Int J Biochem Cell Biol. 1999;31:991–994. doi: 10.1016/s1357-2725(99)00065-5. [DOI] [PubMed] [Google Scholar]
- 18.Garrick MD, et al. DMT1: Which metals does it transport? Biol Res. 2006;39:79–85. doi: 10.4067/s0716-97602006000100009. [DOI] [PubMed] [Google Scholar]
- 19.Dunkov B, Georgieva T. Insect iron binding proteins: Insights from the genomes. Insect Biochem Mol Biol. 2006;36:300–309. doi: 10.1016/j.ibmb.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 20.Dupuy J, et al. Crystal structure of human iron regulatory protein 1 as cytosolic aconitase. Structure. 2006;14:129–139. doi: 10.1016/j.str.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 21.Lauble H, Kennedy MC, Beinert H, Stout CD. Crystal structures of aconitase with isocitrate and nitroisocitrate bound. Biochemistry. 1992;31:2735–2748. doi: 10.1021/bi00125a014. [DOI] [PubMed] [Google Scholar]
- 22.Hempstead PD, et al. Comparison of the three-dimensional structures of recombinant human H and horse L ferritins at high resolution. J Mol Biol. 1997;268:424–448. doi: 10.1006/jmbi.1997.0970. [DOI] [PubMed] [Google Scholar]
- 23.Ong DS, Wang L, Zhu Y, Ho B, Ding JL. The response of ferritin to LPS and acute phase of Pseudomonas infection. J Endotoxin Res. 2005;11:267–280. doi: 10.1179/096805105X58698. [DOI] [PubMed] [Google Scholar]
- 24.von Darl M, Harrison PM, Bottke W. cDNA cloning and deduced amino acid sequence of two ferritins: Soma ferritin and yolk ferritin, from the snail Lymnaea stagnalis L. Eur J Biochem. 1994;222:353–366. doi: 10.1111/j.1432-1033.1994.tb18874.x. [DOI] [PubMed] [Google Scholar]
- 25.Ferreira C, et al. Early embryonic lethality of H ferritin gene deletion in mice. J Biol Chem. 2000;275:3021–3024. doi: 10.1074/jbc.275.5.3021. [DOI] [PubMed] [Google Scholar]
- 26.Smith SR, Ghosh MC, Ollivierre-Wilson H, Hang Tong W, Rouault TA. Complete loss of iron regulatory proteins 1 and 2 prevents viability of murine zygotes beyond the blastocyst stage of embryonic development. Blood Cells Mol Dis. 2006;36:283–287. doi: 10.1016/j.bcmd.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 27.Missirlis F, et al. Homeostatic mechanisms for iron storage revealed by genetic manipulations and live imaging of Drosophila ferritin. Genetics. 2007;177:89–100. doi: 10.1534/genetics.107.075150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vaughan JA, Sonenshine DE, Azad AF. Kinetics of ingested host immunoglobulin G in hemolymph and whole body homogenates during nymphal development of Dermacentor variabilis and Ixodes scapularis ticks (Acari: Ixodidae) Exp Appl Acarol. 2002;27:329–340. doi: 10.1023/a:1023347930746. [DOI] [PubMed] [Google Scholar]
- 29.Sonenshine DE. Biology of ticks. New York: Oxford Univ Press; 1991. pp. 159–188. [Google Scholar]
- 30.de la Fuente J, Kocan KM, Almazan C, Blouin EF. RNA interference for the study and genetic manipulation of ticks. Trends Parasitol. 2007;23:427–433. doi: 10.1016/j.pt.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 31.Jongejan F, Nene V, de la Fuente J, Pain A, Willadsen P. Advances in the genomics of ticks and tick-borne pathogens. Trends Parasitol. 2007;23:391–396. doi: 10.1016/j.pt.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 32.Kohler SA, Henderson BR, Kuhn LC. Succinate dehydrogenase b mRNA of Drosophila melanogaster has a functional iron-responsive element in its 5′-untranslated region. J Biol Chem. 1995;270:30781–30786. doi: 10.1074/jbc.270.51.30781. [DOI] [PubMed] [Google Scholar]
- 33.Charlesworth A, et al. Isolation and properties of Drosophila melanogaster ferritin—molecular cloning of a cDNA that encodes one subunit, and localization of the gene on the third chromosome. Eur J Biochem. 1997;247:470–475. doi: 10.1111/j.1432-1033.1997.00470.x. [DOI] [PubMed] [Google Scholar]
- 34.Zhang D, Albert DW, Kohlhepp P, D-Pham DQ, Winzerling JJ. Repression of Manduca sexta ferritin synthesis by IRP1/IRE interaction. Insect Mol Biol. 2001;10:531–539. doi: 10.1046/j.0962-1075.2001.00293.x. [DOI] [PubMed] [Google Scholar]
- 35.Ong ST, Ho JZ, Ho B, Ding JL. Iron-withholding strategy in innate immunity. Immunobiology. 2006;211:295–314. doi: 10.1016/j.imbio.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 36.Fisher J, et al. Ferritin: A novel mechanism for delivery of iron to the brain and other organs. Am J Physiol Cell Physiol. 2007;293:C641–C649. doi: 10.1152/ajpcell.00599.2006. [DOI] [PubMed] [Google Scholar]
- 37.Sojka D, et al. IrAE: An asparaginyl endopeptidase (legumain) in the gut of the hard tick Ixodes ricinus. Int J Parasitol. 2007;37:713–724. doi: 10.1016/j.ijpara.2006.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kopacek P, Weise C, Gotz P. The prophenoloxidase from the wax moth Galleria mellonella: Purification and characterization of the proenzyme. Insect Biochem Mol Biol. 1995;25:1081–1091. doi: 10.1016/0965-1748(95)00040-2. [DOI] [PubMed] [Google Scholar]
- 39.Sauman I, Reppert SM. Molecular characterization of prothoracicotropic hormone (PTTH) from the giant silkmoth Antheraea pernyi: Developmental appearance of PTTH-expressing cells and relationship to circadian clock cells in central brain. Dev Biol. 1996;178:418–429. doi: 10.1006/dbio.1996.0228. [DOI] [PubMed] [Google Scholar]
- 40.Levashina EA, et al. Conserved role of a complement-like protein in phagocytosis revealed by dsRNA knockout in cultured cells of the mosquito, Anopheles gambiae. Cell. 2001;104:709–718. doi: 10.1016/s0092-8674(01)00267-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.