Abstract
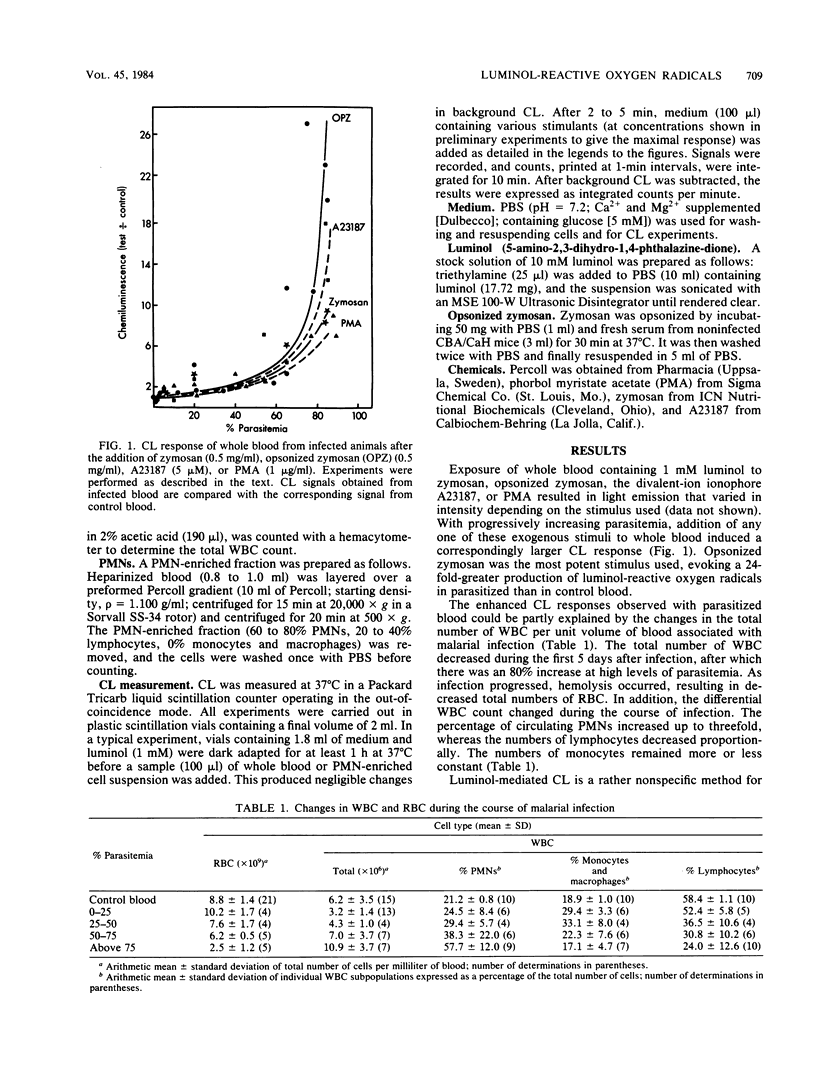
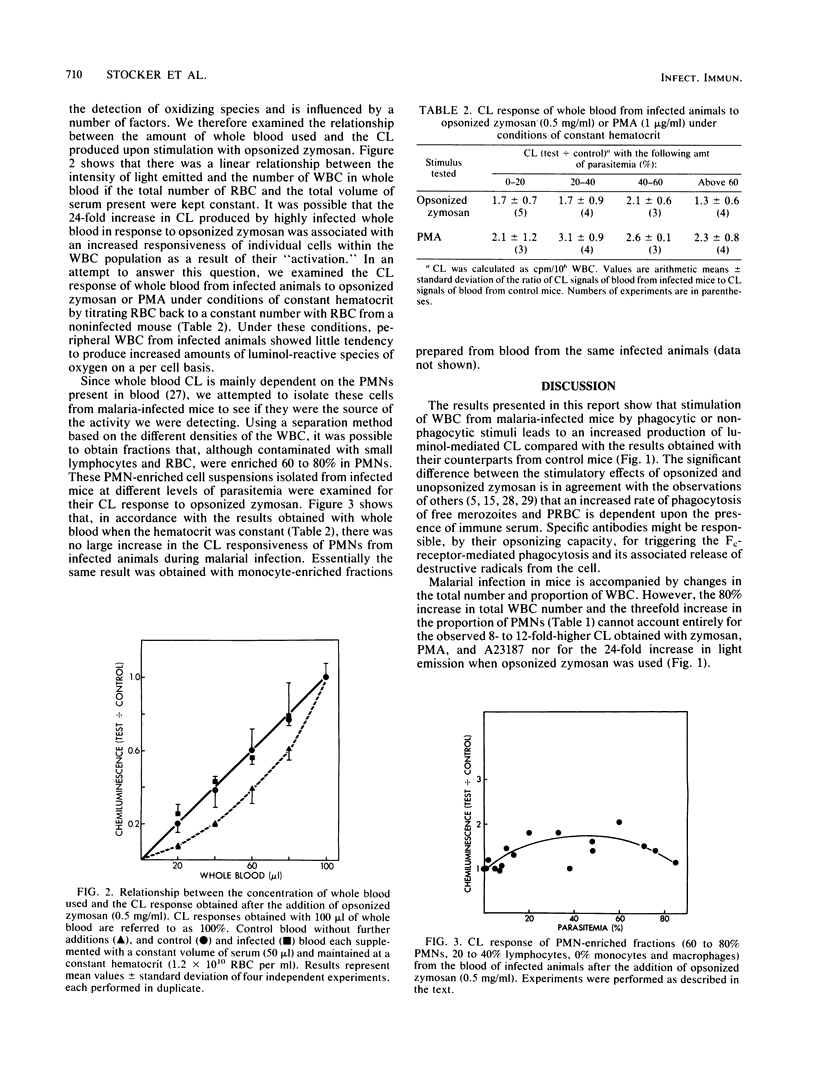
We tested the ability of whole blood and enriched fractions of peripheral blood polymorphonuclear leukocytes obtained from mice during the course of infection with Plasmodium vinckei to produce luminol-mediated chemiluminescence in response to phagocytic and nonphagocytic stimuli. The chemiluminescence response of whole blood to all stimuli increased dramatically and nonlinearly as the infection progressed, and there was a concomitant increase (80%) and decrease (70%) in the total numbers of leukocytes and erythrocytes, respectively. The proportion of polymorphonuclear leukocytes in the total leukocyte population increased threefold. On a per cell basis and at a constant hematocrit, the chemiluminescence response of peripheral leukocytes from infected animals to phorbol myristate acetate or opsonized zymosan was only slightly greater than that of cells from uninfected animals. Polymorphonuclear leukocytes isolated from the blood of infected animals also showed no large increase per cell in chemiluminescence responsiveness. Thus, although leukocyte numbers increase during a murine malarial infection, there appears to be no major change in the capacity of individual peripheral blood leukocytes to produce activated species of oxygen. However, the physiological reduction in the total concentration of hemoglobin at high parasitemia, due to hemolysis and hemoglobin digestion by the parasites, increases the possibility of oxygen radical-mediated damage to tissues and intraerythrocytic parasites as a result of decreased antioxidant protection.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. C., Loose L. D. Phagocytic activation of a luminol-dependent chemiluminescence in rabbit alveolar and peritoneal macrophages. Biochem Biophys Res Commun. 1976 Mar 8;69(1):245–252. doi: 10.1016/s0006-291x(76)80299-9. [DOI] [PubMed] [Google Scholar]
- Babior B. M. Oxygen-dependent microbial killing by phagocytes (first of two parts). N Engl J Med. 1978 Mar 23;298(12):659–668. doi: 10.1056/NEJM197803232981205. [DOI] [PubMed] [Google Scholar]
- Badwey J. A., Karnovsky M. L. Active oxygen species and the functions of phagocytic leukocytes. Annu Rev Biochem. 1980;49:695–726. doi: 10.1146/annurev.bi.49.070180.003403. [DOI] [PubMed] [Google Scholar]
- Carrell R. W., Winterbourn C. C., Rachmilewitz E. A. Activated oxygen and haemolysis. Br J Haematol. 1975 Jul;30(3):259–264. doi: 10.1111/j.1365-2141.1975.tb00540.x. [DOI] [PubMed] [Google Scholar]
- Celada A., Cruchaud A., Perrin L. H. Phagocytosis of Plasmodium falciparum-parasitized erythrocytes by human polymorphonuclear leukocytes. J Parasitol. 1983 Feb;69(1):49–53. [PubMed] [Google Scholar]
- Chaudhry A. N., Santinga J. T., Gabig T. G. The subcellular particulate NADPH-dependent O2.(-)-generating oxidase from human blood monocytes: comparison to the neutrophil system. Blood. 1982 Oct;60(4):979–983. [PubMed] [Google Scholar]
- Clark I. A., Allison A. C., Cox F. E. Protection of mice against Babesia and Plasmodium with BCG. Nature. 1976 Jan 29;259(5541):309–311. doi: 10.1038/259309a0. [DOI] [PubMed] [Google Scholar]
- Clark I. A., Cowden W. B., Butcher G. A. Free oxygen radical generators as antimalarial drugs. Lancet. 1983 Jan 29;1(8318):234–234. doi: 10.1016/s0140-6736(83)92603-x. [DOI] [PubMed] [Google Scholar]
- Clark I. A., Hunt N. H. Evidence for reactive oxygen intermediates causing hemolysis and parasite death in malaria. Infect Immun. 1983 Jan;39(1):1–6. doi: 10.1128/iai.39.1.1-6.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark I. A., Virelizier J. L., Carswell E. A., Wood P. R. Possible importance of macrophage-derived mediators in acute malaria. Infect Immun. 1981 Jun;32(3):1058–1066. doi: 10.1128/iai.32.3.1058-1066.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeChatelet L. R., Shirley P. S. Evaluation of chronic granulomatous disease by a chemiluminescence assay of microliter quantities of whole blood. Clin Chem. 1981 Oct;27(10):1739–1741. [PubMed] [Google Scholar]
- Dockrell H. M., Playfair J. H. Killing of blood-stage murine malaria parasites by hydrogen peroxide. Infect Immun. 1983 Jan;39(1):456–459. doi: 10.1128/iai.39.1.456-459.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easmon C. S., Cole P. J., Williams A. J., Hastings M. The measurement of opsonic and phagocytic function by Luminol-dependent chemiluminescence. Immunology. 1980 Sep;41(1):67–74. [PMC free article] [PubMed] [Google Scholar]
- Jensen J. B., Boland M. T., Allan J. S., Carlin J. M., Vande Waa J. A., Divo A. A., Akood M. A. Association between human serum-induced crisis forms in cultured Plasmodium falciparum and clinical immunity to malaria in Sudan. Infect Immun. 1983 Sep;41(3):1302–1311. doi: 10.1128/iai.41.3.1302-1311.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen J. B., Hoffman S. L., Boland M. T., Akood M. A., Laughlin L. W., Kurniawan L., Marwoto H. A. Comparison of immunity to malaria in Sudan and Indonesia: crisis-form versus merozoite-invasion inhibition. Proc Natl Acad Sci U S A. 1984 Feb;81(3):922–925. doi: 10.1073/pnas.81.3.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khusmith S., Druilhe P., Gentilini M. Enhanced Plasmodium falciparum merozoite phagocytosis by monocytes from immune individuals. Infect Immun. 1982 Mar;35(3):874–879. doi: 10.1128/iai.35.3.874-879.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch R. E., Fridovich I. Effects of superoxide on the erythrocyte membrane. J Biol Chem. 1978 Mar 25;253(6):1838–1845. [PubMed] [Google Scholar]
- Lynch R. E., Fridovich I. Permeation of the erythrocyte stroma by superoxide radical. J Biol Chem. 1978 Jul 10;253(13):4697–4699. [PubMed] [Google Scholar]
- Makimura S., Brinkmann V., Mossmann H., Fischer H. Chemiluminescence response of peritoneal macrophages to parasitized erythrocytes and lysed erythrocytes from Plasmodium berghei-infected mice. Infect Immun. 1982 Aug;37(2):800–804. doi: 10.1128/iai.37.2.800-804.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno Y. I., Hirai K. I., Kanoh T., Uchino H., Ogawa K. Subcellular localization of hydrogen peroxide production in human polymorphonuclear leukocytes stimulated with lectins, phorbol myristate acetate, and digitonin: an electron microscopic study using CeCl3. Blood. 1982 Nov;60(5):1195–1202. [PubMed] [Google Scholar]
- Ohno Y., Hirai K., Kanoh T., Uchino H., Ogawa K. Subcellular localization of H2O2 production in human neutrophils stimulated with particles and an effect of cytochalasin-B on the cells. Blood. 1982 Jul;60(1):253–260. [PubMed] [Google Scholar]
- Picard-Maureau A., Hempelmann E., Krämmer G., Jackisch R., Jung A. Glutathionstatus in Plasmodium vinckei parasitierten Erythrozyten in Abhängigkeit vom intraerythrozytären Entwicklungsstadium des Parasiten. Tropenmed Parasitol. 1975 Dec;26(4):405–416. [PubMed] [Google Scholar]
- Shear H. L., Nussenzweig R. S., Bianco C. Immune phagocytosis in murine malaria. J Exp Med. 1979 Jun 1;149(6):1288–1298. doi: 10.1084/jem.149.6.1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tono-Oka T., Ueno N., Matsumoto T., Ohkawa M., Matsumoto S. Chemiluminescence of whole blood. 1. A simple and rapid method for the estimation of phagocytic function of granulocytes and opsonic activity in whole blood. Clin Immunol Immunopathol. 1983 Jan;26(1):66–75. doi: 10.1016/0090-1229(83)90174-5. [DOI] [PubMed] [Google Scholar]
- Trubowitz S., Masek B. Plasmodium falciparum: phagocytosis by polymorphonuclear leukocytes. Science. 1968 Oct 11;162(3850):273–274. doi: 10.1126/science.162.3850.273. [DOI] [PubMed] [Google Scholar]
- Vernes A. Phagocytosis of P falciparum parasitised erythrocytes by peripheral monocytes. Lancet. 1980 Dec 13;2(8207):1297–1298. doi: 10.1016/s0140-6736(80)92357-0. [DOI] [PubMed] [Google Scholar]
- Weiss S. J. Neutrophil-mediated methemoglobin formation in the erythrocyte. The role of superoxide and hydrogen peroxide. J Biol Chem. 1982 Mar 25;257(6):2947–2953. [PubMed] [Google Scholar]



