In aqueous solution, the water molecules in the layer surrounding an ion rearrange through exchange reactions, in which the attractive hydrogen (H) bond between a water molecule and this ion switches to a new H bond between that water and another water molecule. This exchange and its time scale are critical in a wide range of chemical and biochemical processes. For aqueous reactions such as substitution processes (1) or acid–base mechanisms (2) or for reactions at aqueous interfaces (3), it can be the rate-determining step. It is also key for transport of ionic solutes in water; ionic mobility, for example, depends not only on the ion size but also on the hydration shell lability, which determines whether the ion travels alone or accompanied by its hydration layer (4). Further, this H-bond exchange plays a central role in several physiological contexts. One example is nerve signal transmission, which involves numerous ion transports across cell membranes, where the ions' hydration shells reorganize in the initial and final stages of the membrane-crossing mechanism (5). However, a direct experimental measurement of this ultrafast (picosecond) H-bond exchange has been lacking. In a recent issue of PNAS, Moilanen et al. (6) reported a breakthrough in capturing this exchange reaction with ultrafast infrared (IR) laser pulses.
In the simplest description, an H bond in water is an electrostatic attraction between a partially positively charged H of the hydroxyl group (OH) in the H2O molecule and the partially negatively charged oxygen (O) in another. H bonds are responsible for many of the features that make water so special, e.g., water is able to dissolve a wide range of compounds, solid water, i.e., ice, floats over the liquid, etc. (7). On average, a given water molecule is H-bonded to four other water molecules in a tetrahedral arrangement, with two bonds donated through the given water H atoms and two bonds accepted via the oxygen from two other water molecule H atoms. Water H-bonds to negatively charged ions (anions) are in part responsible for water's power to dissolve, e.g., ordinary table salt, which comprises Na+ and Cl− ions. The number and strength of water H bonds to an anion varies with the anion's size and charge; for example, the first hydration shell of the chloride ion, Cl−, contains ≈6 water molecules. In the aqueous solution of the salt sodium tetrafluoroborate (NaBF4) used in the experiments of ref. 6, the larger BF4− ion hydration shell contains ≈7 water molecules. The partially positively charged H of a hydroxyl group will tend to avoid the positively charged sodium ion, Na+, such that no H bond would be formed (8).
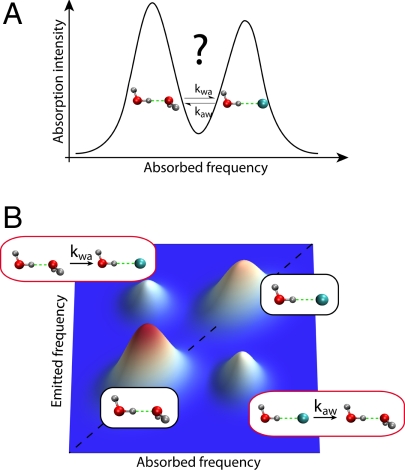
Whether a hydroxyl OH unit in a water molecule is H-bonded to the BF4− anion or to another water molecule is reflected in different hydroxyl IR absorption frequencies, a feature due to the differing H-bond strengths involved and visible in the (time-independent) IR vibrational spectrum (Fig. 1A). This spectrum reflects the fractions of water molecules forming an H bond with the anion vs. another water molecule. However, while the linewidths are partly determined by the exchange time scale, they do not provide unambiguous information on the kinetics underlying this equilibrium. To reveal the dynamics of the exchange reaction between these two states, Moilanen et al. (6) employ an ultrafast two-dimensional (2D) infrared vibrational echo technique to follow the hydroxyl's exchange of H-bonding states (9–11). The similarities and differences of ultrafast vibrational echo spectroscopy and its much older and longer time scale NMR cousin have been particularly well described in ref. 12.
Fig. 1.
Comparison of the one- and two-dimensional infrared spectra of an aqueous salt solution. (A) The one-dimensional absorption spectrum exhibits bands corresponding to the configurations where a water hydroxyl group, OH, donates an H bond either to another water or to an anion. No unambiguous information on the exchange kinetics between these states can be inferred. (B) The (simplified) two-dimensional vibrational echo spectrum for a given population dynamics period during which the system can undergo chemical exchanges. Whereas the diagonal peaks originate from hydroxyls experiencing identical initial and final states, the off-diagonal peaks result from OH groups exchanging between the two states; their growth with the exchange period provides the exchange rate constants for the H-bond switching. (Additional peaks due to transitions involving higher excited states are omitted for clarity. Further, for important technical reasons, the vibrations studied in ref. 6 are not of OH but rather of the OD stretch in partially deuterated water molecules, HOD, dilute in liquid H2O; here and in the text, we ignore this feature for ease of exposition.)
The key relevant feature of the vibrational echo technique is illustrated in Fig. 1B. The respective roles of the three successive laser pulses involved have been nicely summarized by Moilanen et al. (6). The first pulse labels the initial structures, before the second one starts the clock to time the exchange period, ending with the third pulse, which is eventually followed by the emission of the vibrational echo, revealing the nature of the final structure. This sequence leads to a 2D spectrum depending on the absorbed and emitted hydroxyl vibrational frequencies. The simplified representation in Fig. 1B shows the presence of diagonal peaks corresponding to hydroxyls that are in the same state at the beginning and at the end of the exchange period. The key portion of the 2D spectrum lies in the off-diagonal peaks corresponding to hydroxyls with different initial and final states, i.e., OH groups that have undergone a chemical exchange. The kinetics of these peaks provides the exchange forward and backward rate constants.
In fact, there is a panoply of dynamical effects possible for a water molecule in addition to H-bond exchange, and these require attention in the spectroscopic analysis. A partial listing of these effects includes population decay—vibrational energy transfer of an excited water OH stretching vibration, ultimately to the surrounding molecules; spectral diffusion—time evolution of the OH vibrational frequency (without any population decay) due to the fluctuating forces on the OH bond exerted by molecules in its surroundings; and orientational relaxation—H2O angular dynamics due to the torques experienced by the water molecule because of those nearby molecules. These effects, especially spectral diffusion (13) and orientational relaxation (14), are intimately connected to the exchange process. This is why previous experiments have suggested indirect estimates for the exchange time scale through the frequency correlation time or the anisotropy decay time (8, 15). In 2D IR, these effects distort the off-diagonal echo peaks which uniquely reveal the exchange, and were accounted for in ref. 6 to extract the exchange kinetic rate constants, which define the time scales for the H-bond switch.
H bonds are responsible for features that make water special.
The results presented by Moilanen et al. (6) represent a significant advance in the study of H-bond exchange dynamics. Future experiments will undoubtedly lift the current limitations of the technique, such as the high concentrations needed to obtain sufficient signal (a nearly saturated salt solution was used in ref. 6). Further applications of vibrational echo chemical exchange spectroscopy should deepen our understanding of the behavior of ions next to interfaces (16) and of the water dynamics next to extended hydrophobic surfaces such as air/water or water/organic solvent (17) interfaces. This technique should also prove extremely helpful to decipher the water dynamics around biological solutes with exposed charged sites such as phosphate groups in DNA strands (18, 19), and possibly even proteins (20).
Footnotes
The authors declare no conflict of interest.
See companion article on page 375 of issue 2 of volume 106.
References
- 1.Gertner BJ, Whitnell RM, Wilson KR, Hynes JT. Activation to the transition-state: Reactant and solvent energy-flow for a model SN2 reaction in water. J Am Chem Soc. 1991;113:74–87. doi: 10.1021/ja00001a014. [DOI] [PubMed] [Google Scholar]
- 2.Ando K, Hynes JT. Acid-base proton transfer and ion pair formation in solution. Adv Chem Phys. 1999;110:381–430. [Google Scholar]
- 3.Bianco R, Hynes JT. Heterogeneous reactions important in atmospheric ozone depletion: A theoretical perspective. Acc Chem Res. 2006;39:159–165. doi: 10.1021/ar040197q. [DOI] [PubMed] [Google Scholar]
- 4.Ohtaki H, Radnai T. Structure and dynamics of hydrated ions. Chem Rev. 1993;93:1157–1204. [Google Scholar]
- 5.Gouaux E, MacKinnon R. Principles of selective ion transport in channels and pumps. Science. 2005;310:1461–1465. doi: 10.1126/science.1113666. [DOI] [PubMed] [Google Scholar]
- 6.Moilanen DE, Wong D, Rosenfeld DE, Fenn EE, Fayer MD. Ion–water hydrogen-bond switching observed with 2D IR vibrational echo chemical exchange spectroscopy. Proc Natl Acad Sci USA. 2009;106:375–380. doi: 10.1073/pnas.0811489106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eisenberg D, Kauzmann W. The Structure and Properties of Water. London: Oxford Clarendon Press; 1969. [Google Scholar]
- 8.Kropman MF, Bakker HJ. Dynamics of water molecules in aqueous solvation shells. Science. 2001;291:2118–2120. doi: 10.1126/science.1058190. [DOI] [PubMed] [Google Scholar]
- 9.Kim YS, Hochstrasser RM. Chemical exchange 2D IR of hydrogen-bond making and breaking. Proc Natl Acad Sci USA. 2005;102:11185–11190. doi: 10.1073/pnas.0504865102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zheng JR, et al. Ultrafast dynamics of solute-solvent complexation observed at thermal equilibrium in real time. Science. 2005;309:1338–1343. doi: 10.1126/science.1116213. [DOI] [PubMed] [Google Scholar]
- 11.Kwak K, Zheng JR, Cang H, Fayer MD. Ultrafast two-dimensional infrared vibrational echo chemical exchange experiments and theory. J Phys Chem B. 2006;110:19998–20013. doi: 10.1021/jp0624808. [DOI] [PubMed] [Google Scholar]
- 12.Dlott DD. Ultrafast chemical exchange seen with 2D vibrational echoes. Science. 2005;309:1333–1334. doi: 10.1126/science.1117435. [DOI] [PubMed] [Google Scholar]
- 13.Nigro B, Re S, Laage D, Rey R, Hynes JT. On the ultrafast infrared spectroscopy of anion hydration shell hydrogen bond dynamics. J Phys Chem A. 2006;110:11237–11243. doi: 10.1021/jp064846m. [DOI] [PubMed] [Google Scholar]
- 14.Laage D, Hynes JT. Reorientational dynamics of water molecules in anionic hydration shells. Proc Natl Acad Sci USA. 2007;104:11167–11172. doi: 10.1073/pnas.0701699104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kropman MF, Nienhuys HK, Bakker HJ. Real-time measurement of the orientational dynamics of aqueous solvation shells in bulk liquid water. Phys Rev Lett. 2002;88:77601. doi: 10.1103/PhysRevLett.88.077601. [DOI] [PubMed] [Google Scholar]
- 16.Jungwirth P, Winter B. Ions at aqueous interfaces: From water surface to hydrated proteins. Annu Rev Phys Chem. 2008;59:343–366. doi: 10.1146/annurev.physchem.59.032607.093749. [DOI] [PubMed] [Google Scholar]
- 17.Scatena LF, Brown MG, Richmond GL. Water at hydrophobic surfaces: Weak hydrogen bonding and strong orientation effects. Science. 2001;292:908–912. doi: 10.1126/science.1059514. [DOI] [PubMed] [Google Scholar]
- 18.Qu XG, Chaires JB. Hydration changes for DNA intercalation reactions. J Am Chem Soc. 2001;123:1–7. doi: 10.1021/ja002793v. [DOI] [PubMed] [Google Scholar]
- 19.Szyc L, Dwyer JR, Nibbering ETJ, Elsaesser T. Ultrafast dynamics of N–H and O–H stretching excitations in hydrated DNA oligomers. Chem Phys. 2009 doi: 10.1016/j.chemphys.2008.08.013. [DOI] [Google Scholar]
- 20.Ganim Z, et al. Amide I two-dimensional infrared spectroscopy of proteins. Acc Chem Res. 2008;41:432–441. doi: 10.1021/ar700188n. [DOI] [PubMed] [Google Scholar]