Abstract
Glucagon-like peptide-1 (GLP-1) is an endogenous insulinotropic peptide secreted from the gastrointestinal tract in response to food intake. It enhances pancreatic islet β-cell proliferation and glucose-dependent insulin secretion, and lowers blood glucose and food intake in patients with type 2 diabetes mellitus (T2DM). A long-acting GLP-1 receptor (GLP-1R) agonist, exendin-4 (Ex-4), is the first of this new class of antihyperglycemia drugs approved to treat T2DM. GLP-1Rs are coupled to the cAMP second messenger pathway and, along with pancreatic cells, are expressed within the nervous system of rodents and humans, where receptor activation elicits neurotrophic actions. We detected GLP-1R mRNA expression in both cultured embryonic primary cerebral cortical and ventral mesencephalic (dopaminergic) neurons. These cells are vulnerable to hypoxia- and 6-hydroxydopamine–induced cell death, respectively. We found that GLP-1 and Ex-4 conferred protection in these cells, but not in cells from Glp1r knockout (-/-) mice. Administration of Ex-4 reduced brain damage and improved functional outcome in a transient middle cerebral artery occlusion stroke model. Ex-4 treatment also protected dopaminergic neurons against degeneration, preserved dopamine levels, and improved motor function in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) mouse model of Parkinson's disease (PD). Our findings demonstrate that Ex-4 can protect neurons against metabolic and oxidative insults, and they provide preclinical support for the therapeutic potential for Ex-4 in the treatment of stroke and PD.
Keywords: diabetes, exendin-4, neurodegeneration, neuroprotection, stroke
Type 2 diabetes mellitus (T2DM) is emerging as one of the largest health issues worldwide; with some 6% of the world's adult population now affected (1). Although T2DM now occurs more often in the young, the incidence rises dramatically with age, along with that of many of other conditions, including acute and chronic neurologic disorders, exemplified by stroke (2), Parkinson's disease (PD) and Alzheimer's disease (AD) (3,4), which, like T2DM, were once considered relatively infrequent. Indeed, the incidence of stroke, PD, AD, and several other neurologic disorders appears to be higher in persons with T2DM, suggesting that shared mechanisms, such as insulin dysregulation, may underlie these conditions (5). Although associated with different cell types in divergent areas (e.g., cortical and striatal neurons in stroke, substantia nigral and midbrain dopaminergic neurons in PD, pancreatic β-cells in T2DM), parallel biochemical cascades are triggered by specific environmental and genetic signals and lead to the cellular dysfunction and death characteristic of all of these disorders. Consequently, it is possible that an effective treatment strategy for one such disorder may prove beneficial in others as well.
The glucagon-like peptide-1 receptor (GLP-1R) agonist, exendin-4 (Ex-4), is a long-acting analog of the endogenous insulinotropic peptide GLP-1 (supporting information (SI) Fig. S1). GLP-1 is derived from the posttranslational modification of proglucagon and is released from the L cells of the small intestine in response to food ingestion (6,7). GLP-1 and Ex-4 have potent effects on glucose-dependent insulin secretion and insulin gene expression through binding and activation of the G protein–coupled GLP-1R on pancreatic β-cells. Both peptides also have trophic properties, inducing pancreatic β-cell proliferation and inhibiting apoptosis (7,8). Ex-4 has been approved for the treatment of T2DM, in which it has been found to effectively lower plasma glucose levels.
GLP-1R mRNA occurs widely throughout the brains of rodents (9) and humans (6,7,10), and both GLP-1 and Ex-4 can readily enter the brain (11) to modify feeding and satiety (12). We have previously reported that the activation of GLP-1R by GLP-1 and Ex-4 is neurotrophic, inducing neurite outgrowth in PC12 cells and protecting neurons against various insults (6,13–15) through a cascade involving the second messenger, cAMP (13). In light of these neurotrophic actions, the long-term efficacy of Ex-4 in treating T2DM (7), and the elevated risk of cerebrovascular disease and PD in T2DM (1–3,5), we evaluated GLP-1R stimulation in well-characterized cellular and animal models of both stroke and PD to assess its translational potential.
Results
GLP-1R Is Expressed and Functional in Cultured Embryonic Primary Neurons.
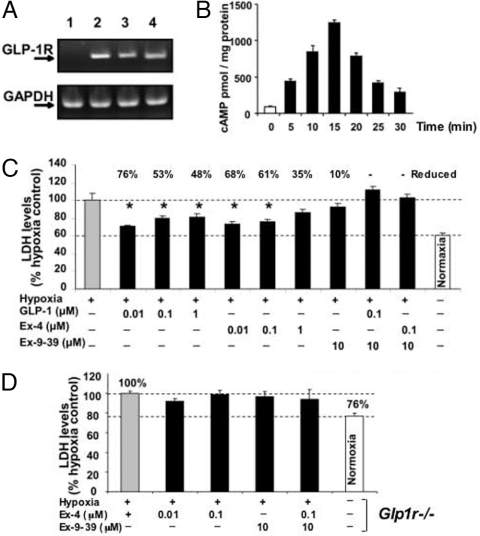
To establish the presence of GLP-1R in primary neurons, cultured rat embryonic cerebral cortical (CC) and ventral mesencephalic (VM) cells were probed for the presence of GLP-1R mRNA by RT-PCR. Both neuron types were found to contain GLP-1R mRNA (Fig. 1A). Incubation of cortical neurons with the natural agonist GLP-1 (10 nM) led to a rapid, transient elevation of intracellular cAMP level. This level peaked within 15 min and then returned toward baseline by 30 min (Fig. 1B), demonstrating the presence of functional GLP-1R in these cells. SH-SY5Y human neuroblastoma cells also expressed GLP-1R at both the mRNA and protein levels, along with increased intracellular cAMP levels, in response to Ex-4 (not shown).
Fig. 1.
(A) One-step RT-PCR shows rat GLP-1R mRNA expression in neuronal cell cultures. The expected RT-PCR product size is 190 bp. GAPDH was used as an external control and showed equal expression across lanes. Lane 1, negative control; lane 2, positive control: RNA from CHO-GLP-1R cells (CHO cells stably transfected with rat GLP-1R); lanes 3 and 4, RNA from primary CC and VM neurons, respectively. (B) GLP-1–stimulated release of cAMP from CC neurons. Time-dependent cAMP levels were assayed after incubation with 10 nM GLP-1 (n = 3). (C) Pretreatment with GLP-1/Ex-4 protects CC neurons from hypoxia-induced loss of cell viability, as indicated by elevated levels of secreted LDH. Compared with normoxia (21% O2, 5% CO2), a 3-h exposure to hypoxia (1% O2, 5% CO2) induced a significant elevation in LDH (P < .05), defined as a 100% response. GLP-1 and Ex-4 (0.01–1.0 μM) protected cells, ameliorating the hypoxia-induced elevation in LDH by up to 76%. This effect was abolished by the GLP-1R antagonist Ex-9–39. n ≥ 5 for each treatment, *P < .05 (1-way ANOVA plus posthoc Dunnett's test) versus hypoxia condition. (D) CC neurons from Glp1−/− mice are vulnerable to hypoxia, as assessed by elevated LDH (P < .05 normoxia vs. hypoxia), and Ex-4 offers no protection (P > .05 vs. hypoxia; 1-way ANOVA plus posthoc Dunnett's test, n ≥ 5).
GLP-1R Stimulation Decreased Hypoxia- and Dopaminergic Toxin–Induced Death of Cultured Primary CC and VM Cells.
Primary neurons are vulnerable to hypoxia, resulting in a loss of viability, as assessed by a significant elevation in lactate dehydrogenase (LDH) level (Fig. 1C) and a decline in (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt) (MTS) level (not shown) compared with cells subjected to normoxia. Incubation with a GLP-1R agonist, GLP-1 or Ex-4 (0.01–1 μM), afforded significant protection against hypoxia, lowering elevated LDH levels by as much as 76%. This effect was lost in the presence of the GLP-1R antagonist Ex-9–39, indicating that the action was mediated through GLP-1R. To confirm this, CC neurons from Glp1r−/− mice were similarly cultured and exposed to hypoxia/normoxia in the presence and absence of Ex-4 and the GLP-1R antagonist. Glp1r−/− primary CC neurons were similarly vulnerable to hypoxia but were not protected by Ex-4 (Fig. 1D).
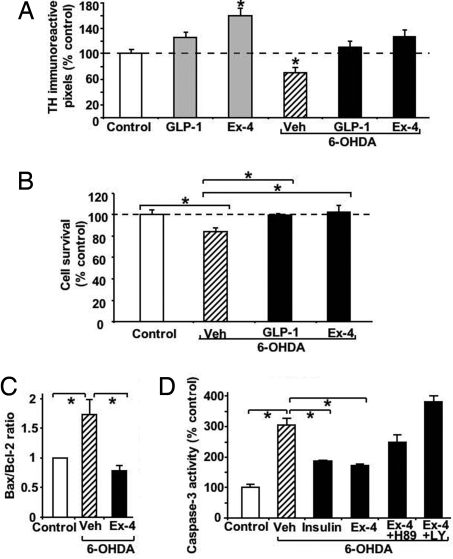
The viability of mesencephalic cell cultures, known to be rich in dopaminergic neurons, was determined by quantifying tyrosine hydroxylase immunoreactivity [TH(IR)] after exposure to the dopaminergic toxin 6-hydroxydopamine (6-OHDA). As expected, 6-OHDA decreased TH(IR) significantly, by 30% (Fig. 2A). GLP-1 and Ex-4 (0.1 μM) fully preserved TH(IR) from 6-OHDA toxicity, and, moreover, Ex-4 elevated TH(IR) in the absence of 6-OHDA by an additional 60%. No significant difference in the number of DAPI-positive nuclei was found among the treatment groups (not shown). To elucidate the universality of these protective effects, parallel studies were performed in SH-SY5Y cells (Fig. 2 B–D). Predictably, exposure to 6-OHDA significantly reduced cell viability (Fig. 2B), with elevations in caspase-3 activity and Bax and declines in Bcl-2 found by Western blot analysis (Fig. 2 C and D). GLP-1 and Ex-4 (0.1 μM) fully protected against 6-OHDA–mediated cell loss and resulted in elevated Bcl-2 and negligible caspase-3 and Bax levels. To define the molecular pathways responsible for the GLP-1R–mediated protection, specific inhibitors of PKA (H89; 10 μM) and PI3K (LY294002; 10 μM) were investigated; these resulted in a loss of protection (Fig. 2D).
Fig. 2.
Pretreatment with GLP-1 or Ex-4 protects TH(IR) of VM primary neurons from 6-OHDA treatment and likewise protects SH-SY5Y cells from 6-OHDA–induced cell death. (A) TH(IR) of primary VM cells pretreated with vehicle (Veh), 0.1 μM GLP-1, or 0.1 μM Ex-4 for 3 h before administration of PBS or 6-OHDA for 90 min. TH-immunostaining was performed 24 h after 6-OHDA. TH(IR) was significantly different versus PBS-treated controls (P < .05; Dunnett's t-test with SNK, n = 5). (B) In a parallel study, SH-SY5Y cells were treated with vehicle (Veh), GLP-1, or Ex-4 (0.1 uμM) for 2 h and then subjected to 6-OHDA (30 μM) for 24 h. Subsequently, cell survival was quantified by MTS assay. Whereas 6-OHDA reduced cell survival to 83% (*P < .05 vs. control), GLP-1 and Ex-4 protected against this 6-OHDA loss of cell viability (*P < .05 vs. vehicle plus 6-OHDA; Dunnett's t-test, n = 6/group). (C and D) In SH-SY5Y cells, markers of apoptosis were elevated by 6-OHDA (30 μM) and lowered by Ex-4 (0.1 μM) after 2 h of pretreatment. This protection was lost in cells treated with inhibitors of PKA (H89; 10 μM) or PI3K (LY294002; 10 μM) and was retained with insulin (0.01 μM; positive control) (*P < .05, Dunnett's t-test, n = 5 vs. vehicle plus 6-OHDA).
Ex-4 Treatment Reduces Infarction Size and Improves Functional Outcome in Stroke.
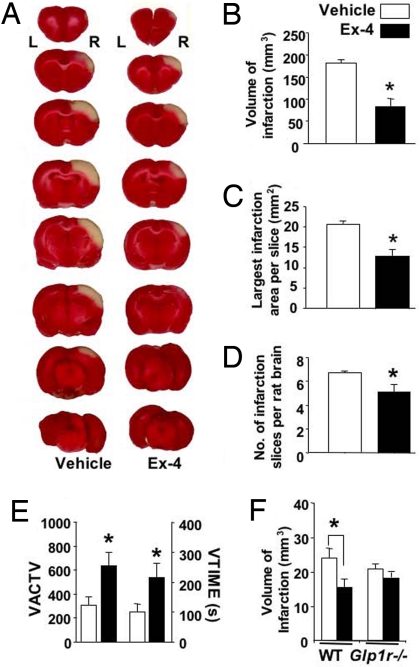
To define the translational potential of our cell culture studies, the protective effect of Ex-4 was evaluated in a well-characterized rodent model of stroke, middle cerebral artery occlusion (MCAo), which mimics the most common type of human stroke. A 1-h transient occlusion produced a well-demarked area of infarction that, as assessed by triphenyltetrazolium chloride (TTC) staining at 48 h, spanned the right frontal, parietal, and occipital cerebral cortices (Fig. 3A). The infarct volume, assessed by measuring the number of 2-mm-thick brain slices affected and the infarct area, was reduced by > 50% in Ex-4–pretreated rats compared with controls. Ex-4 significantly reduced each measured parameter of infarction size (Fig. 3 B–D) and was accompanied by improved functional outcome, as assessed by locomotor activity measures at 2 days (Fig. 3E). Because changes in body temperature, blood pressure, and arterial blood gases may affect the outcome of stroke, these parameters were measured in Ex-4–treated and control rats both before and after treatment (Table S1); no significant changes were found. Likewise, cerebral blood flow remained unchanged before, during, and after Ex-4 administration (Fig. S2). Ex-4's lack of effects on these parameters suggests that its beneficial effects in stroke are due primarily to its central actions. To confirm that these actions are mediated through GLP-1R, parallel studies were performed in wild-type (WT) and Glp1r−/− mice. Ex-4 was found to afford protection in the WT mice, but not in the Glp1r−/− mice (Figs. 3F and S3).
Fig. 3.
Ex-4 markedly reduced cortical infarction induced by transient MCAo. (A) After Ex-4 (1 μM × 20 μL; 83 ng) or vehicle (PBS) administration into the left (L) lateral ventricle, the right (R) middle cerebral artery was ligated for 60 min. At 48 h after ischemia/reperfusion, rats were killed, the brain was sliced into 2-mm sections, and stained with TTC. Marked infarction (white areas) within the right cerebral cortex was found. The size of infarction was significantly decreased in animals treated with Ex-4, compared to vehicle (n = 10/group), with regard to (B) the volume of infarction = [sum of the infarction area in all brain slices (mm2)] × [slice thickness, 2 mm], (C) the area of the largest infarction in a slice, and (D) the number of infarcted slices from each rat brain. (E) Pretreatment with Ex-4 increased locomotor activity in stroke rats, assessed 48 h after ischemia/reperfusion in an activity chamber. Vertical activity (VACTV) and vertical movement time (VTIME) were determined from the number of beam interruptions that occurred in vertical sensors and the time (s) spent in vertical movement during a 30-min test, respectively. (F) Likewise, Ex-4 (1 μM × 5 μL left lateral ventricle) decreased infarct volume in the WT mice but was ineffective in the Glp1r−/− mice (WT control, n = 6; WT Ex4, n = 7; Glp1r−/− control, n = 8; Glp1r−/−, Ex4, n = 7). Overall, *P ≤ .05 1-way ANOVA and Student's t-test.
Ex-4 Treatment Preserves Dopaminergic Cells in a MPTP-Induced PD Model.
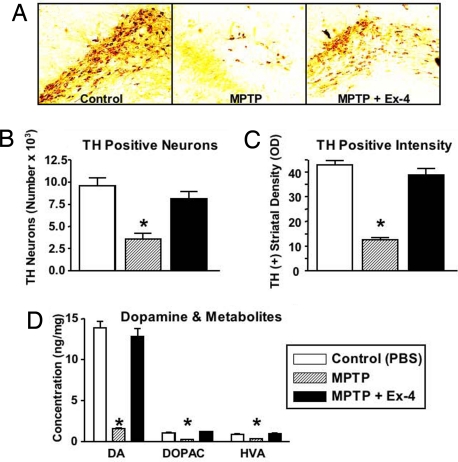
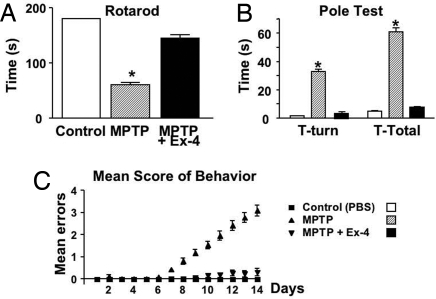
The neuroprotective actions of Ex-4 were quantified in a well-characterized model of PD. Exposure to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) induces a PD-like syndrome in humans, monkeys, and mice. In the brain, MPTP is converted to MPP+, which is selectively transported into dopaminergic neuron axon terminals, causing oxidative stress, mitochondrial dysfunction, and cell death (16). Analyses of dopaminergic markers in mice given MPTP demonstrated a cell loss that culminated in motor function impairment. Ex-4 afforded complete protection against dopaminergic neuron damage and motor impairment. Specifically, compared with controls, MPTP significantly reduced the number of TH-immunopositive (+) neurons within the substantia nigra (SN) by 63%, as assessed by immunohistochemistry (Fig. 4A and B), and depleted TH(+) intensity by 71%, as assessed by immunoblotting (Fig. 4C). In parallel, levels of dopamine (DA) and metabolites [dihydroxyphenylacetic acid (DOPAC) and homovanillic acid (HVA)] were reduced dramatically, and the ratio of metabolites to DA concentration was elevated, consequent to MPTP (Fig. 4D). In contrast, mice given Ex-4 before MPTP showed no differences from controls in terms of the number and intensity of SN TH(+) neurons, as assessed by immunoblotting, and DA and metabolite levels and ratios. Motor function was quantified by several paradigms over multiple days, including mean score of behavior, rotarod, pole test (Fig. 5), beam walk, and open-field activity (Fig. S4); performance in all animals was significantly impaired by MPTP. In contrast, motor function was fully preserved after Ex-4 treatment and for all paradigms was similar to that of controls not treated with MPTP.
Fig. 4.
Mice given Ex-4 (20 nM, 0.25 μL/h in the lateral ventricle over 7 days) were protected from MPTP-induced damage of the dopaminergic system, quantitatively assessed by TH immunohistochemical analysis of the SN and TH immunoblot analyses of the striatum at 7 days. (A) Representative SN sections from control, and MPTP-treated mice with and without Ex-4. (B) Compared with controls, TH(+) cells in SN were reduced by MPTP (*P < .05). Those from mice given Ex-4 and MPTP were no different from controls (P > .05). (C) Similarly, as assessed by immunoblotting in striatum, TH levels were significantly reduced by MPTP (*P < .05 vs. controls) and no different from controls for mice given Ex-4 and MPTP (P > .05, Dunnett's t-test, n = 10/group). (D) Mice given Ex-4 were protected from MPTP-induced depletion of brain DA and metabolites (DOPAC and HVA). DA, DOPAC, and HVA from striatum were quantified by HPLC at 7 days in mice treated with PBS, MPTP, and MPTP plus Ex-4. Levels of each were reduced by MPTP (P < .05 vs. PBS) and preserved by Ex-4 (P > .05 vs. PBS; P < .05 vs. MPTP) compared with controls (Dunnett's t-test, n = 10). The ratios of DOPAC:DA and HVA:DA were 0.08 and 0.065 in controls, 0.16 and 0.31 in MPTP-treated mice, and 0.095 and 0.08 in MPTP plus Ex-4–treated mice.
Fig. 5.
Ex-4 protection of MPTP-induced toxicity of dopaminergic neurons has behavioral consequences. (A) Rotarod: The ability of mice to remain on a rotating rod at 7 days was reduced (67%; P < .05 vs. PBS) by MPTP and preserved by Ex-4 (P > .05 vs. PBS; P < .05 vs. MPTP). (B) Pole test: Assessed on 2 consecutive days, initially 3 h after MPTP. The time taken for mice to turn around (T-Turn) and descend a pole (T-Total) was slower in the MPTP-treated mice (P < .05 vs. PBS and Ex-4 plus MPTP) and no different from PBS controls (P > .05) in the MPTP plus Ex-4 mice. (C) Mean score of behavior: A composite of tests were rated daily. Whereas the MPTP plus Ex-4 mice were no different than the PBS controls, the MPTP mice could be differentiated on and after 7 days (*P < .05 vs. PBS, Dunnett's t-test, n = 10/group).
Discussion
The risk of both stroke and PD is elevated in persons with T2DM (17,18), even in newly treated patients, in whom the short-term risk of stroke is doubled (17). Clearly, an effective neuroprotective strategy would be valuable for this vulnerable patient group, as well as for the general population, given the lack of effective treatments for stroke and PD. Increasing evidence suggests that cortical and dopaminergic neurons die through apoptosis after a stroke and through a related form of programmed cell death during PD (19). Evidence for classic apoptosis in both conditions includes elevated levels of the apoptotic stress-activated protein kinases, caspase-3 (19–21) and of proapoptotic genes and proteins (19,21,22), as was evident in our cell culture studies. Analogous elevations in markers of apoptosis have been described in pancreatic β-cells during T2DM (7,23), one of many commonalities shared by these degenerative conditions. The ability to initiate a degenerative process in different cell types by widely varying insults suggests the existence of a common cell death network that can be entered from different points but, once activated, follows similar interrelated biochemical pathways, with little dependence on the site of entry (22). In such a system, a strategy that effectively halts the death network process in one disease, such as T2DM, may hold promise for another, particularly when the molecular machineries underpinning this action share commonalities.
The incretin, GLP-1, and long-acting Ex-4 induce numerous biological actions in the pancreas, including stimulation of glucose-dependent insulin secretion, elevated insulin synthesis, decreased glucagon levels, and, notably, β-cell proliferation and inhibition of β-cell apoptosis (7,8). These and other actions are mediated through the G protein-coupled GLP-1R, and Ex-4 has demonstrated therapeutic value in T2DM (7). GLP-1R is a member of the class B family of 7-transmembrane-spanning, heterotrimeric G protein-coupled receptors. In humans and rodents, a single structurally identical GLP-1R has been identified that is expressed in a wide range of tissues, including the brain. GLP-1–immunoreactive fibers and GLP-1Rs are widely expressed throughout the brain (6,9,10). Ligand activation of the Gα subunit of GLP-1R on pancreatic β-cells leads to activation of adenylate cyclase activity and production of cAMP, the primary mediator of GLP-1R activation (7).
GLP-1Rs are present in rodent cultured CC and VM primary neurons, as well as hippocampal neurons (14), and also in SH-SY5Y cells. Adding GLP-1 to primary neurons induced a time-dependent elevation in cAMP, indicative of a functional receptor. cAMP-mediated pathways are central to the antiapoptotic actions of GLP-1 in β-cells (6–8), and the neuroprotective effects of cAMP-elevating agents are seen in many neuronal cells, including sensory (24), dopaminergic (25), septal cholinergic (26), cerebellar granule (27,28), and spinal cord motor neurons (29).
Our previous studies have established that a 50% GLP-1R occupancy in primary neurons is achieved by 14 nM GLP-1 (14), a value similar to that for β-cells. Here we show that administration of GLP-1 and Ex-4 to primary CC and VM neurons or SH-SY5Y cells proved to be neuroprotective against insults that modeled stroke and PD. Specifically, these cells were vulnerable to hypoxia and a dopaminergic toxin, as assessed by classic markers of cell viability and the presence of cell death markers. GLP-1 and Ex-4 concentrations as low as 10 nM conferred protection against hypoxia. This effect was lost in the presence of the GLP-1R antagonist Ex-9–39 and was absent in Glp1r−/− neurons, indicating that it is GLP-1R–mediated. Interestingly, not only were VM neurons fully protected from 6-OHDA–induced toxicity by GLP-1 and Ex-4 (100 nM), but also Ex-4 substantially elevated TH(IR) beyond that of untreated controls (Fig. 2A), indicating both neurotrophic and neuroprotective activity. TH also is expressed in catecholamine neurons in the area postrema, and Ex-4 has been shown to significantly elevate TH levels in these neurons by inducing TH gene expression through the TH promoter (30), which contains a cAMP-responsive element (31), representing a further modulatory mechanism that may account in part for the Ex-4–induced rise in TH(IR).
These neurotrophic/protective actions are in accordance with previous findings establishing that GLP-1R stimulation protects hippocampal neurons from amyloid-β peptide–, Fe2+-, and glutamate-induced toxicity (15,32,33). The pathways that underpin the antiapoptotic actions of many endogenous neuroprotective agents commonly converge on activation of the transcription factor cAMP response element–binding protein by phosphorylation. Those mediating GLP-1's antiapoptotic actions in neurons remain to be fully elucidated. Previous work has demonstrated a clear involvement of PKA; neuroprotection by GLP-1 was abolished by Rp-cAMP, which blocks cAMP activation of PKA (13). PI3K and MAPK are other important signaling pathways involved in GLP-1–mediated events. A selective inhibitor of the former (LY294002), but not of the latter (PD98059), has been reported to inhibit GLP-1–mediated protective effects in neuronal cells (13). In the present study, each of these pathways appeared to contribute to the protection afforded by Ex-4 and GLP-1 to SH-SY5Y cells (Fig. 2D). Potential GLP-1 actions mediated through MAPK-independent signaling and growth factor–dependent Ser/Thr kinase AktPKB have been reviewed recently (31–33).
Administration of Ex-4 (10 μg s.c) achieved plasma levels of 200 pg/mL (48 nM) in humans (34), which compare favorably to the doses studied here. To evaluate the translational relevance of the aformentioned cellular effects, the actions of centrally administered Ex-4 were assessed in classical rodent models of stroke and PD. Whereas Ex-4 and GLP-1 readily enter the brain after systemic administration (11), and Ex-4 given by this route has proven effective in alleviating peripheral neuropathy in rodents (35), direct administration into the brain allowed differentiation of centrally mediated GLP-1R actions from numerous systemic ones. Ischemic brain injury activates apoptotic cascades within the ischemic core and penumbra that peak on day 2 after MCAo. p53 mRNA and protein are up-regulated shortly after stroke, leading to p53-dependent programmed cell death in penumbra (36). Administration of Ex-4 substantially decreased infarct size, as assessed by 3 related measures of TTC staining at 48 h in rats (Fig. 3A and B). The reduced stroke volume (≈50%) was similar to that achieved by inhibition of p53-dependent apoptosis (37), suggesting protection from apoptotic rather than necrotic cell death and translating to significant improvements in measures of motor activity. Blood flow, as well as a wide number of physiological parameters (Table S1) that can influence ischemic damage, remained unchanged by Ex-4 administration, supporting a direct central GLP-1R–mediated effect. Parallel studies in WT mice confirm that the neuroprotective actions of Ex-4 in MCAo translate across species, and the loss of this action in Glp1r−/− mice reaffirm that neuroprotection is mediated through GLP-1Rs.
Administration of MPTP in mice induces a consistent dopaminergic cell loss that parallels many aspects of PD (16,20). In the present study, the mice receiving MPTP demonstrated classic reductions in both the number of TH-immunoreactive cells, a marker of dopaminergic cells in the SN (63% loss), and of TH intensity in immunoblot analyses of striatum (71% loss). These animals demonstrated motor function deficits. General behavioral assessment, combining multiple paradigms, detected differences between the MPTP and control animals starting at 7 days after MPTP administration and increasing with time. Specific tests of motor function (i.e., pole test, beam traverse, open-field activity, and rotarod) confirmed MPTP-induced impairment. To correlate reductions in dopaminergic cells with motor function losses, concentrations of DA were quantified at 7 days post-MPTP in striatum; a 89% depletion, accompanied by a 78% drop in DOPAC level and a 75% drop in HVA level, was evident, in line with the results of previous MPTP studies (20). These declines resulted in a 2- to 4-fold elevation in the ratio of metabolite to DA concentration (Fig. 4D). Ex-4 provided complete protection, as assessed by quantification of TH(+) cell number, TH immunoblot analysis results, DA and metabolite levels and ratios, and all behavioral paradigms studied. Overall, the MPTP mice treated with Ex-4 were indistinguishable from controls.
Our findings indicate that the neuroprotective actions of GLP-1R agonists appear to effectively translate across a number of classic cellular and animal models, including stroke and PD, as well as cholinergic ablation (14), kainic acid–induced CA3 hippocampal loss (38), and peripheral neuropathy (35). In contrast, the Glp1r−/− mice demonstrated impaired learning, as well as increased brain injury and associated behaviors after a lesion (32). Together, the findings of these studies suggest a loss of function in -/- mice and a physiological role for GLP-1R activation in the normal brain, as in the pancreas, that can be augmented by pharmacologic concentrations of agonists and inhibited by antagonists. Recent studies have demonstrated that Ex-4 can induce neurogenesis of neural stem cells both in culture and in the subventricular zone of rat brain after a 6-OHDA insult (38,39) and promote differentiation toward a neuronal phenotype (38), as has been reported for PC12 cells (13). Ex-4's ability to improve dopaminergic markers and function when administered a week or more after 6-OHDA– or cytokine-induced apoptosis, rather than at the time of insult as in our study, is indicative of neuroregenerative action (38,39).
In synopsis, the role of GLP-1R stimulation in balancing cell survival versus death in pancreatic cells is well established (7) and, together with the insulinotropic actions of Ex-4, supports its clinical utility in T2DM. Likewise, the parallel GLP-1R–mediated trophic and protective actions of Ex-4 in neurons may be of clinical utility in acute and chronic neurologic disorders, epitomized by stroke and PD. Not only are persons with T2DM at increased risk for stroke and PD, but also several studies have reported a high prevalence of insulin resistance in PD, vascular dementia, and other neurodegenerative conditions (2,4,17), with impaired glucose tolerance seen in 50%–80% of subjects (4,40). Dopaminergic neurons and insulin receptors are both densely localized within the SN, and dopaminergic agents used in PD (e.g, L-DOPA) have been reported to induce hyperglycemia (40). Together, these findings suggest that GLP-1R agonists may exert various useful actions in persons at high risk for stroke or with PD, a hypothesis that is amenable to clinical testing.
Materials and Methods
Cell Cultures.
Primary CC and VM neurons and human SH-SY5Y neuroblastoma cells were probed for GLP-1R mRNA and stimulated with GLP-1 (10 nM) to assess the presence and functionality of GLP-1R. CC cultures were challenged with transient hypoxia (1% O2, 5% CO2, 37 °C, 3 h) followed by normoxia (21%O2, 5% CO2, 37 °C, 48 h). VM cultures were exposed to 6-OHDA (30 μM, 90 min), in the presence and absence of GLP-1 and Ex-4 (10 nM–1.0 μM), and the GLP-1R antagonist Ex-9–39 (10 μM). Cell viability was quantified in hypoxic studies by MTS (Promega) or LDH (Sigma) assays and in 6-OHDA studies by TH immunostaining at 24 h. (For additional details, see SI Materials and Methods.)
Some studies used primary neurons from Glp1r−/− mice, and others used inhibitors of PI3K (LY294002; 10 μM), MAPK (PD98059; 20 μM), and PKA (H89; 10 μM) (13). Biochemical markers of cell death (caspase-3, Bax, and Bcl-2) were assessed by Western blot analysis as described previously (20,41,42).
Animal Studies.
Stroke (MCAo) Model.
At 15 min after left lateral ventricle administration of Ex-4 (1 μM × 20 μL; 83 ng) or vehicle (PBS), adult male Sprague-Dawley rats were subjected to transient (60 min) right-sided MCAo (43). Cerebral blood flow, blood pressure, and related physiological parameters were monitored before, during, and after MCAo. Motor function, assessed in a locomotor activity chamber, and stroke size, defined by TTC staining, were quantified at 48 h (SI Materials and Methods). Likewise, transient (90 min) MCAo was performed in adult male WT and Glp1r−/− ICR mice 15 min after left lateral ventricle administration of Ex-4 (1 μM × 5 μL; 21 ng) or vehicle (PBS), with stroke size determined at 48 h (SI Materials and Methods).
PD (MPTP) Model.
At 2 h after left lateral ventricle administration of Ex-4 (20 nM, 0.25 μL/h over 7 days, using an Alzet pump), adult male C57BL/6 mice were given the dopaminergic toxin MPTP (20 mg/kg in 0.1 mL of PBS i.p. at 2-h intervals × 4 doses MPTP; Sigma) or vehicle (PBS) (20). On day 7, 50-μm sections throughout the SN were processed for immunostaining using TH antibody (T-1299; Sigma), and TH(+) cells were quantified by image analysis. Levels of DA, DOPAC, and HVA were measured by HPLC from striatum, and TH immunoblotting was performed using a TH (phospho S40) antibody (AbCam) (20). Motor function was evaluated by multiple paradigms, including mean score of behaviors, rotarod, pole test, beam walk, and open-field activity as described previously (44,45) (SI Materials and Methods).
Statistics
Dunnett's t-test and 1-way ANOVA with Student-Newman-Keul (SNK) posthoc analysis were used for statistical comparison, with P < .05 considered statistically significant. Data are presented as mean ± SEM.
Supplementary Material
Acknowledgments.
This work was supported in part by the Intramural Research Programs of the National Institute on Aging and the National Institute on Drug Abuse. Animal studies were performed in accordance with approved protocols, in compliance with the National Institutes of Health's Guidelines for Animal Experimentation. Daniel J. Drucker, MD, University of Toronto, kindly provided the Glp1r−/− mice.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0806720106/DCSupplemental.
References
- 1.Sicree R, Shaw J, Zimmet P. In: Diabetes Atlas. 3rd Ed. Gan D, editor. Brussels: International Diabetes Federation; 2006. pp. 15–103. [Google Scholar]
- 2.Hollander M, et al. Incidence, risk, and case fatality of first ever stroke in the elderly population: The Rotterdam Study. J Neurol Neurosurg Psychiatry. 2003;74:317–321. doi: 10.1136/jnnp.74.3.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ott A, et al. Diabetes mellitus and the risk of dementia: The Rotterdam Study. Neurology. 1999;53:1937–1942. doi: 10.1212/wnl.53.9.1937. [DOI] [PubMed] [Google Scholar]
- 4.Arvanitakis Z, Wilson RS, Bienias JL, Evans DA, Bennett DA. Diabetes mellitus and risk of Alzheimer disease and decline in cognitive function. Arch Neurol. 2004;61:661–666. doi: 10.1001/archneur.61.5.661. [DOI] [PubMed] [Google Scholar]
- 5.Craft S, Watson GS. Insulin and neurodegenerative disease: Shared and specific mechanisms. Lancet. 2004;3:169–178. doi: 10.1016/S1474-4422(04)00681-7. [DOI] [PubMed] [Google Scholar]
- 6.Perry T, Greig NH. The glucagon-like peptides: A double-edged therapeutic sword? Trends Pharmacol Sci. 2003;24:377–383. doi: 10.1016/S0165-6147(03)00160-3. [DOI] [PubMed] [Google Scholar]
- 7.Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132:2131–2157. doi: 10.1053/j.gastro.2007.03.054. [DOI] [PubMed] [Google Scholar]
- 8.Li Y, et al. Glucagon-like peptide-1 receptor signaling modulates beta cell apoptosis. J Biol Chem. 2003;278:471–478. doi: 10.1074/jbc.M209423200. [DOI] [PubMed] [Google Scholar]
- 9.Goke R, Larsen PJ, Mikkelsen JD, Sheikh SP. Distribution of GLP-1 binding sites in the rat brain: Evidence that exendin-4 is a ligand of brain GLP-1 binding sites. Eur J Neurosci. 1995;7:2294–2300. doi: 10.1111/j.1460-9568.1995.tb00650.x. [DOI] [PubMed] [Google Scholar]
- 10.Satoh F, et al. Characterization of human and rat glucagon-like peptide-1 receptors in the neurointermediate lobe: Lack of coupling to either stimulation or inhibition of adenylyl cyclase. Endocrinology. 2000;141:1301–1309. doi: 10.1210/endo.141.4.7420. [DOI] [PubMed] [Google Scholar]
- 11.Banks WA, During MJ, Niehoff ML. Brain uptake of the glucagon-like peptide-1 antagonist exendin(9–39) after intranasal administration. J Pharmacol Exp Ther. 2004;309:469–475. doi: 10.1124/jpet.103.063222. [DOI] [PubMed] [Google Scholar]
- 12.Turton MD, et al. A role for glucagon-like peptide-1 in the central regulation of feeding. Nature. 1996;379:69–72. doi: 10.1038/379069a0. [DOI] [PubMed] [Google Scholar]
- 13.Perry T, et al. A novel neurotrophic property of glucagon-like peptide 1: A promoter of nerve growth factor–mediated differentiation in PC12 cells. J Pharmacol Exp Ther. 2002;300:958–966. doi: 10.1124/jpet.300.3.958. [DOI] [PubMed] [Google Scholar]
- 14.Perry T, et al. Protection and reversal of excitotoxic damage by glucagon-like peptide-1 and exendin-4. J Pharmacol Exp Ther. 2002;302:1–8. doi: 10.1124/jpet.102.037481. [DOI] [PubMed] [Google Scholar]
- 15.Perry T, et al. Glucagon-like peptide-1 decreases endogenous Aβ levels and protects hippocampal neurons from death induced by Aβ and iron. J Neurosci Res. 2003;72:603–612. doi: 10.1002/jnr.10611. [DOI] [PubMed] [Google Scholar]
- 16.Javitch JA, D'Amato RJ, Strittmatter SM, Snyder SH. Parkinsonism-inducing neurotoxin, N-methyl-4-phenyl-1,2,3,6 -tetrahydropyridine: Uptake of the metabolite N-methyl-4-phenylpyridine by dopamine neurons explains selective toxicity. Proc Natl Acad Sci U S A. 1985;82:2173–2177. doi: 10.1073/pnas.82.7.2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jeerakathil T, Johnson JA, Simpson SH, Majumdar SR. Short-term risk for stroke is doubled in persons with newly treated type 2 diabetes compared with persons without diabetes: A population-based cohort study. Stroke. 2007;38:1739–1743. doi: 10.1161/STROKEAHA.106.481390. [DOI] [PubMed] [Google Scholar]
- 18.Hu G, Jousilahti P, Bidel S, Antikainen R, Tuomilehto J. Type 2 diabetes and the risk of Parkinson's disease. Diabetes Care. 2007;30:842–847. doi: 10.2337/dc06-2011. [DOI] [PubMed] [Google Scholar]
- 19.Mattson MP. Calcium and neurodegeneration. Aging Cell. 2007;6:337–350. doi: 10.1111/j.1474-9726.2007.00275.x. [DOI] [PubMed] [Google Scholar]
- 20.Duan W, et al. p53 inhibitors preserve dopamine neurons and motor function in experimental parkinsonism. Ann Neurol. 2002;52:597–606. doi: 10.1002/ana.10350. [DOI] [PubMed] [Google Scholar]
- 21.Tatton NA. Increased caspase 3 and Bax immunoreactivity accompany nuclear GAPDH translocation and neuronal apoptosis in Parkinson's disease. Exp Neurol. 2000;166:29–43. doi: 10.1006/exnr.2000.7489. [DOI] [PubMed] [Google Scholar]
- 22.Bredesen DE, Rao RV, Mehlen P. Cell death in the nervous system. Nature. 2006;443:796–802. doi: 10.1038/nature05293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee SC, Pervaiz S. Apoptosis in the pathophysiology of diabetes mellitus. Int J Biochem Cell Biol. 2007;39:497–504. doi: 10.1016/j.biocel.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 24.Rydel RE, Greene LA. cAMP analogs promote survival and neurite outgrowth in cultures of rat sympathetic and sensory neurons independently of nerve growth factor. Proc Natl Acad Sci U S A. 1988;85:1257–1261. doi: 10.1073/pnas.85.4.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mena MA, Caserejos MJ, Bonin A, Ramos JA, Yebenes JG. Effects of dibutyryl cyclic AMP and retinoic acid on the differentiation of dopamine neurons: prevention of cell death by dibutyryl cyclic AMP. J Neurochem. 1995;65:2612–2620. doi: 10.1046/j.1471-4159.1995.65062612.x. [DOI] [PubMed] [Google Scholar]
- 26.Kew JN, Smith DW, Sofroniew MV. Nerve growth factor withdrawal induces the apoptotic death of developing septal cholinergic neurons in vitro: protection by cyclic AMP analogue and high potassium. Neuroscience. 1996;70:329–339. doi: 10.1016/0306-4522(95)00365-7. [DOI] [PubMed] [Google Scholar]
- 27.D'Mello SR, Galli C, Ciotti T, Calissano P. Induction of apoptosis in cerebellar granule neurons by low potassium: Inhibition of death by insulin-like growth factor I and cAMP. Proc Natl Acad Sci U S A. 1993;90:10989–10993. doi: 10.1073/pnas.90.23.10989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Villalba M, Bockaert J, Journot L. Pituitary adenylate cyclase–activating polypeptide (PACAP-38) protects cerebellar granule neurons from apoptosis by activating the mitogen-activated protein kinase (MAP kinase) pathway. J Neurosci. 1997;17:83–90. doi: 10.1523/JNEUROSCI.17-01-00083.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hanson MG, Shen S, Wiemelt AP, McMorris F, Barres BA. Cyclic AMP elevation is sufficient to promote the survival of spinal motor neurons in vitro. J Neurosci. 1998;18:7361–7371. doi: 10.1523/JNEUROSCI.18-18-07361.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamamoto H, et al. Glucagon-like peptide-1–responsive catecholamine neurons in the area postrema link peripheral glucagon-like peptide-1 with central autonomic control sites. J Neurosci. 2003;23:2939–2946. doi: 10.1523/JNEUROSCI.23-07-02939.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lazaroff M, Patankar S, Yoon SO, Chikaraishi DM. The cyclic AMP response element directs tyrosine hydroxylase expression in catecholaminergic central and peripheral nervous system cell lines from transgenic mice. J Biol Chem. 1995;270:21579–21589. doi: 10.1074/jbc.270.37.21579. [DOI] [PubMed] [Google Scholar]
- 32.During MJ, et al. Glucagon-like peptide-1 receptor is involved in learning and neuroprotection. Nat Med. 2003;9:1173–1179. doi: 10.1038/nm919. [DOI] [PubMed] [Google Scholar]
- 33.Perry T, Greig NH. Enhancing central nervous system endogenous GLP-1 receptor pathways for intervention in Alzheimer's disease. Curr Alzheimer Res. 2005;2:377–385. doi: 10.2174/1567205054367892. [DOI] [PubMed] [Google Scholar]
- 34.Calara F, et al. A randomized, open-label, crossover study examining the effect of injection site on bioavailability of exenatide (synthetic Exendin-4) Clin Ther. 2005;27:210–215. doi: 10.1016/j.clinthera.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 35.Perry T, et al. Evidence of GLP-1–mediated neuroprotection in an animal model of pyridoxine-induced peripheral sensory neuropathy. Exp Neurol. 2007;203:293–301. doi: 10.1016/j.expneurol.2006.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li Y, Chopp M, Powers C, Jiang N. Apoptosis and protein expression after focal cerebral ischemia in rat. Brain Res. 1997;765:301–312. doi: 10.1016/s0006-8993(97)00524-6. [DOI] [PubMed] [Google Scholar]
- 37.Leker RR, Aharonowiz M, Greig NH, Ovadia H. The role of p53-induced apoptosis in cerebral ischemia: Effects of the p53 inhibitor pifithrin alpha. Exp Neurol. 2004;187:478–486. doi: 10.1016/j.expneurol.2004.01.030. [DOI] [PubMed] [Google Scholar]
- 38.Bertilsson G, et al. Peptide hormone exendin-4 stimulates subventricular zone neurogenesis in the adult rodent brain and induces recovery in an animal model of Parkinson's disease. J Neurosci Res. 2008;86:326–338. doi: 10.1002/jnr.21483. [DOI] [PubMed] [Google Scholar]
- 39.Harkavyi A, et al. Glucagon-like peptide 1 receptor stimulation reverses key deficits in distinct rodent models of Parkinson's disease. J Neuroinflam. 2008;21:5–19. doi: 10.1186/1742-2094-5-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sandyk R. The relationship between diabetes mellitus and Parkinson's disease. Int J Neurosci. 1993;69:125–130. doi: 10.3109/00207459309003322. [DOI] [PubMed] [Google Scholar]
- 41.Tweedie D, et al. Apoptotic and behavioral sequelae of mild brain trauma in mice. J Neurosci Res. 2007;85:805–815. doi: 10.1002/jnr.21160. [DOI] [PubMed] [Google Scholar]
- 42.Montrose-Rafizadeh CC, et al. Pancreatic glucagon-like peptide-1 receptor couples positively to multiple G proteins and activates mitogen-activated protein kinase pathways in Chinese hamster ovary cells. Endocrinology. 1999;140:1132–1134. doi: 10.1210/endo.140.3.6550. [DOI] [PubMed] [Google Scholar]
- 43.Tomac AC, et al. Effects of cerebral ischemia in mice deficient in Persephin. Proc Natl Acad Sci U S A. 2002;99:9521–9526. doi: 10.1073/pnas.152535899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fernagut PO, et al. Subacute systemic 3-nitropropionic acid intoxication induces a distinct motor disorder in adult C57Bl/6 mice: Behavioural and histopathological characterisation. Neuroscience. 2002;114:1005–1017. doi: 10.1016/s0306-4522(02)00205-1. [DOI] [PubMed] [Google Scholar]
- 45.Matsuura K, Kabuto H, Makino H, Ogawa N. Pole test is a useful method for evaluating the mouse movement disorder caused by striatal dopamine depletion. J Neurosci Methods. 1997;73:45–48. doi: 10.1016/s0165-0270(96)02211-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.