Abstract
Although impaired inhibitory control is linked to a broad spectrum of health problems, including obesity, the brain mechanism(s) underlying voluntary control of hunger are not well understood. We assessed the brain circuits involved in voluntary inhibition of hunger during food stimulation in 23 fasted men and women using PET and 2-deoxy-2[18F]fluoro-D-glucose (18FDG). In men, but not in women, food stimulation with inhibition significantly decreased activation in amygdala, hippocampus, insula, orbitofrontal cortex, and striatum, which are regions involved in emotional regulation, conditioning, and motivation. The suppressed activation of the orbitofrontal cortex with inhibition in men was associated with decreases in self-reports of hunger, which corroborates the involvement of this region in processing the conscious awareness of the drive to eat. This finding suggests a mechanism by which cognitive inhibition decreases the desire for food and implicates lower ability to suppress hunger in women as a contributing factor to gender differences in obesity.
Keywords: amygdala, cognitive inhibition, food stimuli, Orbitofrontal cortex
The ability to control and regulate impulses, emotions, and desires is one of the core features of the self and impaired inhibitory control has been linked to a broad spectrum of psychopathologies and problems including obesity (1). Obesity is associated with an increased risk of all-cause morbidity and mortality, which places a sense of urgency on understanding the processes that have contributed to this epidemic. Of particular relevance is the environment, which has made food not only widely available but also increasingly more varied and palatable. It is likely that a gene-environment interaction, in which genetically susceptible individuals respond to an environment with increased availability of palatable energy-dense foods and reduced opportunities for energy expenditure, contributes to the current high prevalence of obesity.
We have previously shown that the desire for food during presentation of palatable food stimuli was associated with striatal dopamine (DA) release, measured using PET and [11C]raclopride (2). This response was consistent with the role of DA in modulating the motivation for food (3), an effect that is likely mediated by DA's regulation of regions involved with motivation, salience attribution, conditioning, and inhibitory control (4, 5). Using the same experimental paradigm in normal body weight fasting subjects, we found that food presentation increased metabolism in orbitofrontal cortex (OFC) in proportion to the subjective perception of hunger and the desire to eat (6). The OFC is implicated in controlling and planning behaviors and is regulated by DA both through direct as well as indirect striato-thalamo cortical projections. Indeed a recent fMRI study using the blood oxygen level dependent (BOLD) signal showed obese subjects activated striatum and OFC as well as insula (brain region involved with interoception for food signals that is also innervated by DA terminals) while viewing pictures of high-caloric food (7).
Food is a potent natural reinforcer the value of which is further enhanced with food deprivation. Understanding the neurobiological mechanisms underlying the inhibitory control of food intake when one desires it may provide new targets for interventions to help individuals regulate their eating behaviors and maintain a healthy body mass index (BMI). Here we evaluate the responses of the brain when subjects were exposed to appealing food either with or without a prior directive to suppress the desire for food (cognitive inhibition). For this purpose we assessed the regional brain metabolic responses to food stimulation with and without cognitive inhibition using PET and FDG (6). Because there are significant gender differences in the prevalence of obesity and eating disorders (8), we also compared the responses between men and women. We hypothesized that cognitive inhibition would decrease the activation responses of the OFC, striatum, and insula to food presentation; we also hypothesized that the inhibition effects would be stronger in men than in women.
Results
Twenty-three healthy human subjects (13 women and 10 men) were recruited for the study and asked to select their favorite foods among a menu presented to them before the day of the study. The most frequently selected food items were bacon-egg-cheese sandwich, cinnamon bun, pizza, hamburger with cheese, fried chicken, lasagna, barbecue rib, ice cream, brownie, and chocolate cake. The body weight of the subjects was between 115–200 lbs (average: 155.8 ± 24 lbs). The averaged body mass index of the subjects was 24.8 ± 2.7 (range: 20 to 29). There were no significant differences in age, BMI, years of education, social economic background (Table S1), and selected food items between the genders.
Rating Scores of Self-Report Questionnaire.
The food stimulation condition resulted in gradual and significant increases in the ratings of hunger in both women (+43 ± 48%, P < 0.03) and men (+70 ± 76%, P < 0.002) but no significant gender differences. Both women and men also reported significant increases in desire for food (women: +37 ± 42%, P < 0.05; men: +59 ± 80%, P < 0.01) and the value ratings assigned to food (women: +32 ± 33%, P < 0.05; men: +29 ± 28%, P < 0.009) during food stimulation but there were no gender differences on these responses. The food stimulation enhanced self-reports of alertness in men (+33 ± 42%, P < 0.008) but not in women. The cognitive inhibition condition significantly decreased self-reports of hunger (women: P < 0.001; men: P < 0.0006) in both genders while only men reported decreased desire for food (P < 0.0007). The reports of hunger during the cognitive inhibition condition were significantly lower in men than women (P < 0.03). Women showed greater alertness before the food stimulation than men and food stimulation increased alertness more in men not in women. The cognitive inhibition decreased self-reports of alertness in men (P < 0.04) but not in women (Table S2).
PET Measures.
Whole brain metabolism at baseline did not differ between the genders (Table S3). Both women (P < 0.006) and men (P < 0.05) had higher whole brain metabolism in the food stimulation condition than in the baseline conditions and the responses did not differ between the genders.
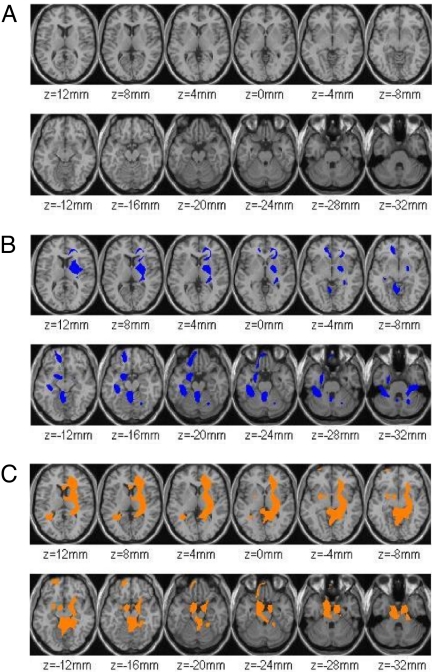
In the women, the Statistical Parametric Mapping (SPM) comparison between food stimulation without cognitive inhibition and the baseline condition (P < 0.005, uncorrected at the cluster level) showed a large contiguous cluster (82133 voxels, 43% of whole brain) in bilateral cortical (inferior frontal, parietal, temporal, occipital and cerebellar regions) and subcortical structures. The comparison between the food stimulation with cognitive inhibition and the baseline condition also showed a significant larger contiguous cluster (1534360 voxels, 83.1% of whole brain) that included similar regions to those obtained with food stimulation without cognitive inhibition. The SPM comparison between the food stimulation without and with cognitive inhibition did not show significant differences (Fig. 1A).
Fig. 1.
SPM images (P < 0.01) of cognitive inhibition during food stimulation (comparison between food stimulation with and without cognitive inhibition) in women (A) and in men (B). Color-coded SPM results are displayed in a transaxial plane superimposed on a structural brain MR image. The results (T value) are presented using the color scale where blue represents deactivation as a result of cognitive inhibition. (C) SPM results (P < 0.01) for the difference between women (A) and men (B), showing the areas where males showed greater decrements with inhibition than females. For none of the regions did females showed greater metabolic decrements than males with inhibition.
In the men, the SPM comparison between baseline and food stimulation without cognitive inhibition yielded two clusters: one in the right parieto-temporal cortex (1679 voxels) and one in the left OFC (499 voxels). The right parieto-temporal cluster encompassed three subclusters that included: the posterior central gyrus (Brodmann's areas 1, −42, −26, 30); the left insula (−44, −6, 2): and the left superior temporal gyrus (−60, −30, −2):. The significance of this cluster was P < 0.004 (uncorrected at the cluster level). The significance for the cluster in the right parieto-temporal cortex (62, −16, 20) was P < 0.05 (uncorrected at the cluster level). The cluster in the left OFC was not significant at the cluster level; however, there were two voxel-level significant regions (−38, 44, 4, P < 0.01; −38, 56, 2, P < 0.02). In contrast, the comparison between the baseline and food stimulation with cognitive inhibition showed no significant differences. SPM comparisons between food stimulation with cognitive inhibition and without inhibition revealed significant decreases in right insula, right striatum, left amygdala, left hypothalamus, left anterior cingulate (BA 24), left hippocampus (BA 28, uncus), left parahippocampal gyrus (BA 36), left OFC (BA 11), and cerebellum (P < 0.01) (Table 1 and Fig. 1B).
Table 1.
Brain regions along with their stereotactic coordinates (Talairach space) where SPM showed significant higher metabolism for the food stimulation without cognitive inhibition than for the food stimulation with cognitive inhibition for the male subjects. None of the regions showed lower metabolism without cognitive inhibition than with inhibition. The corrected p value was set at P < 0.01
| Region | BA | Stereotactic coordinates |
T value | ||
|---|---|---|---|---|---|
| x | y | z | |||
| Left anterior cingulate | 32 | −3 | 33 | −5 | 2.47 |
| Left orbitofrontal cortex | 11 | −19 | 53 | −15 | 2.71 |
| Left amygdala | 25 | 5 | −16 | 2.81 | |
| Left parahippocampus | 36 | −33 | −21 | −25 | 2.44 |
| Left uncus | 28 | −25 | 1 | −25 | 2.67 |
| Right insula | 13 | 43 | −19 | 17 | 2.29 |
| Right putamen | 23 | 1 | 3 | 2.79 | |
| Cerebellar vermis | 1 | −49 | −11 | 3.47 | |
The SPM analyses were set to compare the differences between the genders on the regional brain metabolic changes in the food stimulation conditions. When this comparison occurred in food stimulation with cognitive inhibition, at a significance level of P < 0.01 (voxel-level), the analyses showed the men had greater decreases than the women (Table 2) in amygdala, right striatum, left hypothalamus, left OFC (BA 11), left hippocampus (BA 28), parahippocampus, and cerebellum (Fig. 1C). The subsequent ROI-level analysis confirmed such findings (Table S4).
Table 2.
Regions and their stereotactic coordinates where males showed significantly higher decrements in metabolism between food stimulation without and with cognitive inhibition when compared with females. In none of the regions did females have higher metabolic decrements with inhibition than males. The corrected p value was set at P < 0.01
| Region | BA | Stereotactic coordinates |
T value | ||
|---|---|---|---|---|---|
| x | y | z | |||
| Left orbitofrontal cortex | 11 | −19 | 53 | −15 | 2.33 |
| Left uncus | 28 | 23 | 5 | −23 | 2.24 |
| Left parahippocampus | 36 | −33 | −21 | −25 | 1.89 |
| Left amygdala | 25 | 5 | −16 | 2.48 | |
| Right amygdala | 25 | 5 | −20 | 2.31 | |
| Right putamen | 23 | 1 | 3 | 2.63 | |
| Cerebellar vermis | 1 | −49 | −11 | 2.8 | |
Correlation and Regression Analyses.
Correlation between feeling of hunger and desire for food were done with self-reports of alertness during the food stimulation to assess if the changes reflected nonspecifically activated brain attention/alertness pathways. The changes of alertness were correlated with feeling of hunger (r = 0.65, P < 0.0009) and desire for food (r = 0.61, P < 0.002) in both groups during the food stimulation. Nonetheless, the changes in alertness were not correlated with brain metabolic changes, with hunger, or with desire for food in women or in men during the cognitive inhibition condition. Correlation analysis between the differences in the behavioral and regional brain metabolic changes induced by the food stimulation with and without cognitive inhibition revealed that they were significant for the changes in self-reports of hunger and the changes in the OFC (r = 0.73, P = 0.02); the greater the suppression in metabolism, the greater the decreases in the self-reports of hunger in men. This correlation did not reach significance in women (r = 0.49, P = 0.09) probably due to the modest sample size. A subsequent regression analysis with OFC as the dependent variable and with gender and changes in hunger performed with both groups revealed that the relationship between OFC and decrease in self-report of hunger is still significant (P = 0.01) while the accompanying gender effect is not (P = 0.28). This shows that the insignificant correlation in women could be the result of modest sample size. We also report that no significant correlation was found between BMI and changes in the OFC.
Discussion
This study shows that presentation of appetitive food stimuli to fasting subjects increased self-reports of hunger and desire for food and increased whole brain metabolism to a similar extent in women (25.8 ± 15.9%) and men (28.2 ± 21.9%). This finding is consistent with our prior study in a different group of subjects that also showed significant increases in whole brain metabolism when subjects were exposed to appetitive food stimuli (6). In the current study, we also showed that deliberate cognitive inhibition of the desire for food was effective in reducing the subjective reports of hunger and desire for food in both genders. However, whereas in men the cognitive inhibition resulted in a decrease in activation of limbic and paralimbic regions, in women cognitive inhibition did not affect regional brain metabolism. Specifically, with cognitive inhibition as compared with no inhibition, the male subjects showed significant decreases in left amygdala, left hypothalamus, left OFC, left uncus, right striatum, right insula, anterior cingulate gyrus, parahippocampus, and cerebellum. These regions, which decreased metabolism, had been shown by prior studies to be activated by food stimuli when presented via pictures, smells, taste, recall, or a combination of these (6, 9).
Regional Brain Responses to Cognitive Inhibition During Food Stimulation.
The food stimuli used in this study included a combination of all sensations (visual, smell, taste) and were selected on the basis of an individual's food preferences. This was done to maximize the saliency value of the food stimulus as a reinforcer for the subjects. Thus the deactivation pattern observed with inhibition in the men is likely to reflect the various regions (neuronal circuits) that need to deactivate to overcome the motivational drive to eat the food. These regions are part of the limbic system, which plays an important role in conditioning, habits, emotions, and interception, all of which participate in eating behaviors (10–12). These limbic regions integrate information related to body states into higher-order cognitive, motivational, and emotional processes. A wide network of interconnected brain regions including OFC, ventral striatum, amygdala, hippocampus, parahippocampus, and insula has been widely implicated in many aspect of eating behaviors (i.e., desire for food, food craving) and appears to contribute to compulsive overeating and obesity (13).
Similar to the findings from an fMRI study that reported that cognitive inhibition of sexual arousal upon exposure to an erotic video resulted in decreased activation of the amygdala (14), we also show deactivation of the left amygdala with cognitive inhibition during food stimulation in the male subjects. The amygdala is part of the limbic system and plays a central role in the processing and memory of emotional reactions that are important in the establishment of conditioned responses such as sexual arousal and in the responses to novel stimuli (15, 16). The amygdala is also involved in feeding behavior including learning and recognition of the biological significance of food-related objects (17). Functional neuroimaging studies have shown activation of the amygdala with food-related stimuli, such as tastes and odors as well as with sensations from the gastrointestinal tract (18–20). The amygdala has also been shown to be involved in signaling the impending delivery of glucose and in predicting food odors in appetitive-conditioning paradigms (21, 22). Due to limited spatial resolutions of the imaging modalities, most of these imaging studies did not distinguish among the various amygdaloid nuclei. Experimental lesions in laboratory animals and clinical reports in patients with temporal lobe damage or lobectomies indicate different roles for the various amygdaloid nuclei in obesity and eating behaviors. Using fMRI to assess the brain activation response to gastric distention we reported an association between activation in the left posterior amygdala and the subjective feelings of fullness (20). Indeed, experimental lesions in the most posteriodorsal part of the amygdala induce hyperphagia as well as excessive weight gain in animals (23). These lesions result in extensive retrograde degeneration in the stria terminalis and ventromedial hypothalamic nuclei. In contrast, experimental lesions in the basolateral amygdala, which is involved with the learning of conditioned associations, interrupted conditioned responses to food cues (24). The basolateral amygdala sends a substantial output to the lateral hypothalamus both through a direct connection and through relays in other brain regions such as striatum, prefrontal cortical areas, insula, or hippocampus (25).
This study showed that cognitive inhibition suppressed the activation of various limbic brain regions. Control of the expression of conditioned responses in the amygdala is believed to occur via activation of inhibitory circuits originating from prefrontal cortex, anterior cingulate, hippocampal, and parahippocampal regions (26). The cingulate gyrus and the prefrontal cortex are brain regions that have been implicated in various components of inhibitory control (27), in the control of intentional actions (28), and in self monitoring and in impulsivity (29). These behaviors could influence the ability of an individual to self regulate their eating behavior. Indeed, failure of inhibitory control mechanisms can lead to overexpression of behaviors driven by strong conditioned associations that can appear as pathological states such as drug addiction (30). In fact, we showed that in healthy obese subjects who have reduced striatal dopamine D2 receptor availability (31) these reductions were associated with decreased metabolism in OFC and in anterior cingulate regions (4). Similarly, we have also shown in healthy controls that higher BMI is associated with decreased metabolism in prefrontal cortex (including anterior cingulate and OFC) and with associated impairments in executive function (32). It is possible that decreased activity in prefrontal cortex and anterior cingulate may increase vulnerability to overeat through impairment in inhibitory control processes. In the current study we did not include subjects with BMI greater than 30. Further studies in obese subjects using the same study paradigm is needed to understand if failure for cognitive inhibition to deactivate limbic regions with exposure to palatable food stimuli is associated with their excessive BMI.
When activated by emotional arousal, the amygdala is known to modulate memory storage in the hippocampal and parahippocampal regions (33). Imaging studies have shown that the desire to eat a specific food was associated with activation of the hippocampus, implicating the involvement of this region in storing and retrieving the memories for the desired food (34). Thus hippocampal suppression during cognitive inhibition is likely to contribute to the suppression of the desire for the food and presumably also for the suppression of food intake (35). Indeed, experimental damage to the hippocampus can result in hyperphagia (36). The hippocampus also modulates saliency of stimuli through regulation of DA release in the ventral striatum (37) and is involved in incentive motivation (38) and behavioral control (35). It also regulates activity in prefrontal regions involved with inhibitory control (39). A recent study showed that tasting a liquid meal resulted in decreased activity in posterior hippocampus in obese and previously obese but not in lean subjects implicating the hippocampus in the neurobiology of obesity (40).
The OFC has been identified as a secondary gustatory cortex and is involved with the rapid learning of associations from visual, olfactory, and taste sensations (41). The OFC receives projections from the insula, striatum, and amygdala. These connections place the OFC in a position to process information about the motivational value of gustatory stimuli (42). Using the same food stimulation paradigm, we previously showed enhanced metabolic activity in the OFC during food stimulation, which is in part regulated by DA activity, and we interpreted the activation to reflect downstream effects from DA release (6). Thus, the findings of decreased metabolic activation of the OFC to food stimulation with cognitive inhibition may reflect a decrease in DA cell activation and concomitant regulation of OFC since DA's effects in the OFC in part mediate the influence of DA's involvement in the drive for food consumption. Alternatively, the decreased activation of OFC with cognitive inhibition could decrease the stimulation of DA cell firing in ventral tegmental area (VTA) via cortico-mesencephalic projections, reducing the saliency and motivational value of the food. While the current and prior studies assessed the subjects in a fasting state, the activation of the OFC has also been reported to predict feeding behaviors when subjects are in a fed state (43). The hypothalamus and brain stem are thought to be the principal homeostatic brain regions responsible for the regulation of body weight (44). However, the OFC can be activated during food stimulation even in the presence of postprandial satiety factors (43). Thus the regulation of eating behaviors could be easily switched from a homeostatic state to a hedonic corticolimbic state. The association between suppression of orbitofrontal activation and hunger in men provides further evidence that the OFC plays a key role in the regulation of eating behaviors. Moreover, fMRI studies showing that the OFC is activated with responses associated with immediate reinforcement as opposed to responses associated with temporal delay of the reinforcement (45) also suggest that activation of the OFC may underlie choices that result in immediate food consumption.
Gender Differences in Food Stimulation.
A recent fMRI study reported that women had greater reactivity to food stimuli in brain regions that process visual and taste sensations (46). In our study, we did not find a gender difference in the brain metabolic responses to food stimulation for a significance level of P < 0.01. However, at the significance level of P < 0.05, we observed a greater reactivity in the right anterior frontal (BA 10), left anterior cingulate (BA 9), and left OFC (BA 11) in women than in men during the food stimulation without cognitive inhibition (Fig. S1). The prefrontal dorsolateral cortex (BA 9 and 10) was associated with attention/alertness, working memory, and with inhibition of intentional actions (47), whereas the OFC is involved with salience attribution to reinforcers (e.g., food in this study) (48). The greater activation of the prefrontal cortex and OFC in the women during food stimulation could reflect the modulation of these brain regions by gonadal steroid hormones (49). Indeed in women, activation of the frontal cortex during a working memory task is associated with estradiol levels in the menstrual cycle (50). fMRI studies have also shown that estrogen levels modulate the responses of brain regions that process emotions and reward signals (51). Thus, these findings suggest that estrogen may have contributed to the gender differences in the regional brain responses during food stimulation.
Gender Differences in Cognitive Inhibition.
Here we also report a significant gender difference in the regional brain responses to cognitive inhibition. In women, different from what was observed in men, the decrease in the self-reports of hunger were not paralleled by deactivation in limbic and paralimbic brain regions. Women reported lower subjective feeling of hunger even though the activation of limbic and paralimbic brain regions were similar to when they were not exerting cognitive inhibition. This would appear as dissociation between subjective perception of the motivational saliency of the food and the level of activation of brain regions that assign its motivational value. To our knowledge, there are no prior studies describing gender differences between subjective reports of emotional or motivational states and the associated patterns of regional brain activation/deactivations. This is an area that merits further investigation for if indeed there are differences between the genders on the perception of interoceptive signals, this could also contribute to the observed differences in the prevalence rates of eating disorders and obesity between the genders (52).
Our findings of a lack of response to inhibition in women are consistent with behavioral studies showing significantly higher scores in disinhibition (tendency to overeat in response to food stimuli when presented with palatable food or under emotional distress) in women than in men (53). The decreased inhibitory control in women could underlie their lower success in losing weight while dieting when compared with men (8). Sex hormones directly influence food intake, body weight, and fat distribution and modulate the signaling of leptin and insulin (54). Indeed, female rats are more sensitive to the anorexic effects of leptin than male rats (55). Leptin in the brain enhances the response to satiety signals and decreases perception of food reward during food consumption (56). Leptin also modulates food-related mesolimbic sensitivity to visual food stimulation (57). Furthermore, gonadal steroid hormones affect neurotransmitter turnover in prefrontal regions and in the ventral tegmental area, where DA cells are located (58). Female gonadal hormone levels fluctuate during the rat estrous cycle affecting the release and reuptake of neurotransmitters (i.e., DA, serotonin and norepinephrine) that are involved in satiety and motivation to eat (59).
Limitations.
Our findings are confounded by the fact that we did not control for the time of the menstrual cycle at which the studies were performed nor did we measure gonadal hormones. The menstrual cycle is likely to influence the brain responses to food since the pattern of estradiol secretion during the ovarian cycle has been shown to affect eating behavior; e.g., women eat more during the luteal and menstrual phases than the follicular and peri-ovulatory phases (60). Future studies that compare women in specific phases of the menstrual cycle are warranted to understand hormonal effects on cognitive inhibition and its effect on food cue induced brain activation. Another limitation for this study is the modest sample size; further studies with larger samples are warranted. The effect of food stimulation and cognitive inhibition relied on cooperation of the participants and on self-report measures of subjective effects.
Conclusion
Here we show suppression of metabolic activation in amygdala, hippocampus, insula, OFC, and striatum during food presentation with cognitive inhibition in men but not in women. These regions are involved in the regulation of satiety and motivation to eat, suggesting their involvement in the neurobiological mechanisms underlying cognitive inhibition of the desire for food. The association between suppression of orbitofrontal activation and hunger in men corroborates the involvement of this region in processing motivation for food consumption. Lower cognitive control of brain responses to food stimulation in women compared to men may contribute to gender differences in the prevalence rates of obesity and other eating disorders.
Materials and Methods
Subjects.
The Institutional Review Board at Stony Brook University/Brookhaven National Laboratory approved the protocol. Written informed consents were obtained after the experimental procedure was explained and after the subjects had read the consent form. Twenty-three healthy human subjects (13 women and 10 men, age 32.6 ± 7.5 years old, range: 22–48) with body mass index (kg/m2) less than 30 were recruited for the study. Subjects with the following conditions were excluded from the study: past or present history of eating disorders, surgical/medical treatment for weight control, dependence on alcohol or other drugs of abuse (except for caffeine <5 cups/day or nicotine <1 pack/day), neurological or psychiatric disorder, use of prescription (psychiatric and/or nonpsychiatric) medication(s) that can affect brain function in the past two weeks, medical conditions that may alter cerebral function, cardiovascular disease and diabetes, head trauma with loss of consciousness of more than 30 min. Urine screening tests for psychoactive drugs (including PCP, cocaine, amphetamine, opiates, barbiturates, benzodiazepine, and THC) were performed to corroborate absence of drug use.
The subjects were asked to fill out a questionnaire, which contained the following information on the day of screening: a rating of the subject's overall interest in food; the subject's favorite foods; food smells that stimulate the subject's appetite; food smells that diminish the subject's appetite; a list of foods preferences on a scale from 1 to 10, 10 being the highest. The food items include a variety of popular American meals, snacks, and deserts (e.g., bacon-egg-cheese sandwich, cinnamon bun, pizza, hamburger with cheese, fried chicken, lasagna, barbecue rib, ice cream, brownie and chocolate cake). The 10 food items with the highest ratings were presented to the subject before the food stimulation to confirm their favorite choices and during the food stimulation condition.
Experimental Paradigm.
Subjects were scanned 3 times with 18FDG in 3 different days under the following conditions: i) Day A: food stimulation started 15 min before FDG injection and continued for a total of 45 min in the scanner. When the stimulation completed, the subjects were positioned in the gantry of the PET scanner and image acquisitions were started at 35 min after FDG injection. The subjects were instructed to observe and spontaneously react to the food stimuli. ii) Day B: food stimulation started 15 min before FDG injection and continued for a total of 45 min in the scanner. The image acquisitions started at 35 min after FDG injection. The subjects were instructed to inhibit their desire for food before the presentation of the food stimuli. iii) Day C: baseline condition without stimulation. The sequence was randomized so that for one third of the subjects the first day was day A, for one third of the subjects the first day was day B, and for the other one third of the subjects it was day C.
For the Day A food stimulation condition, the food was warmed to enhance the smell and the subjects were presented with it so that they could view it and smell it. A cotton swab impregnated with the food was placed on their tongues so they could taste it. A given food item was presented for 5 min and then it was exchanged for a new one. The tasting, smelling, and viewing of a given food item were continuous during the stimulation. The subjects were asked to describe their favorite foods and how they like to eat them while they were presented with foods that they had reported as among their favorites. For the Day B food stimulation with cognitive inhibition condition, the food stimulation procedures were the same as the Day A. However, the subjects were asked to inhibit their desire for food and suppress their feelings of hunger while they were presented with foods that they had reported as among their favorite ones. Before the study, the subjects were instructed how to practice their cognitive inhibition (i.e., ignore, shift thoughts) to the food stimulation presented by the investigator (M.J.). Self-report feelings of hunger, desire for food and alertness were recorded before and after food stimulations. For all three conditions, the subjects were asked to have their last meal at 7 p.m. the evening before the day of the study and were studied between 17–19 h after the last meal. We relied on the subjects self-reports to confirm that they had not eaten anything after their last meal as instructed. The subjects arrived at the imaging center at about 8:30 a.m. on the day of the study and a nurse remained with them to ensure their refraining from food or caloric containing drinks before the study, which started after 12 p.m.
PET Imaging.
Subjects were scanned with 2-deoxy-2[18F]fluoro-D-glucose FDG using a Siemens HR+ PET scanner. Details on procedures for positioning of the subject, arterialized venous and venous catheterization, quantification of radiotracer, and transmission and emission scans have been published (6). Briefly, Two i.v. lines were inserted and maintained with saline and heparin. Arterialized venous blood was obtained from a catheter placed in a dorsal hand vein. The hand was prewarmed to 48 °C in a heating box, which ensured the shifting of arterial blood to the vein. The other catheter was in the antecubital region of the opposite arm for tracer injection. One emission scan (20 min) was taken 35 min after an i.v. injection of 4–6 mCi of FDG. During the study, subjects were positioned supine in the PET camera with their eyes open; the room was dimly lit, and noise was kept to a minimum. During the PET studies, participants were instructed to orally respond to each descriptor using a whole number between 1 and 10 for the self-report of “hunger” and “desire of food,” which were obtained before the food presentation and then at 5 min intervals for a total of 45 min. The 18FDG was injected 15 min after food stimulation started and scanning was carried out for 20 min.
Data Analysis.
Both manual drawn preselected regions of interest (ROIs) and Statistical Parametric Mapping (SPM) analyses were used. ROIs in amygdala, anterior cingulate, caudate, frontal cortex, hippocampus, insula, OFC, parietal cortex, putamen, temporal cortex, and thalamus were obtained using a template, which we had previously published (6, 61). We also computed a value for global metabolism by averaging the activity in the 63 planes scanned. Differences in metabolism between the measures obtained in the baseline, food presentation, and food stimulation with cognitive inhibition conditions were tested with paired samples t tests. Pearson product moment correlations were used to assess the relationship between the changes in metabolism (i.e., amygdala, anterior cingulate, hippocampus, striatum, OFC) and the change in the behavioral measures (desire for food, feelings of hunger, ratings of food) between the food presentation and food stimulation with cognitive inhibition interventions.
Differences in metabolism between the 3 conditions were also tested on the voxel-level using the software package for SPM (62). Before the analysis, each subject's PET image was mapped onto the Montreal Neurological Institute template, which closely resembles the Talairach, brain, and smoothed via a Gaussian kernel with full width half maximum (FWHM) at 16 mm. The analysis, which was essentially a paired sample t test performed on each voxel, was performed using the absolute metabolic images. Multiple-test correction was performed via the random field theory less conservative than traditional approaches such as the Bonferroni method (63). Pixels that were significantly different from those in the neutral presentation (P ≤ 0.01) were identified with respect to the Talairach and Tournoux stereotactic coordinates (64) and displayed on the axial magnetic resonance images. The threshold for the cluster size was set at 100 voxels. Only regions with corrected p-values ≤ 0.01 at the cluster-level were considered significantly activated or deactivated.
Supplementary Material
Acknowledgments.
We thank all of the subjects who participated in this study. We also thank K. Torres for Institutional Review Board correspondence and study compliance; D. Schlyer and M. Schueller for Cyclotron operations; D. Alexoff, P. Vaska, and D. Warner for PET operations; C. Shea, Y. Xu, L. Muench, and P. King for radiotracer preparation and analysis; P. Carter and B. Hubbard for patient care and A. Ruggiero for manuscript submission. This work was supported by the Department of Energy OBER (DE-ACO2–98CH10886), National Institute on Drug Abuse (DA6891 & DA6278), National Institute on Alcohol Abuse and Alcoholism (AA9481 & Y1AA3009), and the General Clinical Research Center at Stony Brook University Hospital (NIH MO1RR 10710).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0807423106/DCSupplemental.
References
- 1.van Hout GC, van Oudheusden I, van Heck GL. Psychological profile of the morbidly obese. Obes Surg. 2004;14:579–588. doi: 10.1381/096089204323093336. [DOI] [PubMed] [Google Scholar]
- 2.Volkow ND, et al. “Nonhedonic” food motivation in humans involves dopamine in the dorsal striatum and methylphenidate amplifies this effect. Synapse. 2002;44:175–180. doi: 10.1002/syn.10075. [DOI] [PubMed] [Google Scholar]
- 3.Martel P, Fantino M. Mesolimbic dopaminergic system activity as a function of food reward: A microdialysis study. Pharmacol Biochem Behav. 1996;53:221–226. doi: 10.1016/0091-3057(95)00187-5. [DOI] [PubMed] [Google Scholar]
- 4.Volkow ND, et al. Low dopamine striatal D2 receptors are associated with prefrontal metabolism in obese subjects: Possible contributing factors. NeuroImage. 2008;42:1515–1537. doi: 10.1016/j.neuroimage.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bassareo V, De Luca MA, Di Chiara G. Differential expression of motivational stimulus properties by dopamine in nucleus accumbens shell versus core and prefrontal cortex. J Neurosci. 2002;22:4709–4719. doi: 10.1523/JNEUROSCI.22-11-04709.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang GJ, et al. Exposure to appetitive food stimuli markedly activates the human brain. NeuroImage. 2004;21:1790–1797. doi: 10.1016/j.neuroimage.2003.11.026. [DOI] [PubMed] [Google Scholar]
- 7.Rothemund Y, et al. Differential activation of the dorsal striatum by high-calorie visual food stimuli in obese individuals. NeuroImage. 2007;37:410–421. doi: 10.1016/j.neuroimage.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 8.Keel PK, Baxter MG, Heatherton TF, Joiner TE., Jr A 20-year longitudinal study of body weight, dieting, and eating disorder symptoms. J Abnorm Psychol. 2007;116:422–432. doi: 10.1037/0021-843X.116.2.422. [DOI] [PubMed] [Google Scholar]
- 9.Delamater AR. The role of the orbitofrontal cortex in sensory-specific encoding of associations in pavlovian and instrumental conditioning. Ann NY Acad Sci. 2007;1121:152–173. doi: 10.1196/annals.1401.030. [DOI] [PubMed] [Google Scholar]
- 10.Canli T, et al. Neural correlates of epigenesis. Proc Natl Acad Sci USA. 2006;103:16033–16038. doi: 10.1073/pnas.0601674103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dunsmoor JE, Bandettini PA, Knight DC. Neural correlates of unconditioned response diminution during Pavlovian conditioning. NeuroImage. 2008;40:811–817. doi: 10.1016/j.neuroimage.2007.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koch K, et al. Gender differences in the cognitive control of emotion: An fMRI study. Neuropsychologia. 2007;45:2744–2754. doi: 10.1016/j.neuropsychologia.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 13.Volkow ND, Wang GJ, Fowler JS, Telang F. Overlapping neuronal circuits in addiction and obesity: evidence of systems pathology. Philos Trans R Soc Lond B. 2008;363:3191–3200. doi: 10.1098/rstb.2008.0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beauregard M, Levesque J, Bourgouin P. Neural correlates of conscious self-regulation of emotion. J Neurosci. 2001;21:RC165. doi: 10.1523/JNEUROSCI.21-18-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wright CI, Wedig MM, Williams D, Rauch SL, Albert MS. Novel fearful faces activate the amygdala in healthy young and elderly adults. Neurobiol Aging. 2006;27:361–374. doi: 10.1016/j.neurobiolaging.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 16.Childress AR, et al. Prelude to passion: limbic activation by “unseen” drug and sexual cues. PLoS ONE. 2008;3:e1506. doi: 10.1371/journal.pone.0001506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Petrovich GD, Holland PC, Gallagher M. Amygdalar and prefrontal pathways to the lateral hypothalamus are activated by a learned cue that stimulates eating. J Neurosci. 2005;25:8295–8302. doi: 10.1523/JNEUROSCI.2480-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Small DM, Gerber JC, Mak YE, Hummel T. Differential neural responses evoked by orthonasal versus retronasal odorant perception in humans. Neuron. 2005;47:593–605. doi: 10.1016/j.neuron.2005.07.022. [DOI] [PubMed] [Google Scholar]
- 19.Smeets PA, et al. Effect of satiety on brain activation during chocolate tasting in men and women. Am J Clin Nutr. 2006;83:1297–1305. doi: 10.1093/ajcn/83.6.1297. [DOI] [PubMed] [Google Scholar]
- 20.Wang GJ, et al. Gastric distention activates satiety circuitry in the human brain. NeuroImage. 2008;39:1824–1831. doi: 10.1016/j.neuroimage.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 21.Gottfried JA, O'Doherty J, Dolan RJ. Encoding predictive reward value in human amygdala and orbitofrontal cortex. Science. 2003;301:1104–1107. doi: 10.1126/science.1087919. [DOI] [PubMed] [Google Scholar]
- 22.Kerfoot EC, Agarwal I, Lee HJ, Holland PC. Control of appetitive and aversive taste-reactivity responses by an auditory conditioned stimulus in a devaluation task: A FOS and behavioral analysis. Learn Mem. 2007;14:581–589. doi: 10.1101/lm.627007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.King BM, Cook JT, Rossiter KN, Rollins BL. Obesity-inducing amygdala lesions: Examination of anterograde degeneration and retrograde transport. Am J Physiol. 2003;284:R965–R982. doi: 10.1152/ajpregu.00249.2002. [DOI] [PubMed] [Google Scholar]
- 24.Wellman LL, Gale K, Malkova L. GABAA-mediated inhibition of basolateral amygdala blocks reward devaluation in macaques. J Neurosci. 2005;25:4577–4586. doi: 10.1523/JNEUROSCI.2257-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ramirez DR, Savage LM. Differential involvement of the basolateral amygdala, orbitofrontal cortex, and nucleus accumbens core in the acquisition and use of reward expectancies. Behav Neurosci. 2007;121:896–906. doi: 10.1037/0735-7044.121.5.896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wood JN, Romero SG, Knutson KM, Grafman J. Representation of attitudinal knowledge: Role of prefrontal cortex, amygdala and parahippocampal gyrus. Neuropsychologia. 2005;43:249–259. doi: 10.1016/j.neuropsychologia.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 27.Delgado MR, Gillis MM, Phelps EA. Regulating the expectation of reward via cognitive strategies. Nat Neurosci. 2008:10.1038. doi: 10.1038/nn.2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brass M, Haggard P. To do or not to do: The neural signature of self-control. J Neurosci. 2007;27:9141–9145. doi: 10.1523/JNEUROSCI.0924-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fellows LK. The cognitive neuroscience of human decision making: A review and conceptual framework. Behav Cogn Neurosci Rev. 2004;3:159–172. doi: 10.1177/1534582304273251. [DOI] [PubMed] [Google Scholar]
- 30.Li CS, Sinha R. Inhibitory control and emotional stress regulation: Neuroimaging evidence for frontal-limbic dysfunction in psycho-stimulant addiction. Neurosci Biobehav Rev. 2008;32:581–597. doi: 10.1016/j.neubiorev.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang GJ, et al. Brain dopamine and obesity. Lancet. 2001;357:354–357. doi: 10.1016/s0140-6736(00)03643-6. [DOI] [PubMed] [Google Scholar]
- 32.Volkow ND, et al. Inverse association between body mass index and prefrontal metabolic activity in healthy adults. Obesity. 2008;17:60–65. doi: 10.1038/oby.2008.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith AP, Stephan KE, Rugg MD, Dolan RJ. Task and content modulate amygdala-hippocampal connectivity in emotional retrieval. Neuron. 2006;49:631–638. doi: 10.1016/j.neuron.2005.12.025. [DOI] [PubMed] [Google Scholar]
- 34.Pelchat ML, Johnson A, Chan R, Valdez J, Ragland JD. Images of desire: Food-craving activation during fMRI. NeuroImage. 2004;23:1486–1493. doi: 10.1016/j.neuroimage.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 35.Davidson TL, Kanoski SE, Walls EK, Jarrard LE. Memory inhibition and energy regulation. Physiol Behav. 2005;86:731–746. doi: 10.1016/j.physbeh.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 36.Forloni G, Fisone G, Guaitani A, Ladinsky H, Consolo S. Role of the hippocampus in the sex-dependent regulation of eating behavior: Studies with kainic acid. Physiol Behav. 1986;38:321–326. doi: 10.1016/0031-9384(86)90101-0. [DOI] [PubMed] [Google Scholar]
- 37.Berridge KC, Robinson TE. What is the role of dopamine in reward: Hedonic impact, reward learning, or incentive salience? Brain Res Brain Res Rev. 1998;28:309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- 38.Tracy AL, Jarrard LE, Davidson TL. The hippocampus and motivation revisited: Appetite and activity. Behav Brain Res. 2001;127:13–23. doi: 10.1016/s0166-4328(01)00364-3. [DOI] [PubMed] [Google Scholar]
- 39.Peleg-Raibstein D, et al. Activation of dopaminergic neurotransmission in the medial prefrontal cortex by N-methyl-d-aspartate stimulation of the ventral hippocampus in rats. Neuroscience. 2005;132:219–232. doi: 10.1016/j.neuroscience.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 40.DelParigi A, et al. Persistence of abnormal neural responses to a meal in postobese individuals. Int J Obes Relat Metab Disord. 2004;28:370–377. doi: 10.1038/sj.ijo.0802558. [DOI] [PubMed] [Google Scholar]
- 41.Pritchard TC, et al. Satiety-responsive neurons in the medial orbitofrontal cortex of the macaque. Behav Neurosci. 2008;122:174–182. doi: 10.1037/0735-7044.122.1.174. [DOI] [PubMed] [Google Scholar]
- 42.de Araujo IE, et al. Neural ensemble coding of satiety states. Neuron. 2006;51:483–494. doi: 10.1016/j.neuron.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 43.Batterham RL, et al. PYY modulation of cortical and hypothalamic brain areas predicts feeding behaviour in humans. Nature. 2007;450:106–109. doi: 10.1038/nature06212. [DOI] [PubMed] [Google Scholar]
- 44.Morrison CD, Berthoud HR. Neurobiology of nutrition and obesity. Nutr Rev. 2007;65:517–534. doi: 10.1301/nr.2007.dec.517-534. [DOI] [PubMed] [Google Scholar]
- 45.McClure SM, Ericson KM, Laibson DI, Loewenstein G, Cohen JD. Time discounting for primary rewards. J Neurosci. 2007;27:5796–5804. doi: 10.1523/JNEUROSCI.4246-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Uher R, Treasure J, Heining M, Brammer MJ, Campbell IC. Cerebral processing of food-related stimuli: effects of fasting and gender. Behav Brain Res. 2006;169:111–119. doi: 10.1016/j.bbr.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 47.Bermpohl F, et al. Attentional modulation of emotional stimulus processing: An fMRI study using emotional expectancy. Hum Brain Mapp. 2006;27:662–677. doi: 10.1002/hbm.20209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Porubska K, Veit R, Preissl H, Fritsche A, Birbaumer N. Subjective feeling of appetite modulates brain activity: An fMRI study. NeuroImage. 2006;32:1273–1280. doi: 10.1016/j.neuroimage.2006.04.216. [DOI] [PubMed] [Google Scholar]
- 49.Del Parigi A, et al. Sex differences in the human brain's response to hunger and satiation. Am J Clin Nutr. 2002;75:1017–1022. doi: 10.1093/ajcn/75.6.1017. [DOI] [PubMed] [Google Scholar]
- 50.Schoning S, et al. Functional anatomy of visuo-spatial working memory during mental rotation is influenced by sex, menstrual cycle, and sex steroid hormones. Neuropsychologia. 2007;45:3203–3214. doi: 10.1016/j.neuropsychologia.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 51.Dreher JC, et al. Menstrual cycle phase modulates reward-related neural function in women. Proc Natl Acad Sci USA. 2007;104:2465–2470. doi: 10.1073/pnas.0605569104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kaye W. Neurobiology of anorexia and bulimia nervosa. Physiol Behav. 2008;94:121–135. doi: 10.1016/j.physbeh.2007.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hays NP, Roberts SB. Aspects of eating behaviors “disinhibition” and “restraint” are related to weight gain and BMI in women. Obesity (Silver Spring) 2008;16:52–58. doi: 10.1038/oby.2007.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Woods SC, Gotoh K, Clegg DJ. Gender differences in the control of energy homeostasis. Expl Biol Med. 2003;228:1175–1180. doi: 10.1177/153537020322801012. [DOI] [PubMed] [Google Scholar]
- 55.Clegg DJ, Riedy CA, Smith KA, Benoit SC, Woods SC. Differential sensitivity to central leptin and insulin in male and female rats. Diabetes. 2003;52:682–687. doi: 10.2337/diabetes.52.3.682. [DOI] [PubMed] [Google Scholar]
- 56.Krugel U, Schraft T, Kittner H, Kiess W, Illes P. Basal and feeding-evoked dopamine release in the rat nucleus accumbens is depressed by leptin. Eur J Pharmacol. 2003;482:185–187. doi: 10.1016/j.ejphar.2003.09.047. [DOI] [PubMed] [Google Scholar]
- 57.Farooqi IS, et al. Leptin regulates striatal regions and human eating behavior. Science. 2007;317:1355. doi: 10.1126/science.1144599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Handa RJ, Hejna GM, Lorens SA. Androgen inhibits neurotransmitter turnover in the medial prefrontal cortex of the rat following exposure to a novel environment. Brain Res. 1997;751:131–138. doi: 10.1016/s0006-8993(96)01394-7. [DOI] [PubMed] [Google Scholar]
- 59.Thompson TL, Moss RL. Modulation of mesolimbic dopaminergic activity over the rat estrous cycle. Neurosci Lett. 1997;229:145–148. doi: 10.1016/s0304-3940(97)00450-3. [DOI] [PubMed] [Google Scholar]
- 60.Reed SC, Levin FR, Evans SM. Changes in mood, cognitive performance and appetite in the late luteal and follicular phases of the menstrual cycle in women with and without PMDD (premenstrual dysphoric disorder) Horm Behav. 2008;54:185–193. doi: 10.1016/j.yhbeh.2008.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang GJ, et al. Alcohol intoxication induces greater reductions in brain metabolism in male than in female subjects. Alcohol Clin Exp Res. 2003;27:909–917. doi: 10.1097/01.ALC.0000071740.56375.BA. [DOI] [PubMed] [Google Scholar]
- 62.London: MRC Cyclotron Unit, Hammersmith Hospital; 1999. SPM Software. [Google Scholar]
- 63.Worsley KJ, et al. A unified statistical approach for determining significant signals in images of cerebral activation. Hum Brain Mapp. 1996;4:58–73. doi: 10.1002/(SICI)1097-0193(1996)4:1<58::AID-HBM4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 64.Talairach J, Tournoux PA. Co-planar Stereotaxic Atlas of a Human Brain. New York: Thieme; 1988. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.