Abstract
Stem cell maintenance depends on local signals provided by specialized microenvironments, or niches, in which they reside. The potential role of systemic factors in stem cell maintenance, however, has remained largely unexplored. Here, we show that insulin signaling integrates the effects of diet and age on germline stem cell (GSC) maintenance through the dual regulation of cap cell number (via Notch signaling) and cap cell–GSC interaction (via E-cadherin) and that the normal process of GSC and niche cell loss that occurs with age can be suppressed by increased levels of insulin-like peptides. These results underscore the importance of systemic factors for the regulation of stem cell niches and, thereby, of stem cell numbers.
Keywords: diet, oogenesis, Notch, E-cadherin, aging
The stem cell microenvironment (niche) controls stem cells (1, 2), and niche aging leads to stem cell decline (3–5). The Drosophila germline stem cell (GSC) niche includes terminal filament cells, cap cells, and escort stem cells, and GSC fate and activity require direct contact with cap cells and exposure to niche-derived signals (6). GSCs also respond to systemic signals, such as Drosophila insulin-like peptides (DILPs) (7, 8), which directly modulate their proliferation (9). Increased age leads to decreased niche size and signaling and GSC loss (3). The molecular basis for age-dependent changes in the niche, however, remains poorly understood.
Results and Discussion
Because diet influences aging (10), we examined its effects on GSC maintenance, exploiting the fact that GSCs can be unambiguously identified by their anteriorly anchored fusome (a membranous cytoskeletal structure) and by their juxtaposition to cap cells (11). As previously reported (3, 11, 12), we also observed a decrease in GSC numbers in well-fed females over time. In females on a poor diet, however, the rate of GSC loss was significantly increased (Fig. 1A, B, and E and supporting information (SI) Table S1).
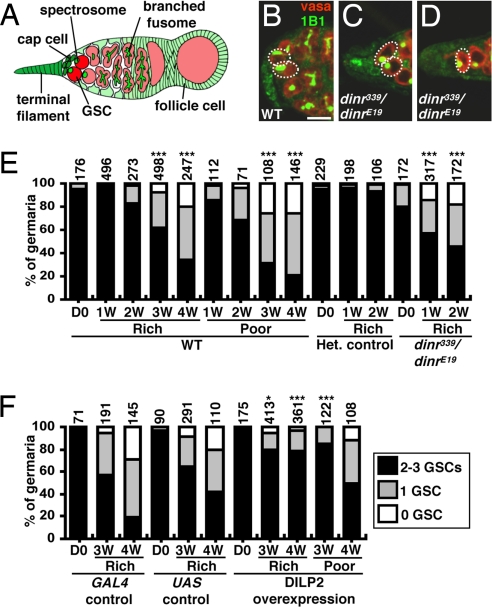
Fig. 1.
GSC maintenance requires insulin signaling. (A) Terminal filament and cap cells form the GSC niche in Drosophila germaria. Each GSC contains a spectrosome/fusome. A GSC division generates another GSC and a cystoblast that leaves the niche and forms a 16-cell cyst enveloped by follicle cells. (B–D) One-week-old wild-type (WT) and dinr339/dinrE19 germaria labeled with vasa (red, germ cells) and 1B1 (green, fusomes). Dashed circles/ovals, GSCs. (Scale bar, 10 μm.) (E and F) GSC numbers in wild-type, dinr339/dinrE19, dinr/+ heterozygous, DILP2-overexpressing, and control females under different diets. D0, newly eclosed; Het., heterozygous; 1W, 2W, 3W, and 4W: 1-, 2-, 3-, and 4-week-old females, respectively. The number of analyzed germaria is shown above each bar. *P < 0.05, ***P < 0.001.
Insulin secretion and signaling respond to diet (13) and diminish in aging humans (14). Using a phosphoinositide 3-kinase reporter (15), we found reduced insulin signaling in older ovaries (Fig. S1). To address if GSC maintenance requires insulin signaling, we measured GSC numbers in Drosophila insulin receptor (dinr) mutants (Fig. 1 C–E and Table S1). The dinr339/dinrE19 females contain slightly fewer GSCs at eclosion and lose them significantly faster than controls. We did not detect GSC death in dinr339/dinrE19 (n = 65) or control (n = 15) germaria, suggesting that GSC loss results from differentiation. The chico1 homozygotes, which lack insulin receptor substrate, a major insulin pathway component, also show increased GSC loss (Table S1). Thus, insulin signaling controls GSC maintenance.
We next tested if DILP expression in germarial somatic cells could counteract the wild-type age-dependent GSC loss. We used the c587-GAL4 driver (see Materials and Methods) to express a UAS-dilp2 transgene, encoding the DILP most closely related to human insulin (16), and thereby increase the local levels of insulin-like signals. GSC loss on rich and poor diets was significantly suppressed by DILP2 overexpression, although this was less pronounced in 4-week-old females on a poor diet (Fig. 1F and Table S1). The less effective rescue on a poor diet could potentially be attributable to lower expression of the c587-GAL4 driver, to the actions of additional diet-dependent signals, or to a combination thereof. Nevertheless, these results suggest that the normal GSC loss observed in wild-type females as their age increases results largely from reduced insulin signaling.
DILPs control GSC division directly, leading to a cell-autonomous dinr requirement (9). We therefore asked whether dinr is required within GSCs for their maintenance. In genetic mosaics, homozygous dinr339 or dinrE19 GSCs are not lost at a higher rate than control GSCs (Fig. 2 A–D), demonstrating that DILPs do not promote GSC maintenance directly.
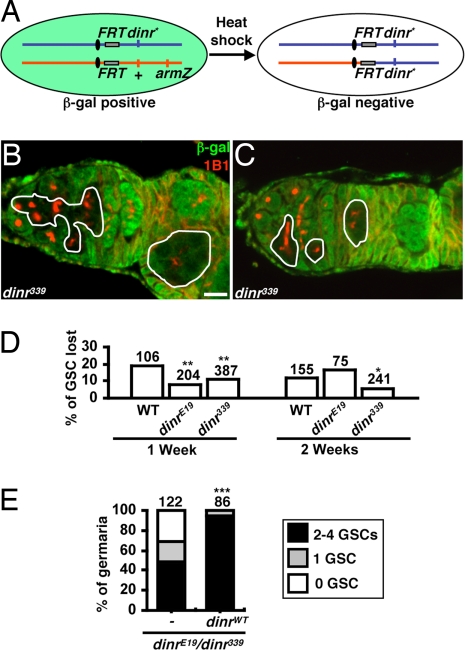
Fig. 2.
dinr is not required cell autonomously for GSC maintenance. (A) FLP/FRT system used to generate homozygous mutant GSCs. Flies carrying a wild-type dinr allele (+) linked to an arm-lacZ transgene in trans to a mutant or wild-type (WT) dinr allele (dinr*) were heat-shocked to induce FLP-mediated recombination between FRT sites. dinr* homozygous GSCs and their progeny are recognized by the absence of β-gal expression. (B) Mosaic germarium in which the dinr339 homozygous GSC and progeny are present. (C) Mosaic germarium in which only the dinr339 homozygous progeny are present, indicating loss of the dinr339 homozygous GSC. In B and C, solid lines outline dinr339 mutant cells. (Scale bar, 10 μm.) (D) Percentage of germaria in which the GSC has been lost. To quantify GSC loss, we calculated the percentage of germaria in which the original dinr mutant GSCs had been lost (instances equivalent to example shown in C) relative to the total number of germaria containing mosaic germline (sum of all instances equivalent to B or C). dinr mutant GSCs are not lost at a higher rate than control GSCs, showing that dinr does not promote GSC maintenance cell autonomously. The slightly lower rate of dinr339 GSC loss is consistent with findings that dinr mutant GSCs spend a higher proportion of their cell cycle displaying a round fusome morphology, which we show coincides with higher levels of E-cadherin at the cap cell–GSC junction (see text and Fig. 6 C and D). (E) GSC number in germaria of 1-week-old control dinrE19/dinr339 mutants (c587/+, CyO/+, dinrE19GAL80ts/dinr339 hh-lacZ) and those expressing wild-type dinr in germarial somatic cells (c587/+, UAS-dinrWT/+, dinrE19GAL80ts/dinr339 hh-lacZ). Somatic expression of dinr rescues the GSC loss phenotype of dinrE19/dinr339 mutants. The number of analyzed germaria is shown above each bar. *P < 0.05, **P < 0.01, **P < 0.001.
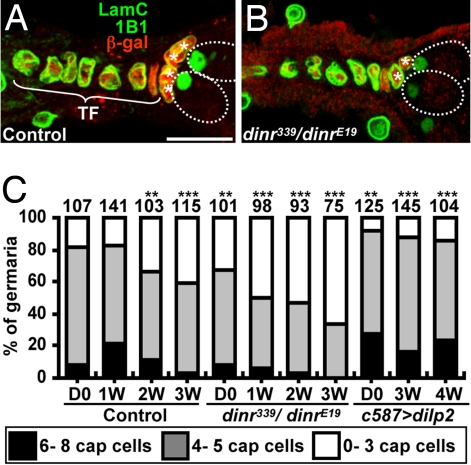
We next hypothesized that insulin signaling may regulate GSC fate via the niche. Indeed, expression of wild-type dinr in somatic cells of dinr339/dinrE19 germaria rescued GSC loss (Fig. 2E). To examine dinr339/dinrE19 niche structure, we counted terminal filament and cap cells (Fig. 3 A and B). Terminal filament cell numbers in dinr339/dinrE19 and control females are similar (Fig. S2). In contrast, dinr339/dinrE19 females eclose with fewer cap cells and also lose them faster over time (Fig. 3C), suggesting that insulin signaling controls cap cell number during development and adulthood. Moreover, DILP2 overexpression suppresses the wild-type age-dependent cap cell number decrease (Fig. 3C). We conclude that DILPs control GSC niche size and that the reduced cap cell numbers observed with increased female age at least in part reflect low insulin signaling levels.
Fig. 3.
Insulin signaling controls GSC niche size. One-week-old dinr339 hh-lacZ/+ (A, control) and dinr339 hh-lacZ/dinrE19 (B) germaria labeled with LamC (green, terminal filament and cap cell nuclear envelopes), 1B1 (green, fusome), and β-gal (red, terminal filament and cap cell nuclei). Dashed ovals indicate GSCs. TF, terminal filament; *, cap cells. (Scale bar, 10 μm.) (C) Cap cell number in control, dinr339/dinrE19, and DILP2-overexpressing females (see Fig. 1 legend). The number of analyzed germaria is shown above each bar. **P < 0.01, ***P < 0.001.
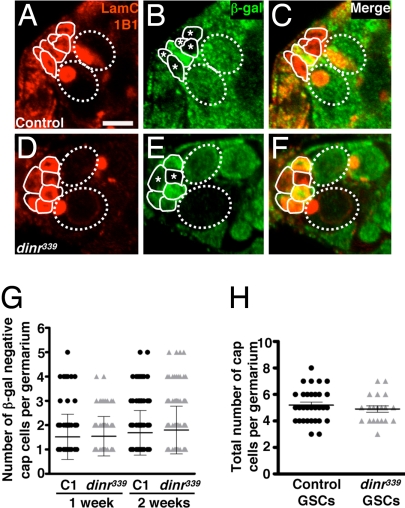
We next asked whether DILPs control cap cell number directly. In mosaic germaria containing β-gal–negative dinr339 or control cap cells (see Materials and Methods), the distribution (and average number) of β-gal–negative cap cells was indistinguishable (Fig. 4 A–G), indicating that dinr does not control cap cell number cell autonomously. It is possible that a second cell type, such as terminal filament cells, produces an intermediate factor; alternatively, cap cells themselves may control their own maintenance via paracrine signaling.
Fig. 4.
Insulin receptor signaling is required in a non–cell autonomous manner to maintain cap cell numbers. Germaria from control (A–C) and dinr339 (D–F) mosaic females labeled with LamC (outlining nuclear envelope of terminal filament and cap cells) and 1B1 (outlining fusome) (A and D), β-gal (B and E), and merged (C and F). Cap cells are outlined by solid lines. GSCs are outlined by dashed ovals. Control (A–C) or dinr339 homozygous mutant (D–F) cap cell clones are recognized by the absence of β-gal (*). (Scale bar, 5 μm.) (G) Similar distribution of number of β-gal–negative cap cells per germarium in control (C1) and dinr339 mosaic females at 1 or 2 weeks after eclosion. (H) Number of cap cells in mosaic germaria containing all β-gal–negative control or dinr339 mutant GSCs in 1-week-old females, showing that dinr function is not required in GSCs to regulate the number of cap cells.
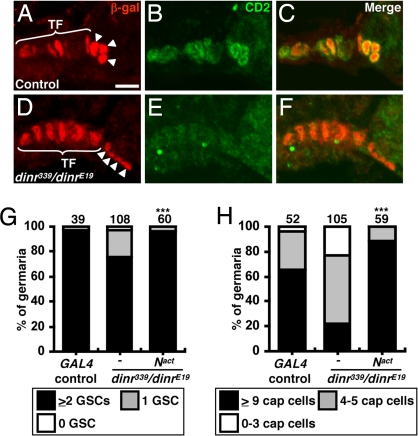
Notch signaling controls cap cell number during niche formation and in adults (17, 18). Notch hyperactivation during development forms ectopic cap cells, leading to excess GSCs. Conversely, defective Notch signaling reduces niche size and GSC number. Notch activation is strongly detected in larval terminal filament and cap cells and is also detected in adult cap cells (17, 18). We examined Notch signaling in dinr mutants using the E(spl)mβ-CD2 reporter (17, 19) (Fig. 5 A–F). Every control germarium (n = 40) had strong CD2 expression in both terminal filament and cap cells. In contrast, CD2 levels were severely reduced in dinr339/dinrE19 germaria (n = 40), indicating that insulin signaling controls Notch activation in the niche.
Fig. 5.
Insulin signaling maintains cap cells via Notch. Control (A–C) and dinr339 hh-lacZ/dinrE19 (D–F) germaria are labeled with β-gal (red, terminal filament and cap cell nuclei) and CD2 [green, E(spl)mβ-CD2 reporter of Notch signaling]. C and F show merge. TF, terminal filament; arrowheads, cap cells. (Scale bar, 5 μm.) GSC (G) or cap cell (H) numbers in 1-week old wild-type GAL4 controls or dinr339/dinrE19 mutants alone or expressing activated Notch (Nact). The number of germaria analyzed is shown above each bar. ***P < 0.001.
We next asked if the reduced cap cell number in dinr mutants was attributable to impaired Notch signaling (Fig. S3). Weak hypomorphic dinrE19/dinr353 females have no reduction in GSC or cap cell number (Tables S1 and S2). Similarly, Notch264–39 heterozygotes (half the Notch dosage) have normal GSC and cap cell numbers. In contrast, dinrE19/dinr353 females heterozygous for Notch264–39 have significantly reduced GSC and cap cell numbers. A decrease in small cap cell number has been reported for Notch264–39 heterozygotes (17); this discrepancy may reflect slightly reduced insulin signaling in the latter study attributable to diet.
To determine if Notch signaling is sufficient to rescue dinr defects, we expressed an activated form of Notch (20) in the somatic cells of dinr339/dinrE19 germaria, and the GSC and cap cell loss phenotypes were rescued (Fig. 5 G and H). These results and the genetic interaction between dinr and Notch are consistent with the insulin pathway acting upstream or in parallel to Notch. Nevertheless, the reduced Notch reporter levels in dinr mutants favor the model that insulin signaling leads to Notch activation, thereby controlling cap cell number and, indirectly, GSC maintenance.
GSCs and terminal filament cells express the Delta ligand for Notch, and removal of Delta function from GSCs has been reported to affect niche activity (18). We reasoned that dinr could be required in GSCs, terminal filament cells, the cap cell population, or a combination thereof to control Delta production and Notch activation. We analyzed dinr mosaic germaria in which all GSCs were dinr339 homozygous (Fig. 4H), and the number of cap cells in those germaria was indistinguishable from control numbers, suggesting that dinr is not required in GSCs for Notch signaling. DILPs may instead regulate Delta within terminal filament or cap cells or, alternatively, act via other intermediate signals to regulate Notch activation within the niche.
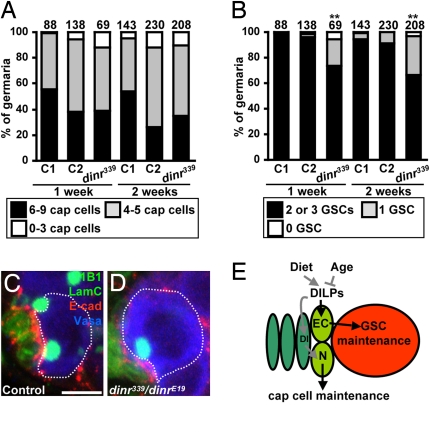
Cap cell and GSC numbers correlate (3, 11). Indeed, in germaria containing control β-gal–negative cap cells (control C1), total cap cell and GSC numbers are roughly proportional (Fig. S4A). Remarkably, despite similar cap cell numbers, a significant fraction of germaria in which dinr mutant cap cells are present contains fewer GSCs relative to control C1 or C2 (i.e., germaria without cap cell clones from dinr mosaics) (Fig. 6 A and B and Fig. S4A). Thus, although dinr does not control cap cell number per se autonomously, it is required within cap cells either for the optimal production and/or secretion of a GSC maintenance factor(s) or to promote GSC attachment.
Fig. 6.
Insulin signaling within cap cells controls GSC maintenance via E-cadherin. (A) Total cap cell numbers in control mosaic germaria (C1) and in germaria without (C2) or with (dinr339) cap cell clones from dinr339 mosaics. The average cap cell number is not statistically different among genotypes. (B) Significant decrease in GSC number in germaria carrying dinr339 cap cells. The number of germaria analyzed is shown above each bar. **P < 0.01. Heterozygous (C) and dinr339/dinrE19 mutant (D) germaria labeled with 1B1 (green, fusome), LamC (green, cap cell nuclear envelopes), and E-cadherin (red). Dashed outlines indicate GSCs. (Scale bar, 5 μm.) (E) DILPs integrate age and diet effects on GSC maintenance. DILPs control niche size via Notch (N) signaling in the niche [potentially via Delta (Dl)] and cap cell–GSC association via E-cadherin (EC). Dark green, terminal filament; light green, cap cells; red, GSC.
Niche-derived bone morphogenetic protein (BMP) signals directly stimulate GSCs to repress differentiation (21–23). To test if insulin signaling controls BMP pathway activation in GSCs, we used the Dad-lacZ reporter (24) (Fig. S4 B and C). Dad-lacZ levels in dinr339/dinrE19 and control females are indistinguishable, showing that dinr does not control BMP signaling. Insulin signaling in cap cells must therefore control another GSC maintenance signal and/or the cap cell–GSC association.
To investigate if dinr controls the physical interaction between cap cells and GSCs, we measured the percentage of dinr339 versus control cap cells directly contacting GSCs in mosaic germaria. Indeed, 21% of dinr339 cap cells (n = 76 β-gal–negative cells analyzed for possible interaction with 102 GSCs) contact GSCs, compared with 50% of control cap cells (n = 30 β-gal–negative cells analyzed for possible interaction with 53 GSCs), indicating that dinr339 cap cells have significantly reduced attachment to GSCs (P < 0.001). These results suggest that insulin signaling in cap cells controls their association with GSCs. Alternatively, insulin signaling may regulate the production of a short-range GSC maintenance signal, such that only GSCs in contact with dinr mutant cap cells are affected.
E-cadherin–mediated adhesion between cap cells and GSCs is required for retaining GSCs in the niche (25). We therefore measured E-cadherin levels at the GSC–cap cell junction. In controls, we found that E-cadherin levels vary with changes in the fusome, a membranous cytoskeletal structure (26). When the fusome is round, its predominant morphology (8), there is a higher intensity of E-cadherin at the junction (2,882 ± 953 arbitrary units, n = 13), although when the fusome is elongated, the intensity is lower (1,618 ± 620 arbitrary units, n = 7, P < 0.001). The intensity of E-cadherin at the junction of cap cells with GSCs displaying elongated fusomes in dinr339/dinrE19 mutants (1,637 ± 429 arbitrary units, n = 6) is similar to that of control. In contrast, the round fusome GSC–cap cell junctions contain significantly lower E-cadherin levels in dinr mutants (2,263 ± 711 arbitrary units, n = 15, P < 0.01) than in controls (Fig. 6 C and D). These results suggest that insulin signaling influences E-cadherin levels at the GSC–cap cell junction and may explain the age-dependent E-cadherin reduction that contributes to GSC loss (3).
Our studies demonstrate that systemic insulin-like signals integrate inputs from diet and age to regulate GSC maintenance via the niche (Fig. 6E). Specifically, we propose that DILPs control cap cell number via Notch and also E-cadherin–mediated GSC retention within the niche. Because diet and insulin signaling control GSC proliferation (7–9), it is also likely that the proliferation decline reported in older females (3) results from reduced insulin signaling. Our results also provide insights into recent findings that systemic factors from young mice can restore Notch activation and skeletal muscle progenitor proliferation and regenerative capacity to old mice in heterochronic parabiotic pairings (27). Finally, our results are intriguing in light of the well-established connection between low insulin signaling, restricted diet, and extended lifespan (28) and of studies in Caenorhabditis elegans suggesting that GSCs may have a negative effect on longevity (29). It is conceivable that excessive stem cell activity in general is deleterious and that slight decreases in stem cell number or activity with age as a result of reduced insulin signaling may actually promote longevity.
Materials and Methods
Drosophila Strains and Culture Conditions.
Drosophila stocks were maintained at 22–25 °C, unless otherwise indicated, on standard cornmeal/molasses/yeast/agar medium. A protein-rich diet consisted of standard medium supplemented with wet yeast paste, and a protein-poor diet consisted of an empty vial containing a Kimwipe (Kimberly Clark, Inc.) soaked in a 5% (vol/vol) molasses solution in water. In both cases, the food was changed daily. yw was used as a wild-type control. The hypomorphic dinrE19 and dinr353 alleles and null dinr339 alleles individually recombined to the FRT82B chromosome have been described (9, 16). The dinrE19/dinr353 heteroallelic females, which have a mild reduction in insulin signaling (see Fig. S1), have a ≈25% reduction in body size and a 5-day developmental delay. The dinrE19/dinr339 heteroallelic females, which have a strong reduction in insulin signaling (see Fig. S1), have half the wild-type body size and a 10–12-day developmental delay. The null chico1 and null Notch264–39 alleles have also been described (7, 17, 30). To express UAS-dilp2 (16), UAS-Notch (Nact) (20), and UAS-dinr (16), we used the c587-GAL4 driver (31), which is expressed in all somatic cells within the pupal and adult germaria (D.D.-B., unpublished data; ref. 31). Flies expressing Nact were raised at 18 °C and shifted to room temperature after eclosion until dissection. Flies expressing UAS-dinr also carried a Gal80ts transgene (32) and were raised at 18 °C (to prevent death of the developing flies attributable to dinr misexpression) and shifted to 29 °C after eclosion until dissection. hh-lacZ, a transgene carrying the hedgehog promoter upstream of the bacterial lacZ gene, was used to label terminal filament and cap cells (33). The tubulin-GPH (15), Dad-LacZ (24), and E(spl)mβ-CD2 (17, 19) reporters were used to monitor insulin, BMP, and Notch signaling, respectively. The hs-flipase (FLP) strain and other genetic elements used are described in Flybase (http://flybase.bio.indiana.edu).
Generation and Analysis of Genetically Mosaic Ovarioles by flipase (FLP)/FLP Recognition Target (FRT)–Mediated Recombination.
Genetic mosaics were generated as described (9). Females of the genotype hs-FLP/+; FRT82B dinr*/FRT82B arm-lacZ were generated by standard crosses (dinr* represents dinr339, dinrE19, or wild-type dinr alleles). To assay the maintenance of homozygous dinr* GSCs, 2-day-old females were heat-shocked for 1 h at 37 °C twice a day for 3 days to induce FLP-mediated mitotic recombination. The dinr mosaic flies and controls were transferred to fresh food with dry yeast daily and kept at 25 °C for 1 or 2 weeks before dissection. The dinr* homozygous clones were identified by the absence of β-gal, as detected by antibody staining, and were analyzed as described (23). To quantify GSC loss, we counted the number of germaria that contained dinr mutant GSCs, along with their dinr mutant progeny, versus similar germaria in which the original dinr mutant GSCs had been lost, and thus only their progeny remained (Fig. 2 B and C). To generate cap cell clones and for analysis of germaria containing all mutant GSCs, early third instar larvae (in which cap cells are still dividing) were heat-shocked at 37 °C twice a day for 2 days. Flies were cultured at room temperature after eclosion.
Immunostaining and Fluorescence Microscopy.
Ovaries were dissected and teased apart in Grace's medium (Cambrex), fixed for 13 min at room temperature in Grace's medium plus 5% (vol/vol) formaldehyde (Ted Pella), washed, and stained as described (34). For terminal filament cell analysis, dissected ovaries were fixed directly and teased apart only after immunostaining. An additional wash in 0.5% Triton X-100 for 30 min was included before incubation with anti–E-cadherin antibodies. The following antibodies were used: mouse monoclonal 1B1 [1:10; Developmental Studies Hybridoma Bank (DSHB)], mouse monoclonal anti-Engrailed (1:50; DSHB), mouse monoclonal anti-Lamin (Lam) C (1:100; DSHB), rat monoclonal anti–E-cadherin (1:3; DSHB), mouse monoclonal anti–β-gal (1:1,000; Promega), rabbit polyclonal anti–β-gal (1:1,000; Cappel), mouse monoclonal anti-CD2 (1:20; Serotec), and rabbit polyclonal anti-vasa (1:1,000; P. Lasko, McGill University, Montreal, Canada). Alexa 488– or Alexa 568–conjugated goat anti-mouse and anti-rabbit secondary antibodies (1:400; Molecular Probes) were used. Samples were incubated in 0.5 μg/mL DAPI (Sigma) for 8 min. Ovaries were mounted in Vectashield (Vector Laboratories). Micrographs were taken using a Zeiss LSM 510 confocal microscope. For quantification of E-cadherin, five to six 0.91-μm-thick optical sections were taken along ≈5 μm of the Z-axis of the region, including the E-cadherin–rich interface between cap cell and GSC. The total intensity of E-cadherin signal for the entire region of contact between cap cell and GSC was measured using ImageJ software.
GSC, Terminal Filament, and Cap Cell Analyses.
We used hh-lacZ expression (recognized by anti–β-gal antibodies) and anti-LamC or anti-Engrailed antibodies to label terminal filament and cap cells, and distinguished between these 2 cell types based on their position and morphology; cap cells are ovoid, whereas terminal filament cells are flatter and disk-like (31, 33) (Fig. 1A). The expression pattern of hh-lacZ, LamC, and Engrailed in terminal filament and cap cells was the same, except that the intensity of the anti-Engrailed staining was weaker; therefore, we used the LamC or hh-lacZ markers for most of our analyses. GSCs were unambiguously identified based on their juxtaposition to cap cells and the morphology and position of their anteriorly anchored fusomes (8, 26) (Fig. 1 A and B). All data were plotted using Microsoft Excel or GraphPad Prism 5 and subjected to Student's t test or χ2 statistical analyses.
Apoptosis Assay.
We used the ApopTag fluorescein direct in situ apoptosis detection kit (Intergen) as described (7, 9) to detect the occurrence of cell death within germaria. In brief, ovaries were dissected in Grace's medium, fixed, and washed as described previously. Ovaries were washed twice for 5 min with 300 μL of equilibration buffer and then incubated for 1 h at 37 °C in 100 μL of terminal deoxynucleotidyl transferase solution. Reactions were stopped in stop/wash solution, and ovaries were rinsed and immunostained as described previously.
Supplementary Material
Acknowledgments.
We thank E. Hafen, L. Dobens, G. Struhl, A. Spradling, P. Lasko, the Bloomington Stock Center, and the Developmental Studies Hybridoma Bank for Drosophila stocks and antibodies; B. Cha and I. Kaverina for help using ImageJ software; and Josh Gamse, David Greenstein, and Anna Means for valuable comments on this manuscript. This work was supported by National Institutes of Health Grant GM 069875 and by American Cancer Society Grant RSG-07-182-01-DDC.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0809144106/DCSupplemental.
References
- 1.Li L, Xie T. Stem cell niche: Structure and function. Annu Rev Cell Dev Biol. 2005;21:605–631. doi: 10.1146/annurev.cellbio.21.012704.131525. [DOI] [PubMed] [Google Scholar]
- 2.Jones DL, Wagers AJ. No place like home: Anatomy and function of the stem cell niche. Nat Rev Mol Cell Biol. 2008;9:11–21. doi: 10.1038/nrm2319. [DOI] [PubMed] [Google Scholar]
- 3.Pan L, et al. Stem cell aging is controlled both intrinsically and extrinsically in the Drosophila ovary. Cell Stem Cell. 2007;1:458–469. doi: 10.1016/j.stem.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 4.Ryu BY, Orwig KE, Oatley JM, Avarbock MR, Brinster RL. Effects of aging and niche microenvironment on spermatogonial stem cell self-renewal. Stem Cells. 2006;24:1505–1511. doi: 10.1634/stemcells.2005-0580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boyle M, Wong C, Rocha M, Jones DL. Decline in self-renewal factors contributes to aging of the stem cell niche in the Drosophila testis. Cell Stem Cell. 2007;1:470–478. doi: 10.1016/j.stem.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 6.Fuller MT, Spradling AC. Male and female Drosophila germline stem cells: Two versions of immortality. Science. 2007;316:402–404. doi: 10.1126/science.1140861. [DOI] [PubMed] [Google Scholar]
- 7.Drummond-Barbosa D, Spradling AC. Stem cells and their progeny respond to nutritional changes during Drosophila oogenesis. Dev Biol. 2001;231:265–278. doi: 10.1006/dbio.2000.0135. [DOI] [PubMed] [Google Scholar]
- 8.Hsu HJ, LaFever L, Drummond-Barbosa D. Diet controls normal and tumorous germline stem cells via insulin-dependent and -independent mechanisms in Drosophila. Dev Biol. 2008;313:700–712. doi: 10.1016/j.ydbio.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.LaFever L, Drummond-Barbosa D. Direct control of germline stem cell division and cyst growth by neural insulin in Drosophila. Science. 2005;309:1071–1073. doi: 10.1126/science.1111410. [DOI] [PubMed] [Google Scholar]
- 10.Guarente L, Kenyon C. Genetic pathways that regulate ageing in model organisms. Nature. 2000;408:255–262. doi: 10.1038/35041700. [DOI] [PubMed] [Google Scholar]
- 11.Xie T, Spradling AC. A niche maintaining germ line stem cells in the Drosophila ovary. Science. 2000;290:328–330. doi: 10.1126/science.290.5490.328. [DOI] [PubMed] [Google Scholar]
- 12.Margolis J, Spradling A. Identification and behavior of epithelial stem cells in the Drosophila ovary. Development. 1995;121:3797–3807. doi: 10.1242/dev.121.11.3797. [DOI] [PubMed] [Google Scholar]
- 13.Ikeya T, Galic M, Belawat P, Nairz K, Hafen E. Nutrient-dependent expression of insulin-like peptides from neuroendocrine cells in the CNS contributes to growth regulation in Drosophila. Curr Biol. 2002;12:1293–1300. doi: 10.1016/s0960-9822(02)01043-6. [DOI] [PubMed] [Google Scholar]
- 14.Shimizu M, et al. Age-related alteration of pancreatic beta-cell function. Increased proinsulin and proinsulin-to-insulin molar ratio in elderly, but not in obese, subjects without glucose intolerance. Diabetes Care. 1996;19:8–11. doi: 10.2337/diacare.19.1.8. [DOI] [PubMed] [Google Scholar]
- 15.Britton JS, Lockwood WK, Li L, Cohen SM, Edgar BA. Drosophila's insulin/PI3-kinase pathway coordinates cellular metabolism with nutritional conditions. Dev Cell. 2002;2:239–249. doi: 10.1016/s1534-5807(02)00117-x. [DOI] [PubMed] [Google Scholar]
- 16.Brogiolo W, et al. An evolutionarily conserved function of the Drosophila insulin receptor and insulin-like peptides in growth control. Curr Biol. 2001;11:213–221. doi: 10.1016/s0960-9822(01)00068-9. [DOI] [PubMed] [Google Scholar]
- 17.Song X, Call GB, Kirilly D, Xie T. Notch signaling controls germline stem cell niche formation in the Drosophila ovary. Development. 2007;134:1071–1080. doi: 10.1242/dev.003392. [DOI] [PubMed] [Google Scholar]
- 18.Ward EJ, et al. Stem cells signal to the niche through the Notch pathway in the Drosophila ovary. Curr Biol. 2006;16:2352–2358. doi: 10.1016/j.cub.2006.10.022. [DOI] [PubMed] [Google Scholar]
- 19.Dobens L, Jaeger A, Peterson JS, Raftery LA. Bunched sets a boundary for Notch signaling to pattern anterior eggshell structures during Drosophila oogenesis. Dev Biol. 2005;287:425–437. doi: 10.1016/j.ydbio.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 20.Struhl G, Fitzgerald K, Greenwald I. Intrinsic activity of the Lin-12 and Notch intracellular domains in vivo. Cell. 1993;74:331–345. doi: 10.1016/0092-8674(93)90424-o. [DOI] [PubMed] [Google Scholar]
- 21.Chen D, McKearin D. Dpp signaling silences bam transcription directly to establish asymmetric divisions of germline stem cells. Curr Biol. 2003;13:1786–1791. doi: 10.1016/j.cub.2003.09.033. [DOI] [PubMed] [Google Scholar]
- 22.Song X, et al. Bmp signals from niche cells directly repress transcription of a differentiation-promoting gene, bag of marbles, in germline stem cells in the Drosophila ovary. Development. 2004;131:1353–1364. doi: 10.1242/dev.01026. [DOI] [PubMed] [Google Scholar]
- 23.Xie T, Spradling AC. Decapentaplegic is essential for the maintenance and division of germline stem cells in the Drosophila ovary. Cell. 1998;94:251–260. doi: 10.1016/s0092-8674(00)81424-5. [DOI] [PubMed] [Google Scholar]
- 24.Kai T, Spradling A. An empty Drosophila stem cell niche reactivates the proliferation of ectopic cells. Proc Natl Acad Sci USA. 2003;100:4633–4638. doi: 10.1073/pnas.0830856100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Song X, Zhu CH, Doan C, Xie T. Germline stem cells anchored by adherens junctions in the Drosophila ovary niches. Science. 2002;296:1855–1857. doi: 10.1126/science.1069871. [DOI] [PubMed] [Google Scholar]
- 26.de Cuevas M, Spradling AC. Morphogenesis of the Drosophila fusome and its implications for oocyte specification. Development. 1998;125:2781–2789. doi: 10.1242/dev.125.15.2781. [DOI] [PubMed] [Google Scholar]
- 27.Conboy IM, et al. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005;433:760–764. doi: 10.1038/nature03260. [DOI] [PubMed] [Google Scholar]
- 28.Tatar M, Bartke A, Antebi A. The endocrine regulation of aging by insulin-like signals. Science. 2003;299:1346–1351. doi: 10.1126/science.1081447. [DOI] [PubMed] [Google Scholar]
- 29.Arantes-Oliveira N, Apfeld J, Dillin A, Kenyon C. Regulation of life-span by germ-line stem cells in Caenorhabditis elegans. Science. 2002;295:502–505. doi: 10.1126/science.1065768. [DOI] [PubMed] [Google Scholar]
- 30.Bohni R, et al. Autonomous control of cell and organ size by CHICO, a Drosophila homolog of vertebrate IRS1–4. Cell. 1999;97:865–875. doi: 10.1016/s0092-8674(00)80799-0. [DOI] [PubMed] [Google Scholar]
- 31.Zhu CH, Xie T. Clonal expansion of ovarian germline stem cells during niche formation in Drosophila. Development. 2003;130:2579–2588. doi: 10.1242/dev.00499. [DOI] [PubMed] [Google Scholar]
- 32.McGuire SE, Mao Z, Davis RL. Spatiotemporal gene expression targeting with the TARGET and gene-switch systems in Drosophila. Sci STKE. 2004;2004:l6. doi: 10.1126/stke.2202004pl6. [DOI] [PubMed] [Google Scholar]
- 33.Forbes AJ, Lin H, Ingham PW, Spradling AC. Hedgehog is required for the proliferation and specification of ovarian somatic cells prior to egg chamber formation in Drosophila. Development. 1996;122:1125–1135. doi: 10.1242/dev.122.4.1125. [DOI] [PubMed] [Google Scholar]
- 34.de Cuevas M, Lee JK, Spradling AC. Alpha-spectrin is required for germline cell division and differentiation in the Drosophila ovary. Development. 1996;122:3959–3968. doi: 10.1242/dev.122.12.3959. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.