Abstract
Recombinase-activating gene-2-deficient (Rag2−/−) mice lacking functional lymphocytes provide a useful model of chronic inflammatory bowel disease-emulating events in human colon cancer. Infection of Rag2−/− mice with Helicobacter hepaticus led to accumulation of macrophages and neutrophils in the colon, a process temporally related to up-regulation of tissue inducible nitric oxide synthase (iNOS) expression at the site of infection and increased nitric oxide (NO) production, as evidenced by urinary excretion of nitrate. Progressive development of increasingly severe inflammation, hyperplasia, dysplasia, and cancer accompanied these changes. Concurrent administration of an iNOS inhibitor prevented NO production and abrogated epithelial pathology and inhibited the onset of cancer. The presence of Gr-1+ neutrophils and elevated tumor necrosis factor-α (TNF-α) expression in colon were required for increased iNOS expression and cancer, whereas interleukin-10 (IL-10) down-regulated TNF-α and iNOS expression and suppressed cancer. Anti-inflammatory CD4+ regulatory lymphocytes also down-regulated iNOS and reduced cancer formation. Collectively, these results confirm essential roles for inflammation, increased TNF-α expression, and elevated NO production in colon carcinogenesis.
Keywords: colorectal cancer, IBD, innate immunity
Chronic Helicobacter pylori infection in humans leads to gastritis and has been established as a causative agent for human gastric cancer (1). Inflammatory bowel diseases (IBDs), such as Crohn's disease and ulcerative colitis, also increase risk for colon cancer (2, 3). Generation of nitric oxide (NO) by inducible NO synthase (iNOS) is a central feature of chronic inflammatory diseases in the gastrointestinal tract (4–9), but precise mechanistic roles for NO in colon cancer development remain undefined.
Colon cancer patients exhibit evidence of nitrosative and oxidative stresses that increase cancer risk (10), resulting from mutagenic reactive oxygen and nitrogen species derived from NO generated by immune cells (6, 11–15). Roles for chronic bacterial infection in IBD and colon cancer have been identified recently in recombinase-activating gene-2-deficient mice (Rag2−/−), which completely lack functional lymphocytes (16–18). We have exploited this mouse model of chronic IBD-associated cancer for studies of the role of NO and its products because it emulates naturally occurring inflammatory events in humans (16, 19, 20).
Rag2−/− mice have been used to assess functions of lymphocytes by adoptive transfer. Populations of CD4+ T cells with low or high expression of CD45RB (17, 21, 22) or CD25 (16, 18, 23, 24) prevent or accelerate colitis in these animals. In wild-type (wt) mice, protection against inflammatory pathology induced by bacterial infection has been attributed to interleukin-10 (IL-10) and IL-10-dependent functions of CD4+ cells (18, 20, 25, 26). Collectively, this evidence forms the rationale for the hypothesis that NO overproduction comprises a linkage between Helicobacter hepaticus-induced inflammation and induction of colon cancer (16), which we further test in this study by assessing protective effects of N-methyl-arginine (NMA), an iNOS inhibitor, administered in the drinking water of infected animals. All experiments were carried out in Rag2−/− mice unless otherwise stated.
Results
Infection and NO Production.
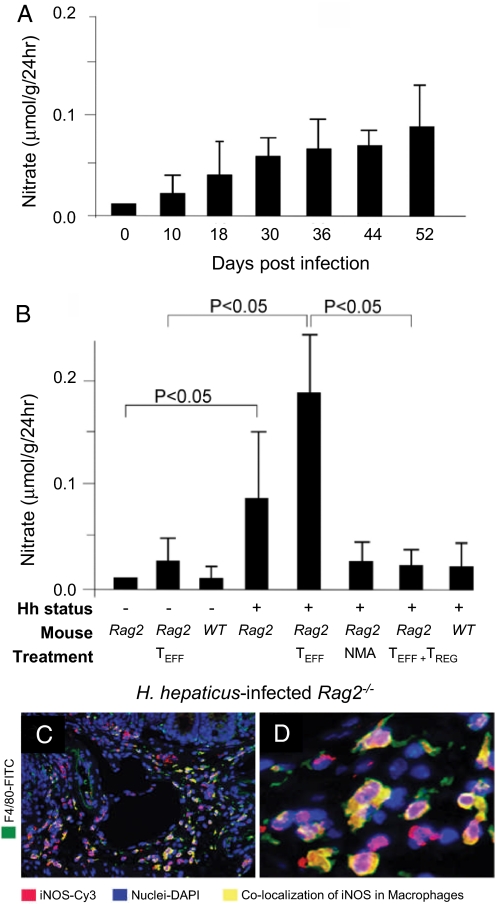
To assess the contribution of NO production to colon cancer development, Rag2−/− and wt mice were infected with H. hepaticus (16, 18). Total NO production was measured by urinary nitrate (NO3−) excretion (11). Infected, but not uninfected, mice showed a time-dependent increase (P < 0.05) in urinary NO3− excretion beginning 2 weeks after infection (Fig. 1A), which was temporally associated with development of IBD and carcinoma (Table 1). In contrast, infected wt mice, showed no elevation of urinary nitrate and did not develop cancer (Fig. 1B) (16), indicating that infection alone was not sufficient to induce cancer.
Fig. 1.
Urinary nitrate excretion after infection with H. hepaticus. (A) Temporal increase in urinary nitrate excretion followed infection with H. hepaticus. (B) Nitrate excretion was elevated in infected Rag2−/− mice with cancer but not WT mice without cancer. Treatment with NMA or TREG cells decreased urinary nitrate levels to that of uninfected Rag2−/− mice and prevented cancer development in infected Rag2−/− mice. (C) FIHC showed colocalization of F4/80 and iNOS proteins in the colon of an H. hepaticus-infected mouse. (Original magnification, 100×.) (D) Higher magnification (1,000×) of C reveals iNOS expression in inflammatory cells.
Table 1.
Induction of colon pathology in immune-deficient Rag2−/− mice by H. hepaticus (Hh) infection and modulating effects of immune cell augmentation
| Hh status | Genotype | Treatment | Severity of colon pathology* |
Carcinoma incidence | Neoplastic invasion | ||
|---|---|---|---|---|---|---|---|
| Inflammation | Hyperplasia | Dysplasia | |||||
| − | wt | − | 0 | 0 | 0 | 0/8 | |
| + | wt | − | 1 (0–2) | 0 (0–1) | 0 | 0/8 | |
| + | wt | − | 0 (0–1) | 0 (0–1) | 0 | 0/8 | |
| − | Rag2−/− | − | 0 | 0 | 0 | 0/8 | |
| + | Rag2−/− | − | 4 (2–4) | 4 (3–4) | 3 (0–4)† | 7/10 | 1/10 |
| + | Rag2−/− | − | 3 (2–4) | 3 (2–4) | 2 (2–3)† | 4/10 | |
| − | Rag2−/− | wt TEFF | 1 (0–2) | 0.5 (0–1) | 0 | 0/8 | 0/10 |
| + | Rag2−/− | wt TEFF | 4 (2–4) | 4 (1–4) | 3 (0–4)† | 6/10 | 4/10 |
| + | Rag2−/− | wt TREG | 0 (0–4) | 0 (0–3) | 0 | 0/10 | |
| + | Rag2−/− | wt TEFF + wt TREG | 0 | 0 | 0 | 0/8 | |
| + | Rag2−/− | wt CD4+ | 1.5 (1–3) | 0 (0–1) | 0 (0–1) | 0/8 | |
| + | Rag2−/− | IL10−/− CD4+ | 4 (0–4) | 4 (0–4) | 3 (3–4)† | 8/8 | 4/8 |
| + | Rag2−/− | IL10-Ig fusion protein | 1.5 (0–4) | 1 (0–3) | 0.5 (0–1) | 0/8 | |
| − | IL10−/−Rag2−/− | − | 1 (0–2) | 1 (0–2) | 0.5 (0–1) | 0/8 | |
| + | IL10−/−Rag2−/− | − | 4 (0–4) | 4 (0–4) | 3 (3–4)† | 10/10 | 1/10 |
| + | IL10−/− | − | 3 (0–3) | 3 (0–3) | 2.5 (2–4)† | 4/8 | 1/10 |
*Median severity score (range) on 0–4 scale.
†P < 0.05 vs. control value by Mann–Whitney U test for nonparametric data
To estimate levels of NO synthesis at the site of infection (20), iNOS expression was examined by quantitative RT-PCR on sections of ascending colon. Tissue from infected mice contained 200-fold higher (P < 0.001) levels of iNOS mRNA than were present in uninfected Rag2−/− or wt controls (Fig. 2A). Expression of iNOS protein in situ in ascending colon was localized by FIHC to epithelial cells and macrophages, and less frequently to granulocytes [Fig. 1 C and D and supporting information (SI) Fig. S1]. Induction of iNOS was associated with the presence of 7/4+ polymorphonuclear cells (Table S1) (18, 20, 27), and also correlated with up-regulation of tumor necrosis factor-α (TNF-α), IL-6, and MIP-2 (Fig. 2A) (28). The iNOS-dependent activities of bone marrow-derived 7/4+ neutrophils have been shown previously to be required for induction of IBD in mice (29).
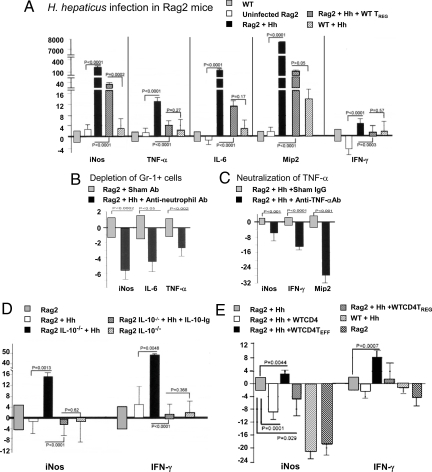
Fig. 2.
Modulation of expression of iNOS and inflammatory cytokines in colon tissue. (A) Expressions of proinflammatory cytokines and iNOS were significantly increased after infection with H. hepaticus (Hh). Depletion of Gr-1+ cells (B) and TNF-α (C) decreased iNOS expression. (D) Infected double-knockout mice showed significant increases in iNOS and IFN-γ gene expression. (E) TEFF cells increased levels of IFN (IFN-γ) and iNOS expression.
Immune Cell Modulation of Infection-Induced NO Production and Colon Pathology.
Myeloid differentiation antigen Gr-1 (or Ly-6G) is expressed on bone marrow precursors and peripheral neutrophils and is considered a good marker for these populations. Treatment with anti-Gr-1 antibody has been shown previously to deplete mature neutrophils and abrogate Helicobacter-induced gastritis in mice (30). For further assessment of the role of neutrophils in colon carcinogenesis in infected Rag2−/− mice, we performed depletion experiments by intraperitoneal injection of anti-Gr-1 antibody thrice weekly for 10 days beginning at 6 weeks after infection. Depletion of Gr-1+ cells significantly (P < 0.01) decreased iNOS gene expression levels (Fig. 2B) and the severity of colon pathology (Table S1) in infected mice compared with infected, sham IgG-treated animals. Interestingly, depletion of Gr1+ cells eliminated 7/4+ neutrophils (μ = 0.4 ± 0.22 cells in treated vs. 28.4 ± 3.33 cells in control mice) and significantly decreased macrophages (6.6 ± 1.88 cells in treated vs. 13.8 ± 1.14 cells in control mice). iNOS expression within epithelia and other inflammatory cells in the colon also was suppressed (Fig. S1 B and D) compared with sham-treated infected mice (Fig. S1 A and C). Depletion also decreased colon mRNA levels of TNF-α (P < 0.001) and IL-6 (P < 0.05) (Fig. 2B), suggesting that Gr-1+ cells may induce iNOS expression through cytokine-mediated processes. Similarly, their depletion decreased the frequency of myeloperoxidase (MPO)-positive cells (21.3 ± 4.3 vs. 4.0 ± 1.3 MPO+ cells per 40× field in sham and anti-Gr-1 mice, respectively; P < 0.01) in the colon (Fig. S1). Taken together, these results demonstrate that Gr-1+ cells are required for colitis-associated cancer in H. hepaticus-infected Rag2−/− mice, and suggest a functional relationship between iNOS and MPO in the carcinogenesis process (31).
Because nuclear factor κB (NF-κB)-mediated inflammatory signaling is dependent upon TNF-α (32), we postulated that this proinflammatory cytokine would be required for recruitment and activation of Gr-1+ neutrophils. We therefore neutralized TNF-α in Rag2−/− mice by injection of anti-TNF antibody, which significantly (P < 0.05) decreased expression of IL-6, MIP-2, and iNOS (Fig. 2C) and reduced bowel pathology (Table S1) compared with uninfected controls. These results suggest a sequence of events in which bacterial infection triggers increased TNF-α expression, leading to up-regulation of iNOS and contributing to development of colon cancer in infected mice.
Anti-inflammatory activities of IL-10 have been shown to inhibit TNF-α-dependent responses to pathogenic bacteria in several mouse models (18, 20, 25). We therefore used Rag2−/−-deficient mice also lacking IL-10 (IL10−/−Rag2−/−) to determine whether IL-10 was capable of preventing iNOS up-regulation triggered by infection. Infected double-knockout mice showed significant (P < 0.01) increases in iNOS gene expression (Fig. 2D), 7/4+ neutrophils, and F4/80+ macrophages (Table S2), as well as inflammatory pathology (Fig. S2 C–E) and colon tumors (P < 0.05; Table 1), when compared with infected Rag2−/− control mice. Uninfected IL10−/−Rag2−/− mice had undetectably low levels of inflammatory cytokines (Fig. 2D) and colon pathology (Table 1). Replacement of IL-10 by injection of IL10-Ig fusion protein restored iNOS and inflammatory cytokine expression to baseline levels (Fig. 2D). Likewise, infected Rag2−/− mice treated with IL10-Ig fusion protein had significantly (P < 0.05) decreased inflammation and tumor development when compared with sham-treated animals (Table 1). Thus, IL-10 suppressed infection-induced iNOS elevation and tumor formation, whereas IL10-deficient mice were highly susceptible to H. hepaticus-induced bowel disease (Table 1 and Table S2).
To study further the role of IL-10 in modulating infection-induced responses, we performed adoptive transfer of purified IL10-producing CD4+ lymphocytes into infected Rag2−/− mice. We found that CD4+ cells collected from wt but not IL10−/− donor mice prevented up-regulation of iNOS (Fig. 2E) and development of cancer (Table 1). To determine whether regulatory T (TREG) cells—i.e., the subset of wt CD4+ cells with low expression of CD45RB that have potent anti-inflammatory properties (16, 18)—would down-regulate TNF-α (18, 27), we performed adoptive i.v. transfer of 3.0 × 105 TREG cells into mice at the time of infection. Mice receiving wt TREG cells had reduced (P < 0.005) epithelial dysplasia in the cecum (Table S3) and colon, as well as decreased inflammatory gene expression (Fig. 2E) 3–4 months after infection. However, this inhibitory effect was not a property of all CD4+ cells. Indeed, infected mice that received the CD4+CD45RBhi (TEFF) subset of cells developed more severe IBD and mucinous carcinoma (Fig. S2 and Table 1), a variant of colorectal cancer in humans that arises in about 20% of patients with IBD (33). TEFF cell recipients also had increased levels of IFN (IFN-γ) and iNOS (Fig. 2E), increased urinary NO3− (Fig. 1B), and a higher frequency of iNOS-bearing F4/80+ and MPO+ cells (data not shown), compared with infected controls. In contrast, uninfected recipients showed minimal pathology (Table 1), in agreement with the previously published findings of others (18). Together, these data indicate that protective properties of IL10-competent lymphocytes reside in the CD45RBlo subset of CD4+ cells. Cotransfer of anti-inflammatory TREG cells along with TEFF cells in infected mice was sufficient to down-regulate urinary NO3− (Fig. 1B) and colon pathology (Table 1), indicating that IL10-competent TREG cells inhibited TNF-α-mediated inflammatory events leading to cancer.
Suppression of NO Production and Colon Pathology.
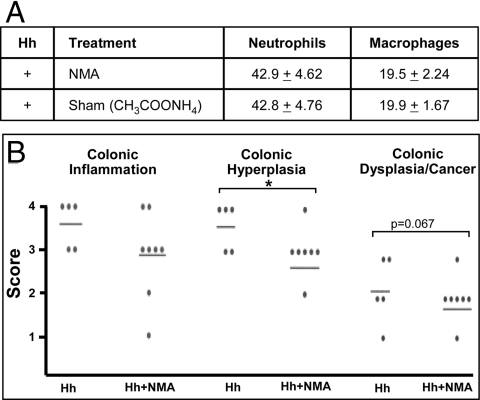
To confirm the role of NO in the induction of colon cancer, mice were administered the iNOS inhibitor NMA in their drinking water continuously, beginning before infection. This treatment resulted in significantly decreased epithelial hyperplasia in colonic (Fig. 3B) and cecal (data not shown) epithelia compared with infected control animals. Decreased epithelial dysplasia was also evident in treated mice at 3–4 months after infection (Fig. 3B), indicative of reduced potential for eventual development of malignancy. Infected animals treated with NMA showed no elevation of NO3− excretion compared with controls (Fig. 1B), confirming that NMA inhibited NO production. Levels of innate immune inflammatory cells (F4/80+ macrophages and 7/4+ neutrophils) remained unchanged in infected mice treated with NMA (Fig. 3A), whereas the number of inflammatory cells expressing iNOS in situ within colonic tissue was reduced by NMA (μ = 4.2 ± 2.86 cells in NMA-treated vs. 7.2 ± 3.35 cells in sham-treated mice). This indicated that inhibition of NO formation by NMA, not a reduction of inflammatory cell counts, was responsible for the observed diminished pathology. Thus, inhibition of NO production in infected mice decreased the probability of colon cancer development.
Fig. 3.
NO synthase inhibitor NMA inhibits development of malignancy in mice. (A) Numbers of 7/4+ neutrophils and F4/80+ macrophages were unchanged by NMA treatment; therefore, protective effects of NMA were not attributable to reduced numbers of inflammatory cells. (B) Histopathologic indices of dysplasia and cancer were reduced by NMA treatment.
Discussion
Observations of Virchow more than a century ago (32) are supported by many epidemiological studies illustrating that chronic inflammation increases cancer risks in humans. The features of colon carcinoma induced in Rag2−/− mice by H. hepaticus infection closely resemble those of colon cancer in humans (34). Epithelial dysplasia and carcinoma arise in situ from inflammatory cell foci and epithelial ulceration, then progress to intramucosal carcinoma, and highly invasive mucinous carcinoma (16, 20, 27). Although a bacterial etiology has not been confirmed for IBD in humans, H. pylori infection has been shown to lead to gastritis-associated gastric cancer (1). Treatment of infected mice with NMA reduced epithelial dysplasia without having an impact on inflammatory cell levels in tissues (Fig. 3), in agreement with previous studies (35, 36). Lack of change in cellular inflammatory cell infiltrates and inflammation in NMA-treated mice (Fig. 3A) points to dysregulation of NO production rather than the presence of inflammatory cells per se in the pathogenesis of colon cancer in this system. The host benefit of blocking NO synthesis beyond IBD-associated colon cancer is suggested by reduced intestinal adenoma formation in mice that undergo spontaneous loss of heterozygosity of the adenomatous polyposis coli (Apc) gene (ApcMin/+ mice) using both inhibitors and mice lacking iNOS (37).
The proinflammatory cytokine TNF-α and bacteria-triggered TNF-α-dependent increases in inflammatory cell infiltrates and NO production have been shown to be required for development of colon cancer in mice (14, 15, 29). Beck et al. (29) revealed the main source of NO to be bone marrow-derived inflammatory cells, in particular iNOS-bearing 7/4+ granulocytes. Neutrophils also have been shown to be required for infection-induced cancer (38, 39). In our experiments, depletion of Gr-1+ cells decreased iNOS expression (Fig. 2B), colitis, and carcinoma (Table 1) and reduced iNOS expression within epithelial cells and other inflammatory cells (Fig. S1 B and D). It also decreased TNF-α (P < 0.001) and IL-6 (P < 0.05) (Fig. 2B), suggesting indirect effects of Gr-1+ neutrophils, such as escalating host inflammatory response (28) and oxidative stressors (11, 29, 40), that promote cancer.
Supplementation with proinflammatory TEFF cells increased IFN-γ-dependent and TNF-α-dependent malignancy in inflammation-driven mouse models (41), confirmed by studies showing increased inflammatory disease after TEFF cell transfer in H. hepaticus-infected Rag2−/− mice (18, 22, 42). Increased IFN-γ secretion and NO (11, 15) have been found in humans with gastrointestinal carcinoma as well (1). Targeting inflammation and NO is clinically relevant, because mucinous colonic carcinoma seen in TEFF cell recipient mice most often affects young humans and has a poor prognosis associated with invasion of adjacent viscera and lymph nodes beyond the pericolonic region (33). Cotransfer of CD4+ TREG and TEFF cells significantly protected against colon carcinogenesis (41), and NO was linked with increased IL-10-mediated recruitment of protective anti-inflammatory TREG cells (43). Prior challenge with H. hepaticus induced IL-10-dependent TREG cells that protected against IBD in mice (26). TREG cells collected from H. hepaticus-infected donors had greater potency to protect against intestinal and mammary cancer than those from uninfected donors (44). Identifying which TREG subsets are most effective in the regulation of TNF-α and NO to prevent or treat infection-associated colon cancer is a future goal.
Our findings suggest a connection between NO and MPO, an enzyme present in granulocytes, in which NO is converted to nitrite, and nitrite is oxidized by MPO to NO2 (31). NO2 is a strong one-electron oxidant capable of both oxidation and nitration of nucleic acids, proteins, and lipids (45–49). Products generated by NO2 oxidation of DNA are toxic and highly mutagenic (50, 51), and lipid peroxidation products yield reactive carbonyls that also modify DNA to form promutagenic bases (52). Studies in MPO−/− mice showed that formation of nitrotyrosine, a product of NO oxidation to peroxynitrite, required MPO following Klebsiella pneumoniae infection that induced primarily neutrophil infiltration (53). Only when both nitrite and MPO were present did nitrotyrosine form.
Our principal findings can be summarized as follows. Infection of Rag2−/− mice with H. hepaticus led to infiltration of macrophages and neutrophils into the colon, which was temporally related to up-regulation of iNOS expression at the site of infection and increased NO production, evidenced by urinary excretion of nitrite. Progressive development of increasingly severe inflammation, hyperplasia, dysplasia, and cancer accompanied these changes. Concurrent administration of an iNOS inhibitor prevented NO production and abrogated the epithelial pathology and inhibited the onset of cancer. The presence of Gr-1+ cells and elevated TNF-α expression in colon were required for increased iNOS expression and cancer, whereas IL-10 down-regulated TNF-α and iNOS expression and suppressed cancer. Anti-inflammatory CD4+ TREG lymphocytes down-regulated iNOS and reduced cancer formation. Collectively, these results confirm essential roles for elevated NO production and TNF-α expression in the carcinogenesis process in this experimental model.
Materials and Methods
Animals.
Experiments were conducted in 129/SvEv Rag2−/− and wt mice (16) housed in Association for Assessment and Accreditation of Laboratory Animal Care-approved facilities in static microisolator cages and were approved by the institutional Animal Care and Use Committee. Each experiment included 6–10 mice per group and was repeated twice (total n = 12–20 mice per group) unless otherwise specified. Treatment groups included equal numbers of males and females unless otherwise specified.
H. hepaticus Infection.
Animals were infected with H. hepaticus (strain 3B1, ATCC 51449) grown and confirmed as pure culture as described previously (16, 54). Mice aged 6–8 weeks received 0.2 mL of fresh inoculum by gavage every other day for a total of 3 doses. Cecum and colon were collected at necropsy and analyzed by PCR using H. hepaticus-specific primers to confirm infection status (16). Subsequent treatments are described below. Time points selected for tissue harvest were based on typical progression of IBD and dysplasia (16). For each adoptive transfer experiment (see below), a cohort of age-matched littermates served as H. hepaticus-infected controls.
Nitrate Excretion.
Quantitation of nitrate excretion as a biomarker of endogenous NO production was performed as described previously (55). A total of 160 mice in 3 separate experiments were fed a low-nitrate diet (AYN-76A; Bioserve) for at least 2 weeks before infection to minimize the background rate of nitrate excretion. Mice were housed individually in metabolic cages, and urine was collected weekly in 0.5 M NaOH to inhibit bacterial growth. Total urinary nitrate concentration was determined as described previously (15, 55). One hundred mice served as Helicobacter-free controls. Forty infected mice were used in the evaluation of NMA effects. At 24 h before infection, 30 mM NMA (EMD Chemicals) replaced drinking water for 10 mice (2 replications; total n = 20) and equimolar ammonium acetate (Sigma Chemical) for 10 mice (2 replications; total n = 20) to serve as sham controls. Mice were euthanized at 3–4 months after infection
Lymphocyte Transfer.
For adoptive transfer of CD4+ lymphocytes, 160 mice (treatment groups of 10 recipient mice, each with 2 replications) underwent the procedure as described previously (16, 41). Single-cell suspensions of CD4+ lymphocytes were prepared as described previously (56, 57) from spleen and mesenteric lymph nodes from Helicobacter-free wild-type or IL10−/− donor mice backcrossed at least 10 generations onto the 129/SvEv genetic background. Reanalysis confirmed purity (>95%) and phenotype of the cells before transfer. Rag2−/− recipients of wt or IL10−/− lymphocytes underwent adoptive transfer 48 h before infection (16). For replacement of IL-10, murine IL-10 was fused to IgG2a by using a PCR cloning strategy and cloned into an adenoviral vector as described previously (27, 57). Serum containing 1 μg of fusion protein was administered by i.p. injection to H. hepaticus-infected IL10−/−Rag2−/− or Rag2−/− mice twice weekly for 6 weeks.
Neutrophil or TNF-α Depletion.
For experiments involving depletion of Gr-1+ cells or neutralization of TNF-α, 40 infected mice were subdivided into groups of 10 each, and each was dosed with 0.2 mL of anti-Gr-1 or anti-TNF antibody, or sham isotype IgG control, as described elsewhere (41). Anti-Gr-1 antibody is widely used to label mature neutrophils. Neutrophils were depleted in 10 infected Rag2−/− mice at 6 weeks after infection by i.p. injection of 500 μg/mouse Ly-6G antibody (BioExpress) thrice weekly for 10 weeks. Ten mice infected with H. hepaticus 6 weeks earlier were injected for 10 days with anti-TNF-α antibody (clone XT-3; BioExpress) at 200 mg per mouse thrice weekly as previously described (44). Mice were euthanized at 3–4 months after infection, and tissues were evaluated as described below.
IL-10 Modulation.
In experiments to determine effects of IL-10 on iNOS expression, 28 IL10−/−Rag2−/− mice were infected and compared with Rag2−/− mice (n = 10 with 2 trials; total n = 20) as described elsewhere. Additionally, 8 IL10−/−Rag2−/− mice were treated by i.p. injection of IL10-Ig fusion protein to restore IL-10 function. Forty mice served as H. hepaticus-free controls. Mice were euthanized at 6–8 weeks after infection.
Histopathology.
For histologic evaluation, formalin-fixed tissues were embedded in paraffin, cut at 5 μm, and stained with hematoxylin and eosin. Lesions were scored by 2 pathologists blinded to sample identity. Hyperplastic and inflammatory lesions were graded on a scale of 0 to 4 with ascending severity as described previously (16, 34). Macrophages and neutrophils were identified in intestinal tissue sections with standard avidin-biotin complex immunohistochemistry and were quantified as described previously (41). Immunofluorescence was performed as described elsewhere (58).
Gene Expression.
Gene expression analysis was conducted on snap-frozen 0.5- to 1.0-cm full-thickness sections of ascending colon by TaqMan analysis (Applied Biosystems) using expression assays designed by Applied Biosystems (Assays-on-Demand: www.appliedbiosystems.com).
Statistical Analysis.
Statistical analyses of colonic lesion scores were performed by using a Mann–Whitney U nonparametric test for ordinal data. Comparisons of frequency of carcinoma between groups were performed by using a 2-sided Fisher exact test. Macrophage and neutrophil counts, urinary nitrates, and cytokine gene expression were compared by using Student's t test. Statistical analyses used Graphpad Prism 4.0 (GraphPad Software, Inc.).
Supplementary Material
Acknowledgments.
We thank the following: S. Xu, N. S. Taylor, J. Cline, E. B. Jarmon, K. Hardy, G. A. Paradis, M. J. Jennings, K. Cormier, J. Bajko, E. Stefanovitch, K. Rydstrom, and C. Boussahamain for technical assistance; and M. Hudelson, E. Robbins, and L. J. Trudel for manuscript preparation. Financial support was provided by National Institutes of Health Grants P01 26736 (to S.R.T., D.B.S., G.N.W., J.G.F., and S.E.E.), R01CA108854 (to S.E.E.), R01CA67529 (to J.G.F.), R01AI51404 (to J.G.F.), R01AI052267 (to S.E.E.), P30 ES02109 (to J.G.F.), and T32RR07036 (to J.G.F.), and from European Union and Greek Ministry of National Education and Religious Affairs Pythagoras II Grant 80860 (to T.P.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0812347106/DCSupplemental.
References
- 1.Fox JG, Wang TC. Inflammation, atrophy, and gastric cancer. J Clin Invest. 2007;117:60–69. doi: 10.1172/JCI30111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Collins RHJ, Feldman M, Fordtran JS. Colon cancer, dysplasia, and surveillance in patients with ulcerative colitis: A critical review. N Engl J Med. 1987;316:1654–1658. doi: 10.1056/NEJM198706253162609. [DOI] [PubMed] [Google Scholar]
- 3.Ekbom A, Helmick C, Zack M, Adami HO. Ulcerative colitis and colorectal cancer: A population-based study. N Engl J Med. 1990;323:1228–1233. doi: 10.1056/NEJM199011013231802. [DOI] [PubMed] [Google Scholar]
- 4.Nguyen T, et al. DNA damage and mutation in human cells exposed to nitric oxide in vitro. Proc Natl Acad Sci USA. 1992;1:3030–3034. doi: 10.1073/pnas.89.7.3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rachmilewitz D, Karmeli F, Okon E, Bursztyn M. Experimental colitis is ameliorated by inhibition of nitric oxide synthase activity. Gut. 1995;37:247–255. doi: 10.1136/gut.37.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wink DA, et al. The multifaceted roles of nitric oxide in cancer. Carcinogenesis. 1998;19:711–721. doi: 10.1093/carcin/19.5.711. [DOI] [PubMed] [Google Scholar]
- 7.Singer II, et al. Expression of inducible nitric oxide synthase and nitrotyrosine in colonic epithelium in inflammatory bowel disease. Gastroenterology. 1996;111:871–885. doi: 10.1016/s0016-5085(96)70055-0. [DOI] [PubMed] [Google Scholar]
- 8.Reynolds PD, Middleton SJ, Hansford GM, Hunter JO. Nitric oxide in ulcerative colitis. Lancet. 1995;345:448. [PubMed] [Google Scholar]
- 9.Kimura H, et al. Increased nitric oxide production and inducible nitric oxide synthase activity in colonic mucosa of patients with active ulcerative colitis and Crohn's disease. Dig Dis Sci. 1997;42:1047–1054. doi: 10.1023/a:1018849405922. [DOI] [PubMed] [Google Scholar]
- 10.Grisham MB. Oxidants and free radicals in inflammatory bowel disease. Lancet. 1994;344:859–861. doi: 10.1016/s0140-6736(94)92831-2. [DOI] [PubMed] [Google Scholar]
- 11.Dedon PC, Tannenbaum SR. Reactive nitrogen species in the chemical biology of inflammation. Arch Biochem Biophys. 2004;423:12–22. doi: 10.1016/j.abb.2003.12.017. [DOI] [PubMed] [Google Scholar]
- 12.Seril DN, Liao J, Yang GY, Yang CS. Oxidative stress and ulcerative colitis-associated carcinogenesis: Studies in humans and animal models. Carcinogenesis. 2003;24:353–362. doi: 10.1093/carcin/24.3.353. [DOI] [PubMed] [Google Scholar]
- 13.Zhang R, Li Y, Beck PL, McCafferty DM. Toll-like receptor 4 regulates colitis-associated adenocarcinoma development in interleukin-10-deficient (IL-10(-/-)) mice. Biochem Soc Trans. 2007;35:1375–1376. doi: 10.1042/BST0351375. [DOI] [PubMed] [Google Scholar]
- 14.Hussain SP, Hofseth LJ, Harris CC. Radical causes of cancer. Nat Rev Cancer. 2003;3:276–285. doi: 10.1038/nrc1046. [DOI] [PubMed] [Google Scholar]
- 15.Gal A, Wogan GN. Mutagenesis associated with nitric oxide production in transgenic SJL mice. Proc Natl Acad Sci USA. 1996;93:15102–15107. doi: 10.1073/pnas.93.26.15102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Erdman SE, et al. CD4(+) CD25(+) regulatory T lymphocytes inhibit microbially induced colon cancer in Rag2-deficient mice. Am J Pathol. 2003;162:691–702. doi: 10.1016/S0002-9440(10)63863-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Erdman SE, Fox JG, Sheppard BJ, Feldman D, Horwitz BH. Regulatory T cells prevent non-B non-T colitis. Gastroenterology. 2001;120(Suppl 1):A524. [Google Scholar]
- 18.Maloy KJ, et al. CD4+CD25+ T(R) cells suppress innate immune pathology through cytokine-dependent mechanisms. J Exp Med. 2003;197:111–119. doi: 10.1084/jem.20021345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rao VP, Poutahidis T, Fox JG, Erdman SE. Breast cancer: Should gastrointestinal bacteria be on our radar screen? Cancer Res. 2007;67:847–850. doi: 10.1158/0008-5472.CAN-06-3468. [DOI] [PubMed] [Google Scholar]
- 20.Erdman SE, et al. CD4(+)CD25(+) regulatory lymphocytes require interleukin 10 to interrupt colon carcinogenesis in mice. Cancer Res. 2003;63:6042–6050. [PubMed] [Google Scholar]
- 21.Powrie F, Leach MW. Genetic and spontaneous models of inflammatory bowel disease in rodents: Evidence for abnormalities in mucosal immune regulation. Ther Immunol. 1995;2:115–123. [PubMed] [Google Scholar]
- 22.Cahill RJ, et al. Inflammatory bowel disease: An immunity-mediated condition triggered by bacterial infection with Helicobacter hepaticus. Infect Immun. 1997;65:3126–3131. doi: 10.1128/iai.65.8.3126-3131.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Powrie F, Maloy KJ. Immunology: Regulating the regulators. Science. 2003;299:1030–1031. doi: 10.1126/science.1082031. [DOI] [PubMed] [Google Scholar]
- 24.Tomczak MF, et al. NF-kappa B is required within the innate immune system to inhibit microflora-induced colitis and expression of IL-12 p40. J Immunol. 2003;171:1484–1492. doi: 10.4049/jimmunol.171.3.1484. [DOI] [PubMed] [Google Scholar]
- 25.Kullberg MC, et al. Helicobacter hepaticus triggers colitis in specific-pathogen-free interleukin-10 (IL-10)-deficient mice through an IL-12- and gamma interferon-dependent mechanism. Infect Immun. 1998;66:5157–5166. doi: 10.1128/iai.66.11.5157-5166.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kullberg MC, et al. Bacteria-triggered CD4(+) T regulatory cells suppress Helicobacter hepaticus-induced colitis. J Exp Med. 2002;196:505–515. doi: 10.1084/jem.20020556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Poutahidis T, et al. Rapid reversal of interleukin-6-dependent epithelial invasion in a mouse model of microbially induced colon carcinoma. Carcinogenesis. 2007;28:2614–2623. doi: 10.1093/carcin/bgm180. [DOI] [PubMed] [Google Scholar]
- 28.Hurst SM, et al. Il-6 and its soluble receptor orchestrate a temporal switch in the pattern of leukocyte recruitment seen during acute inflammation. Immunity. 2001;14:705–714. doi: 10.1016/s1074-7613(01)00151-0. [DOI] [PubMed] [Google Scholar]
- 29.Beck PL, et al. Inducible nitric oxide synthase from bone marrow-derived cells plays a critical role in regulating colonic inflammation. Gastroenterology. 2007;132:1778–1790. doi: 10.1053/j.gastro.2007.01.032. [DOI] [PubMed] [Google Scholar]
- 30.Ismail HF, Fick P, Zhang J, Lynch RG, Berg DJ. Depletion of neutrophils in IL-10(-/-) mice delays clearance of gastric Helicobacter infection and decreases the Th1 immune response to Helicobacter. J Immunol. 2003;170:3782–3789. doi: 10.4049/jimmunol.170.7.3782. [DOI] [PubMed] [Google Scholar]
- 31.van der Vliet A, Eiserich JP, Halliwell B, Cross CE. Formation of reactive nitrogen species during peroxidase-catalyzed oxidation of nitrite. A potential additional mechanism of nitric oxide-dependent toxicity. J Biol Chem. 1997;272:7617–7625. doi: 10.1074/jbc.272.12.7617. [DOI] [PubMed] [Google Scholar]
- 32.Balkwill F, Mantovani A. Inflammation and cancer: Back to Virchow? Lancet. 2001;357:539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 33.Ming SC. In: Pathology of the Gastrointestinal Tract. Ming SC, Goldman H, editors. Baltimore: Williams & Wilkins; 1998. pp. 855–898. [Google Scholar]
- 34.Boivin GP, et al. Pathology of mouse models of intestinal cancer: Consensus report and recommendations. Gastroenterology. 2003;124:762–777. doi: 10.1053/gast.2003.50094. [DOI] [PubMed] [Google Scholar]
- 35.Rao CV, Kawamori T, Hamid R, Reddy BS. Chemoprevention of colonic aberrant crypt foci by an inducible nitric oxide synthase-selective inhibitor. Carcinogenesis. 1999;20:641–644. doi: 10.1093/carcin/20.4.641. [DOI] [PubMed] [Google Scholar]
- 36.Kankuri E, et al. Suppression of acute experimental colitis by a highly selective inducible nitric-oxide synthase inhibitor, N-[3-(aminomethyl)benzyl]acetamidine. J Pharmacol Exp Ther. 2001;298:1128–1132. [PubMed] [Google Scholar]
- 37.Ahn B, Ohshima H. Suppression of intestinal polyposis in Apc(Min/+) mice by inhibiting nitric oxide production. Cancer Res. 2001;61:8357–8360. [PubMed] [Google Scholar]
- 38.Pekarek LA, Starr BA, Toledano AY, Schreiber H. Inhibition of tumor growth by elimination of granulocytes. J Exp Med. 1995;181:435–440. doi: 10.1084/jem.181.1.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tazawa H, et al. Infiltration of neutrophils is required for acquisition of metastatic phenotype of benign murine fibrosarcoma cells: Implication of inflammation-associated carcinogenesis and tumor progression. Am J Pathol. 2003;163:2221–2232. doi: 10.1016/S0002-9440(10)63580-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hierholzer C, et al. Induced nitric oxide promotes intestinal inflammation following hemorrhagic shock. Am J Physiol Gastrointest Liver Physiol. 2004;286:G225–G233. doi: 10.1152/ajpgi.00447.2002. [DOI] [PubMed] [Google Scholar]
- 41.Rao VP, et al. Proinflammatory CD4+ CD45RB(hi) lymphocytes promote mammary and intestinal carcinogenesis in Apc(Min/+) mice. Cancer Res. 2006;66:57–61. doi: 10.1158/0008-5472.CAN-05-3445. [DOI] [PubMed] [Google Scholar]
- 42.Ding X, et al. Inducible nitric oxide synthase-dependent DNA damage in mouse model of inflammatory bowel disease. Cancer Sci. 2005;96:157–163. doi: 10.1111/j.1349-7006.2005.00024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fiorucci S, et al. NCX-1015, a nitric-oxide derivative of prednisolone, enhances regulatory T cells in the lamina propria and protects against 2,4,6-trinitrobenzene sulfonic acid-induced colitis in mice. Proc Natl Acad Sci USA. 2002;99:15770–15775. doi: 10.1073/pnas.232583599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rao VP, et al. Innate immune inflammatory response against enteric bacteria Helicobacter hepaticus induces mammary adenocarcinoma in mice. Cancer Res. 2006;66:7395–7400. doi: 10.1158/0008-5472.CAN-06-0558. [DOI] [PubMed] [Google Scholar]
- 45.Hurst JK. Whence nitrotyrosine? J Clin Invest. 2002;109:1287–1289. doi: 10.1172/JCI15816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Podrez EA, Abu-Soud HM, Hazen SL. Myeloperoxidase-generated oxidants and atherosclerosis. Free Radical Biol Med. 2000;28:1717–1725. doi: 10.1016/s0891-5849(00)00229-x. [DOI] [PubMed] [Google Scholar]
- 47.Shafirovich V, Cadet J, Gasparutto D, Dourandin A, Geacintov NE. Nitrogen dioxide as an oxidizing agent of 8-oxo-7,8-dihydro-2′-deoxyguanosine but not of 2′-deoxyguanosine. Chem Res Toxicol. 2001;14:233–241. doi: 10.1021/tx000204t. [DOI] [PubMed] [Google Scholar]
- 48.Byun J, Henderson JP, Mueller DM, Heinecke JW. 8-Nitro-2′-deoxyguanosine, a specific marker of oxidation by reactive nitrogen species, is generated by the myeloperoxidase-hydrogen peroxide-nitrite system of activated human phagocytes. Biochemistry. 1999;38:2590–2600. doi: 10.1021/bi9822980. [DOI] [PubMed] [Google Scholar]
- 49.Byun J, Mueller DM, Fabjan JS, Heinecke JW. Nitrogen dioxide radical generated by the myeloperoxidase-hydrogen peroxide-nitrite system promotes lipid peroxidation of low density lipoprotein. FEBS Lett. 1999;455:243–246. doi: 10.1016/s0014-5793(99)00893-5. [DOI] [PubMed] [Google Scholar]
- 50.Neeley WL, Essigmann JM. Mechanisms of formation, genotoxicity, and mutation of guanine oxidation products. Chem Res Toxicol. 2006;19:491–505. doi: 10.1021/tx0600043. [DOI] [PubMed] [Google Scholar]
- 51.Niles JC, Wishnok JS, Tannenbaum SR. Peroxynitrite-induced oxidation and nitration products of guanine and 8-oxoguanine: Structures and mechanisms of product formation. Nitric Oxide. 2006;14:109–121. doi: 10.1016/j.niox.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 52.Pang B, et al. Lipid peroxidation dominates the chemistry of DNA adduct formation in a mouse model of inflammation. Carcinogenesis. 2007;28:1807–1813. doi: 10.1093/carcin/bgm037. [DOI] [PubMed] [Google Scholar]
- 53.Gaut JP, et al. Myeloperoxidase produces nitrating oxidants in vivo. J Clin Invest. 2002;109:1311–1319. doi: 10.1172/JCI15021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fox JG, et al. Helicobacter hepaticus sp. nov., a microaerophilic bacterium isolated from livers and intestinal mucosal scrapings from mice. J Clin Microbiol. 1994;32:1238–1245. doi: 10.1128/jcm.32.5.1238-1245.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Green LC, et al. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem. 1982;126:131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- 56.Mihara M, et al. CTLA4Ig inhibits T cell-dependent B-cell maturation in murine systemic lupus erythematosus. J Clin Invest. 2000;106:91–101. doi: 10.1172/JCI9244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tomczak MF, et al. Inhibition of Helicobacter hepaticus-induced colitis by IL-10 requires the p50/p105 subunit of NF-kappa B. J Immunol. 2006;177:7332–7339. doi: 10.4049/jimmunol.177.10.7332. [DOI] [PubMed] [Google Scholar]
- 58.Rogers AB, Cormier KS, Fox JG. Thiol-reactive compounds prevent nonspecific antibody binding in immunohistochemistry. Lab Invest. 2006;86:526–533. doi: 10.1038/labinvest.3700407. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.