Abstract
The differentiation of T cells along different lineages is central to the control of immunity. Here we have used a conditional gene knockout system to delete PKCλ/ι selectively in activated T cells. With this system we have demonstrated that PKCλ/ι is necessary for T-helper cell (Th2) cytokine production and optimal T-cell proliferation and allergic airway inflammation in vivo. Our data demonstrate that the activation of the transcription factors nuclear factor of activated T cells and NF-κB is impaired in PKCλ/ι-deficient activated T cells. In addition, we present genetic knockout evidence in ex vivo experiments with primary T cells that PKCλ/ι is critical for the control of cell polarity during T-cell activation. Therefore PKCλ/ι emerges as a critical regulator of Th 2 activation.
Keywords: Asthma, NF-kappaB, T-cell activation, polarity, NF-AT
CD4+ T cells are central in the control of immunity thanks to their ability to differentiate into different subsets of T-helper (Th) cells (1, 2). It now is well established that naïve CD4+ T cells can differentiate in response to antigen stimulation into 3 distinct subsets of effector cells, Th1, Th2, or Th17 cells, which display distinct cytokine profiles and immune regulatory functions (3). Th17 cells are the latest addition to the group of effector T cells and are induced in vitro by the combined actions of TGFβ and IL-6, are characterized by the secretion of the proinflammatory cytokine IL-17, and have been shown to play an essential role in autoimmunity (4, 5). In contrast, Th1 cells mainly produce IFN-γ and are essential for cell-mediated immune responses against intracellular pathogens. Th2 cells produce a different set of cytokines, including IL-4, IL-5, IL-10, and IL-13, and are important in the control of humoral immunity and allergy (6). In this regard, the pathology of asthma, a chronic lung inflammatory disease with increased prevalence in developed countries (7), is associated with aberrant activation and/or differentiation of CD4+ Th2 lymphocytes (8). The molecular mechanisms controlling Th2 differentiation and function have been the object of much research because of their relevance to inflammation disorders, such as asthma, for which better therapies are sorely needed, and because these mechanisms are a very interesting model system of crosstalk between different signaling cascades during a complex biological process.
IL-4 is important for induction and maintenance of differentiated Th2 cells (9). IL-4 and IL-13 share interactions with the IL-4 receptor chain and activate the transcription factor Stat6 through a Jak1/Jak3 signaling pathway (6, 10). We recently have presented in vitro, ex vivo, and in vivo genetic evidence that the atypical PKC (aPKC) isoform, PKCζ, is necessary for optimal activation of the IL-4 signaling cascade upstream of Jak1 (11). Furthermore, in vivo adoptive transfer experiments using PKCζ−/− mice and cells demonstrated that PKCζ in Th2 cells is required for allergy-induced airway inflammation (11). These findings established PKCζ as an important mediator of Th2 differentiation in vivo in the IL-4 signaling pathway. However, the fact that IL-4 is required for Th2 differentiation but at the same time must be produced by Th2 cells seems paradoxical. It suggests that other signals must trigger the initial activation of the Th2 polarization event, and that the Th2-produced IL-4 serves to amplify and maintain that response. In fact, recent studies propose that activated antigen-presenting cells (APCs) trigger a new set of signals that serve to instruct T cells toward the different differentiated lineages (2). In dendritic cells, for example, Notch ligands, as well as the receptor tyrosine kinase, c-Kit, and its ligand, SCF, signal T lymphocytes to polarize to the Th1 or Th2 lineages, depending on the type of APC-stimulating trigger (12–15).
The role of the other aPKC member, termed PKCλ/ι, in the control of the immune response has not yet been investigated using in vivo genetic systems. PKCλ/ι is highly homologous to PKCζ (16), and because the latter has been shown to be important in Th2 differentiation, our goal in this study was to test whether PKCλ/ι plays a role in this process. Our data shown here clearly establish that PKCλ/ι is required for optimal Th2 cytokine production and allergic airway inflammation and that its loss correlates with impaired activation of key transcriptional factors and cell polarity.
Results
Generation of Conditional PKCλ/ι Knockout Mice on Activated T Cells.
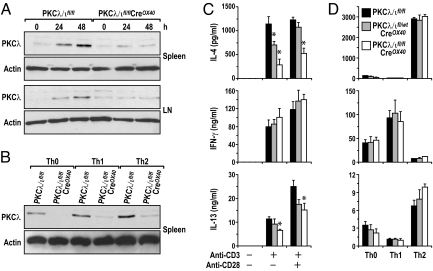
Because PKCλ/ι−/− mice die at very early embryonic stages, probably because of defects in cell polarity (Diaz-Meco, Leitges, and Moscat, unpublished observations), we generated a conditional PKCλ/ι knockout (PKCλ/ιfl/fl) mouse line in which PKCλ/ι is specifically deleted in activated T cells. To do so, we crossed PKCλ/ιfl/fl mice (17) with CreOX40 mice in which the expression of Cre is under the control of the Tnfrsf4 locus (18). OX-40 is expressed almost exclusively in activated T cells, especially CD4+ cells, upon stimulation (18). In this mutant mouse line, PKCλ/ι is expressed at normal levels in immature thymocytes and naïve T cells and, as predicted, is deleted only upon T-cell activation. This strategy is advantageous in that it avoids embryonic lethality and prevents potential confounding effects resulting from the deletion of PKCλ/ι during development or in resting cells. This approach has been used previously to delete the GATA3 gene specifically in activated T cells during Th2 differentiation experiments (18). PCR genotyping was used to screen for homozygous conditional PKCλ/ι-deficient (PKCλ/ιfl/flCreOX40), heterozygous (PKCλ/ιfl/wtCreOX40), and wild-type (PKCλ/ιfl/fl) mice. No Cre-mediated effects were detected when PKCλ/ιwt/wtCreOX40 and PKCλ/ιfl/fl mice were compared (data not shown). The deletion of PKCλ/ι in activated CD4+ T cells was confirmed by Western blot. That is, upon anti-CD3 plus anti-CD28 stimulation under non-skewed conditions or after differentiation of the cells under Th0, Th1, or Th2 conditions, we observed a significant up-regulation of PKCλ/ι in T cells from wild-type mice but not in those from conditional PKCλ/ι-knockout mice (Fig. 1 A and B). The heterozygous activated T cells display PKCλ/ι levels that are intermediate to those of wild-type and PKCλ/ι-deficient activated T cells (supporting information (SI) Fig. S1). Therefore, PKCλ/ι normally is induced during sustained T-cell stimulation and differentiation and is deleted effectively in the mutant cells. This observation in itself is interesting because it suggests that in unstimulated conditions PKCλ/ι levels are very low and that this kinase is induced only when T cells are activated for a relatively long period (Fig. 1 A and B) and that PKCλ/ι might be required for the sustained signaling leading to T-cell differentiation but not for early cell activation.
Fig. 1.
PKCλ/ι is up-regulated upon T-cell activation and differentiation and is required for Th2-cytokine secretion. (A) Purified naïve splenic CD4+ T cells or cells from cervical, axillary, inguinal, and popliteal lymph nodes (LN) were stimulated with anti-CD3/CD28 for 24 or 48 hours. (B) Splenic CD4+ T were differentiated under Th0, Th1, and Th2 conditions for 4 days. Cells in A and B were restimulated with anti-CD3/CD28 for 2 days. Cells were collected for Western blot analysis. Results shown are from a single experiment representative of 2 independent experiments. (C and D) Purified naïve splenic CD4+ T cells were stimulated with (C) anti-CD3/CD28 for 3 days or (D) differentiated under Th0/Th1/Th2 conditions for 4 days and restimulated with plate-bound anti-CD3 for 1 day. Supernatants from cell cultures were collected for ELISA assays to detect cytokines. Data (mean ± SE) are from 4 experiments with triplicated determinations in each sample. * P < 0.05.
Of note, conditional PKCλ/ι-deficient mice appeared to develop normally (data not shown), and the percentages of T and B cells (CD4+, CD8+, and B220+ cells) in the spleens and lymph nodes of these mice were similar to those in wild-type mice (Table S1). The number of double-positive cells (CD4+CD8+) and single-positive cells (CD4+ or CD8+) in the thymus also did not differ in conditional PKCλ/ι-deficient and wild-type mice (Table S2).
Reduced Th2 Cytokine Secretion by CD4+ T Cells from Conditional PKCλ/ι-Deficient Mice.
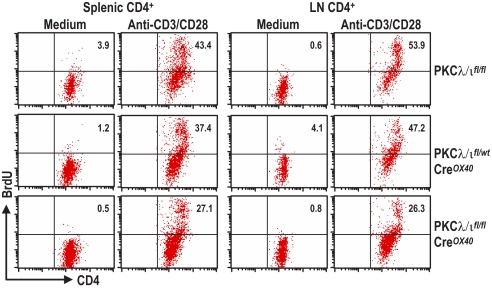
Because PKCζ has been shown to play a critical role during Th2 differentiation (11), and because of the high homology between the 2 aPKCs, we sought to determine whether the conditional deletion of PKCλ/ι in activated T cells would produce an effect similar to that of PKCζ deficiency. To do so, we stimulated purified naïve CD4+ T cells from mice of different genotypes with anti-CD3 in the absence or in the presence of anti-CD28; the levels of different secreted cytokines were detected by ELISA. Interestingly, reduced secretion of IL-4 was observed in PKCλ/ι-deficient cells as compared with wild-type littermate controls when both types of cells were activated with anti-CD3 plus anti-CD28 (Fig. 1C). In the case of heterozygous PKCλ/ι mice (PKCλ/ιfl/wtCreOX40), this reduction was apparent only under anti-CD3 activating conditions (Fig. 1C). These results indicate that PKCλ/ι is required for Th2 cytokine production. Of note, when IFNγ secretion was determined under the same conditions, it was clear that its production was unaffected in the PKCλ/ι-deficient T cells (Fig. 1C). Consistently, secretion of IL-13, another Th2 cytokine, also was reduced in the PKCλ/ι-deficient T cells (Fig. 1C) stimulated under the same conditions. Because these data suggest that PKCλ/ι is required for the control of the balance between Th2 and Th1 cytokines, we next wanted to test whether PKCλ/ι likewise is necessary during Th2/Th1 differentiation in vitro. To address this possibility, isolated CD4+ T cells were cultured for 4 days under non-skewing (Th0) or under Th1 or Th2 conditions. Afterwards, cells were washed and restimulated with anti-CD3 for 24 hours, and cytokine secretion was determined by ELISA. Surprisingly, the loss of PKCλ/ι in activated T cells did not impair IL-4 or IL-13 secretion in Th2-polarized T cells or the secretion of INF-γ in Th1-polarized T cells (Fig. 1D). Collectively these results could be interpreted to mean that PKCλ/ι is required for tilting the balance of Th2 vs. Th1 cytokine secretion but that, under the conditions of in vitro T-cell differentiation, the PKCλ/ι pathway is overridden by the presence of IL-4 (the trigger for Th2 polarization). Of note, as determined by BrdU incorporation, T-cell proliferation in response to stimulation with anti-CD3/CD28 was reduced significantly in both splenic and lymph node PKCλ/ι-deficient CD4+ T cells (Fig. 2). The same results were obtained when PKCλ/ιwt/wtCreOX40 mice were used as a control to rule out any unspecific effect of the Cre line (data not shown).
Fig. 2.
Deletion of PKCλ/ι impairs CD4+ T-cell proliferation. Purified naïve CD4+ T cells from spleens or whole lymph node (LN) cells were stimulated with plate-bound anti-CD3 plus anti-CD28 for 3 days. Then cell proliferation was assessed by BrdU intracellular staining by FACS. Percentages of BrdU-positive cells are shown.
PKCλ/ι Is an Important Mediator in Ovalbumin-Induced Allergic Airway Inflammation.
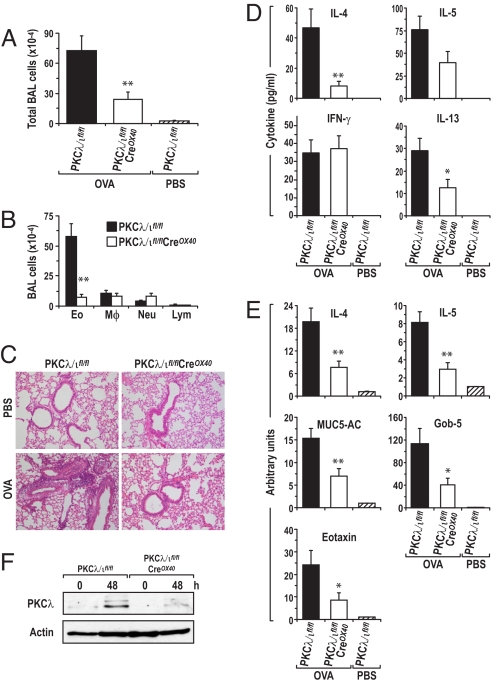
As demonstrated in the previous discussion, although the lack of PKCλ/ι in activated T cells does not impair Th2 differentiation under the standard conditions established in vitro, PKCλ/ι is important for IL-4 and IL-13 secretion in vitro under non-skewing conditions. Therefore, it is conceivable that pathways may be set in motion in vivo that could require PKCλ/ι for an adequate Th2 response. For these reasons, we sought to determine whether PKCλ/ι is necessary for an optimal Th2 inflammatory response in vivo, although it does not seem to be necessary for Th2 differentiation driven by the exogenous addition of IL-4 under standard in vitro conditions. In this regard, it is well established that Th2 cells play a critical role in the development of allergic airway inflammation (11). Because our data show that PKCλ/ι is required for a proper balance of Th2/Th1 cytokines in vitro, we reasoned that PKCλ/ι might affect the airway allergy response in vivo. Therefore, in the next series of experiments we tested the requirement for PKCλ/ι in the ovalbumin (OVA)-induced model of allergic airway inflammation. Thus, wild-type and conditional PKCλ/ι-deficient mice were immunized by i.p. injection of OVA and then challenged 3 times with aerosolized OVA or PBS as a negative control. Twenty-four hours after the last aerosol challenge, mice were killed and subjected to bronchoalveolar lavage (BAL) to determine the recruitment of inflammatory cells. Also, lungs were fixed and examined histologically by H&E staining for cellular infiltration. There was a robust increase in total BAL cell numbers in wild-type mice that had been OVA immunized and challenged with aerosolized OVA, as compared with unimmunized naïve mice (Fig. 3A). Eosinophils accounted for most of this increase in the recruitment of inflammatory cells (Fig. 3B). However, these increases were reduced dramatically in identically treated conditional PKCλ/ι-mutant mice (Fig. 3 A and B). H&E histological analysis of lung sections from these experiments consistently showed that challenged wild-type mice displayed a prominent inflammatory response with massive perivascular and peribronchial infiltration, whereas the conditional PKCλ/ι-mutant mice displayed a highly attenuated response (Fig. 3C). These data indicate that in vitro observations of impaired Th2 cytokine secretion by T cells derived from these mutant mice (see Fig. 1) are relevant to airway inflammation in vivo. Consistent with this idea, ELISA data on the cytokine levels in BAL from these in vivo experiments showed that IL-4, IL-5, and IL-13, which were undetectable in naïve mice, were increased significantly in OVA-challenged wild-type mice but were inhibited in identically treated conditional PKCλ/ι-mutant mice (Fig. 3D). IFN-γ was slightly increased in BAL from PKCλ/ι-mutant mice as compared with identically treated wild-type mice, consistent with the in vitro data of Fig. 1. Interestingly, lung mRNA levels of IL-4, IL-5, and eotaxin also were up-regulated in challenged wild-type mice as compared with unimmunized mice, and this up-regulation was reduced significantly in conditional PKCλ/ι-mutant mice (Fig. 3E).
Fig. 3.
Role of PKC λ/ι in OVA-induced allergic airway inflammation. Conditional PKCλ/ι-deficient mice and their wild-type littermates were immunized with OVA twice and then challenged with aerosolized OVA. Mice were killed 25 hours after the last challenge. (A) The total cells in BAL fluid were counted using a hemocytometer. (B) Differential cell counts of > 300 cells were performed on cytospins stained with Kwik-Diff. The numbers of eosinophils (Eo), macrophages (Mφ), neutrophils (Neu), and lymphocytes (Lym) in BAL are shown. (C) Representative H&E staining of lung histology. (D) Cytokine levels in BAL fluid were determined by ELISA. (E) mRNA levels of various molecules in OVA-induced airway hyperresponsiveness (AHR). Total RNA was extracted from the right lower lobe of the lungs for real-time PCR analysis. The mRNA levels of various molecules are expressed as arbitrary units. All samples were determined by triplicate; the data are normalized to an 18S reference. (F) Representative Western blot results from trachea lymph node cells stimulated with anti-CD3/CD28 for 48 hours from mice with OVA-induced AHR. Results in A, B, D, and E are expressed as mean ± SE from 3 independent experiments with 3–5 mice per group in each experiment. *P < 0.05; **P < 0.01.
Hypersecretion of mucus plays an important role in the pathogenesis and severity of asthma. The primary proteins in mucus are mucin glycoproteins, with MUC-5AC being the primary airway mucin gene. The calcium chloride-activated channel gene hCLCA1 (gob-5 in the mouse) has been suggested to increase MUC-5AC gene expression (19). Both MUC-5AC and gob-5 were increased dramatically in challenged wild-type mice, but, importantly, more than 2/3rds of this increase was lost in the conditional PKCλ/ι-mutant mice (Fig. 3E). Results in Fig. 3F confirm the deletion of PKCλ/ι in this in vivo experiment.
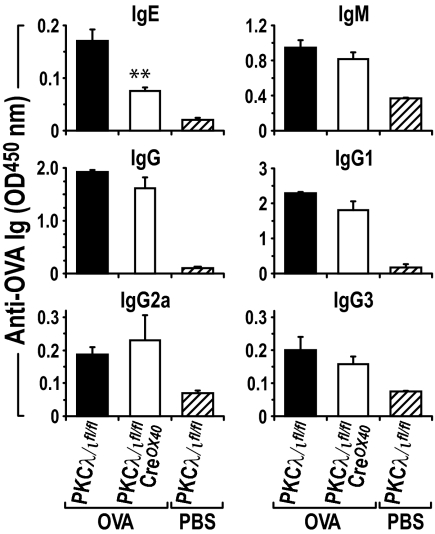
IgE is an important mediator of allergic airway inflammation (7). In mice, IL-4 preferentially induces immunoglobulin isotype switching to IgE and IgG1, whereas IFN-γ preferentially induces switching to IgG2a and IgG3 (7). We reasoned that the reduced IL-4 response observed in OVA-induced allergic airway inflammation in conditional PKCλ/ι-mutant mice might correlate with reduced OVA-specific IgE in vivo. Consistent with this prediction, serum levels of OVA-specific IgE were reduced significantly in conditional PKCλ/ι-mutant mice as compared with their wild-type control littermates (Fig. 4), whereas OVA-specific IgM, total IgG, and IgG1 were reduced only slightly in the PKCλ/ι-deficient mice. OVA-specific total IgG2a and IgG3 did not differ significantly between the 2 mouse genotypes (Fig. 4). Taken together, these results indicate that the loss of PKCλ/ι in activated T cells leads to a significant inhibition of the Th2 response in vivo, consistent with a relevant role for this aPKC in Th2 cell differentiation.
Fig. 4.
Serum OVA-specific IgE is reduced in conditional PKCλ/ι-deficient mice. In the same experiments as Fig. 3, serum levels of OVA-specific IgE, IgM, IgG, and IgG subclasses were assayed by ELISA (mean ± SE). **P < 0.01.
Role of PKCλ/ι in T-Cell Activation.
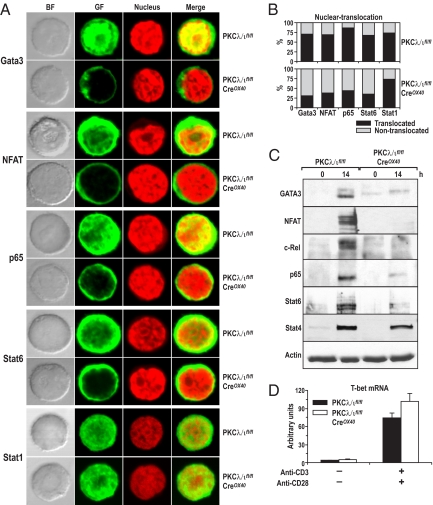
Because PKCλ/ι is important for Th2 cytokine secretion ex vivo and in vivo, we next sought to determine the signaling pathways affected by the loss of PKCλ/ι in activated T cells. Because Th2 cytokine secretion is a long-term process that requires sustained activation of T-cell receptor (TCR) signals, we determined which of the transcription factors reported to be essential for this process were affected by the loss of PKCλ/ι. Specifically, activation of GATA3 (indicated by localization to the nucleus), which is necessary and sufficient to drive T cells to the Th2 lineage, was determined by confocal microscopy in cell cultures of wild-type or PKCλ/ι-deficient CD4+ T cells that had been incubated under non-skewing conditions with anti-CD3 plus anti-CD28 for 14 hours. Fig. 5A shows a representative image demonstrating that GATA3 was localized to the nucleus in activated wild-type CD4+ T cells but was excluded almost entirely from the nucleus in activated PKCλ/ι-deficient cells. Fig. 5B shows the percentage of cells displaying nuclear GATA3 staining based on counts of 400 cells and demonstrates that, although more than 70% of activated wild-type T lymphocytes had nuclear GATA3, nuclear GATA3 was detected in only about 25% of the PKCλ/ι-deficient T cells. These results are very important because they show the relevance of PKCλ/ι for the activation of a Th2-specific transcription factor and are consistent with this kinase being relevant to Th2 cytokine production. Interestingly, when the activation states of other transcription factors important for this process, such as nuclear factor of activated T cells (NFAT), p65, or Stat6, were determined, it was clear that the nuclear levels of all of these transcription factors were reduced severely by the loss of PKCλ/ι (Fig. 5 A and B). NFAT and p65 stimulation are linked to the activation of the TCR (20), whereas activation of Stat6 probably is caused by the secretion of IL-4 in activated T cells. IL-4 production is reduced in PKCλ/ι-deficient activated T cells (see Fig. 1C), and this reduction is likely to account for the reduced nuclear Stat6 levels detected in PKCλ/ι mutant T cells and is consistent with our data demonstrating the lack of a role for PKCλ/ι in IL-4 signaling in Stat6 activation (Diaz-Meco, et al., unpublished data). Immunoblot analysis of a similar experiment demonstrates a dramatic inhibition of GATA3, NFAT, and p65 nuclear levels in the PKCλ/ι-deficient T cells activated as described earlier in this article (Fig. 5C), consistent with the data in Fig. 5 A and B. Interestingly, the nuclear levels of the Th1 transcription factors Stat1 and Stat4 were not affected by the loss of PKCλ/ι in these experiments (Fig. 5 A–C). Of note, the expression of the Th1 master regulatory gene, T-bet, was induced in PKCλ/ι-deficient activated T cells to levels comparable to those of the wild-type cells (Fig. 5D). Results shown in Fig. S2 demonstrate that the OX40-Cre transgene is expressed at 14 hours of stimulation and, consistently, PKCλ/ι is up-regulated at this time point in wild-type T cells and is effectively depleted in the knockout cells. (Fig. S3). The activation of Akt and ERK was not affected by the loss of PKCλ/ι (Fig. S3). Deletion of PKCλ/ι in Jurkat T cells by RNA interference also inhibited NFAT and NF-κB activities (Fig. S4). Collectively, these results could be interpreted to mean that PKCλ/ι plays a decisive role in the TCR activation of p65 and NFAT, which leads to GATA3 activation and the synthesis of IL-4, thus influencing the stimulation of Stat6. Therefore, we conclude that PKCλ/ι is induced during and is required for the sustained activation of key transcriptional events of Th2 differentiation.
Fig. 5.
Deletion of PKCλ/ι in vivo in activated T cells regulates Th2-transcription factors. (A) Purified naïve splenic CD4+ T cells were activated with anti-CD3/CD28 for 14 hours. Cells were harvested and adhered onto polyY-L-lysine–coated coverslips for immunofluorescence staining. Nuclei were stained by propidium iodide. Cells were analyzed by using a confocal microscope. Nuclear translocation of Th2 transcription factors is shown. Images are representative examples from > 300 cells for each staining and are taken from a single experiment that is representative of 3 independent experiments. (B) Cells with nuclear translocation were quantified by cell counting (n > 400) under microscope. T cells were pooled from 7 mice per genotype. (C) Nuclear extracts of T cells treated as above were analyzed by immunoblotting with antibodies for the respective transcription factors. (D) T-bet mRNA levels are shown (arbitrary units). Total RNA was extracted from splenic CD4+ T cells stimulated with anti-CD3/CD28 for 14 hours for real-time PCR analysis. All samples were determined by triplicate; the data are normalized to an 18S reference. BF, bright field; GF, green fluorescence.
Role of PKCλ/ι in T-Cell Polarity.
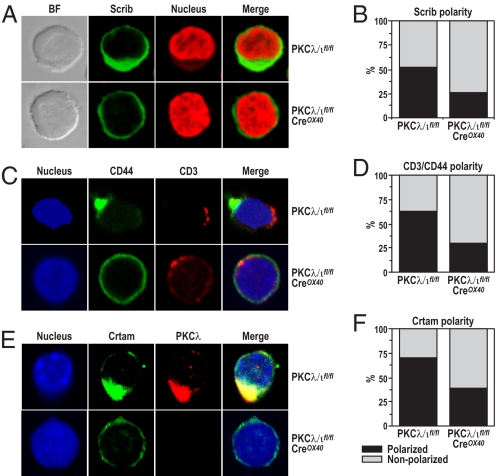
The aPKCs have been implicated in the control of cell polarity in several mammalian in vitro cell-culture experiments (21). More recently, the potential role of polarity in T-cell activation has been shown also (22, 23). However, no genetic evidence from ex vivo experiments has been produced to test directly the involvement of the aPKCs in T-cell polarity. A recent study identified Crtam as a scaffold protein that binds the cell polarity protein Scribble and through this interaction regulates cell polarity, proliferation, and the secretion of cytokines (24). Therefore, a potential explanation for the findings reported here is that PKCλ/ι could be necessary for T-cell proliferation and Th2 cytokine secretion because of its potential role in T-cell polarization. To address this question, we incubated CD4+ T cells from either wild-type or PKCλ/ι-mutant mice with anti-CD3 plus anti-CD28 for 14 hours, following exactly a previously described procedure to induce and assess T-cell polarization (24). We then determined T-cell polarity by monitoring the ability of the polarity marker Scribble to localize to one of the poles of the activated T cell (24). Fig. 6 A and B strongly suggests that the lack of PKCλ/ι during T-cell activation leads to a significant loss of T-cell polarity. To establish this conclusion more firmly, we analyzed the localization of CD44 and CD3 in double immunofluorescence analysis. From the data of Fig. 6C it is clear that CD44 is asymmetrically polarized relative to CD3, consistent with the induction of cell polarity in the activated T cells. More importantly, this polarization is severely impaired in the PKCλ/ι-deficient T cells (Fig. 6 C and D). Interestingly, PKCλ/ι colocalizes with Crtam and is required for its proper polarization in activated T cells (Fig. 6 E and F). Similar conclusions were obtained when the role of PKCλ/ι was investigated in late T-cell polarity using polystyrene beads coated with anti-CD3 plus anti-CD28 (Fig. S5). Of note, the lack of PKCλ/ι does not affect T-cell migration under these experimental conditions (Fig. S6). Collectively these results indicate that PKCλ/ι deficiency in activated T cells leads to impaired polarity during late T-cell activation.
Fig. 6.
PKCλ/ι control cell polarity. (A, C, and E) Purified naïve splenic CD4+ T cells were activated with anti-CD3/CD28 for 14 hours. Cells were treated for immunofluorescence staining as in Fig. 5. Polarity of Scrib (B), CD3/CD44 (D), and Crtam/PKCλ (F) are shown. Images are representative of > 300 cells for each staining. Cells with polarity were quantified by cell counting (n > 400) under a microscope. CD4+ T cells were pooled from 7 conditional PKCλ/ι-deficient mice and their wild-type littermates. The results are from a single experiment that is representative of 2 independent experiments with identical results.
Discussion
Unraveling the signaling pathways that promote T-cell differentiation along the different lineages is of paramount importance on 2 levels: for the general insight it provides on the interplay between different cell-signaling pathways in the regulation of complex cellular processes and, more specifically, for an increased understanding of T-cell biology under normal and pathological conditions. Here we show that PKCλ/ι, 1 of the 2 members of the aPKC family, plays a critical role in allergic airway inflammation in vivo, most likely because it is required for the production of Th2 cytokines. Ex vivo experiments with PKCλ/ι-deficient T cells demonstrate that this kinase is required for the activation of transcription factors critical for adequate Th2 cell function and differentiation. We also provide genetic evidence using conditional knockout cells that PKCλ/ι is essential for T-cell polarity, an event that has been suggested to be relevant to T-cell function (22). Considered collectively, these results suggest that defects in cell polarity caused by the lack of PKCλ/ι in activated T cells, along with alterations in gene expression programs, are responsible for the defects in Th2 cytokine production detected in the ex vivo experiments and account for the impaired lung inflammatory response observed in the PKCλ/ι mutant mice when challenged with an allergic stimulus.
Our findings bring up a number of questions that deserve discussion before a consensus model emerges on the links between cell polarity, signaling, and function, at least in the immune system. How alterations in polarization correlate with defects in gene expression signaling need more in-depth investigation, both in T lymphocytes and in other cell systems in which polarity is essential for relevant cellular functions. In this regard, the identification of a Scribble-interacting partner, Crtam, constitutes an important step in linking polarity and T-cell function in a cause–effect type of experiment (24). Interestingly, the loss of Crtam leads to impaired T-cell polarity, which is accompanied by increased proliferation and decreased production of the Th1 and Th17 cytokines, IFNγ and IL-17, respectively, but not of the Th2 cytokine IL-4 (24). At first glance, these published data could be interpreted to mean that T-cell polarization is essential for T-cell differentiation toward Th1 but not Th2 lineages. However, our results shown here establish that the loss of polarity as a consequence of PKCλ/ι ablation in activated T cells does not correlate with increased proliferation, but rather with reduced proliferation, and leads to the impairment of the production of IL-4 but not of IFNγ.
A potential explanation for these different observations is that PKCλ/ι, in addition to binding the Phox and Bem1p-1 domain (PB1)-containing polarity adapter Par-6 (16), also binds a non-polarity PB1-containing signaling adaptor, termed p62, whose genetic knockout gives a phenotype in vivo and ex vivo very similar to that of PKCλ/ι in terms of T-cell activation (3). This possibility could be interpreted as meaning that PKCλ/ι, because of its ability to bind p62 through their respective PB1 domains (16), will influence late T-cell signaling, ultimately resulting in modulation of Th2 cytokine production and of the response to allergic airway inflammation. On the other hand, probably through its interaction with polarity adapters such as the PB1 scaffold Par-6 and other polarity proteins (16), PKCλ/ι affects the localization of these molecules in polarized regions of the T cell. Therefore, PKCλ/ι generates 2 independent kinds of signals, depending on its different binding partner, and these signals direct PKCλ/ι participation to distinctive signaling cascades. These data are consistent with a model in which the inactivation of different proteins with different polarity would have different cellular consequences because of their association with different signaling complexes.
Materials and Methods
Mice.
PKCλ/ιfl/fl mice were reported previously (17). CreOX40 mice were generated in the laboratory of N. Killeen at the University of California, San Francisco (18). Conditional PKCλ/ι-deficient mice on activated T cells were generated by crossing PKCλ/ιfl/fl mice with CreOX40 mice. Mouse genotyping was performed by PCR using primers for PKCλ/ιfl/fl, OX-40-Cre, and PKCλ/ι deletion to screen homozygous conditional PKCλ/ι-deficient (PKCλ/ιfl/fl CreOX40), heterozygous (PKCλ/ιfl/wt CreOX40), and wild-type (PKCλ/ιfl/fl or PKCλ/ιwt/wt CreOX40) mice. Age- (9–12 weeks) and sex-matched mice were used in each experiment.
CD4+ T-Cell Isolation and Differentiation.
Splenocytes were prepared by disrupting spleens of 9- to12-week-old mice. Naive CD4+ T cells were enriched with a Mouse CD4+ Isolation Kit using an AutoMACS Pro (Miltenyi Biotec). The purity (> 95%) and naïve status of isolated CD4+ T cells were confirmed by FACS staining with conjugated mAbs to CD4, CD8, B220, CD44, and CD62L. Naïve CD4+ T cells (106/ml) were activated with plate-bound anti-CD3 (10 μg/ml) plus soluble anti-CD28 (2 μg/ml) or differentiated into Th0, Th1, or Th2 cells (11). The culture supernatants were collected at different times after activation for cytokine assays by ELISA.
Cytokine Assay.
Cytokines in the supernatants and BAL fluid were assayed by ELISA. IL-4, IL-5, IL-6, IL-10, and IFN-γ were assayed with OptEIA kits (BD PharMingen); and IL-13 and eotaxin were assayed with DuoSet ELISA kits (R&D Systems). ELISA plates were developed with TMB substrate (BD PharMingen), and read by a microplate reader (model 550, Bio-Rad). Cytokine mRNA levels were measured by real-time quantitative PCR.
FACS.
Spleen and lymph node cells or purified CD4+ cells were incubated with anti-CD16/32 (2.4G2) to block FcRγ II/III and then were stained with various conjugated mAbs. Stained cells were analyzed by FACSCalibur with CellQuest software (Becton Dickinson).
Proliferation.
CD4+ T-cell proliferation was assayed in vitro by measuring BrdU incorporation with a BrdU Flow kit (BD PharMingen) following the manufacturers' protocols. Briefly, purified naïve CD4+ T cells or whole lymph node cells were cultured with plate-bound anti-CD3 plus anti-CD28 for 2 to 3 days. BrdU (10 mM) was added to the cultures in the last 18 hours. After fixation, permeabilization, and DNase digestion, cells were stained with APC-conjugated anti-BrdU mAb and analyzed by FACS.
Immunofluorescence Staining.
Purified naïve CD4+ T cells were cultured with plate-bound anti-CD3 plus anti-CD28 for 14 hours. Cells were harvested and adhered onto polyY-L-lysine (Sigma)–coated coverslips for 30 minutes at room temperature, and nonadherent cells were washed off with PBS. Adherent cells were fixed in 4% paraformaldehyde, permeabilized with 0.2% Triton X-100, and incubated with different mAbs. The signals were amplified by the Tyramide Signal Amplification kit (Molecular Probes). For nuclear staining, cells were incubated with propidium iodide (Sigma) or TOPRO-3 (Invitrogen). Coverslips were mounted on Mowiol and examined with a Zeiss LSM-510 Meta Confocal Laser Scanning Microscope (Carl Zeiss MicroImaging). Images from single plane were taken.
Real-Time PCR.
Total RNA was extracted from lung tissues or cultured cells with the RNeasy Mini kit (Qiagen), and cDNA was prepared by the Omniscript Reverse Transcription kit (Qiagen). Quantitative PCR was performed with the SYBR Green PCR Master Mix (Applied Biosystems) on a Mastercycler ep realplex4 apparatus (Eppendorf). The data were normalized to the 18S reference. Primers for IL-4, IL-5, IL-13, eotaxin, MUC-5AC, Gob-5, and T-bet were designed with OLIG 4.0 software.
For more information, please see SI Materials and Methods.
Supplementary Material
Acknowledgments.
We thank Maryellen Daston for editing this manuscript, Glenn Doerman for the artwork, and Hongzhu Liu and Lyndsey Cheuvront for technical assistance. We also thank Dr. Nigel Killeen (University of California, San Francisco) for the generous gift of the CreOX40 mice. This work was funded in part by the University of Cincinnati-CSIC Collaborative Agreement, and by National Institutes of Health Grant R01-AI072581.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0805907106/DCSupplemental.
References
- 1.Reiner SL. Development in motion: Helper T cells at work. Cell. 2007;129:33–36. doi: 10.1016/j.cell.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 2.Kubo M. Notch: Filling a hole in T helper 2 cell differentiation. Immunity. 2007;27:3–5. doi: 10.1016/j.immuni.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 3.Martin P, Diaz-Meco MT, Moscat J. The signaling adapter p62 is an important mediator of T helper 2 cell function and allergic airway inflammation. EMBO J. 2006;25:3524–3533. doi: 10.1038/sj.emboj.7601250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ouyang W, Kolls JK, Zheng Y. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity. 2008;28:454–467. doi: 10.1016/j.immuni.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McGeachy MJ, Cua DJ. Th17 cell differentiation: The long and winding road. Immunity. 2008;28:445–453. doi: 10.1016/j.immuni.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 6.Shuai K, Liu B. Regulation of JAK-STAT signalling in the immune system. Nature Reviews. Immunology. 2003;3:900–911. doi: 10.1038/nri1226. [DOI] [PubMed] [Google Scholar]
- 7.Elias JA, et al. New insights into the pathogenesis of asthma. J Clin Invest. 2003;111:291–297. doi: 10.1172/JCI17748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luster AD, Tager AM. T-cell trafficking in asthma: Lipid mediators grease the way. Nature Reviews. Immunology. 2004;4:711–724. doi: 10.1038/nri1438. [DOI] [PubMed] [Google Scholar]
- 9.Paul WE, Seder RA. Lymphocyte responses and cytokines. Cell. 1994;76:241–251. doi: 10.1016/0092-8674(94)90332-8. [DOI] [PubMed] [Google Scholar]
- 10.O'Shea JJ, Gadina M, Schreiber RD. Cytokine signaling in 2002: New surprises in the Jak/Stat pathway. Cell. 2002;109(Suppl):S121–131. doi: 10.1016/s0092-8674(02)00701-8. [DOI] [PubMed] [Google Scholar]
- 11.Martin P, et al. Control of T helper 2 cell function and allergic airway inflammation by PKC{zeta} Proc Natl Acad Sci USA. 2005;102:9866–9871. doi: 10.1073/pnas.0501202102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee GR, Kim ST, Spilianakis CG, Fields PE, Flavell RA. T helper cell differentiation: Regulation by cis elements and epigenetics. Immunity. 2006;24:369–379. doi: 10.1016/j.immuni.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 13.Sun J, Krawczyk CJ, Pearce EJ. Suppression of Th2 cell development by notch ligands delta1 and delta4. J Immunol. 2008;180:1655–1661. doi: 10.4049/jimmunol.180.3.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wiethe C, et al. Dendritic cell differentiation state and their interaction with NKT cells determine Th1/Th2 differentiation in the murine model of Leishmania major infection. J Immunol. 2008;180:4371–4381. doi: 10.4049/jimmunol.180.7.4371. [DOI] [PubMed] [Google Scholar]
- 15.Amsen D, et al. Instruction of distinct CD4 T helper cell fates by different notch ligands on antigen-presenting cells. Cell. 2004;117:515–526. doi: 10.1016/s0092-8674(04)00451-9. [DOI] [PubMed] [Google Scholar]
- 16.Moscat J, Diaz-Meco MT, Albert A, Campuzano S. Cell signaling and function organized by PB1 domain interactions. Mol Cell. 2006;23:631–640. doi: 10.1016/j.molcel.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 17.Farese RV, et al. Muscle-specific knockout of PKC-lambda impairs glucose transport and induces metabolic and diabetic syndromes. J Clin Invest. 2007;117:2289–2301. doi: 10.1172/JCI31408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu J, et al. Conditional deletion of Gata3 shows its essential function in T(H)1-T(H)2 responses. Nat Immunol. 2004;5:1157–1165. doi: 10.1038/ni1128. [DOI] [PubMed] [Google Scholar]
- 19.Busse PJ, et al. Chronic exposure to TNF-alpha increases airway mucus gene expression in vivo. J Allergy Clin Immunol. 2005;116:1256–1263. doi: 10.1016/j.jaci.2005.08.059. [DOI] [PubMed] [Google Scholar]
- 20.Schulze-Luehrmann J, Ghosh S. Antigen-receptor signaling to nuclear factor κB. Immunity. 2006;25:701–715. doi: 10.1016/j.immuni.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 21.Goldstein B, Macara IG. The PAR proteins: Fundamental players in animal cell polarization. Developmental Cell. 2007;13:609–622. doi: 10.1016/j.devcel.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krummel MF, Macara I. Maintenance and modulation of T cell polarity. Nat Immunol. 2006;7:1143–1149. doi: 10.1038/ni1404. [DOI] [PubMed] [Google Scholar]
- 23.Chang JT, et al. Asymmetric T lymphocyte division in the initiation of adaptive immune responses. Science. 2007;315:1687–1691. doi: 10.1126/science.1139393. [DOI] [PubMed] [Google Scholar]
- 24.Yeh JH, Sidhu SS, Chan AC. Regulation of a late phase of T cell polarity and effector functions by Crtam. Cell. 2008;132:846–859. doi: 10.1016/j.cell.2008.01.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.