Abstract
We examined the status of the neural network mediating the default mode of brain function, which typically exhibits greater activation during rest than during task, in patients in the early phase of schizophrenia and in young first-degree relatives of persons with schizophrenia. During functional MRI, patients, relatives, and controls alternated between rest and performance of working memory (WM) tasks. As expected, controls exhibited task-related suppression of activation in the default network, including medial prefrontal cortex (MPFC) and posterior cingulate cortex/precuneus. Patients and relatives exhibited significantly reduced task-related suppression in MPFC, and these reductions remained after controlling for performance. Increased task-related MPFC suppression correlated with better WM performance in patients and relatives and with less psychopathology in all 3 groups. For WM task performance, patients and relatives had greater activation in right dorsolateral prefrontal cortex (DLPFC) than controls. During rest and task, patients and relatives exhibited abnormally high functional connectivity within the default network. The magnitudes of default network connectivity during rest and task correlated with psychopathology in the patients. Further, during both rest and task, patients exhibited reduced anticorrelations between MPFC and DLPFC, a region that was hyperactivated by patients and relatives during WM performance. Among patients, the magnitude of MPFC task suppression negatively correlated with default connectivity, suggesting an association between the hyperactivation and hyperconnectivity in schizophrenia. Hyperactivation (reduced task-related suppression) of default regions and hyperconnectivity of the default network may contribute to disturbances of thought in schizophrenia and risk for the illness.
Keywords: psychopathology, working memory, fMRI, functional connectivity, genetic risk
Schizophrenia is a severe psychiatric disorder characterized by disturbances of thought and emotion as well as neurocognitive deficits, including attention and working memory (WM) (1). First-degree relatives of persons with schizophrenia often share these cognitive deficits, usually to a milder degree (2). These intermediate deficits, or “endophenotypes,” may reflect the expression of susceptibility genes for schizophrenia in biological relatives (3). Evidence for the genetic basis of schizophrenia comes from a 10-fold increase in the incidence of the disease in first-degree relatives and a 40–65% concordance rate in identical twins, although the molecular genetic basis of the illness remains poorly understood (4). The study of biological relatives offers a window into pathophysiology attributable to familial risk while susceptibility genes are being identified.
There is widespread brain pathology in schizophrenia (5), and a similar but milder pattern of brain abnormalities in first-degree relatives (6). Dysfunction of the dorsolateral prefrontal cortex (DLPFC) appears prominent in schizophrenia by virtue of clinical, neuropsychological, and social deficits characteristic of the disease (7, 8). Functional neuroimaging has revealed DLPFC dysfunction during WM tasks in patients with schizophrenia and also in their first-degree relatives (7, 9). These studies examined task-dependent DLPFC function in patients and relatives, but less is known about task-independent brain function in schizophrenia and nothing is known about such function in first-degree relatives.
Here, we used functional MRI (fMRI) to examine the status of the “default network” in patients with early-phase schizophrenia and in nonpsychotic relatives. The default network comprises regions more active during rest than during a wide range of cognitive tasks, and is therefore thought to mediate task-independent brain function. The default network most consistently includes the medial prefrontal cortex (MPFC) extending to ventral anterior cingulate cortex, the posterior cingulate cortex (PCC) extending to the precuneus, and lateral parietal cortex (10). This network, especially the midline regions of the MPFC and PCC, overlaps with brain regions activated during self-referential cognitive or emotional tasks (11, 12). The overlap suggests that activation in the default network is associated with spontaneous, internally generated, task-independent mentation, which is greatest during rest. The components of the default network appear to operate interactively, exhibiting strong functional connectivity (i.e., temporally correlated patterns of activation) during rest (13).
Activation in the midline default network decreases during task performance (i.e., there is task-related suppression of the default network), when people respond to externally generated stimuli and mentation is task dependent. By contrast, task-dependent activation increases in lateral neocortical networks mediating attention and WM capacities that underlie task performance (11). Indeed, activations in the default network are negatively correlated during rest with activations in regions typically involved in stimulus processing, such as the lateral prefrontal cortex (13, 14). Thus, it appears that the brain alternates between activation of the default network when not engaged in a task and suppression of the default network when engaged in a task. Although there have been some studies of the default network in schizophrenia (15–21), it remains unknown as to whether or not patients with schizophrenia or first-degree relatives exhibit normal task-related suppression of the default network.
The status of the default network is of interest for its potential to reveal the neural substrates of task-independent self-relevant information processing in schizophrenia. Dysfunction of the default network could contribute to both the cognitive deficits, such as diminished attention and WM capacities, and the positive symptoms, such as paranoid ideations and hallucinations, that characterize schizophrenia (22). Greater suppression of the default network is associated with better performance on an attention-demanding task in healthy people (23); thus, failure to suppress the default network in schizophrenia may be related to poor performance on attention-demanding tasks. Core components of the default network (MPFC and PCC) are implicated in self-reference, and disturbance of these regions may promote the assignment of self-relevance to unrelated external events and blur the line between internal thoughts and external events (22). Further, the default system may be overconnected in patients with schizophrenia (21). We hypothesized, therefore, that the default network would be hyperactive (i.e., failure to suppress) and hyperconnected in patients with schizophrenia, and to the extent that such hyperactivation or hyperconnectivity is based on genetic risk factors, that it would also be hyperactive or hyperconnected in relatives.
We compared the status of the default network in 3 carefully matched groups of young adults: patients with early-phase schizophrenia, nonpsychotic first-degree relatives of patients with schizophrenia, and healthy control participants. Patients in the early phase of schizophrenia are of particular interest because their cognitive and neural functioning is less affected by factors that influence functioning in later stages, especially long histories of medication treatment. Further, nonpsychotic relatives provide insight into genetic risk factors for schizophrenia that are independent of the clinical and treatment histories that complicate studies of patients. Participants were examined under three conditions: (i) rest; (ii) an easy WM task (0-back condition), in which participants responded to the letter “X” during sequential letter presentations; and (iii) a difficult WM task (2-back condition), in which participants responded to any letter identical to a letter presented 2 trials previously. We examined the default network at rest relative to WM tasks, the functional connectivity of the default network during rest and task, negative correlations between the default network and DLPFC regions important for WM task performance, and how the status of the default network relates to WM performance and to clinical psychopathology ratings.
Results
Participant Characteristics.
There were no significant differences among groups in age, gender, ethnicity, handedness, or education. Controls had significantly higher parental socioeconomic status than relatives but not patients, and there was no significant difference between relatives and patients. There were no significant group differences in estimated IQ, vocabulary, block design, or oral reading scores [supporting information (SI) Table S1].
WM Performance.
There were no significant group differences on 0-back WM task performance. On the 2-back WM task, controls were significantly more accurate than patients [t (24) = 2.33, P = 0.03] and faster to respond than relatives [t (24) = 2.03, P = 0.05]; patients and relatives did not differ reliably from one another (P > 0.54) (Table S1). One patient performed well below chance on the 2-back WM task; behavioral and imaging analyses remained significant when this participant was excluded.
Psychiatric Symptoms.
Relatives and controls did not differ reliably from one another on a standard psychometric measure of current psychopathology, the Hopkins Symptom Checklist Revised (SCL-90-R) (24).
Neuroimaging.
Within-group analyses were conducted on the whole brain, and between-group comparisons were restricted to a priori anatomically defined MPFC, PCC, and right DLPFC regions. Results were corrected for multiple comparisons within the respective search volumes (Methods).
Task-Related Suppression.
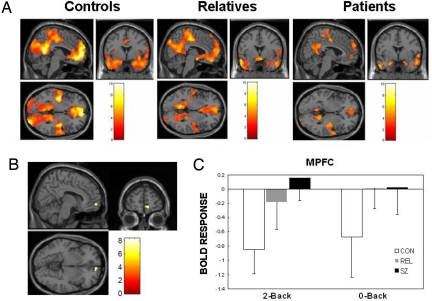
Task-related suppression of the default network appeared todecrease monotonically from controls to relatives to patients (Fig. 1A). A whole-brain ANOVA revealed significant differences among the groups in MPFC [F (2, 36) = 9.69] and in PCC/precuneus [F (2, 36) = 9.06]. After correction for multiple comparisons, the PCC/precuneus difference was marginally significant (peak: [12, −48, 42], P = 0.06), but the MPFC difference remained significant (Fig. 1B). In this MPFC region, controls exhibited the typical pattern of suppression (decreased activation during task) for both 0-back [t (12) = 2.33, P = 0.04] and 2-back [t (12) = 4.98, P < 0.001] tasks. Patients and relatives had significantly less task-related suppression than controls for both the 2-back and 0-back WM tasks [2-back, controls vs. patients: t (24) = 4.28, P < 0.001; 2-back, controls vs. relatives: t (24) = 2.57, P = 0.01; 0-back, controls vs. patients: t (24) = 2.11, P = 0.04; 0-back, controls vs. relatives: t (24) = 2.01, P = 0.05]. Differences between patients and relatives were not significant for either the 2-back or 0-back task (P > 0.20). When differences in 2-back WM accuracy were controlled for statistically with ANCOVA, the results were similar with or without exclusion of 1 patient with poor performance (Fig. S1).
Fig. 1.
Task-related suppression in default network regions. (A) Greater activation during rest than task (2-back WM) in PCC/precuneus and MPFC for controls (CON), relatives (REL), and patients (SZ). (B) MPFC region (peak: [12, 57, −6]) showing significant task-related suppression differences among groups. (C) Task-related suppression (with 95% confidence intervals) of MPFC region during 2-back and 0-back conditions. There was significantly more task suppression for controls than for relatives and patients on both WM tasks. The [x, y, z] locations are listed in Montreal Neurological Institute coordinates.
Task-Related Activation.
For task-related activation, there was widespread activation in regions typically associated with WM, including DLPFC, in each group. Between-group differences were found in right DLPFC, where patients and relatives exhibited significantly greater activation than controls (Fig. S2).
Functional Connectivity of Default Network During Rest.
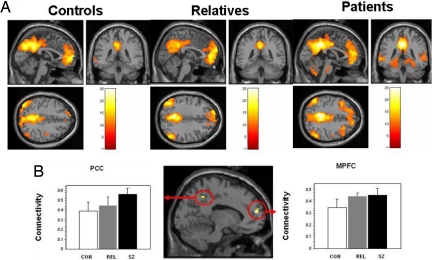
We examined functional connectivity during rest between 4 default seed regions defined by the literature (MPFC, PCC/precuneus, and bilateral parietal cortices) (14) and every voxel in the brain. Second-level within-group analyses performed on the average z-maps from the 4 source region of interest (ROI) seeds revealed widespread connectivity with the default network regions, with an apparent increase in the extent or strength of connectivity from controls to relatives to patients (Fig. 2A) (within-group analyses for individual MPFC and PPC seeds are shown in Fig. S3). An ANOVA revealed significant differences among groups in MPFC and in PCC/precuneus (Fig. 2B). Post hoc t tests on these clusters revealed greater connectivity between the default network and MPFC for patients [t (24) = 2.22, P = 0.04] and relatives [t (24) = 2.44, P = 0.02] than for controls and greater connectivity between the default network and PCC for patients than for controls [t (24) = 2.99, P = 0.01]. Differences between patients and relatives were not significant in MPFC (P = 0.81) and were marginally significant in PCC [t (24) = 2.05, P = 0.05].
Fig. 2.
Functional connectivity with default network during rest. (A) Areas showing positive connectivity with default network areas (averaged across 4 ROI seeds) in controls (CON), relatives (REL), and patients (SZ). (B) (Middle) Regions within default network showing significant connectivity differences between groups. Connectivity with default network (with 95% confidence intervals) in MPFC (Right, peak: [−12, 54, 15]) and PCC/precuneus (Left, peak: [−9, −51, 48]). There was significantly more connectivity with MPFC for relatives and patients than for controls and significantly more connectivity with PCC/precuneus for patients than for controls.
Functional Connectivity of Default Network During Task.
When analyzing default network connectivity during task performance, a similar pattern was found with significantly greater default network connectivity with MPFC and PCC in patients and with MPFC in relatives than in controls (Fig. S4).
Anticorrelations with Default Network.
We examined areas showing negative connectivity during rest and task (i.e., anticorrelations) with MPFC. The largest anticorrelation difference among groups occurred in right DLPFC in a cluster overlapping with the peak task activation response (peak DLPFC voxel of the entire group task activation, n = 39). During both rest and task, controls exhibited significantly greater anticorrelations between DLPFC and MPFC than patients [rest: t (24) = 2.49, P = 0.020; task: t (24) = 3.16, P = 0.004] and relatives [rest: t (24) = 2.31, P = 0.02; task: t (24) = 2.36, P = 0.026) (Fig. S5).
Correlations Between Default Network and WM Performance.
Hyperactivation (reduced suppression) and hyperconnectivity of the default network were associated with inferior WM performance in both patients and relatives. Task-related suppression in MPFC correlated positively with 2-back WM task accuracy among patients (r = 0.72, P = 0.006) and among relatives (r = 0.75, P = 0.003). There were significant negative correlations between task-phase MPFC connectivity with 2-back WM accuracy in patients (peak: precuneus, BA7 [−3, −57, 51], r = −0.74, P = 0.004) and relatives (peak: precuneus, BA7 [−3, −63, 48], r = −0.80, P < 0.001).
Correlations Between Default Network and Psychopathology.
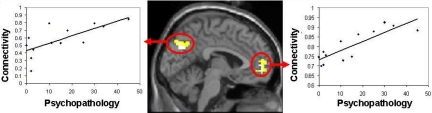
Hyperactivation and hyperconnectivity of the default network were associated with greater psychopathology in controls, relatives, and patients. Task suppression in MPFC correlated negatively with the SCL-90-R scores of psychopathology among controls (r = −0.73, P = 0.005) and among relatives (r = −0.71, P = 0.006). Among patients, (i) task suppression in MPFC correlated negatively with both negative (r = − 0.68, P = 0.01) and positive (r = −0.76, P = 0.003) composite symptom scores on the Scale for the Assessment of Negative and Positive Symptoms (SANS/SAPS) ratingsof current psychopathology and (ii) connectivity with the MPFC seed region during rest (Fig. 3) and task (Fig. S6) correlated positively with SAPS scores.
Fig. 3.
Functional connectivity during rest correlated with psychopathology in patients. (Middle) Whole-brain correlation between severity of psychopathology (composite SAPS score of positive symptomology) and strength of connectivity with the MPFC seed z-maps. Peaks in PCC/precuneus BA7 [−3, −57, 36], P = 0.003, r = 0.76 (Left) and in MPFC [−6, 51, 6], P < 0.001, r = 0.88 (Right).
Relation of Task Suppression and Connectivity in Patients.
There was a negative correlation between task suppression and resting connectivity (average z-maps) in MPFC (r = −0.76, P = 0.003) and PCC (r = −0.75, P = 0.003) (Fig. S7).
Discussion
Patients with schizophrenia and relatives of patients with schizophrenia exhibited functional pathology of the default mode of brain function. Patients and relatives exhibited hyperactivation of MPFC and hyperconnectivity of the default network. Controls exhibited the typical task-related suppression of the default network during WM performance. Patients and relatives exhibited significantly less task-related suppression of the default network during WM performance, resulting in tonic hyperactivation of the default network. The magnitude of default-network hyperactivation was associated with both cognitive and clinical variation. MPFC suppression correlated positively with WM accuracy among both patients and relatives. MPFC suppression correlated negatively with psychiatric symptomatology within all 3 groups. Further, during both rest and task, patients and relatives exhibited hyperconnectivity of the default network. The magnitude of default network hyperconnectivity was associated with clinical severity in the patients and with WM accuracy in patients and relatives. Also, during rest and task, patients had reduced anticorrelations between the default network (MPFC) and the right DLPFC, a region where patients also showed hyperactivation during task performance. These correlations suggest that hyperactivation and hyperconnectivity of the default network may play an important role in the cognitive and clinical symptomatology of schizophrenia, and the disturbance of the default network in the relatives suggests a genetic basis for that disturbance.
Our study demonstrates both hyperactivation and hyperconnectivity of the default network in schizophrenia and also in first-degree relatives of persons with schizophrenia. Differences in default network functioning occurred despite many similarities among the groups, including age, education, estimated IQ, and reading capacity. The 3 groups also performed similarly on the easier (0-back) WM task, but there were performance differences on the more difficult (2-back) WM task. There are, however, 3 reasons to believe that differences in the default network were not simply secondary to performance differences. First, reduced suppression occurred in patients and relatives for the 0-back task, for which there were no reliable performance differences. Second, when differences in 2-back performance were controlled for statistically, patient and relative groups still exhibited reduced suppression. Third, hyperconnectivity in patients and relatives was present during rest, when no task was being performed. Therefore, dysfunction of the default network cannot be attributed as secondary to deficits in performance.
Hyperactivation of the default network in patients is in accord with a study reporting abnormal activation during WM in schizophrenia that was published before the current conceptualization of the default network (25). Patients exhibited greater activation than controls for a 2-back WM task than for a 0-back WM task in areas now considered to be components of the default network (e.g., MPFC). This finding is equivalent to the hyperactivation reported in the present study. Two studies reported a tendency for patients to show hyperactivation, but neither study reported significant differences between patients and controls (15, 19). The authors speculated that the ease of the passive auditory odd-ball task used in those studies may have contributed to the lack of group differences.
The observed hyperconnectivity of the default network in schizophrenia may be compared with other studies of this network during rest in patients, and also contrasted to prior analyses of connectivity in task-related brain regions. One study found that patients demonstrated reduced regional homogeneity of activation clusters during rest in many brain regions (17). Another study, using an automated whole-brain analytical method that did not identify ROIs, reported widespread abnormal functional connectivity in patients with schizophrenia (16). Aberrant default-mode functional connectivity, analyzed by independent components analysis, was reported in a study where patient and control groups performed an auditory odd-ball task (15). The only other study using a whole-brain seed-driven analysis, however, also reported hyperconnectivity of the default system during rest (21). In contrast, studies examining connectivity in task-related regions during task performance have often observed reduced connectivity in patients with schizophrenia (e.g., 25–28).
Thus, our findings are broadly consistent with prior reports of abnormal connectivity in schizophrenia but offer several insights. First, there is a specific hyperconnectivity of the default network in schizophrenia using the same sort of analysis that has defined the normal characteristics of the default system (14). Second, the hyperconnectivity persists through rest and task. Third, patients exhibit reduced anticorrelations during both rest and task between the default network (MPFC) and DLPFC regions broadly implicated in WM, specifically exhibiting abnormal activation in patients during the 2-back WM task in this study. Fourth, the magnitude of hyperconnectivity was associated with the severity of both cognitive and psychiatric disorders in schizophrenia. Finally, there was a relation in patients between the hyperactivation and hyperconnectivity in both MPFC and PCC.
The relatives in this study exhibited dysfunction of the default network similar to that of patients, which indicates that default network dysfunction is associated with genetic risk for schizophrenia. Like patients, relatives exhibited hyperactivation of MPFC, significant correlations between the magnitude of hyperactivation and both cognitive and clinical measures, hyperconnectivity during rest and task, reduced anticorrelations between MPFC and DLPFC, and increased activation for 2-back WM performance in DLPFC. The similar pattern in patients and relatives, who were all unmedicated and had never been psychotic, suggests that the findings in patients were not secondary to disease history, which typically includes medication treatment and many psychosocial consequences of schizophrenia, such as social isolation. These functional abnormalities are consistent with reduced MPFC cortical thickness in relatives compared with controls (29). In contrast, whereas patients and controls differed by multiple measures in PCC, relatives were similar to controls in PCC by these same measures. This suggests that MPFC dysfunction is related to risk for the disease, whereas PCC dysfunction is related either to greater risk for the disease or to the expression of the illness.
Hyperactivation and hyperconnectivity of the default network may be involved in the core cognitive symptoms of schizophrenia. In the healthy brain, weaker task-related suppression of the default network is associated with inferior cognitive performance [e.g., inferior memory formation (30), lapses of attention (23), and worse learning of cognitive skill (31)]. Further, as WM demands are increased, default network suppression increases (32), indicating that suppression of the default network becomes increasingly important with increasing cognitive demands. In the present study, both failure to suppress the default network and hyperconnectivity of the default network correlated with impaired performance on the 2-back WM task in both patients and relatives. Importantly, increased connectivity of the default network and decreased anticorrelation between MPFC and DLPFC persisted during task performance. Thus, hyperactivation and hyperconnectivity of the default network in schizophrenia may constantly divide or misdirect attentional resources when patients attempt to perceive, think about, or act on the external world. Impaired performance on attentional and WM tasks has long been noted in schizophrenia (33, 34), and this impairment has been attributed to hypoactivation and hypoconnectivity of relevant frontotemporal and frontohippocampal systems (27, 35). These findings raise the possibility that functional pathology in the default network reflects an inability of patients to allocate resources away from internal thoughts and feelings and towards external stimuli in order to adaptively perform difficult tasks.
Functional pathology in the default network may also contribute to psychopathological symptoms in schizophrenia. The magnitudes of hyperactivity and hyperconnectivity during both rest and task correlated with measures of disease severity among the patients. Further, the magnitude of MPFC suppression correlated with quantitative measures of psychopathology not only in relatives but in controls. The relation between MPFC suppression and variation in behaviors among people without disease is consistent with evidence that among healthy people, lesser suppression of the default network is associated with increased mind-wandering (31). These results relate variation in default network function to variation in personality. It is striking that the personality variations associated with MPFC in controls lie along the same dimensions as those that characterize schizophrenia.
Hyperactivation of the default network may blur the normal boundary between internal thoughts and external perceptions. Constant overengagement of the default network could lead to an exaggerated focus on one's own thoughts and feelings as well as an ambiguous integration between one's own thoughts and feelings with events in the environment. Thus, neutral events would seem to be imbued with exaggerated self-relevance, and the boundary between the internal world of reflection and feeling and the external world of perception and action would be weakened. Indeed, many symptoms of schizophrenia involve an exaggerated sense of self-relevance in the world, such as paranoid ideation that individuals and groups are conspiring against the patient, and a blurring of internal reflection and external perception, such as hallucinations. Thus, hyperactivation and hyperconnectivity may contribute fundamentally to the severe disturbances of thought that characterize schizophrenia.
Methods
Participants.
Participants were 13 persons with a diagnosis of Diagnostic and Statistical Manual of Mental Disorders Revised Fourth Edition (DSM-IV) schizophrenia or schizoaffective or schizophreniform disorder (“patients”), 13 nonpsychotic first-degree relatives of persons with a DSM-IV diagnosis of schizophrenia (“relatives”), and 13 control participants (“controls”) without a personal or family history of psychotic illness. Ten patients, but none of the relatives or controls, were receiving psychotropic medication.
fMRI: WM Tasks.
Participants performed 2 runs of a block-designed visual N-back WM task with blocks of rest, 0-back trials, and 2-back trials as described previously (9).
Statistical Analysis.
All (non-fMRI) variables were compared using independent sample t tests performed in the Statistical Package for the Social Sciences (SPSS) software (standard version 11.0.1; SPSS, Inc.). fMRI data were analyzed using Statistical Parametric Mapping (SPM)-2 software (Wellcome Trust Centre for Neuroimaging, University College of London) and in-house software running under the MATLAB environment (Mathworks, Inc.).
Functional Activation
First-Level Analyses.
For each participant, functional images were realigned, normalized to the Montreal Neurological Institute template supplied with SPM-2, and smoothed with an 8-mm Gaussian kernel. Within-subject analyses used a block-based general linear model. Each block (2-back, 0-back, and rest) was modeled using a boxcar function convolved with a canonical hemodynamic response function. Estimated motion correction parameters were included as additional covariates. As a quality control measure, stimulus-correlated motion was calculated for each condition and each motion parameter for each subject (SI Text). Contrasts were created for each subject for rest >2-back (task-related suppression), rest >0-back (task-related suppression during 0-back), and 2-back > rest (task-related activation) conditions and were submitted to second-level random-effects analyses.
Second-Level Analyses.
Within-group effects were tested using single-sample t tests on contrast images for each group separately. Between-group differences were tested using an ANOVA F test with variances assumed unequal between groups. To characterize among group differences, post hoc two-tailed t tests were performed on parameter estimates (levels of task-related suppression) in 2-back and 0-back conditions from significant clusters. Addressing the possibility that activation differences between groups reflected performance differences between groups, we performed an ANCOVA in which accuracy was included as a covariate.
Functional Connectivity
First-Level Analyses.
Default network regions defined from the literature (14) were used as seeds for whole-brain functional connectivity analyses during rest. Time series from a 10-mm sphere centered around MPFC (−1, 47, −4), PCC (−5, −49, 40), and lateral parietal (−45, −67, 36) peak foci were extracted from the rest periods and temporally band pass filtered (0.004 < f < 0.08 Hz) to reduce effects of low-frequency drift and high-frequency noise. Pearson's correlation coefficients were calculated between the mean time series of each ROI and every voxel in the brain, for each subject separately, and converted to z-scores using a Fisher transform. To obtain a quantitative measure of overall default network connectivity, average z-maps from the 4 source ROI seeds (resulting in 1 mean z-map per subject) were calculated, reflecting the average degree of association with the 4 default network regions for each voxel.
Second-Level Analyses.
Second-level analyses of these average z-maps were performed as for the functional activation analyses described previously. Methods for removing spurious sources of variance as well as connectivity analyses for anticorrelations are described in SI Text. Because the most consistent differences among groups in both task suppression and mean default connectivity were within the MPFC, anticorrelations and correlations with psychopathology and accuracy were performed on the connectivity maps from the MPFC seed region.
Correlations with Psychopathology and Accuracy.
To evaluate the association between current psychopathology levels in patients and the previously discussed task suppression and connectivity measures, second-level whole-brain voxel-wise correlations were performed between the SAPS score, which reflects a composite rating of score of Hallucinations, Delusions, Bizarre Ideation, Formal Thought Disorder, and Inappropriate Affect, and both task-related suppression (rest >2-back contrast) and connectivity with MPFC during rest. To characterize within-group task-related suppression and task connectivity relation to WM performance, within-group second-level correlation analyses were performed between task-related suppression as well as connectivity with MPFC during task and 2-back WM tasks.
Relation of Task Suppression and Connectivity.
To investigate the association between functional activation and connectivity, second-level whole-brain voxel-wise correlations between task-related suppression in the MPFC region (Fig. 1) and connectivity (average z-maps) measures were calculated for the patients.
False-Positive Control in Second-Level Analyses.
Voxel-wise inferences for all within-group analyses were corrected for multiple comparisons using a whole-brain false discovery rate of P < 0.05. Cluster-level inferences for all between-group analyses were restricted to a priori anatomical ROIs as defined by Wake Forest University Pickatlas (36, 37) [default areas (n = 10): MPFC (BA10) and PCC/precuneus (BA30, BA31, and BA7); task-related areas (n = 9): right DLPFC (BA9 and BA46)]. Results surviving family-wise error correction for multiple comparisons, with cluster-level P < 0.05, were reported and further analyzed with post hoc t tests. For 2 subsidiary analyses, we used alternative approaches. For the ANOVA/ANCOVA comparison controlling for task performance, we used a liberal threshold of P = 0.001 uncorrected to compare better the effect of controlling for WM performance (Fig. S1). For the task activation ANOVA, because we were replicating prior results, we used anatomically constrained inference (38) to the DLPFC voxel reported from a prior study (9).
Supplementary Material
Acknowledgments.
We thank the participants and their families and the project staff for their contributions to the study. Staff members included Ariel Brown, Ann Cousins, Lisa Gabel, Anthony Giuliano, Stephen Glatt, Jennifer Koch, Marc Korczykowski, Erica Lee, Nikos Makris, Virna Merino, Elon Mesholam, Raquelle Mesholam-Gately, Shay Moses, Caroline Patterson, Nicole Peace, Sari Reisner, William Stone, and Sharon White. We thank Anthony Wagner and Russell Poldrack for helping in the initial task design. This study was supported by a grant from the Mental Illness and Neuroscience Discovery Institute (to L.J.S.), by the National Association of Research in Schizophrenia and Depression Stone Award (to L.J.S.); by National Institute of Mental Health Grants MH 43518 and MH 65562 (to M.T.T. and L.J.S.), Grant MH 63951 (to L.J.S.), and Grant R25 MH 60485 (to H.W.T., Training Principal Investigator: Ming T. Tsuang, MD, PhD); by the Commonwealth Research Center, Massachusetts Department of Mental Health (L.J.S.); by the Poitras Center for Affective Disorders Research (S.W.G. and J.D.E.G.); and by National Institute of Mental Health Grant MH 40799 (to R.W.M.), Grant MH 50740 (to M.E.S.), and Grant MH 62157 (to A.I.G.). This work was also supported in part by the National Center for Research Resources (Grant P41RR14075).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0809141106/DCSupplemental.
References
- 1.Heinrichs RW, Zakzanis KK. Neurocognitive deficit in schizophrenia: A quantitative review of the evidence. Neuropsychology. 1998;12:426–445. doi: 10.1037//0894-4105.12.3.426. [DOI] [PubMed] [Google Scholar]
- 2.Snitz BE, Macdonald AW, 3rd, Carter CS. Cognitive deficits in unaffected first-degree relatives of schizophrenia patients: A meta-analytic review of putative endophenotypes. Schizophr Bull. 2006;32:179–194. doi: 10.1093/schbul/sbi048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tsuang MT. Genotypes, phenotypes, and the brain: A search for connections in schizophrenia. Br J Psychiatry. 1993;163:299–307. doi: 10.1192/bjp.163.3.299. [DOI] [PubMed] [Google Scholar]
- 4.Cardno AG, Gottesman II. Twin studies of schizophrenia: From bow-and-arrow concordances to star wars Mx and functional genomics. Am J Med Genet. 2000;97:12–17. [PubMed] [Google Scholar]
- 5.Harrison PJ. The neuropathology of schizophrenia: A critical review of the data and their interpretation. Brain. 1999;122:593–624. doi: 10.1093/brain/122.4.593. [DOI] [PubMed] [Google Scholar]
- 6.Boos HB, Aleman A, Cahn W, Pol HH, Kahn RS. Brain volumes in relatives of patients with schizophrenia: A meta-analysis. Arch Gen Psychiatry. 2007;64:297–304. doi: 10.1001/archpsyc.64.3.297. [DOI] [PubMed] [Google Scholar]
- 7.Weinberger DR, et al. Prefrontal neurons and the genetics of schizophrenia. Biol Psychiatry. 2001;50:825–844. doi: 10.1016/s0006-3223(01)01252-5. [DOI] [PubMed] [Google Scholar]
- 8.Goldman-Rakic PS. Cellular and circuit basis of working memory in prefrontal cortex of nonhuman primates. Prog Brain Res. 1990;85:325–335. doi: 10.1016/s0079-6123(08)62688-6. discussion: 335–326. [DOI] [PubMed] [Google Scholar]
- 9.Seidman LJ, et al. Altered brain activation in dorsolateral prefrontal cortex in adolescents and young adults at genetic risk for schizophrenia: An fMRI study of working memory. Schizophr Res. 2006;85:58–72. doi: 10.1016/j.schres.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 10.Raichle ME, et al. A default mode of brain function. Proc Natl Acad Sci USA. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: Relation to a default mode of brain function. Proc Natl Acad Sci USA. 2001;98:4259–4264. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kelley WM, et al. Finding the self? An event-related fMRI study. J Cognit Neurosci. 2002;14:785–794. doi: 10.1162/08989290260138672. [DOI] [PubMed] [Google Scholar]
- 13.Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: A network analysis of the default mode hypothesis. Proc Natl Acad Sci USA. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fox MD, et al. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garrity AG, et al. Aberrant “default mode” functional connectivity in schizophrenia. Am J Psychiatry. 2007;164:450–457. doi: 10.1176/ajp.2007.164.3.450. [DOI] [PubMed] [Google Scholar]
- 16.Liang M, et al. Widespread functional disconnectivity in schizophrenia with resting-state functional magnetic resonance imaging. NeuroReport. 2006;17:209–213. doi: 10.1097/01.wnr.0000198434.06518.b8. [DOI] [PubMed] [Google Scholar]
- 17.Liu H, et al. Decreased regional homogeneity in schizophrenia: A resting state functional magnetic resonance imaging study. NeuroReport. 2006;17:19–22. doi: 10.1097/01.wnr.0000195666.22714.35. [DOI] [PubMed] [Google Scholar]
- 18.Bluhm RL, et al. Spontaneous low-frequency fluctuations in the BOLD signal in schizophrenic patients: Anomalies in the default network. Schizophr Bull. 2007;33:1004–1012. doi: 10.1093/schbul/sbm052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Calhoun VD, Maciejewski PK, Pearlson GD, Kiehl KA. Temporal lobe and “default” hemodynamic brain modes discriminate between schizophrenia and bipolar disorder. Hum Brain Mapp. 2008;29:1265–1275. doi: 10.1002/hbm.20463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu Y, et al. Disrupted small-world networks in schizophrenia. Brain. 2008;131:945–961. doi: 10.1093/brain/awn018. [DOI] [PubMed] [Google Scholar]
- 21.Zhou Y, et al. Functional disintegration in paranoid schizophrenia using resting-state fMRI. Schizophr Res. 2007;97:194–205. doi: 10.1016/j.schres.2007.05.029. [DOI] [PubMed] [Google Scholar]
- 22.Anselmetti S, et al. Psychopathological and neuropsychological correlates of source monitoring impairment in schizophrenia. Psychiatry Res. 2007;150:51–59. doi: 10.1016/j.psychres.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 23.Weissman DH, Roberts KC, Visscher KM, Woldorff MG. The neural bases of momentary lapses in attention. Nat Neurosci. 2006;9:971–978. doi: 10.1038/nn1727. [DOI] [PubMed] [Google Scholar]
- 24.Derogatis LR. Symptom Checklist-90-Revised (SCL-90-R): Administration, Scoring and Procedures Manual. Towson, MD: Clinical Psychometric Research; 1983. [Google Scholar]
- 25.Meyer-Lindenberg A, et al. Evidence for abnormal cortical functional connectivity during working memory in schizophrenia. Am J Psychiatry. 2001;158:1809–1817. doi: 10.1176/appi.ajp.158.11.1809. [DOI] [PubMed] [Google Scholar]
- 26.Friston KJ. Schizophrenia and the disconnection hypothesis. Acta Psychiatr Scand Suppl. 1999;395:68–79. doi: 10.1111/j.1600-0447.1999.tb05985.x. [DOI] [PubMed] [Google Scholar]
- 27.Fletcher P, McKenna PJ, Friston KJ, Frith CD, Dolan RJ. Abnormal cingulate modulation of fronto-temporal connectivity in schizophrenia. NeuroImage. 1999;9:337–342. doi: 10.1006/nimg.1998.0411. [DOI] [PubMed] [Google Scholar]
- 28.Ford JM, Mathalon DH, Whitfield S, Faustman WO, Roth WT. Reduced communication between frontal and temporal lobes during talking in schizophrenia. Biol Psychiatry. 2002;51:485–492. doi: 10.1016/s0006-3223(01)01335-x. [DOI] [PubMed] [Google Scholar]
- 29.Seidman LJ, et al. Altered cortical thickness in adolescents and young adults at genetic risk for schizophrenia. Schizophr Bull. 2007;33:354. [Google Scholar]
- 30.Daselaar SM, Prince SE, Cabeza R. When less means more: Deactivations during encoding that predict subsequent memory. NeuroImage. 2004;23:921–927. doi: 10.1016/j.neuroimage.2004.07.031. [DOI] [PubMed] [Google Scholar]
- 31.Mason MF, et al. Wandering minds: The default network and stimulus-independent thought. Science. 2007;315:393–395. doi: 10.1126/science.1131295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McKiernan KA, Kaufman JN, Kucera-Thompson J, Binder JR. A parametric manipulation of factors affecting task-induced deactivation in functional neuroimaging. J Cognit Neurosci. 2003;15:394–408. doi: 10.1162/089892903321593117. [DOI] [PubMed] [Google Scholar]
- 33.Nuechterlein KH, Dawson ME. Information processing and attentional functioning in the developmental course of schizophrenic disorders. Schizophr Bull. 1984;10:160–203. doi: 10.1093/schbul/10.2.160. [DOI] [PubMed] [Google Scholar]
- 34.Gur RE, et al. Visual attention circuitry in schizophrenia investigated with oddball event-related functional magnetic resonance imaging. Am J Psychiatry. 2007;164:442–449. doi: 10.1176/ajp.2007.164.3.442. [DOI] [PubMed] [Google Scholar]
- 35.Meyer-Lindenberg AS, et al. Regionally specific disturbance of dorsolateral prefrontal-hippocampal functional connectivity in schizophrenia. Arch Gen Psychiatry. 2005;62:379–386. doi: 10.1001/archpsyc.62.4.379. [DOI] [PubMed] [Google Scholar]
- 36.Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- 37.Lancaster JL, et al. Automated Talairach atlas labels for functional brain mapping. Hum Brain Mapp. 2000;10:120–131. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Friston KJ. Testing for anatomically specified regional effects. Hum Brain Mapp. 1997;5:133–136. doi: 10.1002/(sici)1097-0193(1997)5:2<133::aid-hbm7>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.