Abstract
Spatiotemporal control of transgene expression is of paramount importance in gene therapy. Here, we demonstrate the use of magnetic resonance temperature imaging (MRI)-guided, high-intensity focused ultrasound (HIFU) in combination with a heat-inducible promoter [heat shock protein 70 (HSP70)] for the in vivo spatiotemporal control of transgene activation. Local gene activation induced by moderate hyperthermia in a transgenic mouse expressing luciferase under the control of the HSP70 promoter showed a high similarity between the local temperature distribution in vivo and the region emitting light. Modulation of gene expression is possible by changing temperature, duration, and location of regional heating. Mild heating protocols (2 min at 43°C) causing no tissue damage were sufficient for significant gene activation. The HSP70 promoter was shown to be induced by the local temperature increase and not by the mechanical effects of ultrasound. Therefore, the combination of MRI-guided HIFU heating and transgenes under control of heat-inducible HSP promoter provides a direct, noninvasive, spatial control of gene expression via local hyperthermia.
Keywords: gene therapy, local control gene expression, MRI-guided HIFU
The objective of gene therapy is to express a therapeutic gene in the region where therapy is required (i.e., spatial control) and for the duration necessary (i.e., temporal control) to achieve a therapeutic effect while minimizing systemic toxicity. Several approaches for controlling gene expression have been proposed (1). Tissue-specific or disease-specific promoters can provide spatial control of gene expression (2, 3), whereas small molecule-dependent gene switches give temporal control (4, 5). Alternatively, physical stimuli, such as ionizing radiation (6) and heat (7), offer the possibility of activating gene expression in deep tissue with excellent spatial definition, and they allow temporal control of the start of gene activation. Ionizing radiation may be a limiting factor when repeated activation is required. Local activation of gene expression with the use of heat in combination with thermoinducible promoters, such as the heat shock protein (HSP) promoters, has already been successfully used (8). In vitro experiments showed that the human HSP70B promoter is suitable for this purpose because it shows high heat-induced expression amplification (9, 10). In addition, its magnitude of induction depends on temperature as well as duration of the induced hyperthermia (11, 12). Similar promoter characteristics were reported in vivo in a number of organs, such as skin (12), muscle (13), prostate (14), and liver (15), that were genetically modified using either a virus or a plasmid to express a reporter gene under the control of the HSP promoter. Noninvasive in vivo local heating, and thus local activation of gene expression, could be achieved with high-intensity focused ultrasound (HIFU) (15–17), but a subtle compromise has to be found in the heating strategy, because excessive tissue heating can induce tissue damage and necrosis. Local temperature distribution can be monitored with magnetic resonance temperature imaging (MRI) to evaluate the efficacy of heat-induced gene activation technique as well as safety. Furthermore, recent developments in MRI-guided HIFU techniques allow automatic control of the HIFU energy deposition in order that the local tissue temperature at the targeted location follow a predefined temperature evolution (18, 19). This fully automated approach has been developed and tested in vivo for tumor models expressing a fluorescent protein reporter gene under transcriptional control of the HSP70B promoter (16). Unfortunately, because of limitations of currently available fluorescence imaging capabilities in deep tissues, the kinetics of gene expression are difficult to assess in vivo. In addition, tumor models show a high variability in the level of gene expression because of the spatial heterogeneity of cancerous tissue and physiology. As a consequence, a quantitative spatiotemporal correlation between MR temperature maps and transgene expression could not be established. Moreover, the presence of necrosis complicated the evaluation of the noninvasiveness of the method.
To overcome these limitations, in the present work a healthy transgenic mouse strain was chosen to compare spatiotemporal control of gene expression with different heating patterns under reproducible conditions. This animal model, expressing the firefly luciferase reporter gene under control of an HSP promoter, allowed in vivo assessment of the spatial extent and time course of gene expression via bioluminescence imaging. In addition, the influence of the mechanical and thermal stress of HIFU on HSP promoter activation was compared. Finally, a systematic evaluation of the damage induced by HIFU hyperthermia in the targeted tissue was performed to evaluate the safety of this gene activation strategy.
Results
In Vitro Characterization of the Heat-Sensitive Mouse Promoter.
The NLF-1 mouse strain contains 3 copies of the firefly luciferase gene under the control of a mouse HSP70 promoter (the HSPa1b type; ref. 20) per haploid genome. The purpose of the in vitro characterization of the heat-sensitive mouse promoter was to provide a reference for the temporal window and the induction levels of the promoter. The promoter's activity was assessed with respect to temperature and duration of hyperthermia in homogeneous bone marrow cell suspensions obtained from the femoral bones of NLF-1 mice. It is shown (Fig. 1) that expression of the reporter gene can be modulated by the heating parameters. At 42°C, a weak expression is observed for heating durations longer than 16 min only. At 43 °C and 44 °C, higher levels of expression are observed, with maximal intensities observed for 32 min and 16 min of heating, respectively. The levels of emitted light remain comparable for these 2 temperatures upon reduction of relative exposure times (i.e., 16 min at 43 °C/8 min at 44 °C). At 45 °C, the maximum light intensity (obtained at 8 min) was lower than for 43 °C and 44 °C, and it decreased to zero with increasing exposure time. Cell viability was measured at different time points after applying various heating protocols (20 min at 43 °C, 10 min at 44 °C, and 5 min at 45 °C). At 5 h after heating, a clear decline in cell viability (85%) was noticed for the 45 °C heating protocol compared with the 43 °C (95%) and 44 °C (95%) heating protocols. The decreased viability at the higher temperatures provides an explanation for the decreased light intensities at those temperatures.
Fig. 1.
HSP70 promoter activity in bone marrow cells 5 h after heating at different temperatures (42 °C, 43 °C, 44 °C, and 45 °C) and for different durations (0, 1, 2, 4, 8, 16, and 32 min). Promoter activity was measured as emitted light by luciferase by using an in vitro enzymatic assay.
Accuracy of Automatic Temperature Control In Vivo in the Leg Muscle of Mouse.
Fig. 2A illustrates the accuracy of the MRI-based temperature control at the HIFU focal point during 2 min of heating at 43 °C in thigh muscle. The standard deviation of the MRI-measured temperature was 0.61 °C at normal body temperature (observed during repetitive temperature mapping). The average difference between target profile at the plateau and experimental data was 0.66 °C during heating, demonstrating the precision of the automatic feedback coupling to ensure the predefined temperature evolution. Fig. 2B displays a typical MRI thermal map obtained during automatic temperature control. A color-coded image superimposed on a gray-level magnitude MRI image shows the temperature induced by HIFU heating. The temperature increase is localized at the HIFU focal point and diffuses out because of heat conduction.
Fig. 2.
Typical time course of the temperature evolution during a heating experiment (A), with the target temperature (in black) and the measured temperature at the focal point (in red). (B) MRI temperature map (color-coded) superimposed on an anatomical MRI image (grayscale) of the mouse leg.
Comparative Analysis of MR Temperature Mapping and Bioluminescence Imaging.
Fig. 3 A and B shows MRI temperature maps (5 min after the start of the experiment) of 2 mice heated with the same protocol; i.e., 8 min of heating at 43 °C in the focal point. Fig. 3 C and D shows bioluminescence images of the same 2 mice taken 6 h after heating and following i.p. injection of luciferin. Although both mice underwent the same heating protocol, the resulting temperature distribution in the leg appears clearly different, whereas the temperature evolution in the focal point was identical. This spatial difference is also observed in the distribution of emitted photons for both mice (Fig. 3 C and D). These results demonstrate the close correspondence between spatial distribution of temperature and expression. Because heat conduction is spatially heterogeneous, the temperature distribution can be expected to be different from animal to animal, despite an identical evolution of the temperature in the focal point.
Fig. 3.
MRI temperature maps of 2 mice heated with the same protocol, i.e., 8 min of heating at 43 °C at the focal point (A and B). The colors in A and B show the local temperature distribution in the mouse leg in the range of 38 °C (blue) to 42 °C and higher (red). (C and D) Bioluminescence images of the same 2 mice taken 6 h after the heating protocol. The colors in C and D show the light intensity measured with an optical CCD camera in the range of 320 photons per second (blue) to 2,000 photons per second and more (red).
Nature of Gene Activation Stimulus.
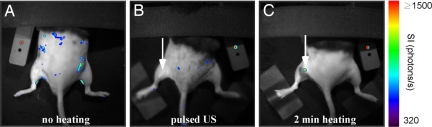
HIFU exposure results in thermal and nonthermal mechanical stress to the tissue. From the literature, it is known that inducible HSP70 promoters are predominantly sensitive to temperature but may also be activated by other stimuli, like hypoxia (21), ischemia (22), and mechanical stress (23). To use MRI thermometry as a reliable method to predict local transgene activation, gene induction should not be affected by the mechanical part of ultrasound. To evaluate the influence of mechanical stress, a set of experiments was performed on a separate group of animals, applying the same amount of total acoustical energy and the same pressure amplitude, but with varying duty cycle. A 20% HIFU duty cycle and 0.7-W acoustical power (comparable to the mean power found in the heating experiments) resulted in a negligible temperature increase. Fig. 4 compares bioluminescence images obtained without HIFU application (Fig. 4A), after MRI-monitored pulsed HIFU experiment with 20% duty cycle (Fig. 4B), and after MRI-controlled HIFU heating (43 °C, 2 min) (Fig. 4C). As expected, local light emission was observed at the spot where the leg of the animal was heated (Fig. 4C, white arrow). The average acoustical energy deposited for this experiment was 116 J (n = 4). In contrast, no light was detected at the target location following pulsed HIFU with 125 J of energy deposition (Fig. 4B, white arrow). Low-intensity light emission appeared at random locations (≈160 photons/s) in all cases, attributed to very low basal activity of the HSP70 promoter.
Fig. 4.
Bioluminescence images taken before (A) and 6 h after (B) depositing 125 J of energy with pulsed ultrasound, and 6 h after heating at 43 °C for 2 min (C). The arrows indicate the locations of HIFU application. The color coding represents the light intensity measured with the CCD camera in photons per second.
Kinetics of Light Intensity After Heating in Vivo.
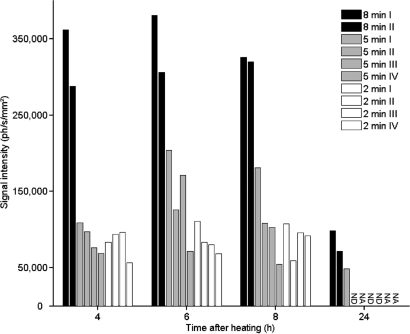
The results of the in vitro experiments (Fig. 1) suggest that the level of expression of the transgene is transient and depends on the heating amplitude and duration. Using bioluminescence images (BLIs), the kinetics of light emission was followed for several heating conditions in the leg muscle. Fig. 5 shows the evolution of light intensity for 10 mice heated at 43 °C for 2, 5, and 8 min, respectively. Light was observed in all cases from 4 h up to 24 h after heating. Light intensity remained nearly constant between 4 and 8 h, and it decreased to basal levels at 24 h. Weak emission levels persisted at 24 h for the 8-min protocols and for a single 5-min experiment. Increasing heating time influenced the level of expression, with a small increase between 2- and 5-min heating experiments, whereas substantially higher (3- to 4-fold) expression was detected for the 8-min heating protocols. Some variability in light emission may have arisen because of small differences in the depth of focal point and in heat diffusion. As expected from in vitro experiments, the heat-induced light production in vivo was found to be transient, with its intensity varying according to the heating protocol.
Fig. 5.
Time course of gene expression in vivo upon hyperthermia. The leg muscle of transgenic mice was heated at 43 °C for 2 min (n = 4), 5 min (n = 4), or 8 min (n = 2). The mean light intensity is reported in photons per second per mm2. Light emitted by luciferase was measured at 4, 6, 8, and 24 h after heating. Light emission below the quantification threshold was marked as ND (not detectable), and measurements not performed are marked as NA (not available).
It would be conceivable that the heating protocols have an influence on light intensity kinetics via indirect physiological events, such as perfusion, substrate availability, etc. To evaluate this possibility, studies were done with a transgenic Tet-Off mouse expressing the luciferase gene constitutively in the absence of tetracycline. Heating protocols with HIFU (n = 2) and water bath (n = 3) showed no influence of the heating procedure on BLI light intensity.
Histological Analysis of Heated Muscle.
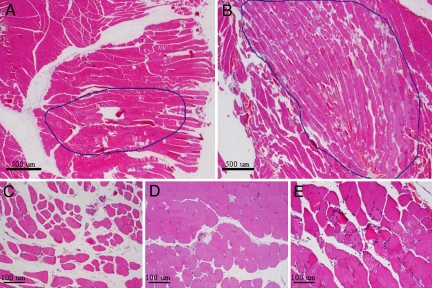
The HIFU-induced tissue damage was investigated by histological analysis. No muscular damage was noticed in muscle tissue with the pulsed HIFU protocol and following 2 min of heating at 43 °C. However, for 5-min protocols at the same temperature, a modest variability in fiber diameters without specific pattern and moderate interstitial edema were noted, as was the presence of a few necrotic fibers with centralized nuclei. Few atrophic fibers and no inflammation were observed. The region with abnormalities did not exceed 3 mm in diameter (Fig. 6 A and C). When increasing the heating duration to 8 min, the muscular damage was more extensive and corresponded to a region measuring up to 11 mm in diameter. In this case, a marked variability was observed in fiber diameters with clearly necrosed fibers and severe interstitial edema, without fascicular or perifascicular pattern. Necrotic fibers were accompanied by inflammatory infiltrates (lymphocytes and polynuclear cells) (Fig. 6 B and E).
Fig. 6.
H&E-stained histology sections of excised muscle tissue 24 h after heating at 43 °C for 5 min (A and C) and 8 min (B and E). (D) After 5 min of heating, a modest variability in fiber diameters and interstitial edema was observed compared with the unheated muscle. The muscular damage was more extensive after 8 min of heating, including necrotic fibers and inflammatory infiltrates. (Magnification: A and B, 2×; C–E, 10×.)
Discussion
Spatial and temporal control of transgene expression is an important requirement for gene therapy. In the present paper, using MRI-guided HIFU in a transgenic mouse expressing luciferase under the control of a heat-sensitive promoter, a high similarity is shown between the local temperature distribution in vivo and the region emitting light. Control of transgene expression was achieved by automatic adjustment of the HIFU power based on continuous MRI thermometry to force the temperature to follow a predefined temperature evolution. The good spatial correspondence between increased temperature and gene expression was demonstrated by comparing MRI temperature maps and bioluminescence images. This observation is based on qualitative comparison between images of 2 different imaging modalities obtained on living animals, and it demonstrates the great potential for MRI-guided HIFU to predict and control the region of gene expression. Future research should investigate the role of absolute temperature and thermal dose on the spatial distribution and level of gene activation.
The spatial positioning of the focal point of the focused ultrasound and the quantity of deposited energy can be completely controlled by the operator. However, the resulting local temperature distribution, and thus the region of gene activation, are not completely predictable and may depend on several parameters, such as tissue absorption of ultrasound and heat conduction. These parameters can be spatially heterogeneous and may be affected by temperature increase itself. Thus, the resulting heated area may vary from animal to animal when using an identical heating protocol, as shown in Fig. 3 A and B. However, temperature maps of each animal clearly correspond with light-emitting regions.
The in vitro characterization shows that the thermoinducible HSP70a1b promoter possesses the same features as the thermoinducible HSP70B human promoter already described in the literature (9, 11). At temperatures higher than 42 °C, the promoter activity has the tendency to be dose-dependent. However, cell death is observed in vitro at prolonged exposure at 45 °C, and promoter activity is lowered.
From the in vivo results, shown in Fig. 5, we derived a similar dose-dependent promoter activity that allowed modulation of the gene expression level through appropriate choices in heating conditions. Furthermore, it was found that the promoter has a low level of basal expression, because light intensity measured in animals not submitted to HIFU heating was very weak (≈160 photons/s). In contrast, the level of induced activity increased at least 10-fold, even with mild activation protocols; i.e., heating for 2 min at 43 °C (Fig. 4C). We also demonstrated that the influence of mechanical stress of HIFU on gene activation was negligible, in agreement with the results from Liu et al. (17). In addition, indirect effects of HIFU heating on BLI light intensity were excluded on the basis of tests on transgenic mice expressing the luciferase gene constitutively.
The induction of the luciferase gene was transient, with maximal protein activity occurring 6–8 h after heating. The time course of activation and subsequent deactivation is an intrinsic property of the promoter, as is mRNA and protein processing, and therefore does not allow temporal switch-off control by external factors. Because expression returns to near-basal activity at 24 h following heat shock, repetitive heatings at the same location may allow for activation during prolonged periods. Alternatively, temporal regulation may be achieved via the use of 2- or 3-component systems comprising (i) a small molecule-dependent transactivator whose expression is placed under the dual control of an HSP70 promoter and a transactivator-responsive promoter, and (ii) a transactivator-responsive promoter to which a transgene of interest is linked (24).
The time evolution of luciferase expression upon different activation protocols is comparable to in vitro observations made by other groups (10, 12). However, absolute quantification of the resulting light intensity as a marker of protein activity remains difficult, because the bioluminescence imaging of the reporter gene used here was a 2-dimensional projection method (resulting in depth-weighted projection), whereas multislice MRI thermometry was used for guidance of HIFU.
The noninvasiveness of the proposed approach was evaluated by a systematic histological analysis of the heated muscles. For short-duration hyperthermia (i.e., 2 min at 43 °C), no damage was observed but, as expected, increasing the duration of the hyperthermia resulted in an increase of induced damage in the leg muscles, with substantial alteration of the muscle appearance after 8-min hyperthermia. This illustrates the importance of the choice of the heating conditions in vivo to observe sufficient induction of expression avoiding tissue damage. This justifies the fundamental importance of a precise, noninvasive, and quantitative temperature measurement and control system provided by the combination of MRI thermometry and HIFU heating.
Methods
Animals.
Male homozygote transgenic mice (NLF-1) ages 17–26 weeks were used in this study (20). NLF-1 mice contain a transgene that allows firefly luciferase expression under control of the HSP70 promoter 1B (Hspa1b). Each individual mouse was tested with regard to its phenotype by heating both front legs in a water bath at constant temperature (43 °C) for 5 min 3 weeks before the start of the protocol. BLIs were acquired to verify the presence of light emission in the heated area (see below for details of the BLI protocol). The posterior part of the mouse was shaved with a clipper and depilatory cream to improve ultrasound and light propagation before HIFU studies. Male homozygote transgenic mice (Tet-Off, n = 5) with the luciferase gene expressed constitutively in the absence of tetracycline were used to evaluate the effects of HIFU heating protocols on bioluminescence intensity. All procedures were performed according to protocols approved by the French law and the rules of animal care of the institute.
Bone Marrow Cell Extraction and Heating.
The HSP70 promoter was characterized in vitro using bone marrow cells from NLF-1 mice. Animals were first anesthetized (2% isoflurane in air) and then rapidly decapitated. Bone marrow cells were flushed out of the femoral bone shafts by using RPMI medium 1640 (Invitrogen) supplemented with 2% FCS (Invitrogen). Bone marrow mononuclear cells were isolated on Ficoll by centrifugation (400 × g, 20 min) and washed 3 times in RPMI medium 1640 plus 10% FCS (300 × g, 5 min). Viable cells were counted with Trypan blue exclusion dye and plated (3.5 × 105 cells/50 μL per tube) in RPMI medium 1640 supplemented with 10% FCS and mIL-3 (5 ng/mL). Cells were heated by using a thermal cycler (T gradient; Biometra) at different temperatures (42 °C, 43 °C, 44 °C, and 45 °C) and for different durations (0, 2, 4, 8, 16, and 32 min), placed in a water bath (37 °C, 10 min), and finally incubated at 37 °C in a 5% CO2 incubator. The luciferase activity was measured 5 h after heating by using the luciferase assay system (Promega) using a luminometer apparatus (Berthold Technologies).
HIFU Device.
Ultrasound experiments were performed with an in-house-designed, single-channel focused ultrasound transducer (built by Imasonic SA) incorporated in the bed of the 1.5-T magnetic resonance imaging system. The transducer had a focal length of 80 mm and external radius aperture of 60 mm. The sinusoidal signal (1.5 MHz) was generated by using a Yokogawa FG110 wave generator and amplified by using a Kalmus KMP 170F amplifier. The dimensions of the focal point were 1 × 1 × 3 mm3 (at −3 dB). The wave generator was remotely controlled via in-house written software running under Windows (Microsoft).
Animal Preparation for MRI-Guided HIFU Heating.
Animals were sedated with isoflurane (2% in air) and received muscle relaxants (100 μL of lidocaine, 2.5 mg/mL) in 3 different positions intramuscularly) in the left hind leg to prevent unwanted muscle contraction during sonication. Injection of 200 μL of the local anesthetic ropivacaine (0.5 mg/mL) was performed s.c. in 2 different positions and 500 μL of ketamine (1.67 mg/mL) was administered intraperitoneally as an analgesic (t = 0). The animal was positioned over a heating plate to maintain the animal body temperature at 35 °C. At t = 10–15 min, the animal was positioned prone into the MRI, above the focused ultrasound transducer with the hind legs immersed in a thermoregulated (35 °C) water bath with 0.1% (wt/vol) manganese. At t = 30 min, the deposition of energy by ultrasound was started.
MRI Thermometry During HIFU Sonication.
Online monitoring of temperature distribution was performed with MRI thermometry by using the proton resonance frequency technique (25). Dynamic MR temperature imaging was performed on a Philips Achieva 1.5 Tesla with a segmented Echo Planar Imaging (EPI) sequence (EPI factor = 5, echo time = 15 ms, repetition time = 34 ms, flip angle = 16°, field of view = 64 × 56 mm2, matrix = 64 × 64, 3 slices), resulting in continuous and volumetric acquisition of gradient echo images with a resolution of 1 × 1 × 2 mm3 every 1.6 seconds. A 23-mm surface receiver coil was positioned above the mouse hind leg to provide sufficient signal-to-noise ratio on MRI images. MRI temperature maps were calculated using the phase information in the gradient echo images and displayed online. Potential drift of the temperature (caused by drift in time of the phase of the MRI signal not related to temperature) was compensated by subtracting the average temperature in a reference region of interest (ROI) selected within the muscle outside the heated area. Short (≈10 s), low-power HIFU was performed to verify the position of the focal point. The maximal tissue temperature increase resulting from this test did not exceed 4 °C.
Heating Protocols.
HIFU heating experiments were performed with automatic feedback regulation of the tissue temperature at the HIFU focal point based on dynamic analysis of the MRI temperature images to adjust the output power of the HIFU system (18). Three heating conditions were investigated, all consisting of a ramp time of 1 min, followed by a plateau at 43 °C of either 2, 5, or 8 min. The required temperature increase was determined for each animal based on measurement of the rectal temperature by using an MRI-compatible thermometer (Luxtron). The electric power was registered for each protocol. The efficiency of the transducer in water was used to calculate acoustical power levels. The equivalent amount of energy then was applied to a separate group of animals at constant power (equal to the mean value found in the initial set of experiments with automatic feedback) in a pulsed manner (20% duty cycle at 1 Hz, increasing the duration by a factor of 5) to investigate the influence of the mechanical stress induced by HIFU on the resulting gene activation. These experiments also were performed under MR temperature imaging with identical imaging parameters but without automatic MRI-guided feedback controlling the HIFU power.
Bioluminescence Image Acquisition and Analysis.
Bioluminescence images of mice were acquired by using a NightOWL LB 981 system equipped with an NC 100 CCD camera (Berthold Technologies). Mice were injected intraperitoneally with d-luciferin (2.9 mg in 100 μL of sterile phosphate buffer; Promega). Two minutes later, mice were sedated with isoflurane (2% in air), and BLIs (2-min integration period, 2 × 2 binning) were taken 7 and 10 min after the luciferin injection in prone and supine positions, respectively. A low-light-emitting standard (Glowell; LUX Biotechnology) was placed next to the animal during each image acquisition to provide a constant reference for the resulting images. Grayscale body-surface reference images were collected for superposition of BLI images on anatomical maps. Pseudocolor luminescent images representing the spatial distribution of emitted photons were generated by using IDL programming language (ITT). BLI analysis was done semiautomatically by first placing a small ROI in the region corresponding with the heated region. Then, a region-growing algorithm was used to extend this ROI automatically to find the region that corresponded to 320 photons/s per mm2. This threshold was chosen to exclude signal intensity related to the background and basal activities, and was determined by analyzing the histogram of the number of pixels as function of the signal intensity for 10 heated animals. The mean light intensity then was evaluated within the resulting ROI.
Histology.
Mice were killed 24 h after heating by cranial dislocation. Muscle samples were obtained from both muscles (gluteal and rectus femoris) by dissection. The nonheated muscle samples served as negative control for muscle damage. Samples were fixed in 10% neutral-buffered formalin, followed by paraffin embedding and micrometer-thick sectioning (3 μm). Each paraffin block, containing the whole muscle sample, was totally sliced until exhausted. Then, H&E staining was performed for routine qualitative and quantitative examination of tissue morphology of the entire sample.
Histological examination was done by a confirmed pathologist for skeletal muscle in a blinded manner, with systematic attention to the following parameters: muscle fiber diameters (degree and distribution of atrophy), alterations in muscle fibers (centralization of subsarcolemmal nuclei, changes in contour), cell necrosis, inflammatory infiltrate, and size of damage, if any (measured with a microscopic lens).
Acknowledgments.
We thank Karine Nouette-Gaulain for animal anesthesia. This work is supported by the Ligue National Contre le Cancer, the Conseil Régional d'Aquitaine, InNaBioSante Foundation (project ULTRAFITT), MediTrans EC-FP6-project NMP4-CT-2006-026668, Diagnostic Molecular Imaging EC-FP6-project LSHB-CT-2005-512146, the Ministère de la Recherche, and Philips Medical Systems.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Vilaboa N, Voellmy R. Regulatable gene expression systems for gene therapy. Curr Gene Ther. 2006;6:421–438. doi: 10.2174/156652306777934829. [DOI] [PubMed] [Google Scholar]
- 2.Gorski K, Carneiro M, Schibler U. Tissue-specific in vitro transcription from the mouse albumin promoter. Cell. 1986;47:767–776. doi: 10.1016/0092-8674(86)90519-2. [DOI] [PubMed] [Google Scholar]
- 3.Melo LG, et al. Endothelium-targeted gene and cell-based therapies for cardiovascular disease. Arterioscler Thromb Vasc Biol. 2004;24:1761–1774. doi: 10.1161/01.ATV.0000142363.15113.88. [DOI] [PubMed] [Google Scholar]
- 4.Rendahl KG, et al. Regulation of gene expression in vivo following transduction by two separate rAAV vectors. Nat Biotechnol. 1998;16:757–761. doi: 10.1038/nbt0898-757. [DOI] [PubMed] [Google Scholar]
- 5.Wang Y, DeMayo FJ, Tsai SY, O'Malley BW. Ligand-inducible and liver-specific target gene expression in transgenic mice. Nat Biotechnol. 1997;15:239–243. doi: 10.1038/nbt0397-239. [DOI] [PubMed] [Google Scholar]
- 6.Hallahan DE, et al. Spatial and temporal control of gene therapy using ionizing radiation. Nat Med. 1995;1:786–791. doi: 10.1038/nm0895-786. [DOI] [PubMed] [Google Scholar]
- 7.Madio DP, et al. On the feasibility of MRI-guided focused ultrasound for local induction of gene expression. J Magn Reson Imaging. 1998;8:101–104. doi: 10.1002/jmri.1880080120. [DOI] [PubMed] [Google Scholar]
- 8.Vekris A, et al. Control of transgene expression using local hyperthermia in combination with a heat-sensitive promoter. J Gene Med. 2000;2:89–96. doi: 10.1002/(SICI)1521-2254(200003/04)2:2<89::AID-JGM90>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 9.Gerner EW, et al. Heat-inducible vectors for use in gene therapy. Int J Hyperthermia. 2000;16:171–181. doi: 10.1080/026567300285367. [DOI] [PubMed] [Google Scholar]
- 10.Huang Q, et al. Heat-induced gene expression as a novel targeted cancer gene therapy strategy. Cancer Res. 2000;60:3435–3439. [PubMed] [Google Scholar]
- 11.Borrelli MJ, Schoenherr DM, Wong A, Bernock LJ, Corry PM. Heat-activated transgene expression from adenovirus vectors infected into human prostate cancer cells. Cancer Res. 2001;61:1113–1121. [PubMed] [Google Scholar]
- 12.Smith RC, Machluf M, Bromley P, Atala A, Walsh K. Spatial and temporal control of transgene expression through ultrasound-mediated induction of the heat shock protein 70B promoter in vivo. Hum Gene Ther. 2002;13:697–706. doi: 10.1089/104303402317322267. [DOI] [PubMed] [Google Scholar]
- 13.Xu L, Zhao Y, Zhang Q, Li Y, Xu Y. Regulation of transgene expression in muscles by ultrasound-mediated hyperthermia. Gene Ther. 2004;11:894–900. doi: 10.1038/sj.gt.3302254. [DOI] [PubMed] [Google Scholar]
- 14.Silcox CE, et al. MRI-guided ultrasonic heating allows spatial control of exogenous luciferase in canine prostate. Ultrasound Med Biol. 2005;31:965–970. doi: 10.1016/j.ultrasmedbio.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 15.Plathow C, et al. Focal gene induction in the liver of rats by a heat-inducible promoter using focused ultrasound hyperthermia: Preliminary results. Invest Radiol. 2005;40:729–735. doi: 10.1097/01.rli.0000184763.62578.06. [DOI] [PubMed] [Google Scholar]
- 16.Guilhon E, et al. Spatial and temporal control of transgene expression in vivo using a heat-sensitive promoter and MRI-guided focused ultrasound. J Gene Med. 2003;5:333–342. doi: 10.1002/jgm.345. [DOI] [PubMed] [Google Scholar]
- 17.Liu Y, Kon T, Li C, Zhong P. High intensity focused ultrasound-induced gene activation in solid tumors. J Acoust Soc Am. 2006;120:492–501. doi: 10.1121/1.2205129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salomir R, Vimeux FC, de Zwart JA, Grenier N, Moonen CT. Hyperthermia by MR-guided focused ultrasound: accurate temperature control based on fast MRI and a physical model of local energy deposition and heat conduction. Magn Reson Med. 2000;43:342–347. doi: 10.1002/(sici)1522-2594(200003)43:3<342::aid-mrm4>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 19.Vimeux FC, et al. Real-time control of focused ultrasound heating based on rapid MR thermometry. Invest Radiol. 1999;34:190–193. doi: 10.1097/00004424-199903000-00006. [DOI] [PubMed] [Google Scholar]
- 20.Christians E, Campion E, Thompson EM, Renard JP. Expression of the HSP 70.1 gene, a landmark of early zygotic activity in the mouse embryo, is restricted to the first burst of transcription. Development. 1995;121:113–122. doi: 10.1242/dev.121.1.113. [DOI] [PubMed] [Google Scholar]
- 21.Patel B, et al. Hypoxia induces HSP 70 gene expression in human hepatoma (HEP G2) cells. Biochem Mol Biol Int. 1995;36:907–912. [PubMed] [Google Scholar]
- 22.Richard V, Kaeffer N, Thuillez C. Delayed protection of the ischemic heart–from pathophysiology to therapeutic applications. Fundam Clin Pharmacol. 1996;10:409–415. doi: 10.1111/j.1472-8206.1996.tb00595.x. [DOI] [PubMed] [Google Scholar]
- 23.Xu Q, Schett G, Li C, Hu Y, Wick G. Mechanical stress-induced heat shock protein 70 expression in vascular smooth muscle cells is regulated by Rac and Ras small G proteins but not mitogen-activated protein kinases. Circ Res. 2000;86:1122–1128. doi: 10.1161/01.res.86.11.1122. [DOI] [PubMed] [Google Scholar]
- 24.Vilaboa N, Fenna M, Munson J, Roberts SM, Voellmy R. Novel gene switches for targeted and timed expression of proteins of interest. Mol Ther. 2005;12:290–298. doi: 10.1016/j.ymthe.2005.03.029. [DOI] [PubMed] [Google Scholar]
- 25.Ishihara Y, et al. A precise and fast temperature mapping using water proton chemical shift. Magn Reson Med. 1995;34:814–823. doi: 10.1002/mrm.1910340606. [DOI] [PubMed] [Google Scholar]