Abstract
TRPA1 functions as an excitatory ionotropic receptor in sensory neurons. It was originally described as a noxious cold-activated channel, but its cold sensitivity has been disputed in later studies, and the contribution of TRPA1 to thermosensing is currently a matter of strong debate. Here, we provide several lines of evidence to establish that TRPA1 acts as a cold sensor in vitro and in vivo. First, we demonstrate that heterologously expressed TRPA1 is activated by cold in a Ca2+-independent and Ca2+ store-independent manner; temperature-dependent gating of TRPA1 is mechanistically analogous to that of other temperature-sensitive TRP channels, and it is preserved after treatment with the TRPA1 agonist mustard oil. Second, we identify and characterize a specific subset of cold-sensitive trigeminal ganglion neurons that is absent in TRPA1-deficient mice. Finally, cold plate and tail-flick experiments reveal TRPA1-dependent, cold-induced nociceptive behavior in mice. We conclude that TRPA1 acts as a major sensor for noxious cold.
Keywords: cold sensing, pain, sensory neurons, TRP channels
Sensing the environmental temperature is essential for animals to maintain thermal homeostasis and to avoid prolonged contact with harmfully hot or cold objects (1). Our understanding of the molecular basis of thermosensation has made great strides with the discovery that several members of the transient receptor potential (TRP) cation channel family exhibit highly temperature-sensitive gating and are expressed in cells of the sensory system (1). Mice lacking specific temperature-sensitive TRP channels illustrate how these channels serve as molecular thermometers in the peripheral sensory system (2). At least 3 heat-activated members of the TRPV subfamily (TRPV1, TRPV3, and TRPV4) are critically involved in sensing hot temperatures. TRPM8, a channel activated by cold temperatures and cooling compounds, such as menthol, plays a major role in cold sensing (1). Importantly, although TRPM8-deficient mice exhibit significant deficits in cold sensing in the temperature range between 28°C and 15°C, they retain a normal response to noxious cold temperatures, demonstrating the existence of TRPM8-independent mechanisms to detect noxious cold (3–5). TRPA1 has been put forward as a potential candidate to mediate detection of noxious cold, based on its expression in nociceptive neurons, and on the finding that heterologously expressed TRPA1 in CHO cells is activated by cold temperatures with a lower temperature threshold for activation than TRPM8 (6–8).
At this point, however, the role of TRPA1 in (noxious) cold sensing is highly controversial. First, there is no consensus as to whether TRPA1 is directly gated by cold temperatures. Two groups have reported that they failed to detect cold-induced activation of heterologously expressed TRPA1 (9, 10), and a third report suggested that cold-induced activation of TRPA1 in overexpression systems is an indirect effect, caused by cold-induced Ca2+ release from intracellular stores and subsequent Ca2+-dependent activation of the channel (11). Second, several studies have shown a lack of correlation between mustard oil (MO) responses and cold sensitivity in somatosensory neurons, which led to the conclusion that many TRPA1-expressing neurons are not cold-sensitive (9, 12, 13). Third, behavioral experiments with Trpa1−/− mice did not provide unequivocal evidence for an in vivo role in (noxious) cold sensing: whereas one study reported mild and sex-dependent alterations in the behavioral response to prolonged exposure to noxious cold in Trpa1−/− mice (14), a second study found no signs for altered cold sensitivity in these mice (13).
In the present study we have reevaluated the role of TRPA1 in cold sensation, and we provide several novel lines of evidence to demonstrate that TRPA1 acts a noxious cold sensor. First, we establish Ca2+-independent and Ca2+ store-independent activation of heterologously expressed TRPA1 by cold, and we show that the temperature-dependent gating of TRPA1 is mechanistically similar to that of other temperature-sensitive TRP channels. Second, we identify a subset of cold-sensitive trigeminal ganglion (TG) neurons that rely on TRPA1 for their cold responses. Finally, we provide behavioral evidence showing that TRPA1 is required for the normal nociceptive response to noxious cold.
Results and Discussion
Cold Activation of Heterologous TRPA1.
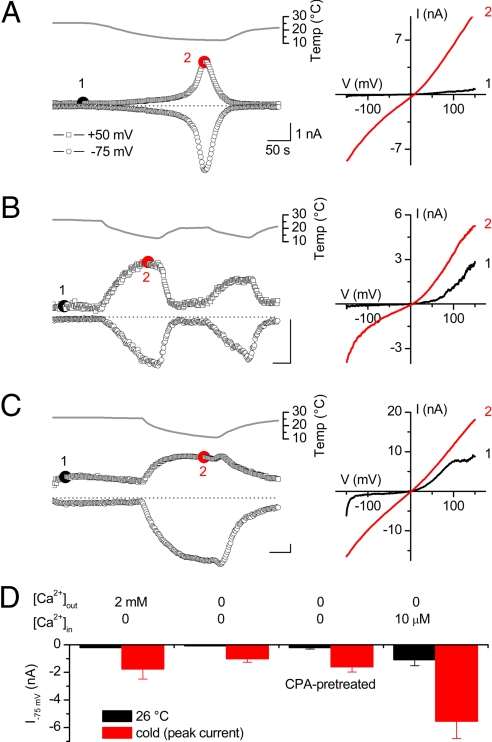
We investigated whether heterologously expressed TRPA1 is activated by cooling by using whole-cell patch clamp recordings on murine TRPA1-expressing CHO cells. At 26°C and in the presence of extracellular Ca2+ (2 mM), voltage ramps from −150 to +150 mV elicited sizable, strongly outwardly rectifying TRPA1 currents (Fig. 1A). Consistent with previous reports (6, 7), we found that cooling to 10°C resulted in a robust increase of both outward and inward currents (Fig. 1A). In contrast, the small background current in nontransfected CHO cells was linear, and its amplitude did not increase upon cooling (data not shown). The time course of the TRPA1 current during cooling in the presence of extracellular Ca2+ typically showed 3 phases: a phase of slow current activation, followed by a phase with more rapid activation and, finally, rapid current decay (Fig. 1A). In line with previous studies, we attribute the second phase of rapid current activation to Ca2+-dependent TRPA1 activation by Ca2+ ions entering through the channel pore, and the decay phase to Ca2+-induced channel desensitization (10, 15).
Fig. 1.
Ca2+-independent and Ca2+ store-independent activation of heterologously expressed TRPA1 by cold. (A) Time course of whole-cell TRPA1 currents at +50 and −75 mV during cooling in extracellular solution containing 2 mM Ca2+ (Left). Current–voltage relations were obtained at the indicated time points (Right). (B) Same as for A, but using Ca2+-free intracellular and extracellular solutions. (C) Same as for A, but now using a Ca2+-free extracellular solution and an intracellular solution containing 10 μM free Ca2+. (D) Average inward current amplitudes at −75 mV for the conditions shown in A, B, and C and for cells preincubated for 30 min with 10 μM CPA in Ca2+-free solution.
TRPA1 is directly activated by intracellular Ca2+ ions (11, 15), which has led to the hypothesis that cold-induced activation of TRPA1 represents Ca2+-induced channel activation secondary to cold-induced Ca2+ release from intracellular stores (11). To investigate this possibility, we first tested cold sensitivity of TRPA1 in the absence of Ca2+ by omitting extracellular Ca2+ and including 10 mM 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetate (BAPTA) into the pipette. After allowing BAPTA to diffuse into the cell for 180 s, yielding an intracellular BAPTA concentration of at least 8 mM, we could still measure robust cold activation of TRPA1 currents [Fig. 1B and supporting information (SI) Fig. S1]. Simultaneous monitoring of Fura-2 demonstrated that under this condition, cold did not evoke an increase in intracellular Ca2+ (Fig. S1). Note, however, that the second phase of rapid current activation and the subsequent current decay were no longer observed, in line with the notion that these 2 phases represent Ca2+-dependent processes (10, 15). Importantly, we also found that cooling-induced activation of TRPA1 was fully preserved in cells pretreated for 30 min in Ca2+-free medium supplemented with the SERCA pump inhibitor cyclopiazonic acid (CPA) to deplete intracellular Ca2+ stores before cooling (Fig. 1D). Taken together, these data demonstrate that cold activation of TRPA1 does not require Ca2+ release from intracellular Ca2+ stores.
Although these results show that Ca2+ is not required for cold activation of TRPA1, they do not exclude that Ca2+ and cold act via the same mechanism to activate TRPA1. If this is the case, activation of TRPA1 by high intracellular Ca2+ would mask subsequent activation by cold. To investigate this, we tested the cold sensitivity of TRPA1 in cells dialyzed with a pipette containing 10 μM free Ca2+. In line with previous reports, we measured strongly outwardly rectifying Ca2+-activated TRPA1 currents (11, 15), and subsequent cooling caused a further current increase, especially at negative potentials (Fig. 1C). These data indicate that elevated intracellular Ca2+ does not compromise subsequent cold activation of TRPA1. Moreover, the maximum amplitude of the cold-induced current was larger with high than with low [Ca2+]i (Fig. 1D), indicating that the effects of Ca2+ and cold on channel activity are at least partially additive.
Taken together, these data establish that cooling from 26°C to 10°C activates TRPA1 in a Ca2+-independent and Ca2+ store-independent manner, and that incoming Ca2+ can further potentiate channel activity. There are notable differences in the shape of the whole-cell TRPA1 current–voltage relations, ranging from strongly outwardly rectifying at 26°C to moderately outwardly rectifying at 16°C under Ca2+-free conditions, and virtually linear at 16°C with 10 μM intracellular Ca2+ (Fig. 1 A–C). We attribute these different degrees of rectification to shifts in the voltage dependence of channel activation induced by Ca2+ ions (11) and cold (see below).
Cooling-induced activation of TRPA1 was also consistently measured in cell-attached patch-clamp recordings, even when Ca2+ was omitted from the extracellular and pipette solutions (Fig. S2). Channel activity (quantified as NPopen) at 50 mV increased ≈10-fold upon cooling from 26°C to 16°C (10.3 ± 1.5-fold increase; n = 4). As expected for ion diffusion through a pore and consistent with a previous report (8), we observed a substantial decrease in the single-channel amplitude upon cooling. The single-channel conductance decreased from 91 ± 4 pS at 25°C to 40 ± 2 pS at 10°C, corresponding to a Q10 of 1.7.
Effect of Temperature on TRPA1 Gating.
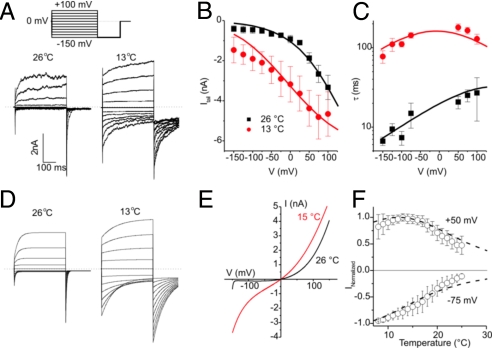
Thermal activation of certain TRP channels, including the cold-activated TRPM8 and the heat-activated TRPV1, TRPM4, and TRPM5, reflects a temperature-induced shift of their voltage-dependent activation curve, and the effects of temperature on channel gating can be approximated by a 2-state model (2, 16, 17). Because TRPA1 also exhibits voltage-dependent activation (11, 18, 19), we analyzed whether cold activation of TRPA1 responds to the same general mechanism and whether the 2-state model can be used to describe cold activation of TRPA1. We determined the voltage dependence as well as the kinetics of channel activation and deactivation at different temperatures by measuring whole-cell currents during a voltage step protocol consisting of 400-ms voltage steps to test potentials ranging from −150 mV to +100 mV, followed by an invariant step to −150 mV. These experiments were performed in Ca2+-free conditions to exclude the influence of Ca2+ on the voltage dependence of TRPA1 (11).
TRPA1 currents in response to the voltage step protocol applied at 26°C and 13°C are shown in Fig. 2A. From these data we measured the peak inward tail current at −150 mV (Fig. 2B), which revealed that the voltage dependence of channel activation is shifted toward more negative voltages upon cooling, similar to what has been shown previously for TRPM8. In addition, we determined the relaxation time constants at different voltages by fitting a monoexponential function to the current traces (Fig. 2C). Similarly to the behavior of TRPM8, cooling caused a drastic slowing of current relaxation, especially for the closing transition at negative voltages (Fig. 2C). Next, we performed a global fit of the 2-state model to the time constants and tail current amplitudes at different voltages and temperatures, taking into account the effect of temperature on the single-channel conductance. This analysis resulted in values for the changes in enthalpy and entropy associated with channel opening and closing (Fig. S2). A gating charge (z) of 0.375 e0 was obtained by fitting Boltzmann equations to the plots of tail current amplitudes as a function of test voltage, and was assumed to be constant over the investigated temperature range. Using these parameters, we simulated the kinetics of TRPA1 channel activation and inactivation during voltage steps at different temperatures (Fig. 2D), current–voltage (I–V) curves as obtained during voltage ramps (Fig. 2E), and steady-state currents at different voltages as a function of temperature (Fig. 2F), and found a good agreement with the corresponding experimental data.
Fig. 2.
Effects of cooling on the voltage-dependent gating and kinetics of TRPA1. (A) Whole-cell currents in Ca2+-free intracellular and extracellular solutions in response to the indicated voltage step protocol applied at 26°C and 13°C. (B) Average peak inward tail currents at −150 mV (n = 8) at 26°C and 13°C. (C) Average time constants obtained from monoexponential fits to the time course of current relaxation at different voltages and temperatures. Solid lines in B and C represent a global fit of the 2-state model to the experimental data. (D) Model predictions of TRPA1 currents at 26°C and 13°C in response to the voltage step protocol in A. (E) Model predictions of TRPA1 currents during 400-ms voltage ramps, such as those used in Fig. 1. (F) Average TRPA1 currents at different temperatures and at −75 and +50 mV, normalized to the maximal current in the tested temperature range. Dotted lines represent the corresponding model prediction.
In essence, activation of TRPA1 is associated with a decrease in entropy and enthalpy, similar to what has been determined for TRPM8 (16) (Fig. S3). Consequently, the rate of activation of these cold-sensitive channels is much less temperature-sensitive than the rate of channel deactivation, leading to an increase in open probability upon cooling. A 2-state model is obviously a simplification of the complex gating behavior of TRPA1 and, for instance, does not account for the effects of intracellular Ca2+ or other ligands. However, we have previously shown that the use of more complex models to describe the temperature sensitivity of TRP channels (20) yields very similar values for the changes in enthalpy and entropy during channel gating (21).
We conclude that the effects of temperature on TRPA1 gating are mechanistically similar to what has been described for other temperature-sensitive TRP channels.
Combined Effects of Cold and MO on TRPA1.
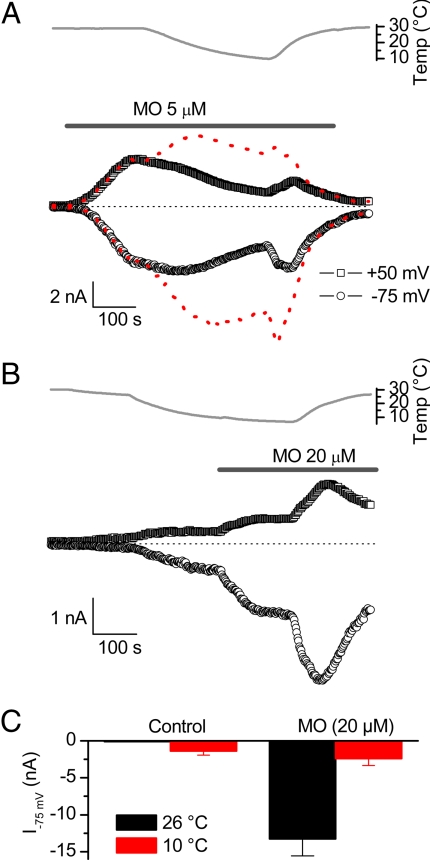
Agonists such as MO, acrolein, and cinnamaldehyde activate TRPA1 via covalent modification of cysteine and/or lysine residues in the cytosolic part of the channel (18, 22). Given that this activation mechanism is fundamentally distinct from cold-induced activation, we investigated the combined effects of cold and MO on TRPA1. Consistent with a previous study (9), we found that cooling reduced the amplitude of TRPA1 currents preactivated by MO (Fig. 3A). However, this does not necessarily imply that the effect of cold on TRPA1 gating is absent in the presence of MO. Indeed, cooling results in a substantial decrease in the single-channel conductance of TRPA1, which may explain the observed decrease in whole-cell currents. To quantify this, we divided each individual data point of the inward and outward whole-cell currents in Fig. 3A by the single-channel amplitude at the corresponding temperature, yielding the time course of NPopen (Fig. 3A, red dashed line). Interestingly, this analysis revealed that even after stimulation with MO, cooling increases the open probability of TRPA1.
Fig. 3.
Combined effects of cold and MO on TRPA1 currents. (A) Time course of inward and outward TRPA1 currents during application of MO. Cooling leads to a decrease in the MO-activated current. The red dotted line represents the same data points normalized to the single-channel current amplitude at the corresponding temperature, which yields a direct measure of NPopen. Note the increase in NPopen upon cooling. (B) Time course of inward and outward TRPA1 currents cooling and subsequent stimulation with MO. (C) Average inward TRPA1 currents evoked by MO and/or cooling.
Next, we examined the MO sensitivity of TRPA1 preactivated by cold. As shown in Fig. 3B, MO caused only a modest increase of inward and outward TRPA1 currents when applied at 10°C. The amplitude of the MO-induced current at 10°C was ≈10-fold lower than when MO was applied at room temperature (Fig. 3C). This difference cannot be explained solely by the effect of cooling on the single-channel amplitude of TRPA1, indicating that cooling interferes with the process of MO-induced TRPA1 activation. Moreover, we found that rewarming of the solution in the continued presence of MO caused a drastic increase in the amplitude of inward and outward currents (Fig. 3B). One possible explanation could be that the covalent binding of MO to the reactive cysteines on TRPA1, which occurs rapidly at room temperature, is strongly attenuated at 10°C.
From these data we conclude that stimulation of TRPA1 with MO does not abolish the effects of cold on channel gating. However, cooling reduces the amplitude of MO-induced TRPA1 currents, both by lowering the single-channel current amplitude and by inhibiting the process of MO-induced channel activation. Comparison of TRPA1 current amplitudes upon stimulation with either cold or MO also reveals that MO is a much stronger stimulus than cold: maximal MO-induced inward TRPA1 currents at −75 mV were 10-fold larger than TRPA1 currents activated by a 10°C cold stimulus (Fig. 3C). The relatively small amplitude together with the substantial effect of cooling on the TRPA1 single-channel conductance may explain why cold-activated TRPA1 currents have escaped detection in certain expression systems and experimental conditions (9, 10). Moreover, the substantial difference in potency has to be taken into account when comparing cold- and MO-induced responses in TRPA1-expressing sensory neurons.
TRPA1-Mediated Cold Responses in TG Neurons.
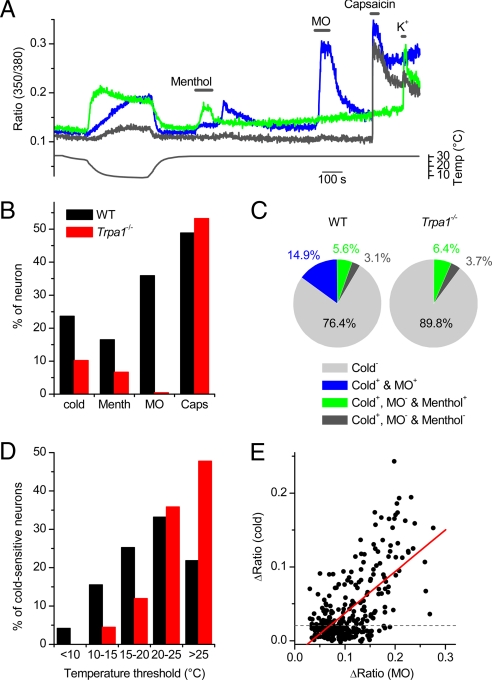
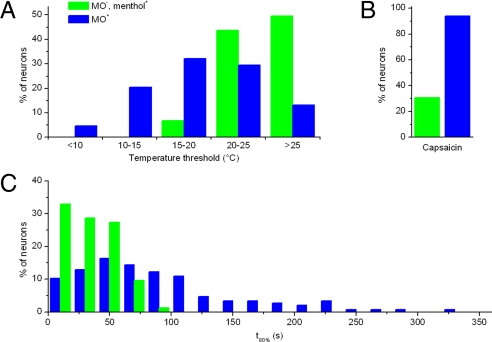
To investigate the contribution of TRPA1 to the cold sensitivity of sensory neurons, we performed intracellular Ca2+ recordings on TG neurons from 29 WT and 19 Trpa1−/− mice, aged between 8 and 12 weeks. Some representative examples of [Ca2+]i traces from cold-sensitive WT neurons are shown in Fig. 4A. TG neurons were also tested for their sensitivity to menthol, MO, and capsaicin, knowing that menthol ((-)-menthol; 100 μM) activates both TRPM8-expressing and TRPA1-expressing neurons (19), whereas responses to MO (100 μM) are almost exclusively limited to TRPA1-expressing neurons (13, 19), and capsaicin activates exclusively TRPV1-expressing neurons (23, 24). In line with previous studies (13, 19), we observed a ≈60% reduction in the fraction of menthol-sensitive cells and a ≈99% reduction in the fraction of MO-sensitive cells in preparations from Trpa1−/− mice, whereas the responsiveness to capsaicin remained unaltered (Fig. 4B). Notably, we found a significant reduction of the total fraction of cold-sensitive neurons from 23.6% (211 of 894) in WT mice to 10.2% (49 of 481 mice; P < 0.001) in Trpa1−/− mice (Fig. 4B).
Fig. 4.
TRPA1-dependent cold responses in TG neurons. (A) Ratiometric measurement of changes in intracellular Ca2+ in response to cold, menthol (100 μM), MO (100 μM), or capsaicin (1 μM), illustrating the 3 types of cold-sensitive TG neurons. (B) Comparison of the percentage of cells responding to cold and chemical stimuli in preparations from WT and Trpa1−/− mice. (C) Pie charts showing the percentage of cold-insensitive and the 3 types of cold-sensitive neurons in TG from WT and Trpa1−/− mice. (D) Histogram comparing temperature thresholds in cold-sensitive TG from WT and Trpa1−/− mice. The temperature threshold was defined as the temperature at which the ratio was increased by 10% of the maximal cold-induced increase. (E) Correlation of the amplitude of the MO and cold responses in MO-sensitive neurons from WT mice (n = 177). The solid line represents a linear fit to the data.
Cold-sensitive neurons from WT mice could be categorized into 3 groups: (i) MO-sensitive neurons, implying expression of TRPA1 (TRPA1+); (ii) MO-insensitive and menthol-sensitive neurons (TRPM8+/TRPA1−); and (iii) MO- and menthol-insensitive neurons (TRPM8−/TRPA1−) (Fig. 4C). Note that many neurons in the TRPA1+ group also respond to menthol, which we interpret as activation of TRPA1 by menthol (19, 25). In preparations from Trpa1−/− mice, the fractions of cold-sensitive neurons that could be categorized as TRPM8+/TRPA1− or TRPM8−/TRPA1− were not significantly altered (Fig. 4C), suggesting that the reduced number of cold-sensitive neurons in Trpa1−/− mice is due to the selective elimination of a population of TRPA1+ cold-sensitive neurons. The distribution of temperature thresholds in cold-sensitive TG neurons from Trpa1−/− mice was significantly shifted toward higher temperatures compared with WT (P < 0.001; Fig. 4D), indicating the elimination of cold-sensitive neurons with a threshold temperature lower than 20°C.
Our analysis revealed that a substantial fraction of MO-sensitive (TRPA1+) TG neurons were insensitive to a cold stimulus (Fig. 4B), in line with findings from previous studies (9, 12, 13). One possible explanation for this apparent inconsistency lies in the strong difference in the potency of MO and cold to activate TRPA1 (Fig. 3C): In cells expressing a relatively low number of TRPA1 channels, a weaker stimulus, such as cold or menthol, may not be sufficient to evoke a measurable Ca2+ response, in contrast to a stronger stimulus, such as MO. Indeed, when we plotted the amplitude of the cold response in MO-positive cells in function of the amplitude MO response (ΔRatioMO), we found a clear positive correlation (r = 0.641, n = 295; P < 0.0001; Fig. 4E). When we analyzed only those cells that belonged to the upper 20% of MO responses (ΔRatioMO > 0.15), we found that more than 90% (53 of 59) showed a significant cold response. In contrast, a cold response was found in only 37% (22 of 59) of the cells that belonged to the lower 20% of MO responses (ΔRatioMO < 0.06). Thus, the odds of detecting cold responses in TRPA1-expressing neurons increase with the level of functional TRPA1 expression. Note that TRPA1 expression is low at birth and increases strongly during the first postnatal weeks (26), which may explain why TRPA1-mediated cold responses have escaped detection in studies that used TG or DRG neurons isolated from neonatal or young animals (9, 13). In line with this notion, we found that only 4.3% of TG neurons prepared from young WT mice (first postnatal week) could be classified as TRPA1+ cold-sensitive (Fig. S4), compared with 14.9% in preparations from adult WT mice (Fig. 4C).
Comparison of TRPA1+ and TRPM8+ Cold-Sensitive TG Neurons.
A further comparison of cold-sensitive TG neurons from WT mice revealed several distinctive properties of cells expressing TRPM8+ (MO-insensitive, menthol-sensitive) and TRPA1+ (MO-sensitive) (Fig. 5).
Fig. 5.
Comparison of TRPM8- and TRPA1-dependent, cold-sensitive neurons. (A) Histogram comparing the temperature threshold in MO-sensitive TG neurons (n = 88) and MO-insensitive, menthol-sensitive TG neurons (n = 65). (B) Histogram obtained from the same cells as for A, comparing the time needed to reach 80% of the maximal cold response (t80%). (C) Comparison of the capsaicin sensitivity between MO-insensitive, menthol-sensitive (n = 52), and MO-sensitive (n = 133) cold-sensitive neurons.
First, TRPA1+ TG neurons were characterized by a significantly lower (colder) temperature threshold (18.9 ± 0.4°C; n = 152) than TRPM8+ neurons (25.0 ± 0.3°C; n = 61; P < 10−5; Fig. 5A). The lower temperature threshold of TRPA1+ neurons also underlies the altered distribution of temperature thresholds in TG neurons from Trpa1−/− mice (Fig. 4D).
Second, the time course of the cold response in TRPA1+ neurons was clearly slower than in TRPM8+ neurons. Analysis of the time needed to reach 80% of the maximal response (t80%; Fig. 5B) revealed a swift response to a cold stimulus in all TRPM8+ TG neurons (t80% = 33 ± 2 s; n = 73), whereas the rate of Ca2+ increase in TRPA1+ neurons was much more variable and significantly slower (t80% = 84 ± 6 s; n = 146; P < 10−5). The slow time course of the cold-induced Ca2+ signal in TRPA1+ neurons may also help to explain why previous studies using cold stimuli of short duration (<60 s) failed to detect consistent cold responses in MO-positive somatosensory neurons (9, 13, 27).
Third, TRPA1+ and TRPM8+ cold-sensitive neurons differed in their sensitivity to capsaicin, which is generally used as a marker of nociceptor neurons (Fig. 5C). Only approximately one third of TRPM8+ neurons were sensitive to capsaicin, in line with recent GFP labeling studies (28, 29). In contrast, ≈94% (125 of 133) of the TRPA1+ cold-sensitive neurons responded to capsaicin. Concomitantly, 36.6% (164 of 448) of all capsaicin-responsive neurons from WT mice showed sensitivity to cold, compared with only 9.8% (38 of 386) of capsaicin-sensitive neurons from Trpa1−/− mice.
Finally, TRPA1+ and TRPM8+ cold-sensitive neurons exhibited opposite sensitivity to the antimycotic drug clotrimazole (CLT). We reported recently that CLT is a potent inhibitor of TRPM8-mediated menthol responses, whereas it has an agonistic effect on TRPA1 (25). In line with this, we found that cold responses in TRPM8+ TG neurons were inhibited by 10 μM CLT (85 ± 3% inhibition of the cold response; n = 5; Fig. S5), whereas cold responses in TRPA1+ neurons were potentiated by CLT (to 145 ± 25% of the cold response; n = 7; Fig. S5).
Taken together, these data demonstrate that TRPA1 and TRPM8 act as primary cold sensors in 2 distinct subsets of TG neurons. When compared to TRPM8+ neurons, TRPA1+ cold-sensitive TG neurons exhibit a lower temperature threshold, a slower cold response, a more general capsaicin sensitivity, and an inverse response to CLT. In line with previous studies (13, 30, 31), we also found evidence for a subset of cold-sensitive TG neurons that do not rely on TRPM8 and TRPA1 for their cold response. The molecular mechanisms underlying the cold sensitivity of these neurons are currently unknown, but they may involve other thermosensitive ion channels, such as TREK-1 (32), the epithelial sodium channel ENaC (33), background potassium channels (34, 35), and the voltage-gated Na+ channel NaV1.8 (36, 37).
Cold-Induced Nocifencive Behavior in Trpa1−/− Mice.
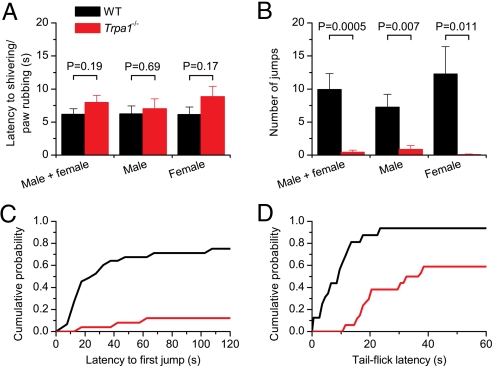
The results from the Ca2+ imaging experiments on TG neurons indicate that Trpa1−/− mice have significantly fewer cold-sensitive, capsaicin-positive nociceptors. To investigate whether this leads to altered cold-induced nociception in vivo, we compared WT and Trpa1−/− mice in 2 behavioral assays. In a first assay, WT and Trpa1−/− mice were placed on a metal cold plate set at a temperature of 0°C, and their behavior was observed. During prolonged exposure to noxious cold stimuli, we could distinguish at least 2 distinct phases in the behavioral response: an acute cold response corresponding with shivering and rubbing together of the paws, followed by brisk lifting of the hind paws and other cold avoidance behaviors (38). Given that a previous report (14) showed significant sex-related differences in cold sensitivity, male and female mice were analyzed separately. In line with a previous study (13), we did not observe a significant difference between genotypes or sex in the latency to the first cold-related response (e.g., rubbing of the forepaws or shivering) (Fig. 6A). However, when left on the cold plate for a 2-min period, we found that Trpa1−/− mice showed significantly less nocifensive behavior than the WT mice, consistent with findings in a previous report (14). Importantly, we observed a striking difference in the type of pain-related behavior between genotypes. Most WT mice (10 of 13 males and 9 of 12 females) started jumping, suggesting that the cold plate induces significant pain (Fig. 6 B and C, and Movie S1). In contrast, only 3 of 25 Trpa1−/− mice (1 female and 2 males) jumped at least once, and for both sexes the total number of jumps was significantly lower in knockout mice of both sexes (Fig. 6B and Movie S1). Moreover, the latency to the first jump was significantly longer in the Trpa1−/− mice (Fig. 6C). These data confirm that Trpa1−/− mice are still able to sense cold, but also indicate that the behavioral response to noxious cold is significantly reduced in the absence of TRPA1. Note that we never observed such cold-induced jumping behavior in WT or Trpa1−/− mice when they were placed on a cold plate set at 10°C (0 of 12 mice tested for each genotype). Moreover, in line with previous work (14), we did not observe differences between WT and Trpa1−/− mice in their nociceptive behavior when placed on a hot plate at 55°C (data not shown), confirming that Trpa1−/− mice still feel pain.
Fig. 6.
Altered cold-induced nociceptive behavior in Trpa1−/− mice. (A) Latency to the first behavior reaction to cold upon placement on a cold plate at 0°C in mice of different sex and genotype. The numbers of mice tested on the cold plate were as follows: WT male (n = 13), WT female (n = 12), Trpa1−/− male (n = 15), and Trpa1−/− female (n = 13). (B) Average number of jumps during a 2-min period on a cold plate at 0°C. No jumps were observed when the plate was set to 30°C. (C) Cumulative probability plot showing the latency to the first jump off the cold plate in WT and Trpa1−/− mice. (D) Tail-flick latency upon tail immersion in a water–methanol mixture at −10°C in WT (n = 32) and Trpa1−/− (n = 34) male mice.
To exclude the possibility that the deficit in cold-induced jumping behavior in Trpa1−/− mice is caused by an effect of TRPA1 gene targeting or unidentified environmental factors on higher neural structures, we used the cold tail-flick test, whereby the latency to tail withdrawal was measured upon immersion of the distal part of the tail in a solution at −10°C. This procedure is known to induce acute pain (39), and tail-flicking in such a condition is considered a spinal reflex (38). For WT mice, we obtained a tail-flick latency of 11.8 ± 2.5 s (n = 32), with 2 of 32 animals (6%) that did not respond before the cutoff time (60 s; Fig. 6D). Trpa1−/− mice exhibited significantly longer tail-flick latencies (P < 0.0001), with a mean latency of 38.1 ± 3.4 s (n = 34), and 14 of 34 animals (41%) did not respond before the cutoff time. The difference between genotypes was again sex-independent (data not shown). Taken together, these behavioral data demonstrate that TRPA1 plays an important role in noxious cold sensing in vivo.
It should be noted that the difference in nociceptive behavior between WT and Trpa1−/− mice was only observed at temperatures that are significantly lower than what is needed to see TRPA1 current activation in CHO cells or trigeminal neurons. An explanation for this lies probably in the fact that the temperature at the cold-sensitive nerve ends is not equal to the temperature of the cold plate or cold solution because of the isolating effect of the skin and the constant circulation of blood at 37°C throughout the body. Similar temperature differences between in vitro TRP channel activation and in vivo TRP channel-dependent nociceptive effects have also been reported for TRPV1- and TRPV3-deficient mice (23, 40).
A recent study reported that mice in which all NaV1.8-expressing sensory neurons are eliminated by diphtheria toxin A (DTA mice) show strong resistance to noxious cold, as assayed using a cold plate set at 0°C (36), similar to what we observed in the Trpa1−/− mice. TRPM8 expression and the TRPM8-mediated behavioral response to acetone cooling are not affected in these DTA mice (36). Importantly, the DTA mice exhibit a strongly reduced expression of TRPA1 in DRG neurons and lack TRPA1-mediated nociceptive responses to formalin (36). Based on our present results, the loss of a noxious cold response in the DTA mice can be fully attributed to the loss of TRPA1-expressing sensory neurons. Thus, noxious cold sensing in vivo requires somatosensory neurons that express both NaV1.8 and TRPA1.
Final Conclusions.
Our data establish the function of TRPA1 as a cold sensor in vitro and in vivo. In addition, we provided data and analyses that may explain why several previous studies could not detect a significant role for TRPA1 as a cold sensor in heterologous expression systems, sensory neurons, or awake-behaving animals. TRPA1 represents a promising target for the prevention or treatment of cold-induced pain.
Materials and Methods
Cells and Animals.
We used a tetracycline-regulated system for inducible expression of TRPA1 in CHO cells, as described previously (6). Naïve CHO cells were used as controls. TG neurons from adult (postnatal weeks 8–12) or newborn (postnatal week 1) mice were cultured as described previously (19). Trpa1−/− mice (14) were backcrossed 7 times in the C57BL/6J background, resulting in mice that exhibited 99.2% heterozygosity to the C57BL/6J strain, and WT C57BL/6J mice were used as controls. Because we did not use littermates in our study, we cannot fully exclude that subtle environmental effects (e.g., whether the animals were born to a WT or Trpa1−/− mother) may have influenced the outcome of some of our experiments. However, given that WT and Trpa1−/− mice exhibited undistinguishable cellular and behavioral responses to various TRPA1-independent sensory stimuli (e.g., heat), we consider it highly unlikely that factors other than the lack of TRPA1 would underlie the observed phenotypic differences. All animal experiments were carried out in accordance with the European Union Community Council guidelines and were approved by the local ethics committee.
Cellular Recordings.
Ionic currents were recorded in the whole-cell and the cell-attached configurations of the patch-clamp technique. Fura-2-based intracellular Ca2+ measurements were performed as described previously (25). See SI Methods for details of the recording parameters and solutions.
Behavioral Tests.
Cold-induced nocifensive behavior was tested by placing the mice on a metal cold plate or by submerging the distal half of the tail in a cold water–methanol mixture. See SI Methods for apparatus and data acquisition details.
Data Analysis.
Data analysis, model simulations, and data display were performed by using Origin 7.0 (OriginLab Corporation) or Igor Pro 4.0 (Wavemetrics). Group data are expressed as mean ± SEM from n independent experiments. Significance between groups was tested by using the unpaired or paired Student's t tests, the χ2 test, or the Kolmogorov–Smirnov, as appropriate.
Supplementary Material
Acknowledgments.
We thank Dr. Nils Damann and the members of our laboratories for helpful discussions, and J. Prenen and M. Benoit for technical assistance. The TRPA1-expressing CHO cell line was kindly provided by A. Patapoutian (Scripps Research Institute, La Jolla, CA). W.E. is a doctoral fellow of the Research Foundation–Flanders. This work was supported by grants from Interuniversity Attraction Poles Program–Belgian State–Belgian Science Policy (P6/28), by Research Foundation–Flanders Grants G.0172.03 and G.0565.07, by Research Council of the Katholieke Universiteit Leuven Grant GOA 2004/07, and by the Flemish government (Excellentiefinanciering, EF/95/010).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0808487106/DCSupplemental.
References
- 1.Caterina MJ. Transient receptor potential ion channels as participants in thermosensation and thermoregulation. Am J Physiol Regul Integr Comp Physiol. 2007;292:R64–R76. doi: 10.1152/ajpregu.00446.2006. [DOI] [PubMed] [Google Scholar]
- 2.Talavera K, Nilius B, Voets T. Neuronal TRP channels: Thermometers, pathfinders and life-savers. Trends Neurosci. 2008;31:287–295. doi: 10.1016/j.tins.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 3.Dhaka A, et al. TRPM8 is required for cold sensation in mice. Neuron. 2007;54:371–378. doi: 10.1016/j.neuron.2007.02.024. [DOI] [PubMed] [Google Scholar]
- 4.Colburn RW, et al. Attenuated cold sensitivity in TRPM8 null mice. Neuron. 2007;54:379–386. doi: 10.1016/j.neuron.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 5.Bautista DM, et al. The menthol receptor TRPM8 is the principal detector of environmental cold. Nature. 2007;448:204–208. doi: 10.1038/nature05910. [DOI] [PubMed] [Google Scholar]
- 6.Story GM, et al. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell. 2003;112:819–829. doi: 10.1016/s0092-8674(03)00158-2. [DOI] [PubMed] [Google Scholar]
- 7.Bandell M, et al. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron. 2004;41:849–857. doi: 10.1016/s0896-6273(04)00150-3. [DOI] [PubMed] [Google Scholar]
- 8.Sawada Y, et al. Cold sensitivity of recombinant TRPA1 channels. Brain Res. 2007;1160:39–46. doi: 10.1016/j.brainres.2007.05.047. [DOI] [PubMed] [Google Scholar]
- 9.Jordt SE, et al. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature. 2004;427:260–265. doi: 10.1038/nature02282. [DOI] [PubMed] [Google Scholar]
- 10.Nagata K, Duggan A, Kumar G, Garcia-Anoveros J. Nociceptor and hair cell transducer properties of TRPA1, a channel for pain and hearing. J Neurosci. 2005;25:4052–4061. doi: 10.1523/JNEUROSCI.0013-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zurborg S, et al. Direct activation of the ion channel TRPA1 by Ca2+ Nat Neurosci. 2007;10:277–279. doi: 10.1038/nn1843. [DOI] [PubMed] [Google Scholar]
- 12.Babes A, Zorzon D, Reid G. Two populations of cold-sensitive neurons in rat dorsal root ganglia and their modulation by nerve growth factor. Eur J Neurosci. 2004;20:2276–2282. doi: 10.1111/j.1460-9568.2004.03695.x. [DOI] [PubMed] [Google Scholar]
- 13.Bautista DM, et al. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell. 2006;124:1269–1282. doi: 10.1016/j.cell.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 14.Kwan KY, et al. TRPA1 contributes to cold, mechanical, and chemical nociception but is not essential for hair-cell transduction. Neuron. 2006;50:277–289. doi: 10.1016/j.neuron.2006.03.042. [DOI] [PubMed] [Google Scholar]
- 15.Doerner JF, Gisselmann G, Hatt H, Wetzel CH. Transient receptor potential channel A1 is directly gated by calcium ions. J Biol Chem. 2007;282:13180–13189. doi: 10.1074/jbc.M607849200. [DOI] [PubMed] [Google Scholar]
- 16.Voets T, et al. The principle of temperature-dependent gating in cold- and heat-sensitive TRP channels. Nature. 2004;430:748–754. doi: 10.1038/nature02732. [DOI] [PubMed] [Google Scholar]
- 17.Talavera K, et al. Heat activation of TRPM5 underlies thermal sensitivity of sweet taste. Nature. 2005;438:1022–1025. doi: 10.1038/nature04248. [DOI] [PubMed] [Google Scholar]
- 18.Macpherson LJ, et al. Noxious compounds activate TRPA1 ion channels through covalent modification of cysteines. Nature. 2007;445:541–545. doi: 10.1038/nature05544. [DOI] [PubMed] [Google Scholar]
- 19.Karashima Y, et al. Bimodal action of menthol on the transient receptor potential channel TRPA1. J Neurosci. 2007;27:9874–9884. doi: 10.1523/JNEUROSCI.2221-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brauchi S, Orio P, Latorre R. Clues to understanding cold sensation: Ghermodynamics and electrophysiological analysis of the cold receptor TRPM8. Proc Natl Acad Sci USA. 2004;101:15494–15499. doi: 10.1073/pnas.0406773101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Voets T, et al. TRPM8 voltage sensor mutants reveal a mechanism for integrating thermal and chemical stimuli. Nat Chem Biol. 2007;3:174–182. doi: 10.1038/nchembio862. [DOI] [PubMed] [Google Scholar]
- 22.Hinman A, Chuang HH, Bautista DM, Julius D. TRP channel activation by reversible covalent modification. Proc Natl Acad Sci USA. 2006;103:19564–19568. doi: 10.1073/pnas.0609598103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Caterina MJ, et al. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- 24.Davis JB, et al. Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature. 2000;405:183–187. doi: 10.1038/35012076. [DOI] [PubMed] [Google Scholar]
- 25.Meseguer V, et al. Transient receptor potential channels in sensory neurons are targets of the antimycotic agent clotrimazole. J Neurosci. 2008;28:576–586. doi: 10.1523/JNEUROSCI.4772-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hjerling-Leffler J, Alqatari M, Ernfors P, Koltzenburg M. Emergence of functional sensory subtypes as defined by transient receptor potential channel expression. J Neurosci. 2007;27:2435–2443. doi: 10.1523/JNEUROSCI.5614-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fajardo O, Meseguer V, Belmonte C, Viana F. TRPA1 channels mediate cold temperature sensing in mammalian vagal sensory neurons: pharmacological and genetic evidence. J Neurosci. 2008;28:7863–7875. doi: 10.1523/JNEUROSCI.1696-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takashima Y, et al. Diversity in the neural circuitry of cold sensing revealed by genetic axonal labeling of transient receptor potential melastatin 8 neurons. J Neurosci. 2007;27:14147–14157. doi: 10.1523/JNEUROSCI.4578-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dhaka A, Earley TJ, Watson J, Patapoutian A. Visualizing cold spots: TRPM8-expressing sensory neurons and their projections. J Neurosci. 2008;28:566–575. doi: 10.1523/JNEUROSCI.3976-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Babes A, Zorzon D, Reid G. A novel type of cold-sensitive neuron in rat dorsal root ganglia with rapid adaptation to cooling stimuli. Eur J Neurosci. 2006;24:691–698. doi: 10.1111/j.1460-9568.2006.04941.x. [DOI] [PubMed] [Google Scholar]
- 31.Munns C, AlQatari M, Koltzenburg M. Many cold sensitive peripheral neurons of the mouse do not express TRPM8 or TRPA1. Cell Calcium. 2007;41:331–342. doi: 10.1016/j.ceca.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 32.Maingret F, et al. TREK-1 is a heat-activated background K(+) channel. EMBO J. 2000;19:2483–2491. doi: 10.1093/emboj/19.11.2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Askwith CC, Benson CJ, Welsh MJ, Snyder PM. DEG/ENaC ion channels involved in sensory transduction are modulated by cold temperature. Proc Natl Acad Sci USA. 2001;98:6459–6463. doi: 10.1073/pnas.111155398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reid G, Flonta M. Cold transduction by inhibition of a background potassium conductance in rat primary sensory neurones. Neurosci Lett. 2001;297:171–174. doi: 10.1016/s0304-3940(00)01694-3. [DOI] [PubMed] [Google Scholar]
- 35.Viana F, de la Pena E, Belmonte C. Specificity of cold thermotransduction is determined by differential ionic channel expression. Nat Neurosci. 2002;5:254–260. doi: 10.1038/nn809. [DOI] [PubMed] [Google Scholar]
- 36.Abrahamsen B, et al. The cell and molecular basis of mechanical, cold, and inflammatory pain. Science. 2008;321:702–705. doi: 10.1126/science.1156916. [DOI] [PubMed] [Google Scholar]
- 37.Zimmermann K, et al. Sensory neuron sodium channel Nav1.8 is essential for pain at low temperatures. Nature. 2007;447:855–858. doi: 10.1038/nature05880. [DOI] [PubMed] [Google Scholar]
- 38.Le Bars D, Gozariu M, Cadden SW. Animal models of nociception. Pharmacol Rev. 2001;53:597–652. [PubMed] [Google Scholar]
- 39.Wang JJ, Ho ST, Hu OY, Chu KM. An innovative cold tail-flick test: The cold ethanol tail-flick test. Anesth Analg. 1995;80:102–107. doi: 10.1097/00000539-199501000-00018. [DOI] [PubMed] [Google Scholar]
- 40.Moqrich A, et al. Impaired thermosensation in mice lacking TRPV3, a heat and camphor sensor in the skin. Science. 2005;307:1468–1472. doi: 10.1126/science.1108609. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.