Abstract
Animal studies suggest that diets low in calories and rich in unsaturated fatty acids (UFA) are beneficial for cognitive function in age. Here, we tested in a prospective interventional design whether the same effects can be induced in humans. Fifty healthy, normal- to overweight elderly subjects (29 females, mean age 60.5 years, mean body mass index 28 kg/m2) were stratified into 3 groups: (i) caloric restriction (30% reduction), (ii) relative increased intake of UFAs (20% increase, unchanged total fat), and (iii) control. Before and after 3 months of intervention, memory performance was assessed under standardized conditions. We found a significant increase in verbal memory scores after caloric restriction (mean increase 20%; P < 0.001), which was correlated with decreases in fasting plasma levels of insulin and high sensitive C-reactive protein, most pronounced in subjects with best adherence to the diet (all r values < −0.8; all P values <0.05). Levels of brain-derived neurotrophic factor remained unchanged. No significant memory changes were observed in the other 2 groups. This interventional trial demonstrates beneficial effects of caloric restriction on memory performance in healthy elderly subjects. Mechanisms underlying this improvement might include higher synaptic plasticity and stimulation of neurofacilitatory pathways in the brain because of improved insulin sensitivity and reduced inflammatory activity. Our study may help to generate novel prevention strategies to maintain cognitive functions into old age.
Keywords: aging, cognition, diet, unsaturated fatty acids
Because of the constant growth of the elderly population in today's societies worldwide (1), the search for new prevention and treatment strategies to maintain higher brain functions throughout life is of major economic and medical importance (see for example ref. 2). In the last 3 decades, numerous studies suggested that modifiable lifestyle factors including a low-calorie diet (caloric restriction, CR), and specific micro- and macronutrients like unsaturated fatty acids (UFA), might exert beneficial effects on the aging brain (3–7). In animal models of aging and neurodegenerative diseases, CR protected hippocampal, striatal, and cortical neurons, and ameliorated functional decline (8–18). In longitudinal observations in humans, it was found that a CR diet, as consumed by residents of the city of Okinawa, Japan, contributed to healthy aging and longevity (19). Conversely, obesity as a result of high energy intake has been shown to increase the risk of age-related cognitive decline (20).
A diet rich in mono- and polyUFA has been demonstrated to enhance cognitive performance in rats (21). It has been further proposed by epidemiological studies in humans that UFA, provided e.g., by olive oil and sea-fish in the traditional mediterranean diet, exert a risk-lowering effect for AD and cognitive impairment (22–27). Recently, 2 interventional studies reported a significant cognitive improvement in patients suffering from mild cognitive impairment (MCI) after intake of omega-3 polyUFA supplements vs. placebo (28, 29). However, inconclusive or negative findings from animal and observational studies have also been reported for CR (e.g., 30, 31, 32) and UFAs (33, 34).
Taken together, potential benefits of specific “brain-healthy diets” have been proposed, but have not been confirmed unequivocally by animal experiments and human epidemiological studies. Evidence drawn from prospective interventional trials in humans is still missing (CR) or scarce (UFA, 28, 29). Therefore, the aim of the present study was to elucidate cognitive effects of a diet low in calories or high in UFAs in healthy elderly individuals (for a flowchart, see Fig. 1). Because memory impairment is an early indication of AD and its precursor, MCI (35), we considered the ability to remember and learn new contents as our primary outcome measure, in accordance with previous studies on lifestyle interventions (36, 37). Moreover, we tried to identify potential mechanisms underlying the positive effects of these dietary interventions. Metabolic factors like insulin-resistance or low-grade inflammation might contribute to age-related cognitive impairments (38, 39), and improvement of metabolic state should result in acute improvement of cognition, in addition to long-term deceleration of cognitive decline. Therefore, we assessed peripheral blood levels for insulin, glucose, and markers of inflammation. Neuronal function may also be enhanced via neurotrophic factors (4), which are suggested to be activated by moderate stressors like CR via adaptive cellular stress response pathways (5). This possibility was tested by assessing neurotrophic levels in peripheral blood.
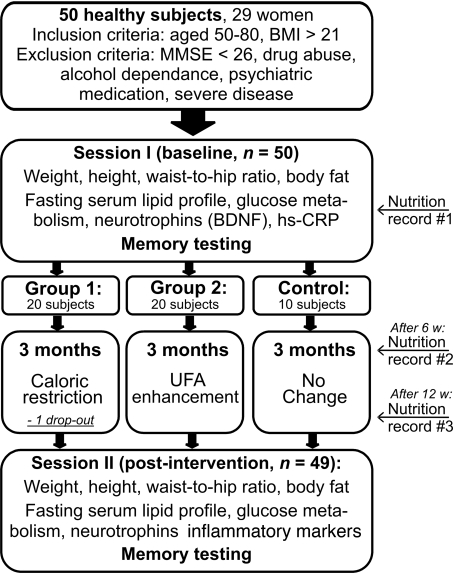
Fig. 1.
Flow-chart of the study. 50 healthy elderly subjects were initially included in the study and performed baseline measurements of physiological parameters, fasting serum levels, and memory tests (session I). Based on age, sex, and BMI, subjects were stratified into 3 groups to follow either a specific diet, namely caloric restriction (n = 20, group 1) or unsaturated fatty acids (UFA) enhancement (n = 20, group 2), or not to change previous eating habits (control, n = 10). Dietary instructions were provided by clinical dieticians. One women from group 1 was not available for posttesting. After a period of 3 months, participants again underwent measurements of physiological parameters, fasting serum levels, and memory tests (session II). At baseline, after 6 and after 12 weeks, subjects additionally completed nutrition diaries over 7 consecutive days.
Results
Dietary Compliance.
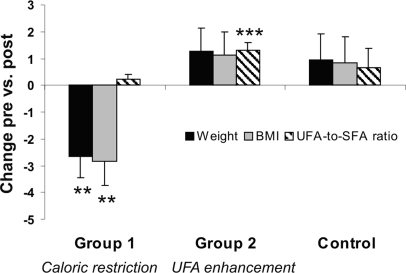
Details of physiological measures and serum levels at baseline and after intervention are shown in Table S1. As intended by the intervention, there was a significant weight loss (F(2, 46) = 7.25, P = 0.002; t (18) = 3.24, P = 0.005; Fig. 2; Table S1) and body mass index (BMI) reduction (F(2, 46) = 7.24, P = 0.002; t (18) = 3.33, P = 0.004; Fig. 2, Table S1) in the CR group (group 1). In addition to significant weight loss and reduction of fasting insulin levels, CR subjects' postintervention questionnaire on adherence to dietary guidelines demonstrated that they followed the instructions (16 of 18 answered “definitely yes,” or “predominantly yes”). The remaining 2 subjects of the CR group (n = 18, 1 subject did not complete the questionnaire) answered they changed their dietary habits “at least half of the time. Asked whether they changed their physical activity during the study, 18 of 18 answered with ”no.“ No significant changes emerged for body fat and waist-to-hip ratio in this group, nor for parameters of lipid metabolism (P > 0.05; Table S1). For the UFA enhancement group (group 2) and for the control group no significant changes emerged for weight, BMI, body fat, and waist to-hip ratio, nor for serum levels of triglycerides, cholesterol, HDL, LDL, or their ratio (all P > 0.05; Fig. 2; Table S1).
Fig. 2.
Percentage changes in weight (black bars), BMI (gray bars), and changes in unsaturated fatty acids (UFA)-to-saturated fatty acids (SFA) ratio (striped bars) after caloric restriction (group 1), UFA enhancement (group 2), and control condition. Note that caloric restriction led to a significant decrease in weight, BMI, and UFA enhancement to a significant increase in the UFA-to-SFA-ratio. Error bars indicate standard error. ***, P < 0.001; **, P < 0.01 according to ANOVARM posthoc testings.
Dietary intake at baseline and after the intervention (self-reported) is shown in Table S2. Dietary records revealed that all groups increased the proportional intake of UFAs significantly (F(1,46) = 92.45, P < 0.001; t(48) = −10.06, P < 0.001; Table S2), yet with highest increase in the UFA enhancement group (+18%, close to the aim of + 20%). The UFA-to-saturated fatty acids ratio was significantly improved only in the UFA enhancement group (F(2, 46) = 3.32, P = 0.045; t (19) = −4.58, P < 0.001; Fig. 2; Table S2). Considering marine sources of omega-3 UFAs, intake of eicosapentaenoic acid (EPA) and docosahexaeinoic acid (DHA) was relatively low at baseline (mean 0.2 g/day; max. 1.6 g/day) and did not increase over the intervention (P > 0.05).
Intervention Effects.
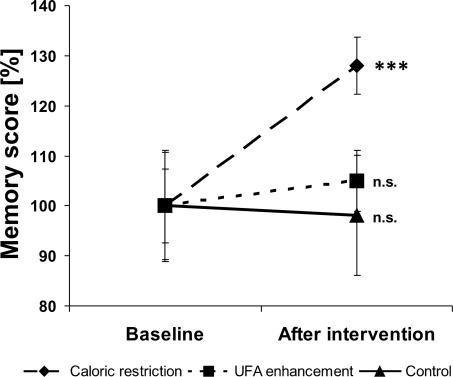
ANOVARM showed a significant TIME x GROUP interaction on memory scores (F(2, 46) = 5.42, P = 0.008). Subsequent t tests revealed that this was due to significant differences in memory scores in the CR group before and after intervention, with higher scores after the intervention (t (18) = −4.73, P = 0.0002; Fig. 3). Likewise, there was a significant difference for memory scores uncorrected for false-positive misidentifications (t(18) = −2.85, P = 0.011) and for “number of false-positives” (t (18) = 2.62, P = 0.018). In summary, subjects remembered more words and made fewer mistakes after caloric restriction. No differences were shown for memory scores in the UFA enhancement group (all P values ≥ 0.31) or in the control group (all P values > 0.62).
Fig. 3.
Percentage memory scores normalized to baseline values before and after caloric restriction (dashed line), unsaturated fatty acid (UFA) enhancement (dotted line), and control (solid line). Note that after caloric restriction, a highly significant improvement in memory scores can be seen. Baseline memory scores were not significantly different. Dots give means, bars indicate standard error. ***, P < 0.001.
In the CR group, inverse associations emerged between changes in several laboratory parameters [insulin, fasting glucose, hs-CRP, and tumor necrosis factor-alpha (TNF-α)] and changes in memory score:
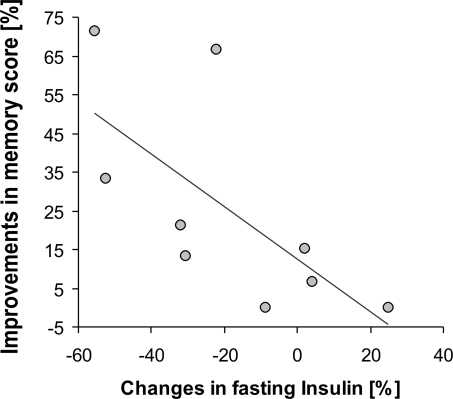
Increases in memory score were correlated with decreases in insulin levels (r = −0.45, P = 0.06). Focusing the analysis on those individuals with best adherence to the intervention (defined as weight loss >1 SD of mean weight loss of the control group, resulting in a weight loss of >2 kg, n = 9), a highly significant inverse association emerged (Bonferroni corrected, r = −0.78, P = 0.014; Fig. 4). In addition, there was a trend for a significantly reduced mean value of fasting glucose serum levels in the CR group after the intervention (t (18) = 1.82, P = 0.086), yet ANOVARM failed to reach significance (F(2, 46) = 1.98; P = 0.15).
Fig. 4.
Inverse correlation (Spearman, r = −0.81, P = 0.014) between changes in insulin levels and memory score improvements after caloric restriction in those subjects with best adherence to the diet (n = 9). Line indicates regression fit.
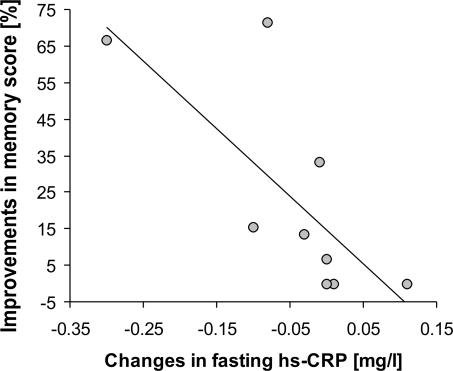
Furthermore, increases in memory score were correlated with decreases in hs-CRP levels (trend; r = −0,41, P = 0.083). Again, if including only those subjects with best adherence to the diet, a highly significant inverse association emerged (Bonferroni corrected, r = −0.83, P = 0.005; Fig. 5). A weak correlation was also found for increases in memory score and decreases in TNF-α in the CR group (r = −0.39, P = 0.102), more obvious in those subjects with best adherence to the diet (r = −0.59, P = 0.094).
Fig. 5.
Inverse correlation (Spearman, r = −0.83, P = 0.005) between changes in high sensitive C-reactive protein (hs-CRP) and memory score improvements after caloric restriction in those subjects with best adherence to the diet (n = 9). Line indicates regression fit.
For serum levels of BDNF, IGF-1, and IL-1β, no significant correlations with memory scores emerged (P > 0.05), nor for these or any of the other parameters in the UFA enhancement group or in the control group (P > 0.05). Likewise, no significant effects for GROUP × TIME was detected by ANOVARM.
Discussion
In this prospective interventional study in healthy normal to overweight elderly individuals, we found a significant improvement in memory performance after a caloric restricted diet (CR) over a period of 3 months. Memory improvement was correlated with decreases in fasting insulin and hs-CRP, most pronounced in those individuals with best adherence to the CR diet. In contrast, no significant changes in memory performance emerged after a diet rich in UFA or after control conditions.
Caloric Restriction.
The findings of this interventional trial in humans support experimental animal studies (4) and epidemiological observations in humans (19, 40) that have suggested beneficial effects of CR on the aging brain. For example, CR has been demonstrated to enhance spatial memory performance in rats (17), and even CR over a period of 4 months sufficed to reduce age-related impairments in motor- and learning tasks in mice (9). Moreover, Fontan-Lozano and colleagues (41) reported that a CR diet, using an intermittent fasting regime enhanced learning and consolidation processes in mice, probably via higher expression of an NMDA-receptor subunit in the hippocampus. Interestingly, adult-onset short-term CR over 7 weeks in rats attenuated the effects of excitotoxic insults in hippocampal slices compared with ad libitum control diet (8). These results concur with the current study, because we found that even moderate CR over a period of 3 months improves cognition in healthy elderly subjects.
Unsaturated Fatty Acids.
Considering UFA, observational studies in elderly cohorts (23, 25, 42) and small clinical trials in patients suffering from MCI or AD (43, 44) suggested that a diet high in mono- or omega-3 UFA might postpone cognitive decline. In rats, it has been demonstrated that a diet rich in mono- and di-UFA enhanced spatial memory performance (21). Conversely, higher intake of omega 3-fatty acids did not improve cognitive performance in epidemiological studies (for example, see ref. 33). The latter results are consistent with our study that failed to detect a beneficial effect of a 3-month dietary intervention high in UFA on cognitive performance. Several possible explanations may account for these negative findings: First, our results could be due to low adherence to the UFA diet, or to insufficiently high UFA dosage in the dietary protocol, rather than to a lack of positive effects of UFA on cognition per se. Because our dietary protocol promoted a self-prepared diet arranged independently at home, the present study does not allow us to distinguish between these possibilities. According to dietary records, however, there was a significant increase in the UFA-to-saturated fatty acids ratio in the UFA enhancement group, which nearly met the protocol aim of 20% UFA increase. However, self-reported dietary information is prone to errors (45).
Second, the amount of marine omega-3 UFA (mainly EPA, DHA) did not increase over the intervention period according to dietary records, mainly because there was no significant increase in fatty seafish meals. Because these sources of omega-3 fatty acids are suggested to be most effective in delaying cognitive decline (23, 28, 29), a low EPA/DHA-intake might have contributed to our negative findings, yet the evidence is still unequivocal (see e.g., 46 for positive results on olive oil, however, this might also depend on other, non-UFA ingredients in olive oil). Therefore, future studies have to further evaluate the effects of different UFAs on cognitive functions on the aging brain in health and disease.
Mechanisms of Diet-Induced Cognitive Changes.
Insulin.
In the present study, we found a decrease of fasting peripheral insulin in the CR group, in accordance with studies in healthy rats (47) and monkeys (48–50) after CR, and with clinical data in obese patients (e.g., 51). Reducing peripheral insulin levels should result in increased insulin sensitivity and central insulin levels (3), because higher levels of peripheral insulin lead to a down-regulation of insulin transport at the bloodbrain-barrier and thus to central hypoinsulinemia (52, 53). Importantly, improved insulin signaling in the brain has been suggested to have neuroprotective effects (38, 52, 54,), whereas increased peripheral circulating insulin may promote the development of cognitive impairments and AD (52, 54, 55).
Furthermore, the observed correlation between decrease in peripheral insulin and increase in memory points to a possible role of insulin in mediating the beneficial effects of CR on memory functions (for review, see ref. 7). Levels of insulin, insulin receptors, and insulin-regulated pathways in the brain are involved in glutamate- and GABA-mediated synaptic plasticity and in gene expressions required for long-term memory consolidation (38, 56). For example in the hippocampus, insulin has been shown to induce NMDA receptor phosphorylation (57), and to increase channel activities of NMDA receptors (58), which play an important role in learning and memory formation (for example, see ref. 59). Thus, it has been convincingly demonstrated that insulin signaling exerts neuroprotective and neuromodulatory effects in the brain, although the molecular machinery linking insulin and cognitive improvement, for example the exact role of kinase molecules in learning and memory, needs to be further elucidated (38).
In summary, the present study lends experimental support to a model derived from animal studies in which reduced fasting insulin levels due to CR led to lower insulin resistance, higher insulin sensitivity, subsequently to improved insulin signaling in the brain and to increased synaptogenesis and neuronal survival (3). Higher insulin sensitivity due to CR in our subjects, with subsequently improved insulin signaling in the brain, would be a plausible explanation for the observed memory improvements in the current study.
Neurotrophic factors.
Neuronal function may also be enhanced via neurotrophic factors (5, 38), which are suggested to be activated by moderate stressors like exercise and CR via adaptive cellular stress response pathways (e.g., heat shock protein 70; for details, see refs. 4 and 60). Neurotrophic factors, such as IGF-1 and BDNF, are widely known to be involved in neuronal growth and neurogenesis and might also protect mature neurons from degeneration (61). IGF-1 is also a ligand for insulin receptors (62), thus activating insulin pathways in the brain. Both IGF-1- (63) and BDNF-levels (64) have been suggested to be enhanced after CR in rodents. Our results did not show a significant difference in either IGF-1 or BDNF induced by dietary interventions. One explanation might be that we could only assess peripheral levels of IGF-1 and BDNF. Even though both peripheral IGF-1 (65) and BDNF (66) have been shown to pass the blood–brain barrier, these measures may not be a perfect reflection of brain concentrations. In addition, other neurotrophic molecules such as glia-derived neurotrophic factor (GDNF) and nerve growth factor (NGF) might have changed in adaptation to CR (3), which were not assessed in the current study. To clarify these issues, measurements of these factors could be additionally assessed in future studies.
Inflammation.
CR has been shown to exert anti-inflammatory effects (4), including down-regulation of hs-CRP levels in rodents (67) and TNF-α in humans (68, 69), in line with the present data. With regard to cognition, several studies have proposed that “inflammatory activity,” as indicated by serum markers of inflammatory responses, is negatively correlated with neuropsychological performance and cognitive decline (70, 71). For example, an observational study by Teunissen and colleagues (72) found significant inverse correlations of serum levels of CRP and haptoglobin with performance in a verbal learning task (congruent to the memory test used in the current study) in healthy elderly individuals. The current study is the first to confirm these findings, and to extend the proposed association for TNF-α in an interventional design. However, anti-inflammatory pathways linking CR and memory remain to be further elucidated (4), e.g., by including a larger number of subjects with elevated levels of inflammatory markers at baseline, and testing an extended range of markers. Interestingly, TNF-α has been demonstrated to promote insulin resistance in experimental animal studies (73, 74). Therefore, a reduction of TNF-α by CR might additionally contribute to maintain cognitive functions via improved insulin signaling (3).
Limitations.
Several limitations should be considered when interpreting our findings. First, dietary habits were self-reported only and thus prone to over- or underestimation (45). However, in the CR group, weight loss and BMI reduction demonstrated adherence to the intended dietary regime. Second, individuals in the control group did not receive the same amount of attention by dietary counsellors, and interaction with group members, as participants in the CR group. Better memory performance may thus be due to a Hawthorne (75) effect or an effect of “enhanced environmental enrichment” by social interaction in the CR group. However, the finding that individuals in the UFA enhancement group, receiving a similar amount of attention and social interaction, did not show memory improvements, renders this explanation highly unlikely.
Conclusion
To our knowledge, the current results provide first experimental evidence in humans that caloric restriction improves memory in the elderly. Our findings further point to increased insulin sensitivity and reduced inflammatory activity as mediating mechanisms, leading to higher synaptic plasticity and stimulation of neuroprotective pathways in the brain. Future studies incorporating measurements of additional neurotrophic and inflammatory markers, and brain imaging to assess structural changes (for example, see ref. 36), should provide further insights into potential mediators of improved cognition by changes in dietary habits.
The present findings may help to develop new prevention and treatment strategies for maintaining cognitive health into old age (3).
Materials and Methods
Subjects.
Fifty healthy elderly subjects (age: 60.5 years ± 7.6 SD, BMI: 28 kg/m2 ± 3.7 SD; 29 females) were recruited via newspaper advertisement. Inclusion criteria were age between 50 and 80 years, a BMI > 21 to exclude potential underweight after intervention, and postmenopausal status for women. At screening visit, participants underwent a routine medical and neurological examination. Exclusion criteria were severe cardiac and pulmonary disease, diabetes or other metabolic disorder, psychiatric disorders, memory impairment based on a score of <26 on the MiniMental State Examination (MMSE; 76), and drug abuse, including alcohol dependence and heavy smoking. Psychiatric comorbidity was additionally monitored using the Beck's Depression Inventory (BDI, German version; 77) and Spielberger's State Trait Angst Inventar (STAI 1 and 2, German version; 78). One woman was not available for postassessment, leaving 49 participants for final analyses. Based on age, sex, and BMI, subjects were stratified into 3 groups: (i) Caloric restriction, (ii) increase of the amount of UFA (“UFA enhancement”), and (iii) control group; for details on groups see below. Demographic variables at baseline are given in Table S3. The study was conducted at the Department of Neurology at the University of Münster, Germany. All subjects provided written informed consent and received reimbursement for participation. The research protocol was approved by the Ethics Committee of the University Hospital of Muenster, Germany.
Caloric Restriction.
According to previous recommendations based on studies of rodents and rhesus monkeys (49, 60), participants (n = 19) were instructed to reduce caloric intake aiming at a 30% reduction relative to previous habits, over a period of 3 months. The intended individual caloric intake was calculated a priori based on individual dietary records, because the aim of the caloric restriction intervention was to reduce each subject's individual caloric intake by 30%, compared with pretrial levels. To avoid cognitive changes due to malnutrition (79), minimal intake was set to 1,200 kcal per day.
Unsaturated Fatty Acids Enhancement.
According to previous recommendations based on studies of rodents (21), participants (n = 20) were instructed to enhance intake of UFAs aiming at a 20% increase compared with previous habits, over a period of 3 months. They were instructed to keep the amount of total fat intake unchanged.
All subjects assigned to 1 of the 2 dietary interventions were trained on how to follow their respective diet by experienced clinical dieticians blinded to the underlying study hypothesis. Therefore, after completing baseline measurements, participants attended a 2-h tutorial (maximum 12 persons each). Additionally, they received dietary instructions in written forms. Group 1 additionally underwent 1-h individual schooling at baseline and a second 1-h tutorial carried out by the clinical dieticians after a period of 6 weeks. Moreover, subjects of the 2 intervention groups received supplementary dietary counseling via telephone if needed, so that any problems in adhering to the intervention could rapidly be addressed during the entire intervention period. To provide optimal supervision, dieticians obtained information about individual nutritional intake from the nutrition diaries and personal interviews. Adherence to the intervention was monitored by measures of weight, BMI, waist-to-hip ratio, amount of body fat, and fasting serum levels of triglycerides, cholesterol, and hs-CRP, because these parameters have been shown to decrease after caloric restriction and/or after a dietary enhancement of UFA (80–83). In addition, information on adherence to the diet was collected by a postintervention Questionnaire, and self-reported nutritional records were collected in the course of the intervention period.
Control.
Participants (n = 10) were instructed not to change previous eating habits over a period of 3 months. No specific dietary counselling was administered to avoid self-chosen/self-administered changes in dietary patterns that have been reported after any dietary counselling (for example, ref. 84). For simplicity this condition is subsequently referred to as control intervention.
Nutritional records.
Supply of nutrients, caloric-, and UFA-intake were documented by nutritional records at the beginning of the study (record 1), after 6 weeks intervention (record 2), and after 12 weeks intervention (record 3). Each record encompassed 7 days of protocol. For nutritional records, all subjects had to plot, on a daily basis, all food and drink intake in an in-house developed standard nutrition diary (University of Münster, Department of Internal Medicine) similar to records used in other studies (see e.g., 45). The diary contained numerous nutritional items presented in standard servings, and additional free lines to describe foods not listed in the diary. Subjects had to mark the respective items, with the possibility to adjust for individual servings. Nutrients, amount of calories, and amount of UFAs where quantified using the software EBISpro (Erhardt).
Physiological Parameters and Blood Sampling.
Before and after 3 months of intervention (sessions I and II; see Fig. 1), the following variables were assessed: Weight (in kilograms; measured), height (in meters; self-reported), waist-to-hip ratio (in centimeters/centimeters, measured), body fat (percentage, measured), diastolic and systolic blood pressure, heart rate, fasting serum levels of triglyceride, total-, HDL- and LDL-cholesterol, insulin, glucose, insulin-like growth factor 1 (IGF-1), brain derived neurotrophic factor (BDNF), catecholamines (85), markers of inflammation [i.e., high sensitive-C-reactive protein (hs-CRP) and tumor necrosis factor-alpha (TNF-α)], and routine parameters (sodium, potassium, calcium, phosphate, protein, creatinine, urea; data not shown), for details, see SI Text.
Neuropsychological Testing.
Before and after 3 months of intervention, subjects were tested on memory performance using the German version of the Rey Auditory Verbal Learning Task (AVLT) (84). The test was performed by a trained clinical neuropsychologist. Participants were asked to learn as many words as possible out of a list of 15 words. As primary outcome measure “memory score,” we considered the total number of retrieved words after 30 min (delayed memory), adjusted for false positive misidentifications (86, 87), in line with previous studies (36, 37, 88), and with signal detection theory (89). Additionally, analysis was conducted for total number of retrieved words without adjustment for false-positives, and for total numbers of false-positive errors. Two different but congruent versions were presented at the 2 test sessions to avoid test-retest effects.
To assess potential differences in attention or working memory due to the intervention, subjects completed trail making tests (TMT) A and B (90), and forward/backward digit span (WMS-R, 91), before and after the intervention. No significant differences emerged between pre and post intervention test sessions (all P > 0.5).
Statistical Analysis.
Before data analysis, normal or near-normal distribution and homogeneity of variances were tested by the Kolmogorov–Smirnov test and the Levene's Test.
To monitor dietary compliance, individual physiological parameters and serum levels and dietary intake before and after the intervention were compared by a repeated measures analysis of variance (ANOVARM) with TIME (“baseline,” “after intervention period”), and between factor GROUP (“caloric restriction,” “UFA enhancement,” “control”), followed by post hoc paired t tests (2-tailed) as appropriate.
To assess intervention effects, an ANOVARM on the outcome variable “memory scores” was conducted, with TIME and between factor GROUP. Depending on significance, post hoc paired t tests (two-tailed) were performed as appropriate.
Correlations between changes in memory score and changes in physiological parameters and serum levels from baseline to post intervention assessment were assessed using Spearman's correlation coefficient.
Significance was set at P < 0.05, all data are presented as mean with standard error of the mean, unless indicated otherwise.
Supplementary Material
Acknowledgments.
We thank N. Rösser and C. Willemer for help with subject recruitment. This work was supported by Deutsche Forschungsgemeinschaft Grant Fl 379–4/1 (to A. F.), Interdisziplinäres Zentrum für Klinische Forschung Grant Floe/3/004/08 (to A.F.), Bundesministerium für Forschung und Bildung Grant 01GW0520 (to A.F. and S.K.), and Innovative Medizinische Forschung Münster Grant FL110605 (to A. F.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0808587106/DCSupplemental.
References
- 1.Pressley JC, Trott C, Tang M, Durkin M, Stern Y. Dementia in community-dwelling elderly patients: A comparison of survey data, medicare claims, cognitive screening, reported symptoms, and activity limitations. J Clin Epidemiol. 2003;56:896–905. doi: 10.1016/s0895-4356(03)00133-1. [DOI] [PubMed] [Google Scholar]
- 2.Dwyer J. Starting down the right path: Nutrition connections with chronic diseases of later life. Am J Clin Nutr. 2006;83:415S–420S. doi: 10.1093/ajcn/83.2.415S. [DOI] [PubMed] [Google Scholar]
- 3.Martin B, Mattson MP, Maudsley S. Caloric restriction and intermittent fasting: Two potential diets for successful brain aging. Ageing Res Rev. 2006;5:332–353. doi: 10.1016/j.arr.2006.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mattson MP, Duan W, Wan R, Guo Z. Prophylactic activation of neuroprotective stress response pathways by dietary and behavioral manipulations. NeuroRx. 2004;1:111–116. doi: 10.1602/neurorx.1.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stranahan A, Mattson M. Impact of Energy Intake and Expenditure on Neuronal Plasticity. Neuromol Med. 2008 doi: 10.1007/s12017-008-8043-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parrott MD, Greenwood CE. Dietary influences on cognitive function with aging: From high-fat diets to healthful eating. Ann NY Acad Sci. 2007;1114:389–397. doi: 10.1196/annals.1396.028. [DOI] [PubMed] [Google Scholar]
- 7.Gomez-Pinilla F. The influences of diet and exercise on mental health through hormesis. Ageing Res Rev. 2008;7:49–62. doi: 10.1016/j.arr.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Youssef FF, Ramchandani J, Manswell S, McRae A. Adult-onset calorie restriction attenuates kainic acid excitotoxicity in the rat hippocampal slice. Neurosci Lett. 2008;431:118–122. doi: 10.1016/j.neulet.2007.11.064. [DOI] [PubMed] [Google Scholar]
- 9.Ingram DK, Weindruch R, Spangler EL, Freeman JR, Walford RL. Dietary restriction benefits learning and motor performance of aged mice. J Gerontol. 1987;42:78–81. doi: 10.1093/geronj/42.1.78. [DOI] [PubMed] [Google Scholar]
- 10.Duan W, Lee J, Guo Z, Mattson MP. Dietary restriction stimulates BDNF production in the brain and thereby protects neurons against excitotoxic injury. J Mol Neurosci. 2001;16:1–12. doi: 10.1385/JMN:16:1:1. [DOI] [PubMed] [Google Scholar]
- 11.Patel NV, et al. Caloric restriction attenuates Abeta-deposition in Alzheimer transgenic models. Neurobiol Aging. 2005;26:995–1000. doi: 10.1016/j.neurobiolaging.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 12.Wang J, et al. Caloric restriction attenuates beta-amyloid neuropathology in a mouse model of Alzheimer's disease. FASEB J. 2005;19:659–661. doi: 10.1096/fj.04-3182fje. [DOI] [PubMed] [Google Scholar]
- 13.Duan W, Mattson MP. Dietary restriction and 2-deoxyglucose administration improve behavioral outcome and reduce degeneration of dopaminergic neurons in models of Parkinson's disease. J Neurosci Res. 1999;57:195–206. doi: 10.1002/(SICI)1097-4547(19990715)57:2<195::AID-JNR5>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 14.Bruce-Keller AJ, Umberger G, McFall R, Mattson MP. Food restriction reduces brain damage and improves behavioral outcome following excitotoxic and metabolic insults. Ann Neurol. 1999;45:8–15. [PubMed] [Google Scholar]
- 15.Zhu H, Guo Q, Mattson MP. Dietary restriction protects hippocampal neurons against the death-promoting action of a presenilin-1 mutation. Brain Res. 1999;842:224–229. doi: 10.1016/s0006-8993(99)01827-2. [DOI] [PubMed] [Google Scholar]
- 16.Maswood N, et al. Caloric restriction increases neurotrophic factor levels and attenuates neurochemical and behavioral deficits in a primate model of Parkinson's disease. Proc Natl Acad Sci USA. 2004;101:18171–18176. doi: 10.1073/pnas.0405831102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stewart J, Mitchell J, Kalant N. The effects of life-long food restriction on spatial memory in young and aged Fischer 344 rats measured in the 8-arm radial and the Morris water mazes. Neurobiol Aging. 1989;10:669–675. doi: 10.1016/0197-4580(89)90003-1. [DOI] [PubMed] [Google Scholar]
- 18.Fontan-Lozano A, et al. Caloric restriction increases learning consolidation and facilitates synaptic plasticity through mechanisms dependent on NR2B subunits of the NMDA receptor. J Neurosci. 2007;27:10185–10195. doi: 10.1523/JNEUROSCI.2757-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Willcox BJ, et al. Caloric restriction, the traditional Okinawan diet, and healthy aging: The diet of the world's longest-lived people and its potential impact on morbidity and life span. Ann NY Acad Sci. 2007;1114:434–455. doi: 10.1196/annals.1396.037. [DOI] [PubMed] [Google Scholar]
- 20.Knecht S, Ellger T, Levine JA. Obesity in neurobiology. Prog Neurobiol. 2008;84:85–103. doi: 10.1016/j.pneurobio.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 21.Wong KL, Murakami K, Routtenberg A. Dietary cis-fatty acids that increase protein F1 phosphorylation enhance spatial memory. Brain Res. 1989;505:302–305. doi: 10.1016/0006-8993(89)91456-x. [DOI] [PubMed] [Google Scholar]
- 22.Scarmeas N, Stern Y, Tang MX, Mayeux R, Luchsinger JA. Mediterranean diet and risk for Alzheimer's disease. AnnNeurol. 2006;59:912–921. doi: 10.1002/ana.20854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Gelder BM, Tijhuis M, Kalmijn S, Kromhout D. Fish consumption, n-3 fatty acids, and subsequent 5-y cognitive decline in elderly men: The Zutphen Elderly Study. Am J Clin Nutr. 2007;85:1142–1147. doi: 10.1093/ajcn/85.4.1142. [DOI] [PubMed] [Google Scholar]
- 24.Issa AM, et al. The efficacy of omega-3 fatty acids on cognitive function in aging and dementia: A systematic review. Dement Geriatr Cogn Disord. 2006;21:88–96. doi: 10.1159/000090224. [DOI] [PubMed] [Google Scholar]
- 25.Panza F, et al. Mediterranean diet and cognitive decline. Public Health Nutr. 2004;7:959–963. doi: 10.1079/phn2004561. [DOI] [PubMed] [Google Scholar]
- 26.Barberger-Gateau P, et al. Fish, meat, and risk of dementia: Cohort study. BMJ. 2002;325:932–933. doi: 10.1136/bmj.325.7370.932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morris MC, Evans DA, Tangney CC, Bienias JL, Wilson RS. Fish consumption and cognitive decline with age in a large community study. Arch Neurol. 2005;62:1849–1853. doi: 10.1001/archneur.62.12.noc50161. [DOI] [PubMed] [Google Scholar]
- 28.Chiu C-C, et al. The effects of omega-3 fatty acids monotherapy in Alzheimer's disease and mild cognitive impairment: A preliminary randomized double-blind placebo-controlled study. Progr Neuropsychopharmacol Biol Psychiatry. 2008;32:1538–1544. doi: 10.1016/j.pnpbp.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 29.Freund-Levi Y, et al. Omega-3 fatty acid treatment in 174 patients with mild to moderate Alzheimer disease: OmegAD study: A randomized double-blind trial. Arch Neurol. 2006;63:1402–1408. doi: 10.1001/archneur.63.10.1402. [DOI] [PubMed] [Google Scholar]
- 30.Bellush LL, Wright AM, Walker JP, Kopchick J, Colvin RA. Caloric restriction and spatial learning in old mice. Physiol Behav. 1996;60:541–547. doi: 10.1016/s0031-9384(96)80029-1. [DOI] [PubMed] [Google Scholar]
- 31.Yanai S, Okaichi Y, Okaichi H. Long-term dietary restriction causes negative effects on cognitive functions in rats. Neurobiol Aging. 2004;25:325–332. doi: 10.1016/S0197-4580(03)00115-5. [DOI] [PubMed] [Google Scholar]
- 32.Matochik JA, et al. Age-related decline in striatal volume in rhesus monkeys: Assessment of long-term calorie restriction. Neurobiol Aging. 2004;25:193–200. doi: 10.1016/s0197-4580(03)00092-7. [DOI] [PubMed] [Google Scholar]
- 33.Larrieu S, Letenneur L, Helmer C, Dartigues JF, Barberger-Gateau P. Nutritional factors and risk of incident dementia in the PAQUID longitudinal cohort. J Nutr Health Aging. 2004;8:150–154. [PubMed] [Google Scholar]
- 34.Arendash GW, et al. A diet high in omega-3 fatty acids does not improve or protect cognitive performance in Alzheimer's transgenic mice. Neuroscience. 2007;149:286–302. doi: 10.1016/j.neuroscience.2007.08.018. [DOI] [PubMed] [Google Scholar]
- 35.Blacker D, et al. Neuropsychological measures in normal individuals that predict subsequent cognitive decline. Arch Neurol. 2007;64:862–871. doi: 10.1001/archneur.64.6.862. [DOI] [PubMed] [Google Scholar]
- 36.Pereira AC, et al. An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proc Natl Acad Sci USA. 2007;104:5638–5643. doi: 10.1073/pnas.0611721104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Floel A, et al. Lifestyle and Memory in the Elderly. Neuroepidemiology. 2008;31:39–47. doi: 10.1159/000137378. [DOI] [PubMed] [Google Scholar]
- 38.Zhao W-Q, Chen H, Quon MJ, Alkon DL. Insulin and the insulin receptor in experimental models of learning and memory. Eur J Pharmacol. 2004;490:71–81. doi: 10.1016/j.ejphar.2004.02.045. [DOI] [PubMed] [Google Scholar]
- 39.Stranahan AM, et al. Diet-induced insulin resistance impairs hippocampal synaptic plasticity and cognition in middle-aged rats. Hippocampus. 2008;18:1085–1088. doi: 10.1002/hipo.20470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beydoun MA, Beydoun HA, Wang Y. Obesity and central obesity as risk factors for incident dementia and its subtypes: A systematic review and meta-analysis. Obesity Rev. 2008;9:204–218. doi: 10.1111/j.1467-789X.2008.00473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fontan-Lozano A, et al. Caloric restriction increases learning consolidation and facilitates synaptic plasticity through mechanisms dependent on NR2B subunits of the NMDA receptor. J Neurosci. 2007;27:10185–10195. doi: 10.1523/JNEUROSCI.2757-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Solfrizzi V, et al. Dietary fatty acids intake: Possible role in cognitive decline and dementia. Exp Gerontol. 2005;40:257–270. doi: 10.1016/j.exger.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 43.Yehuda S, Rabinovtz S, Carasso RL, Mostofsky DI. Essential fatty acids preparation (SR-3) improves Alzheimer's patients quality of life. Int J Neurosci. 1996;87:141–149. doi: 10.3109/00207459609070833. [DOI] [PubMed] [Google Scholar]
- 44.Kotani S, et al. Dietary supplementation of arachidonic and docosahexaenoic acids improves cognitive dysfunction. Neurosci Res. 2006;56:159–164. doi: 10.1016/j.neures.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 45.Kroke A, et al. Validation of a self-administered food-frequency questionnaire administered in the European Prospective Investigation into Cancer and Nutrition (EPIC) Study: Comparison of energy, protein, and macronutuient intakes estimated with the doubly labeled water, urinary nitrogen, and repeated 24-h dietary recall methods. Am J Clin Nutr. 1999;70:439–447. doi: 10.1093/ajcn/70.4.439. [DOI] [PubMed] [Google Scholar]
- 46.Solfrizzi V, et al. High monounsaturated fatty acids intake protects against age-related cognitive decline. Neurology. 1999;52:1563–1569. doi: 10.1212/wnl.52.8.1563. [DOI] [PubMed] [Google Scholar]
- 47.Masoro EJ, McCarter RJ, Katz MS, McMahan CA. Dietary restriction alters characteristics of glucose fuel use. J Gerontol. 1992;47:B202–208. doi: 10.1093/geronj/47.6.b202. [DOI] [PubMed] [Google Scholar]
- 48.Kemnitz JW, et al. Dietary restriction increases insulin sensitivity and lowers blood glucose in rhesus monkeys. Am J Physiol. 1994;266:E540–547. doi: 10.1152/ajpendo.1994.266.4.E540. [DOI] [PubMed] [Google Scholar]
- 49.Lane MA, et al. Calorie restriction lowers body temperature in rhesus monkeys, consistent with a postulated anti-aging mechanism in rodents. Proc Natl Acad Sci USA. 1996;93:4159–4164. doi: 10.1073/pnas.93.9.4159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cefalu WT, et al. A study of caloric restriction and cardiovascular aging in cynomolgus monkeys (Macaca fascicularis): A potential model for aging research. J Gerontol A Biol Sci Med Sci. 1997;52:B10–19. doi: 10.1093/gerona/52a.1.b10. [DOI] [PubMed] [Google Scholar]
- 51.Wycherley TP, Brinkworth GD, Noakes M, Buckley JD, Clifton PM. Effect of caloric restriction with and without exercise training on oxidative stress and endothelial function in obese subjects with type 2 diabetes. Diabetes Obesity Metabol. 2008;10:1062–1073. doi: 10.1111/j.1463-1326.2008.00863.x. [DOI] [PubMed] [Google Scholar]
- 52.Craft S. Insulin resistance syndrome and Alzheimer's disease: Age- and obesity-related effects on memory, amyloid, and inflammation. Neurobiol Aging. 2005;26:65–69. doi: 10.1016/j.neurobiolaging.2005.08.021. [DOI] [PubMed] [Google Scholar]
- 53.Baura GD, et al. Insulin transport from plasma into the central nervous system is inhibited by dexamethasone in dogs. Diabetes. 1996;45:86–90. doi: 10.2337/diab.45.1.86. [DOI] [PubMed] [Google Scholar]
- 54.Cole GM, Frautschy SA. The role of insulin and neurotrophic factor signaling in brain aging and Alzheimer's Disease. Exp Gerontol. 2007;42:10–21. doi: 10.1016/j.exger.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 55.Taguchi A, White MF. Insulin-like signaling, nutrient homeostasis, and life span. Annu Rev Physiol. 2008;70:191–212. doi: 10.1146/annurev.physiol.70.113006.100533. [DOI] [PubMed] [Google Scholar]
- 56.McNay EC. Insulin and ghrelin: Peripheral hormones modulating memory and hippocampal function. Curr Opin Pharmacol. 2007;7:628–632. doi: 10.1016/j.coph.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 57.Christie JM, Wenthold RJ, Monaghan DT. Insulin causes a transient tyrosine phosphorylation of NR2A and NR2B NMDA receptor subunits in rat hippocampus. J Neurochem. 1999;72:1523–1528. doi: 10.1046/j.1471-4159.1999.721523.x. [DOI] [PubMed] [Google Scholar]
- 58.Skeberdis VA, Lan J, Zheng X, Zukin RS, Bennett MV. Insulin promotes rapid delivery of N-methyl-d- aspartate receptors to the cell surface by exocytosis. Proc Natl Acad Sci USA. 2001;98:3561–3566. doi: 10.1073/pnas.051634698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nakazawa K, et al. Requirement for hippocampal CA3 NMDA receptors in associative memory recall. Science. 2002;297:211–218. doi: 10.1126/science.1071795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mattson MP. Neuroprotective signaling and the aging brain: Take away my food and let me run. Brain Res. 2000;886:47–53. doi: 10.1016/s0006-8993(00)02790-6. [DOI] [PubMed] [Google Scholar]
- 61.Connor B, Dragunow M. The role of neuronal growth factors in neurodegenerative disorders of the human brain. Brain Res Rev. 1998;27:1–39. doi: 10.1016/s0165-0173(98)00004-6. [DOI] [PubMed] [Google Scholar]
- 62.Kitamura S, et al. Ghrelin concentration in cord and neonatal blood: Relation to fetal growth and energy balance. J Clin Endocrinol Metab. 2003;88:5473–5477. doi: 10.1210/jc.2002-021350. [DOI] [PubMed] [Google Scholar]
- 63.Niedernhofer LJ, et al. A new progeroid syndrome reveals that genotoxic stress suppresses the somatotroph axis. Nature. 2006;444:1038–1043. doi: 10.1038/nature05456. [DOI] [PubMed] [Google Scholar]
- 64.Lee J, Seroogy KB, Mattson MP. Dietary restriction enhances neurogenesis and up-regulates neurotrophin expression in the hippocampus of adult mice. J Neurochem. 2002;80:539–547. doi: 10.1046/j.0022-3042.2001.00747.x. [DOI] [PubMed] [Google Scholar]
- 65.Carro E, Torres-Aleman I. Serum insulin-like growth factor I in brain function. Keio J Med. 2006;55:59–63. doi: 10.2302/kjm.55.59. [DOI] [PubMed] [Google Scholar]
- 66.Pan W, Banks WA, Fasold MB, Bluth J, Kastin AJ. Transport of brain-derived neurotrophic factor across the blood-brain barrier. Neuropharmacology. 1998;37:1553–1561. doi: 10.1016/s0028-3908(98)00141-5. [DOI] [PubMed] [Google Scholar]
- 67.Kalani R, Judge S, Carter C, Pahor M, Leeuwenburgh C. Effects of caloric restriction and exercise on age-related, chronic inflammation assessed by C-reactive protein and interleukin-6. J Gerontol Ser A. 2006;61:211–217. doi: 10.1093/gerona/61.3.211. [DOI] [PubMed] [Google Scholar]
- 68.Johnson JB, et al. Alternate day calorie restriction improves clinical findings and reduces markers of oxidative stress and inflammation in overweight adults with moderate asthma. Free Radical Biol Med. 2007;42:665–674. doi: 10.1016/j.freeradbiomed.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jung SH, et al. Effect of weight loss on some serum cytokines in human obesity: Increase in IL-10 after weight loss. The J Nutr Biochem. 2008;19:371–375. doi: 10.1016/j.jnutbio.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 70.Dik MG, et al. Contribution of metabolic syndrome components to cognition in older individuals. Diabetes Care. 2007;30:2655–2660. doi: 10.2337/dc06-1190. [DOI] [PubMed] [Google Scholar]
- 71.Yaffe K, et al. Inflammatory markers and cognition in well-functioning African-American and white elders. Neurology. 2003;61:76–80. doi: 10.1212/01.wnl.0000073620.42047.d7. [DOI] [PubMed] [Google Scholar]
- 72.Teunissen CE, et al. Inflammation markers in relation to cognition in a healthy aging population. J Neuroimmunol. 2003;134:142–150. doi: 10.1016/s0165-5728(02)00398-3. [DOI] [PubMed] [Google Scholar]
- 73.Feinstein R, Kanety H, Papa MZ, Lunenfeld B, Karasik A. Tumor necrosis factor-alpha suppresses insulin-induced tyrosine phosphorylation of insulin receptor and its substrates. J Biol Chem. 1993;268:26055–26058. [PubMed] [Google Scholar]
- 74.Stephens JM, Lee J, Pilch PF. Tumor necrosis factor-alpha-induced insulin resistance in 3T3–L1 adipocytes is accompanied by a loss of insulin receptor substrate-1 and GLUT4 expression without a loss of insulin receptor-mediated signal transduction. J Biol Chem. 1997;272:971–976. doi: 10.1074/jbc.272.2.971. [DOI] [PubMed] [Google Scholar]
- 75.Adair G. The Hawthorne effect: A reconsideration of the methodological artifact. J Appl Psychol. 1984;69:334–335. [Google Scholar]
- 76.Folstein M. Mini-Mental and son. Int J Geriatric Psychiatry. 1998;13:290–294. [PubMed] [Google Scholar]
- 77.Hautzinger M. The Beck Depression Inventory in clinical practice. [Der Beck-Depressionsinventar (BDI) in der Klinik] Nervenarzt. 1991;62:689–696. [PubMed] [Google Scholar]
- 78.Laux L, Glanzmann P, Schaffner P, Spielberger CD. The State-Trait-Angst Inventory. Theoretical Backgrounds and Manual. Weinheim, Germany: Beltz Test; 1981. [Das State-Trait-Angst Inventar Theoretischer Hintergrund und Manual] [Google Scholar]
- 79.Goodwin JS, Goodwin JM, Garry PJ. Association Between Nutritional-Status and Cognitive-Functioning in A Healthy Elderly Population. J Am Med Assoc. 1983;249:2917–2921. [PubMed] [Google Scholar]
- 80.Balk EM, et al. Effects of omega-3 fatty acids on serum markers of cardiovascular disease risk: A systematic review. Atherosclerosis. 2006;189:19–30. doi: 10.1016/j.atherosclerosis.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 81.Harris WS. n-3 fatty acids and serum lipoproteins: Human studies. Am J Clin Nutr. 1997;65:1645S–1654S. doi: 10.1093/ajcn/65.5.1645S. [DOI] [PubMed] [Google Scholar]
- 82.Walford RL, Mock D, MacCallum T, Laseter JL. Physiologic changes in humans subjected to severe, selective calorie restriction for two years in biosphere 2: Health, aging, and toxicological perspectives. Toxicol Sci. 1999;52:61–65. [PubMed] [Google Scholar]
- 83.Heilbronn LK, et al. Effect of 6-month calorie restriction on biomarkers of longevity, metabolic adaptation, and oxidative stress in overweight individuals—A randomized controlled trial. J Am Med Assoc. 2006;295:1539–1548. doi: 10.1001/jama.295.13.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Brownell KD, Cohen LR. Adherence to dietary regimens. 2: Components of effective interventions. Behav Med (Washington, DC) 1995;20:155–164. doi: 10.1080/08964289.1995.9933732. [DOI] [PubMed] [Google Scholar]
- 85.Maycock PF, Frayn KN. Use of alumina columns to prepare plasma samples for liquid-chromatographic determination of catecholamines. Clin Chem. 1987;33:286–287. [PubMed] [Google Scholar]
- 86.Helmstaedter C, Kurthen M. Memory and epilepsy: Characteristics, course, and influence of drugs and surgery. Curr Opin Neurol. 2001;14:211–216. doi: 10.1097/00019052-200104000-00013. [DOI] [PubMed] [Google Scholar]
- 87.Strauss E, Sherman EM, Spreen O. A Compendium of Neuropsychological Tests. Administration, Norms, and Commentary. Oxford, UK: Oxford Univ Press; 2006. [Google Scholar]
- 88.Knecht S, et al. High-normal blood pressure is associated with poor cognitive performance. Hypertension. 2008;51:663–668. doi: 10.1161/HYPERTENSIONAHA.107.105577. [DOI] [PubMed] [Google Scholar]
- 89.Hochhaus L. A table for the calculation of d′ and Beta. Psychol Bull. 1972;77:375–376. [Google Scholar]
- 90.Reitan RM, Herring S. A short screening device for identification of cerebral dysfunction in children. J Clin Psychol. 1985;41:643–650. doi: 10.1002/1097-4679(198509)41:5<643::aid-jclp2270410510>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 91.Markowitsch HJ, Harting C. Interdependence of priming performance and brain-damage. Int J Neurosci. 1996;85:291–300. doi: 10.3109/00207459608986690. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.