Abstract
The visual system, with its ability to perceive motion, is crucial for most animals to walk or fly steadily. Theoretical models of motion detection exist, but the underlying cellular mechanisms are still poorly understood. In this issue of Neuron, Rister and colleagues dissect the function of neuronal subtypes in the optic lobe of Drosophila to reveal their role in motion detection.
The visual system of animals discriminates different aspects of the visual world, including color, form, and depth. Perhaps the most important feature of the visual system is its ability to detect movement. Motion perception is an important prerequisite for flight control in flies and thus for their ability to search for food, to pursue mates, or even to escape from predators or a fly swatter. Large flies such as Calliphora and Musca, and more recently the fruit fly Drosophila, have been excellent model systems with which to study the principles of motion detection, although the cellular basis of this behavior remains unclear (Borst and Haag, 2002).
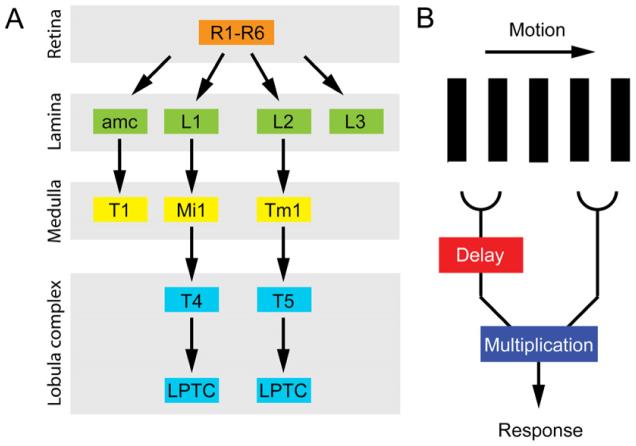
Visual information is first received in the retina. In the Drosophila compound eye, the retina contains about 800 ommatidia, each of which consists of six outer photoreceptors, R1–R6, and two inner photoreceptors, R7 and R8 (Hardie, 1985). While the inner photo-receptors are specialized for color vision, the outer photoreceptors are involved in a variety of tasks, including brightness detection, orientation behavior, and motion detection. R1–R6 project their axons to the lamina region of the optic lobe, where they each synapse onto the monopolar neurons L1, L2, and L3, as well as the amacrine cell amc (Figure 1A) (Meinertzhagen and Sorra, 2001). The terminations of photoreceptors and their target neurons in the lamina form so-called “cartridges” that parallel the organization of ommatidia in the retina (Braitenberg, 1967; Trujillo-Cenoz, 1965). These anatomical structures do not exactly represent individual ommatidia but are the “visual sampling units” of motion detection. L1–L3 project to a deeper region of the optic lobe, the medulla, whereas amc contacts the lamina processes of the medulla cell T1 (Fisch-bach and Dittrich, 1989).
Figure 1. Proposed Neural Pathways and Theoretical Model for Motion Detection.
(A) Proposed neural pathways (Bausenwein et al., 1992) originating from outer photoreceptors R1—R6. Neurons are color coded according to the location of their cell bodies within the optic lobes (photoreceptors in orange, lamina neurons in green, medulla neurons in yellow, T4/5 neurons and lobula complex neurons in blue).
(B) Model of the correlation-type motion detector (after Borst and Egelhaaf, 1989).
Despite the detailed description of the lamina neural network (Meinertz-hagen and Sorra, 2001), the function of individual cell types is not well understood. Theoretically, motion detection requires two inputs that are compared to provide information about the direction and speed of motion (Borst and Egelhaaf, 1989). These requirements are met by a correlation-type elementary motion detector (EMD) (Figure 1B). In its most basic form, the signal from one ommatidium (or more precisely a visual sampling unit) is delayed and then compared with the nondelayed signal from a neighboring unit. The introduced delay introduces asymmetry to the system and can thus inform about the direction of the motion.
Neural correlates for the EMD have not yet been identified, but experimental evidence shows that the so-called lobula plate tangential cells (LPTCs) in a deeper region of the optic lobe respond to visual motion in a direction-sensitive manner (Hausen, 1982; Hengstenberg, 1982), suggesting that the EMD directly provides input into LPTCs. Two different pathways have been proposed to instruct these cells (Figure 1A) (Bausenwein et al., 1992; Strausfeld, 1984). In the first pathway, the signal from R1–R6 appears to propagate from L1 through Mi1 and T4 to LPTC (Figure 1A). In the other pathway, R1–R6 are thought to activate L2, which subsequently relays the information through Tm1 and T5 to LPTC. Thus, L1 and L2 could represent the two offset inputs into the EMD (Braitenberg and Hauser-Holschuh, 1972). Alternatively, L1 and L2 might each be part of distinct EMDs that act redundantly or have specialized functions such as different velocity optima, directional sensitivities, or contrast sensitivities. Experimental evidence for or against any of these hypotheses is still lacking. Furthermore, none of these models propose where and how the delay is introduced. In this issue, Rister et al. (2007) investigate the function of the lamina neurons L1 and L2 and suggest a possible contribution of the amc/T1 pathway in motion detection and other related tasks.
To study the role of various lamina neurons and address possible parameters of the EMD, Rister and colleagues used a variety of sophisticated motion-detection assays as well as genetic manipulations. They evaluated the response to patterns of stripes rotating around individual flies in flying or walking flies. To be able to manipulate different lamina cell types, the authors identified GAL4 driver lines that show expression in both L1 and L2 neurons, as well as lines that specifically label L1, L2, or T1 neurons in the lamina. With these lines, synaptic transmission could be blocked in any combination of these neurons. Furthermore, the authors also used flies mutant in the ort gene, which encodes a histamine receptor subunit. As hista-mine is the exclusive neurotransmitter of photoreceptors, these flies are defective in neural transmission from photoreceptors to secondary neurons. By driving expression of wild-type ort with the specific GAL4 lines, Rister and colleagues could restore function only in selected secondary neurons and thus test for sufficiency of these neurons.
Using these tools, Rister and colleagues show that both L1 and L2 are involved in the response to moving striped patterns in stationary and walking flies, as well as the landing response in flight. Do these cells function redundantly, or are they both necessary? The answer depends on the context. When the patterns have a high contrast, L1 and L2 appear to be redundant and to support motion detection individually, as rescuing only L1 or only L2 function in ort mutant flies is sufficient to support motion detection. Interestingly though, at low contrast, both L1 and L2 pathways are required simultaneously, suggesting an interaction between them.
At intermediate contrast, which is prevalent in the environment, L1 and L2 appear to be more specialized. They have different sensitivities to pattern contrast or background light intensity: L2 can function at intermediate contrast or low light intensity, whereas L1 requires slightly higher pattern contrast and higher light intensity. Does this mean that L1 plays an accessory role to L2? This does not seem to be the case. When Rister and colleagues tested directional motion stimuli, they found that L1 mediates the detection of back-to-front motion (i.e., detecting objects catching up on the fly) at intermediate pattern contrast, whereas L2 is specialized in front-to-back motion (i.e., optic flow when the fly is moving) in the same conditions. Thus, for intermediate pattern contrast, the two pathways are differentially sensitive to the direction of motion.
Interestingly, the connectivity pattern of another lamina monopolar neuron, L4, suggests a mechanism for how input from neighboring ommatidia could be computed in the L2 pathway. L4 contacts the L2 neuron within the same cartridge as well as the L2 neurons in the adjacent posterodorsal and postero-ventral cartridges (Meinertzhagen and O’Neil, 1991); thus, L4 is ideally positioned to participate in the EMD, maybe introducing the required delay. Clearly, this is speculative, but it should be possible to address this issue through a better understanding of the connectivity of these cells.
Motion detection appears in fact even more complex, as the amc-T1 pathway is also involved in this process. Although inhibiting T1 alone does not affect motion detection at any pattern contrast, T1 apparently contributes to L1 function at intermediate pattern contrast, as interfering with synaptic transmission in both L2 and T1 significantly reduces the response to motion as compared to blocking L2 alone. How could the T1 pathway support L1 function? T1 does not constitute an independent pathway for motion detection, as it cannot support motion vision in the absence of L1 and L2 function. Therefore, it is likely to modulate the L1 pathway. However, T1 and L1 do not synapse in the lamina (Meinertzhagen and Sorra, 2001) and do not arborize in the same layers in the medulla (Fischbach and Dittrich, 1989). Thus, the T1 pathway might feed into the L1 pathway downstream of L1.
This study took advantage of the power of Drosophila molecular genetics to provide a uniquely detailed analysis of the first level of motion processing in Drosophila, shedding light onto the underlying neural mechanisms and identifying the cells involved in early stages of motion detection. Until now, conflicting models attempted to explain how motion is processed, but experimental data were missing. Although the problem is still not fully understood, this work provides the first steps toward a detailed description. Importantly, the previously suggested model wherein L1 and L2 form the two input channels into the motion detector is not correct for most of the conditions analyzed. Instead, L1 and L2 are part of specialized systems, at least at intermediate conditions.
Most importantly, Rister and colleagues provide a thorough framework for further analysis of the motion-detection pathway. It will be interesting to conduct similar analyses for the other lamina cell types and their medulla targets. The synaptic connections in the lamina are not as simple as described above, as many of the cells have multiple postsynaptic contacts, a prominent example being the photoreceptor-L2-L4 synapse (Meinertzhagen and Sorra, 2001). The function of L4 has not been addressed, but it will be important to test its function, especially as L4 interacts closely with L2. In addition, there are many feedback synapses from L2 and amacrine cells onto the photoreceptors as well as from “back-propagating” cells from the medulla. Finally, it will be important to unravel whether L1 and L2 signal through the proposed pathways involving T4 and T5, respectively (Bausenwein et al., 1992; Strausfeld, 1984).
In conclusion, this work offers an elegant paradigm for how the power of fly genetics allows for in-depth analysis of neural circuits. It may also inspire similar studies in other animal models.
REFERENCES
- Bausenwein B, Dittrich AP, Fischbach KF. Cell Tissue Res. 1992;267:17–28. doi: 10.1007/BF00318687. [DOI] [PubMed] [Google Scholar]
- Borst A, Egelhaaf M. Trends Neurosci. 1989;12:297–306. doi: 10.1016/0166-2236(89)90010-6. [DOI] [PubMed] [Google Scholar]
- Borst A, Haag J. J. Comp. Physiol. [A] 2002;188:419–437. doi: 10.1007/s00359-002-0316-8. [DOI] [PubMed] [Google Scholar]
- Braitenberg V. Exp. Brain Res. 1967;3:271–298. doi: 10.1007/BF00235589. [DOI] [PubMed] [Google Scholar]
- Braitenberg V, Hauser-Holschuh H. Exp. Brain Res. 1972;16:184–209. doi: 10.1007/BF00233996. [DOI] [PubMed] [Google Scholar]
- Fischbach KF, Dittrich AP. Cell Tissue Res. 1989;258:441–475. doi: 10.1007/BF00318687. [DOI] [PubMed] [Google Scholar]
- Hardie RC. In: Sensory Physiology 5. Ottoson D, editor. Springer-Verlag; Berlin, Heidelberg, New York, Tokyo: 1985. pp. 1–79. [Google Scholar]
- Hausen K. Biol. Cybern. 1982;46:67–79. [Google Scholar]
- Hengstenberg R. J. Comp. Physiol. [A] 1982;149:179–193. [Google Scholar]
- Meinertzhagen IA, O’Neil SD. J. Comp. Neurol. 1991;305:232–263. doi: 10.1002/cne.903050206. [DOI] [PubMed] [Google Scholar]
- Meinertzhagen IA, Sorra KE. Prog. Brain Res. 2001;131:53–69. doi: 10.1016/s0079-6123(01)31007-5. [DOI] [PubMed] [Google Scholar]
- Rister J, Pauls D, Schnell B, Ting C-Y, Lee C-H, Sinakevitch I, Morante J, Strausfeld NJ, Ito K, Heisenberg M. Neuron. 2007;56(this issue):155–170. doi: 10.1016/j.neuron.2007.09.014. [DOI] [PubMed] [Google Scholar]
- Strausfeld NJ. In: Photoreception and Vision in Invertebrates. Ali MA, editor. Plenum Press; New York: 1984. pp. 483–522. [Google Scholar]
- Trujillo-Cenoz O. J. Ultrastruct. Res. 1965;13:1–33. doi: 10.1016/s0022-5320(65)80086-7. [DOI] [PubMed] [Google Scholar]