Summary
In all living cells regulated passage across membranes of specific proteins occurs through a universally conserved secretory channel. In bacteria and chloroplasts, the energy for the mechanical work of moving polypeptides through that channel is provided by SecA, a regulated ATPase. Here we use site-directed spin labeling and electron paramagnetic resonance (EPR) spectroscopy to identify the interactive surface used by SecA for each of the diverse binding partners encountered during the dynamic cycle of export. Although the binding sites overlap, resolution at the level of aminoacyl sidechains allows us to identify contacts that are unique to each partner. Patterns of constraint and mobilization of residues on that interactive surface suggest a conformational change that may underlie the coupling of ATP hydrolysis to precursor translocation.
Keywords: SecA, EPR, translocon, SecB, precursors
Introduction
The general secretory system of Escherichia coli1 translocates polypeptides that are devoid of stable tertiary structure; precursors must be bound by components of the export apparatus before they fold. In some cases the nonnative precursor can be captured directly by SecA, but often SecB, a small cytosolic chaperone (molar mass 69 kDa), first interacts with the ligand. Subsequently a ternary complex is formed by binding SecA. SecA has high affinity for SecY and in this way the precursor is delivered to the translocon at the membrane. In a dynamic process that is not yet understood the ligand is passed from SecB to SecA and subsequently through the translocon channel. When SecA carrying a precursor, either with or without SecB in the complex, binds the translocon its ATPase activity is stimulated to couple the energy of hydrolysis to the mechanical work which moves the precursor through the channel. In vivo once the process has been started translocation can be completed by the energy of protonmotive force.
Movement of the precursor through the channel occurs in multiple, successive steps while SecA undergoes cycles of binding and hydrolysis of ATP. The transduction of the chemical energy to mechanical work involves conformational changes in SecA. Evidence for such changes has been presented1; however, the precise nature of the molecular movement remains undefined. Here we use site-directed spin labeling in combination with EPR spectroscopy to probe the surface of SecA that is in contact with each of the binding partners encountered during export. Our goal is to provide a detailed description at the resolution of aminoacyl sidechains of the interactions between the binding partners and the molecular movements that result from such interactions.
Results and Discussion
We created a collection of SecA variants, each carrying a nitroxide spin label (structure of spin label shown in Fig. 4) at one of 47 different positions. We have successfully applied this methodology to define the surface on spin-labeled SecB that interacts with its precursor polypeptide ligands2 as well as that which specifically binds SecA3. Those publications contain a detailed discussion of the information contained in EPR spectral line shapes. Briefly stated, the line shape reflects mobility of the sidechain, both the internal motion of the nitroxide and local fluctuations of the backbone on the nanosecond time scale. When spin-labeled SecA forms a complex, there are two ways in which mobility of the probe might change. If the sidechain were either directly in contact with a binding partner or were a near neighbor of an aminoacyl group that was, the motion of the nitroxide would be constrained resulting in broadening of the spectrum. On the other hand, as contacts are made with an element of structure of SecA conformational changes might be induced either at a distance or nearby that would result in release of constraints that existed in the free SecA protein. This would manifest itself as an increase in mobility of the nitroxide and would be observed as a narrowing of the spectrum. How changes in spectral line shape are deemed to be significant is further explained in Material and Methods.
We began our survey by random selection of positions on the surface of SecA. When a change in spectral line shape was observed upon formation of a complex we substituted neighboring aminoacyl residues in order to obtain patterns of constraint or mobilization. Every spin-labeled variant of SecA was examined in complex with each of the binding partners which include SecB, the precursor forms of two natural ligands for the export system, the periplasmic galactose-binding protein (pGBP) and the outer membrane protein OmpA (proOmpA) as well as the mature, but unfolded, form of galactose-binding protein. In addition, EPR spectra of SecA in complex with inverted membrane vesicles carrying the translocon, SecYEG, were compared with spectra of spin-labeled SecA in complex with liposomes to distinguish the specific effects of binding to SecY from those of binding to phospholipids.
In the course of this study approximately 700 spectral pairs were generated. Therefore we have chosen to summarize all of the data in Venn diagrams (Fig. 1a) and to show selected examples of spectra. The Venn diagrams clearly illustrate the degree of overlap among the residues that are constrained or mobilized by more than one of the diverse binding partners. Some residues in the Venn diagram are represented by a script font to indicate that either a constraint or mobilization is seen depending on binding partner (S600, M607, K609, Q644) or on temperature (D601, L645, S636). Residues for which no change in spectral line shape was observed with any of the binding partners are listed in Figure 1a and representative examples of spectra that display no change are shown in Figure 2. The distribution of the 47 residues that were examined is displayed as CPK models on the protomer of SecA (Figs. 1b and 1c) in an open conformation (see discussion below).
Fig. 1.
Constraints and mobilizations. For key to the color scheme see text. (a) Venn Diagrams. Script font indicates residues constrained or mobilized depending on ligand. (b) Constrained sites, displayed as CPK models. (c) View in (b) rotated 180° around the x-axis. (d) Sites that are mobilized by lipids (orange) or by both lipids and SecB (blue-gray) displayed as CPK models. Note, residue K4 is not resolved in this structure. These structures and the subsequent structures, except those in Fig. 3, are PDB code 2FSF with the PBD modeled in, based on the B. subtilis SecA PBD (PDB code 1TF5)8.
Fig. 2.
Positions on SecA that show no significant change when in complex with any of the binding partners. Black traces, spin-labeled SecA alone; red traces, SecA with binding partner indicated. A subset of residues with the binding partners as indicated was chosen to illustrate the precise overlay of spectra. All spectra were acquired at 6 °C.
For sites that showed constraint the color scheme used indicates the structural subdomain in which they reside (the Venn diagrams use the same color scheme). Residues that showed no constraints are displayed in gray. The subdomains of SecA include two nucleotide-binding folds, NBF1 (yellow) and NBF2 (light brown), a precursor binding domain, PBD (pink), a long α-helix, the helical scaffold domain, HSD (blue), a helical wing domain, HWD (purple), an intramolecular regulator, IRA1 (dark brown), and a short 10 residue α-helix (residues 600 – 609, green) that links NBF2 to HSD. The amino-terminal residues 1 – 8 and the C-terminal domain (residues 836 – 901) were not resolved in the structure.
In the case of mobilization, all positions that showed changes upon the binding of inverted membrane vesicles containing SecYEG also showed mobilization when bound to liposomes (Fig. 1a). Therefore it seems likely that it is binding to phospholipids, not binding to SecYEG, that results in mobilization. Figure 1d displays those residues mobilized by lipids in orange, and residues mobilized by SecB as well as by liposomes in blue-gray.
SecA has been crystallized in five dimeric forms which differ in interfacial contacts4; 5; 6; 7; 8. The protomer structures are very closely related and display only two different conformations, the open state9 and the closed state5. We have chosen to display the contact sites on the open conformation for two reasons. Firstly, an NMR study by Gelis et al.10 provides evidence that in solution E. coli SecA is 90% in the open form and only 10% closed. Secondly, the line shape of residue S350 indicates a degree of mobility that is consistent with its position in the open form where it is on the surface (Fig. 3) and could move freely. In the closed state it is buried (Fig. 3) and movement would be restricted (for further discussion of line shape see Materials and Methods). A 60° rigid body rotation of the PBD (Fig. 3, pink) relates the open conformation (Fig. 3, domains colored individually) to the closed (Fig. 3, green). This movement breaks the interface between the PBD and HSD (blue) and HWD (purple) resulting in the formation of a groove between these structural elements. Osborne and Rapoport9 suggested that precursors bind into this groove, based on examination of the structure. However, the interactive surface that we have defined in this study for all ligands examined (Fig. 1b, all constraints), i.e., polypeptide ligands (>Fig. 7), SecB (Fig. 9) and SecY (Fig. 10) lies to the opposite side of the PBD.
Fig. 3.
Comparison of the two conformations of SecA: closed, green, PDB code 1M6N; open, colored by domain, PDB code 1TF5. The PBD is shown in CPK to illustrate the change in accessibility of the nitroxide at position S350 (E. coli residue number). The nitroxide is colored by atom: carbon, gray; oxygen, red; nitrogen, blue; sulfur, yellow.
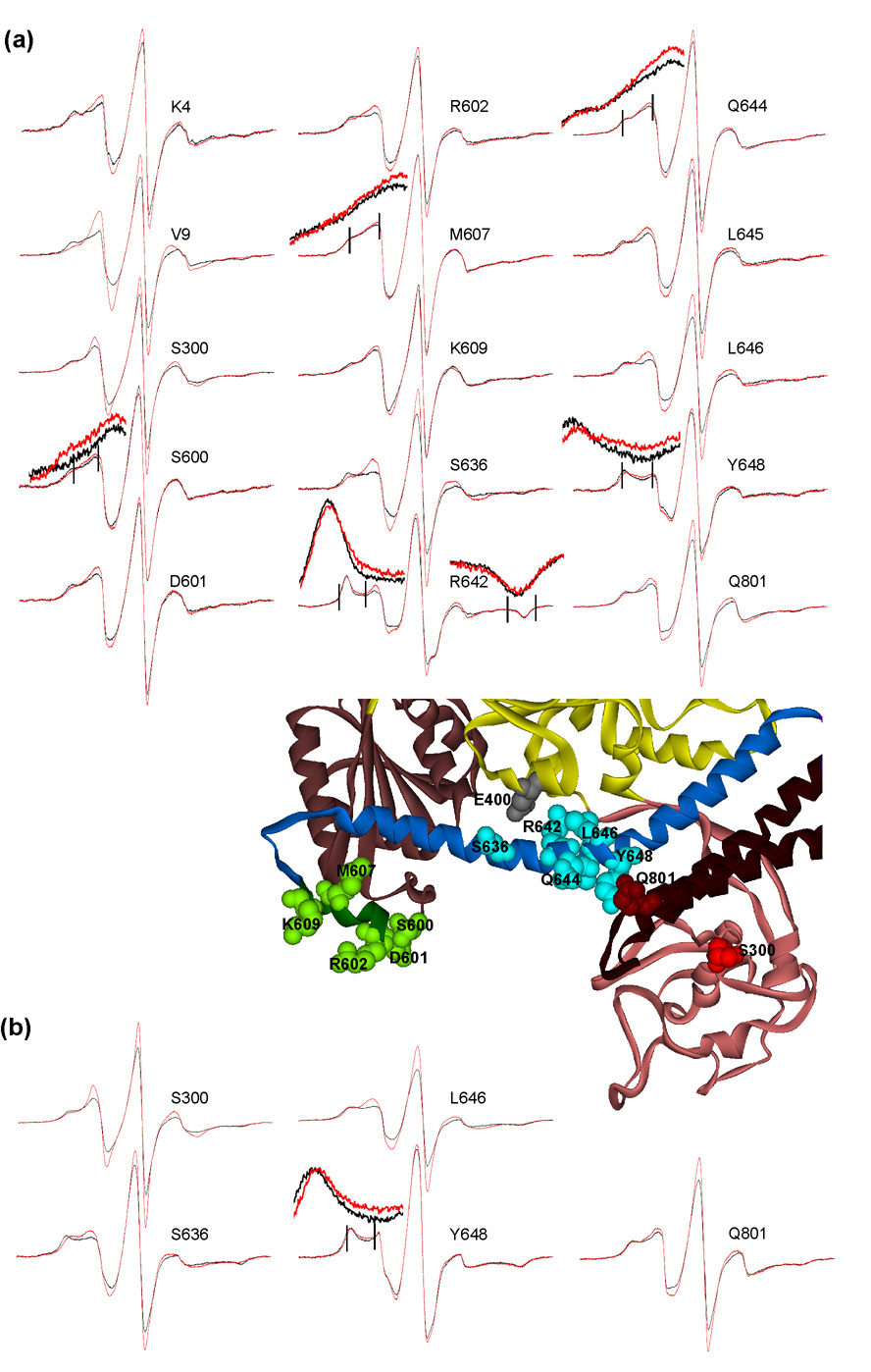
A selection of the spectra of the nitroxide probes at sites that showed constraints are displayed in Figure 4 and Figure 6. All remaining spectra that showed constraints are displayed in Supplementary Data. Visual inspection of the spectra of residues I641 (Fig. 4a) and G11 (Fig. 4b) with and without binding partners illustrates the changes that result from constraint. The broadening of a spectrum manifests itself by a drop in amplitude of the central line of the normalized spectra and movement of intensity outward to the hyperfine extrema, most easily seen on the low field side (indicated by arrow). The residues that are constrained (with the exception of A52) lie on one face of SecA (Fig. 1b) located either on the long α-helix of the HSD (blue CPK), on the short linker helix (green CPK) or near the amino terminus (dark yellow CPK). The interactive surface extends from this face into a wide cleft which contains sites of contact in the PBD (red CPK)
Fig. 4.
Spectra of constrained residues. Black traces, spin-labeled SecA alone; red traces, spin-labeled SecA in complex with ligand. (a) Binding of polypeptide ligands as indicated. The arrows indicate position of low field hyperfine extrema for residues I642 and G11. Spectra acquired at 6°C. (b) Binding of SecB. Temperature of acquisition indicated. The insets show the region of the spectra, indicated by vertical lines, magnified by a factor of four for both the intensity and the field.
Let us consider the details of each of the binding surfaces in turn, beginning with that for precursors. In defining the binding site for an unfolded polypeptide ligand we used two natural precursors which carry signal sequences and also the mature form of one, galactose-binding protein. Each spin-labeled SecA species was tested with each of the polypeptide ligands. We have chosen one ligand to display for each residue in Figure 4a. All polypeptide ligands, including the mature form, caused the same degree of change in spectral line shape for each of the residues. Therefore, the site we have defined is not specific for signal sequences but rather interacts with the unfolded polypeptide ligand. If the polypeptide is folded into a stable tertiary structure it shows no interaction, even if it carries a signal sequence. This was demonstrated by addition of the folded forms of the precursor and mature galactose-binding to spin-labeled SecA. No change in line shape was observed (Fig. 5). The specific binding of the unfolded state of a polypeptide is similar to the well-characterized interaction of unfolded polypeptides with the chaperone SecB11. Polypeptide ligands incubated with SecA in the absence or in the presence of SecB (data not shown for presence of SecB) showed constraints at the same residues and of the same magnitude; therefore, we conclude that the same surface on SecA provides contact sites for polypeptides independent of the means of delivery of the ligand.
Fig. 5.
Constraints require unfolded ligands. (a) Precursor galactose-binding protein. (b) Mature galactose-binding protein. Left panels: Black traces are SecAI641 alone; red traces are SecAI641 with ligand in 1 mM EGTA which maintains the ligands in an unfolded state. Right panels: black traces are SecA alone and green traces are SecAI641 with the ligands after addition of 3 mM CaCl2 which causes the ligands to partition to a folded state.
Consideration of our data in addition to that provided by other investigators allows us to propose a model for the interaction of precursor polypeptides with SecA. The structure of a complex between a synthetic signal peptide and SecA determined by NMR10 shows contact with 13 aminoacyl residues (Fig. 7, orange stick representation) that lie on one side of the groove (Fig. 7a, arrow) that was originally suggested by Osborne and Rapoport as a potential binding site9. In addition a signal sequence carrying a photoreactive group has been used to label a peptide of SecA (residues 269 – 322, orange ribbon, Fig. 7a) that is in this region12. Contact sites that we have identified (Fig. 7a, green and blue CPK) define the opening to a large cleft (indicated by the diamond) that lies to the side of the PBD opposite the groove discussed above. Rotation of the structure by 90° around the x-axis provides a view of the floor of the cleft (Fig. 7b) making visible the two β-strands that are the stem of the PBD (bright pink ribbons). This stem has been suggested to bind signal peptides by several groups5; 8; 13. In addition to these β-strands, Chou and Gierasch14 postulated interaction with residues that lie nearby (Fig. 7, residues 218, 226, 228 and 229, pink stick representation). Residue S350 (red CPK), which showed a strong constraint in this study, is on the wall of the cleft formed by PBD. The opposite wall is formed by NBD2. We suggest that the signal sequence interacts with the narrow groove as defined by others and that the mature portion of the polypeptide crosses over the IRA1 to make contact on HSD and the linker helix as it enters the wide cleft. The residues within this same cleft that were proposed by others to bind signal sequences5; 8; 13; 14 might instead function to bind regions within the mature portion of polypeptide ligands. Whether SecA functions as a dimer during export is currently controversial, however, a polypeptide ligand might easily occupy the binding site shown here on one protomer and extend into the corresponding site on a second protomer if a dimer were the active species.
Addition of SecB alone to the collection of spin-labeled SecA variants defined the SecB binding site. Examination of the Venn diagram (Fig. 1a) shows that two residues which bind SecB, V9 and S350, make contacts with all binding partners and three others (two in the linker helix and one in HSD) are shared by precursors and SecB. It is not surprising that SecB and the polypeptide ligands show overlapping binding sites since SecB most likely delivers the polypeptides to the same surface on SecA that SecA uses to bind precursors directly. Two residues in the linker helix that make contact with SecB also serve as contact sites for SecY. Only one residue, M607, is specific for SecB, since G11 is also a contact site for phospholipids (see discussion below).
We have previously shown that SecA and SecB form two types of complexes differing in stoichiometry depending on whether or not zinc is present in the zinc-binding domain in the C terminus15. If zinc is present, this domain binds onto the flat β-sheets that form the sides of SecB and a complex is formed that contains two protomers of SecA bound to a tetramer of SecB (we refer to this as A2:B4). If zinc is lacking, only one protomer binds yielding a complex of stoichiometry A1:B415. The spin-labeled SecA variants that contained a single cysteine for modification have had the zinc-coordinating cysteines removed. As expected these species form complexes with SecB that have a stoichiometry A1:B4 (Fig. 8a, zincless SecA spin labeled at S350 is shown as an example). To determine that the same contacts are present in the A2:B4 complexes the residues that showed constraint with SecB (Fig. 1a) were engineered into a species of SecA which retained the three zinc-coordinating cysteines. All of these spin-labeled variants were shown to form A2:B4 complexes by size-exclusion column chromatography (Fig. 8b, SecA containing zinc spin labeled at S350 is shown as an example) therefore substitution by the spin label did not displace the zinc. When SecA containing zinc was in complex with SecB all residues, with the exception of G11 which will be investigated in a future study, showed the same degree of constraint as that seen in complexes between SecB and the SecA species which lacked zinc. Therefore, we conclude that the contact surface in complexes is the same whether one or two protomers of SecA are bound to a tetramer of SecB.
Fig. 8.
Comparison of spin-labeled species of SecA with and without zinc in complex with SecB. Samples containing 6 µM SecA dimer and 6 µM SecB tetramer were subjected to size-exclusion chromatography and the eluent was monitored to determine protein concentration by change in refractive index. Molar mass was determined by total intensity light scatter. The traces represent concentration and the symbols represent molar mass. (a) Complexes with zincless SecA. Samples applied were SecB (gray-green); zincless SecA (red); zincless SecA spin-labeled at S350 (green); SecB and zincless SecA (blue); SecB and zincless SecA spin-labeled at S350 (brown). (b) Complexes with SecA species containing zinc. Samples applied were SecB (gray-green); SecA containing zinc (red); SecA containing zinc labeled at S350 (green); SecB and SecA containing zinc (blue); SecB and SecA containing zinc spin-labeled at S350 (brown).
The binding site on SecA for SecB defined by these constraints is shown in Figure 9>. As seen in the view on the right, the floor of the cleft is formed by NBD1 (yellow) whereas NBD2 forms one wall (light brown) and PBD forms the other. The movies included in Supplementary Data gives an impression of the depth of this cleft. The central structure in Figure 9 shows one possible orientation of SecB bound to SecA. The CPK models on SecB are the residues identified as contact sites for SecA in our previous EPR study3. For simplicity we show only one protomer of SecA bound to SecB. SecB is organized structurally as a dimer of dimers. Because of steric considerations it is likely that the second protomer of SecA docks onto the upper surface of SecB, as it is oriented in Figure 9, making contacts with the dimer shown colored blue-green. The C-terminal region of SecB, residues 142 to 155, has been shown to bind SecA within the first 11 residues15. In the structure shown, resolution ends at residue 146 (Fig. 9, arrow) so the missing nine residues might easily extend to contact V9 and G11 (golden yellow CPK) on SecA. This docking model differs from that which we previously proposed in a study which had the nitroxide probes on SecB3. At that time we docked both protomers of SecA in closed conformation onto one surface of SecB in anti-parallel fashion. This choice of orientation of SecA was based on the position of the C termini, which are known contacts for SecB, in the crystal structure of the Bacillus subtilis closed form. The E. coli structure had not yet been solved. As noted here we feel the mobility indicated by the line shapes and the pattern of constraints is more consistent with binding to the open form.
Complexes between SecA and SecY show a pattern of constraints similar to that seen with both polypeptide ligands and SecB. The constrained residues include those that lie at the entrance to the cleft in the HSD and in the linker helix as well as S350, which is within the cleft. Four other residues that lie within the cleft (R220, T221, H339 and M344) make contact with SecY, but not with other binding partners. Thus it seems that SecY penetrates the cleft more deeply than do SecB or the precursors. Figure 10 shows the contact surface for SecYEG in stereo to emphasize the depth of the cleft. To display all surfaces the structure is rotated sequentially 90° around the x-axis away from the viewer (see movies in Supplementary Data for impression of the depth of the cleft as well as distribution of residues which show no changes (gray) on all surfaces). Likely candidates for binding partners in SecY that could penetrate into the cleft of SecA are the cytoplasmic loops between helices TM6 and TM7 and between TM8 and TM916; 17. An X-ray structure of a ribosome bound to a SecY complex suggests that during cotranslational export these cytoplasmic loops insert into the polypeptide exit tunnel of the ribosome18. Thus the loops of SecY might function analogously to capture the precursor from either SecA or the ribosome.
The residues constrained show that SecY engages SecA through the same interactive face as do the other ligands. This conclusion is in agreement with an elegant, in vivo study by Jilaveanu and Oliver19 that identified this same face of SecA as the surface that is in contact with the aqueous periplasmic compartment through the translocon pore. These conclusions do not necessarily mean that in order to engage SecY, SecA must release SecB. Experimental evidence is to the contrary. SecA has high affinity for SecB when SecA is bound to the translocon20, and SecB is released only when SecA binds ATP21. Not only does SecA interact with SecB and SecY through the same surface, but indeed four of the same residues (V9, S350, S604, G605) are used by both. This apparent contradiction is resolved if we consider that within the complex between SecB and SecA the binding interactions are asymmetric even though both components have two-fold symmetry15. In addition the interfacial contacts which stabilize the antiparallel dimer of SecA are blocked from forming15. Thus one protomer of SecA could be released from a complex with SecB by breaking a subset of interactions freeing the sites on SecA for binding SecY. If release from SecB were accompanied by transfer of the precursor from SecB to SecA then one protomer might bind the translocon to deliver the precursor while the other protomer might stay associated with SecB to bind the next more distal segment of the precursor. In this way stepwise translocation of the precursor might be achieved.
SecA binds phopholipids in the absence of the translocon. This lipid binding is thought to be of physiological significance since strains depleted for acidic phospholipids are defective in protein export22. Candidates for lipid binding sites on SecA are G11, near the amino terminus and K609 in the linker helix. Constraint of these residues was observed at 6°C (Fig. 6) and therefore contact is likely to be with the lipid head groups since the bilayer would be in an ordered condensed state. At 27°C, G11 remained constrained but K609 became mobilized. All other sites that showed changes in spectral line shape upon interaction with liposomes (15 out of the total 47 examined) demonstrated mobilization (Fig. 11). Interaction of SecA with SecB increases mobility of a subset of the residues mobilized by lipids (Fig. 1d, blue-gray, mobilized by SecB or lipid; orange, mobilized by lipid only, Fig. 11). This subset of residues were also engineered into the SecA species containing zinc and shown to display the same degree of mobilization in the A1:B4 and the A2:B4 complexes (data not shown). For residues mobilized by binding SecB, the extent of change is higher at 23°C (Fig. 11) than at 6°C (data not shown) and is the same as that seen for binding lipids at the higher temperature. It is likely that a conformational change would occur more readily at the higher temperature because flexibility of SecA would be increased. Although SecB binding does not appear to mobilize the linker helix, it should be noted that SecB makes contacts with five of the ten residues and therefore increased mobility resulting from a change in conformation might be obscured.
Fig. 6.
Spectra of constrained residues. (a) Binding of SecY. (b) Binding of lipid. Traces and insets are as described in Fig. 4.
The conformational change in SecA that results in mobilization of residues might be caused by direct propagation of movement from the sites of contact to the elements mobilized or indirectly by disruption of interfacial contacts of a SecA dimer which in turn causes further changes. We presently cannot distinguish these possibilities. Both of the residues identified as contact sites for SecB and lipids are involved in interfacial contacts in the reported B. subtilis dimeric structures: G11 in the antiparallel dimer5 and K609 in the intertwined dimer4. We have reported that binding SecB disrupts interfacial contacts15 and it has been observed that at low concentrations binding liposomes renders SecA monomeric23. However, at concentrations near those used in this study SecA remained dimeric when associated with lipids unless nucleotides were present24.
The observed pattern of mobilization (Fig. 11) could be explained by a rolling movement of the long α-helix in HSD away from the NBDs freeing residues within that helix as well as residue Q801 (dark brown), which makes contact with HSD. Movement of the linker helix away from direct contact with the body of the protein would relieve constraints at S600, D601 and M607. However, residues R602 and K609, which point away from NBF1, also show increased mobility. If the conformational change disrupted the helical nature of this region, the backbone would become more flexible thereby increasing mobility of all residues. The mobilized residues that lie outside of the HSD and the linker helix are in the PBD (S300) and IRA1 (Q801). Thus the change in conformation is propagated to elements that are likely to be involved in the movement of polypeptides through the translocon.
The rolling movement we propose to explain the increase in mobility is similar to that proposed by Mori and Ito25. Variants of SecA with substitutions at E400 (Fig. 11, gray CPK) and R642 that could not form a salt bridge were defective in export in vivo and in vitro and this defect was suppressed by the mutational change M607T in the linker helix. These observations led to the proposal that a crucial conformational change involved movement of HSD and the linker helix away from the NBDs.
Further support for the relevance of the movement observed here is found in an NMR study26 which showed that the coupling of ATP binding and hydrolysis to translocation involves a shift in equilibrium between disorder and order of the nucleotide binding cleft. This shift is allosterically propagated to the PBD. The binding of ATP favors the ordered state of the interface of NBD1 and NBD2 whereas after hydrolysis bound ADP favors disorder. The high ATPase activity characteristic of SecA poised for translocation is suppressed by interactions between the NBDs and both HSD and IRA127. Thus, the rolling movement of HSD away from the NBDs induced by binding SecB or lipids could account for their demonstrated ability to activate SecA ATPase28; 29. After translocation is initiated, the cycle of order-disorder transitions coupled to ATP binding and hydrolysis could result in the cyclic rolling away of HSD followed by rebinding. These movements of the HSD and linker helix, which are binding sites for precursors and for SecY, could serve as the structural transducer of the chemical energy of ATP hydrolysis to the mechanical work of moving precursors from SecA to SecY and through the translocon.
It is crucial to the function of SecA that one interactive surface serves to bind multiple partners. Passage of a precursor captured by the chaperone SecB to SecA occurs in a ternary complex at the interface between SecA and SecB2. Delivery of precursor from SecA to SecY would also occur most efficiently if SecY were bound over the same surface that holds the precursor. The disposition of the residues specific for each partner on the common surface shows characteristic patterns that would allow simultaneous binding of precursor and either SecB or SecY to facilitate transfer. Figure 12 displays residues 636 through 648 of the HSD and residues 600 through 609 of the linker helix as helical wheels. One face of the HSD (Fig. 12a) is packed against the NBD’s. Residues 636, 647 and 640 that are at the top of the wheel would be facing up into solution from the surface of the long HSD (blue) as displayed in Figure 7a, Figure 9(right side), Figure 10a and Figure 11. The residues on the right-hand side of the wheel point into the entrance of the cleft and residues 638 and 645 at the bottom of the wheel lie on the floor of the cleft (Fig. 7b and Fig. 10b) near the entrance.
Fig. 7.
Binding surface for polypeptide ligand. Contact sites identified in this study, CPK models; sites identified by others, ball and stick representations. The view in (b) is related to that in (a) by a 90° rotation about the x-axis away from the viewer. The arrow indicates the position of the groove originally suggested by Osborne and Rapoport as a potential binding site. The asterisk indicates the opening to the large cleft that we show interacts with ligands.
The residue on the HSD identified as a contact site for SecB is located at the upper surface (residue 636, light blue) whereas sites that contact precursor polypeptides (green) are found not only on the upper surface but wrap around the helix and extend into the cleft. The SecYEG binding site overlaps with that of the precursors, starting at the mouth of the cleft and extending to residues in the helix that lie on the floor. The remaining contact sites for SecYEG are in the PBD and lie deep within the cleft on the floor (R220) or the walls (T221, H339 and M344). Only S350 on the wall of the cleft makes contact with all binding partners.
The helical wheel in Figure 12b displays the residues of the linker in the same orientation as the wheel in Figure 12a, i.e., the residues at the top would extend upward into solution. This helix is not tightly packed against other structural elements but is positioned along side of the HSD above NBD2 which forms the wall of the cleft (see Fig. 7, Fig. 9, Fig. 10a and 10b). The disposition of contact sites around this helix is not as distinctly defined as for the HSD. Although sites for SecYEG are confined to the lower half, the contact sites for both precursor and SecB overlap with those for SecYEG. The sites for precursor are skewed to the face opposite those for SecYEG and sites for SecB are found distributed around the entire helix. It should be noted that this helix contains residues that show mobilization by contact of SecA with lipids and thus might not remain in a helical structure during the functional cycle of SecA.
Incorporation of all data presented here in addition to data of others suggest a model for the interactions of SecA with its binding partners. When SecA receives a precursor polypeptide from SecB or binds it directly, the signal sequence would be held in the narrow groove and the polypeptide would cross over the IRA1 to interact at HSD and the linker helix at the mouth of the cleft. When SecY binds SecA on this same surface the precursor would lie at the interface, as it did in the SecA:SecB complex. As SecA undergoes a conformational change the rolling of the HSD and the coupled movements of the linker helix, IRA1 and PBD might bring the precursor further into the cleft and alter the accessibility of sidechains resulting in release of the precursor from SecA and transfer to SecY.
The knowledge of the location and relationships of the binding sites on SecA established here sets the foundation for further elucidation of the dynamic interplay between SecA and its binding partners during conversion of chemical energy to the mechanical work of protein translocation.
Materials and Methods
Mutagenesis and Protein Purification
The strategy of site-directed mutagenesis requires that only one cysteine be available for modification. Therefore as our base protein we used a SecA species in which the four native cysteines were replaced with serines. The plasmid pT7SecAC4 (from D. Oliver, Wesleyan University) carrying the gene for the base protein under the T7 promoter was modified by standard recombinant DNA techniques (Quickchange, Stratagene) to generate the single cysteine variants used here. The plasmids were transformed into BL21(DE3) and the SecA variants purified from the appropriate strains as described15 with the following exceptions: the harvested cells were suspended at 2g/mL in 20 mM HEPES (KOH), pH 7.6 and stored at −80 °C; the cell suspension was thawed on ice and the following additions were made: dithiothreitol (DTT) to 2 mM, DNase to 10µg/mL, Mg(OAc)2 to 3 mM and phenylmethylsulfonylfluoride (PMSF) to1 mM final concentrations. Cells were mechanically disrupted using a French pressure cell at 8000 psi in two successive passes with DTT (2 mM) added between each pass and centrifuged at 65,000 rpm for 2 hours (Ty65 rotor, Beckman) at 4 °C. The supernatant was filtered with a syringe filter containing a polyethersulfone membrane, size 0.2 um, and applied to a 5 mL HiTrap Blue HP affinity column (GE Healthcare) equilibrated in 10 mM HEPES (KOH), 2 mM DTT, pH 7.6. The column was washed with the same buffer containing 0.4 M NaCl. SecA was then eluted with 10 mM HEPES (KOH), 50% ethylene glycol, 1.5 M NaCl, 2 mM DTT, pH 7.6. The pool containing SecA was concentrated and stored at −80 °C until spin-labeling (see below). For the subset of SecA species that showed constraint or mobilization with SecB the cysteine substitutions were also put into a SecA gene that retained the three native cysteines that coordinate zinc.
Precursor galactose-binding protein30 and proOmpA31 were purified as described. The protein concentrations were determined spectrophotometrically at 280 nm using extinction coefficients of 78,900 M−1cm−1 for SecA monomer, 47,600 M−1cm−1 for SecB tetramer, 37,410 M−1cm−1 for precursor galactose-binding protein and 52,955 M−1cm−1 for proOmpA.
Inner Membrane Vesicles and Liposomes
Inner membrane vesicles containing high levels of SecYEG were prepared as described32. As a control that constraints seen with complexes between SecA and inner membrane vesicles were specific for SecYEG, we obtained vesicles from D. Amin (G. Hazelbauer) that did not overproduce SecYEG. Liposomes were prepared from E. coli polar lipids (Avanti) by extrusion using a LiposoFast apparatus (Avestin, Inc.) with a 100 nm pore polycarbonate membrane. The lipid concentration in both vesicles and liposomes was determined as described on the Avanti Polar Lipids Website (www.avantilipids.com) and used at a final concentration of 15 mM.
Spin Labeling of SecA Variants
Immediately before labeling with the nitroxide reagent (1-oxyl-2,2,5,5-tetramethylpyrroline-3-methyl)-methanethiosulfonate (Toronto Research Chemicals Inc.), the reducing agent, DTT, was removed by exchange into 10 mM Hepes-HAc, 300 mM KOAc (pH 6.7) using a Nap10 column (Amersham). The proteins were concentrated using a Nanosep Centrifugal Device with Omega membrane, 30K MWCO (Pall). Typically 0.2 mL of each SecA variant, at a concentration of approximately 0.44 mM monomer, was incubated with a 2.5 fold molar excess of the spin labeling reagent. For labeling of species in the SecA variants that contained the three cysteines which ligate zinc as well as the cysteine to be labeled the spin label was added at 1.2 molar equivalence. This resulted in the preferentially labeling of the substituted cysteine without reaction of the zinc-coordinating cysteines. The reagent was prepared as a stock of 100 mM in acetonitrile and stored in the dark at −80 °C. Acetonitrile was kept below 2% of the total volume in the incubation reaction. The reaction was allowed to proceed on ice in the dark for 1 – 2 h. To remove excess spin label the entire reaction mixture was applied to either a Nap5 or Nap10 column (Amersham) equilibrated in 10 mM HEPES (HAc), 300 mM KOAc, 5 mM Mg(OAc)2, 1 mM EGTA (pH 6.7) and eluted in ten 150 uL fractions. The fractions were examined by EPR spectroscopy and were pooled to minimize free spin. If necessary, the protein was concentrated using a Nanosep Centrifugal Device with Omega membrane, 30K MWCO (Pall) and stored at −80 °C. The spin-labeled variants are designated by the amino acid and residue number that has been substituted by cysteine and modified by the nitroxide side-chain.
EPR Measurements
EPR spectroscopy was performed on an X-band Bruker EMX spectrometer with a high sensitivity resonator. Protein samples of 5 µL were loaded into synthetic silica capillaries (0.6 mm i.d.× 0.84 mm o.d.) sealed at one end. Spectra were acquired using incident microwave power at 20 mW, and a 100 kHz field modulation of 1 to 3 gauss as appropriate. Spectra were recorded with a scan width of 100 gauss centered at 3356 gauss. For each spectral line 15 scans were acquired. The molecular tumbling of SecA (monomeric mass, 102 kDa), either free or in complex with the binding partners studied here, is too slow to be averaged into the spectra. All spectra were normalized and further analyzed using the Labview programs written by Christian Altenbach (UCLA).
For each experiment SecA was used at 30 µM dimer (60 µM spin label) and the binding partners added as follows: SecB tetramer, 60 µM; precursor ligands, 60 µM; inner membrane vesicles or liposomes to a final concentration of 15 mM lipid. The basic buffer was 10 mM Hepes-HAc, 300 mM KOAc, 5 mM Mg(OAc)2, pH 6.7. Additional components introduced with the precursor ligands were also added to the solution of SecA alone so that conditions with and without ligand were the same. Spectra were acquired for all complexes both at 6 °C and near room temperature.
To form complexes with precursors the ligands, unfolded in denaturant (1 M GnHCl or 4 M urea) were added by rapid dilution into a solution held on ice containing SecA so that the final concentration of denaturant was 0.17 M for GnHCl or 0.4 M for urea. Galactose-binding protein is an ideal ligand for these studies because the rate of folding is drastically decreased in the absence of calcium30. Therefore for experiments with galactose-binding protein 1 mM EGTA was included in the buffers. All spectra were collected at 6 °C to slow the rate of folding. To demonstrate that folded ligands do not interact with SecA the ligands were diluted from the denaturant into a solution containing 3 mM CaCl2 (in addition to 1 mM EGTA) and incubated for 15 minutes at room temperature. This results in the folding of both mature and precursor forms of galactosebinding protein. The mixture was returned to ice, spin-labeled SecA was added and the spectrum acquired at 6 °C. When spectra were acquired for polypeptide ligands at 23 °C very little constraint was seen even for proOmpA, which does not partition to folded but aggregates and does not bind.
Analysis of EPR spectral line shapes
The line shape reflects the mobility of a nitroxide. As the probe goes from highly mobile to constrained two features change. The central line width broadens which is also seen as a decrease in intensity of the central line since all spectra are normalized. Second, the overall spectral breadth increases, that is, the total intensity is spread over a wider range of the magnetic field. It is this change in line shape that indicates a significant constraint. As intensity moves from the center out toward the extrema it also becomes broadened. Therefore the observed increase at the extrema appears to be smaller than the decrease in amplitude of the central line. Spectra are conventionally shown normalized to the same area of total spin. However, during analysis we always examine a “same amplitude” comparison as well which forces the central lines to the same amplitude so that changes in line shape are more obvious. For example, the small change in the center line of the spectra shown in Fig. 5A is not significant since the same amplitude analysis showed that the lines overlay exactly.
The line shape also provides information about the position of a residue within a structure. If a residue makes many contacts within the tertiary structure it will be very constrained as seen for residue I642 (Fig. 11). Notice the very distinct hyperfine extrema which are indicative of a highly ordered spin (i.e., low mobility). Residue S350, on the other hand, has a spectrum consistent with a surface exposed residue. Note the similarity of the line shape of residue S350 with residues S600, D601, K609 and S636 shown in Fig. 11. These are all characteristic of surface exposed residues in α-helices. The characteristic line shapes for isotropic, anisotropic, ordered and rapid motion were described in our earlier paper Crane et al.3 and further in depth discussions can be found in Columbus et al.33.
Mobilization
In our study we have interpreted narrowing of spectra and increase in amplitude of the central line as increase in mobility of the nitroxide probe. It must be noted that if two nitroxides are in close proximity, less than ~25 Å apart, dipolar coupling will decrease the amplitude of the spectra. Therefore if the residues moved apart the amplitude would increase, however, there would be no change in line shape. The intensity would not move in from the outer extrema toward the center. All the changes in the spectra that we attribute to mobilization show changes in line shape and therefore do not result from a decrease in spin coupling.
Activity Assays
To ascertain that neither removal of the native cysteines nor subsequent introduction of the nitroxide at newly substituted cysteines disrupted structure or function all variants were subjected to analysis by high performance liquid chromatography on a TSK 3000SW (TosoHaas) in 10 mM Hepes-KOH pH 6.7, 300 mM KOAc, 5 mM Mg(OAc)2 at 7 °C15. The absolute molar mass of proteins was determined directly using static light scatter by passing the eluent through a multi-angle laser light scatter detector followed by a differential refractometer (DAWN-EOS and Optilab Rex, respectively; using a specific refractive index increment (dn/dc) of 0.2 mL/gm and the Debye plotting formalism of the Astra software supplied with the instrument. All SecA variants were folded, demonstrated a monomer-dimer equilibrium (monomer molar mass 102 kDa), and were shown to bind SecB. Figure 8 shows examples of spin-labeled SecA species compared to unlabeled SecA. The different species of SecA all show elution profiles characteristic of a self-associating system, an asymmetric peak with a sharp leading edge and a tail. The mass is highest at the apex and decreases as the concentration drops. Complexes between SecB and SecA species containing zinc elute well ahead of the complexes with the zincless SecA species and show higher molar mass characteristic of A2:B4. For demonstration that these eluting complexes differ in stoichiometry and not in affinity see our previous study15. The exception was SecA with a nitroxide at I642. The protein showed no aggregation, but the equilibrium was shifted to dimer. It did not bind SecB, but bound precursor and SecYEG.
The spin-labeled species that contain zinc were shown to be active in an in vitro translocation assay15 using proOmpA. For the SecA species that do not contain zinc, representative residues from each element of structure as well as from the three classifications of interaction (constraint, no change and mobilization) were assessed for ATPase activity34 and shown to have the expected level of stimulation of membrane ATPase by addition of proOmpA.
Generation of Movies
The movies in Supplementary Data were generated using the eMovie plugin35 within the PyMOL molecular graphics system (http://www.pymol.org).
Supplementary Material
Two movies associated with this article can be found in the online version.
Supplementary Fig. 1. Spectra of constrained residues. Black traces, spin-labeled SecA alone; red traces, spin-labeled SecA in complex with ligand. (a) Binding of polypeptide ligands as indicated. Spectra acquired at 6°C. (b) Binding of SecB. Temperature of acquisition indicated. The insets show the region of the spectra, indicated by vertical lines, magnified by a factor of four for both the intensity and the field.
Supplementary Fig. 2. Spectra of constrained residues. Binding of SecY. Traces and insets are as described in Supplementary Fig. 1.
Movies. Contact sites for SecYEG. The domains are colored as described in the main text. Spin-labeled residues on SecA showing constraints by interaction with SecYEG are colored red. The residues shown in gray are those that showed no change with any binding partner. Movie 1 rotates around the x-axis. Movie 2 rotates around the y-axis.
Fig. 9.
Binding surfaces for SecB on SecA. Views in right panels are related to those in the left by a 90° rotation away from the viewer about the x-axis. In the left panel sidechains of residues 337 through 339, which masked S350 visually, were removed. The central structure illustrates one possible way SecB could bind to SecA and make contacts at the residues which are constrained. The arrow indicates position 146 at which the resolution of the X-ray structure of SecB ends. Nine residues are missing.
Fig. 10.
Stereo view of contact sites on SecA for SecYEG. The CPK models are colored by domain as described in the main text. Spin-labeled residues on SecA showing constraints by interaction with SecYEG are colored light green in the linker helix, bright blue in the helical scaffold domain, red in the precursor binding domain and light brown in the nucleotide binding domains. The views in (a) through (d) are rotated sequentially 90° around the x-axis away from the viewer to display all surfaces. The residues shown in gray are those that showed no change with any binding partner.
Fig. 11.
Spectra of mobilized residues. (a) Lipids. (b) SecB. Mobilized residues are shown as CPK models and colored by domain, except for E400, gray, which is discussed in the text. All spectra were gathered at 27 °C. The insets are as described in Fig. 4.
Acknowledgements
We thank Samuel Grinter for help in protein purification, Divya Amin for the inner membrane vesicles that do not contain SecYEG, Wing-Cheung Lai for Figure 12, and Chunfeng Mao for assistance with the in vitro activity assays. We are grateful to Anastassios Economou for providing the coordinates of E. coli SecA with the PBD. This work was supported by NIH grant GM29798 and an endowment from the Hugo Wurdack Trust at the University of Missouri.
Fig. 12.
Helical wheel showing disposition of constrained residues. The color scheme is: white, residues not tested; gray, residues that showed no constraint; red, residues constrained in complexes with SecYEG; green, residues constrained in complexes with precursor polypeptides; light blue, residues constrained in complexes with SecB. (a) Residues 636 through 648. The helix is oriented with the long axis from amino to carboxyl terminus pointing into the page (indicated by the X). NBD represents the nucleotide binding folds that are in contact with the HSD. The curved arrow within the wheel indicates the proposed direction of the rolling of the helix. (b) Residues 600 through 609. The helix is oriented with the long axis from amino to carboxyl terminus pointing out toward the reader (indicated by the dot).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Papanikou E, Karamanou S, Economou A. Bacterial protein secretion through the translocase nanomachine. Nat. Rev. Microbiol. 2007;5:839–851. doi: 10.1038/nrmicro1771. [DOI] [PubMed] [Google Scholar]
- 2.Crane JM, Suo Y, Lilly AA, Mao C, Hubbell WL, Randall LL. Sites of interaction of a precursor polypeptide on the export chaperone SecB mapped by site-directed spin labeling. J. Mol. Biol. 2006;363:63–74. doi: 10.1016/j.jmb.2006.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crane JM, Mao C, Lilly AA, Smith VF, Suo Y, Hubbell WL, Randall LL. Mapping of the docking of SecA onto the chaperone SecB by site-directed spin labeling: insight into the mechanism of ligand transfer during protein export. J. Mol. Biol. 2005;353:295–307. doi: 10.1016/j.jmb.2005.08.022. [DOI] [PubMed] [Google Scholar]
- 4.Zimmer J, Li W, Rapoport TA. A novel dimer interface and conformational changes revealed by an X-ray structure of B. subtilis SecA. J. Mol. Biol. 2006;364:259–265. doi: 10.1016/j.jmb.2006.08.044. [DOI] [PubMed] [Google Scholar]
- 5.Hunt JF, Weinkauf S, Henry L, Fak JJ, McNicholas P, Oliver DB, Deisenhofer J. Nucleotide control of interdomain interactions in the conformational reaction cycle of SecA. Science. 2002;297:2018–2026. doi: 10.1126/science.1074424. [DOI] [PubMed] [Google Scholar]
- 6.Sharma V, Arockiasamy A, Ronning DR, Savva CG, Holzenburg A, Braunstein M, Jacobs WR, Jr, Sacchettini JC. Crystal structure of Mycobacterium tuberculosis SecA, a preprotein translocating ATPase. Proc. Natl. Acad. Sci. U. S. A. 2003;100:2243–2248. doi: 10.1073/pnas.0538077100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vassylyev DG, Mori H, Vassylyeva MN, Tsukazaki T, Kimura Y, Tahirov TH, Ito K. Crystal structure of the translocation ATPase SecA from Thermus thermophilus reveals a parallel, head-to-head dimer. J. Mol. Biol. 2006;364:248–258. doi: 10.1016/j.jmb.2006.09.061. [DOI] [PubMed] [Google Scholar]
- 8.Papanikolau Y, Papadovasilaki M, Ravelli RB, McCarthy AA, Cusack S, Economou A, Petratos K. Structure of dimeric SecA, the Escherichia coli preprotein translocase motor. J. Mol. Biol. 2007;366:1545–1557. doi: 10.1016/j.jmb.2006.12.049. [DOI] [PubMed] [Google Scholar]
- 9.Osborne AR, Clemons WM, Jr, Rapoport TA. A large conformational change of the translocation ATPase SecA. Proc. Natl. Acad. Sci. U. S. A. 2004;101:10937–10942. doi: 10.1073/pnas.0401742101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gelis I, Bonvin AM, Keramisanou D, Koukaki M, Gouridis G, Karamanou S, Economou A, Kalodimos CG. Structural Basis for Signal-Sequence Recognition by the Translocase Motor SecA as Determined by NMR. Cell. 2007;131:756–769. doi: 10.1016/j.cell.2007.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Randall LL, Hardy SJS. High selectivity with low specificity: how SecB has solved the paradox of chaperone binding. Trends Biochem. Sci. 1995;20:65–69. doi: 10.1016/s0968-0004(00)88959-8. [DOI] [PubMed] [Google Scholar]
- 12.Musial-Siwek M, Rusch SL, Kendall DA. Selective photoaffinity labeling identifies the signal peptide binding domain on SecA. J. Mol. Biol. 2007;365:637–648. doi: 10.1016/j.jmb.2006.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Papanikou E, Karamanou S, Baud C, Frank M, Sianidis G, Keramisanou D, Kalodimos CG, Kuhn A, Economou A. Identification of the preprotein binding domain of SecA. J. Biol. Chem. 2005;280:43209–43217. doi: 10.1074/jbc.M509990200. [DOI] [PubMed] [Google Scholar]
- 14.Chou YT, Gierasch LM. The conformation of a signal peptide bound by Escherichia coli preprotein translocase SecA. J. Biol. Chem. 2005;280:32753–32760. doi: 10.1074/jbc.M507532200. [DOI] [PubMed] [Google Scholar]
- 15.Randall LL, Crane JM, Lilly AA, Liu G, Mao C, Patel CN, Hardy SJS. Asymmetric binding between SecA and SecB two symmetric proteins: implications for function in export. J. Mol. Biol. 2005;348:479–489. doi: 10.1016/j.jmb.2005.02.036. [DOI] [PubMed] [Google Scholar]
- 16.Mori H, Ito K. An essential amino acid residue in the protein translocation channel revealed by targeted random mutagenesis of SecY. Proc. Natl. Acad. Sci. U.S.A. 2001;98:5128–5133. doi: 10.1073/pnas.081617398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mori H, Ito K. Different modes of SecY-SecA interactions revealed by site-directed in vivo photo-cross-linking. Proc. Natl. Acad. Sci. U.S.A. 2006;103:16159–16164. doi: 10.1073/pnas.0606390103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Menetret JF, Schaletzky J, Clemons WM, Jr, Osborne AR, Skanland SS, Denison C, Gygi SP, Kirkpatrick DS, Park E, Ludtke SJ, Rapoport TA, Akey CW. Ribosome binding of a single copy of the SecY complex: implications for protein translocation. Mol. Cell. 2007;28:1083–1092. doi: 10.1016/j.molcel.2007.10.034. [DOI] [PubMed] [Google Scholar]
- 19.Jilaveanu LB, Oliver DB. In vivo membrane topology of Escherichia coli SecA ATPase reveals extensive periplasmic exposure of multiple functionally important domains clustering on one face of SecA. J. Biol. Chem. 2007;282:4661–4668. doi: 10.1074/jbc.M610828200. [DOI] [PubMed] [Google Scholar]
- 20.Hartl FU, Lecker S, Schiebel E, Hendrick JP, Wickner W. The binding cascade of SecB to SecA to SecY/E mediates preprotein targeting to the E. coli plasma membrane. Cell. 1990;63:269–279. doi: 10.1016/0092-8674(90)90160-g. [DOI] [PubMed] [Google Scholar]
- 21.Fekkes P, van der Does C, Driessen AJ. The molecular chaperone SecB is released from the carboxy-terminus of SecA during initiation of precursor protein translocation. EMBO J. 1997;16:6105–6113. doi: 10.1093/emboj/16.20.6105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Vrije T, de Swart RL, Dowhan W, Tommassen J, de Kruijff B. Phosphatidylglycerol is involved in protein translocation across Escherichia coli inner membranes. Nature. 1988;334:173–175. doi: 10.1038/334173a0. [DOI] [PubMed] [Google Scholar]
- 23.Or E, Boyd D, Gon S, Beckwith J, Rapoport T. The bacterial ATPase SecA functions as a monomer in protein translocation. J. Biol. Chem. 2005;280:9097–9105. doi: 10.1074/jbc.M413947200. [DOI] [PubMed] [Google Scholar]
- 24.Bu Z, Wang L, Kendall DA. Nucleotide binding induces changes in the oligomeric state and conformation of Sec A in a lipid environment: a small-angle neutron-scattering study. J. Mol. Biol. 2003;332:23–30. doi: 10.1016/s0022-2836(03)00840-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mori H, Ito K. The long alpha-helix of SecA is important for the ATPase coupling of translocation. J. Biol. Chem. 2006;281:36249–36256. doi: 10.1074/jbc.M606906200. [DOI] [PubMed] [Google Scholar]
- 26.Keramisanou D, Biris N, Gelis I, Sianidis G, Karamanou S, Economou A, Kalodimos CG. Disorder-order folding transitions underlie catalysis in the helicase motor of SecA. Nat. Struct. Mol. Biol. 2006;13:594–602. doi: 10.1038/nsmb1108. [DOI] [PubMed] [Google Scholar]
- 27.Karamanou S, Vrontou E, Sianidis G, Baud C, Roos T, Kuhn A, Politou AS, Economou A. A molecular switch in SecA protein couples ATP hydrolysis to protein translocation. Mol. Micro. 1999;34:1133–1145. doi: 10.1046/j.1365-2958.1999.01686.x. [DOI] [PubMed] [Google Scholar]
- 28.Lill R, Dowhan W, Wickner W. The ATPase activity of SecA is regulated by acidic phospholipids, SecY, and the leader and mature domains of precursor proteins. Cell. 1990;60:271–280. doi: 10.1016/0092-8674(90)90742-w. [DOI] [PubMed] [Google Scholar]
- 29.Miller A, Wang L, Kendall DA. SecB modulates the nucleotide-bound state of SecA and stimulates ATPase activity. Biochem. 2002;41:5325–5332. doi: 10.1021/bi025639p. [DOI] [PubMed] [Google Scholar]
- 30.Topping TB, Randall LL. Chaperone SecB from Escherichia coli mediates kinetic partitioning via a dynamic equilibrium with its ligands. J. Biol. Chem. 1997;272:19314–19318. doi: 10.1074/jbc.272.31.19314. [DOI] [PubMed] [Google Scholar]
- 31.van der Does C, de Keyzer J, van der Laan M, Driessen AJ. Reconstitution of purified bacterial preprotein translocase in liposomes. Methods Enzymol. 2003;372:86–98. doi: 10.1016/s0076-6879(03)72005-9. [DOI] [PubMed] [Google Scholar]
- 32.Woodbury RL, Topping TB, Diamond DL, Suciu D, Kumamoto CA, Hardy SJS, Randall LL. Complexes between protein export chaperone SecB and SecA. Evidence for separate sites on SecA providing binding energy and regulatory interactions. J. Biol. Chem. 2000;275:24191–24198. doi: 10.1074/jbc.M002885200. [DOI] [PubMed] [Google Scholar]
- 33.Columbus L, Kalai T, Jeko J, Hideg K, Hubbell WL. Molecular motion of spin labeled side chains in alpha-helices: analysis by variation of side chain structure. Biochem. 2001;40:3828–3846. doi: 10.1021/bi002645h. [DOI] [PubMed] [Google Scholar]
- 34.Levit MN, Grebe TW, Stock JB. Organization of the receptor-kinase signaling array that regulates Escherichia coli chemotaxis. J. Biol. Chem. 2002;277:36748–36754. doi: 10.1074/jbc.M204317200. [DOI] [PubMed] [Google Scholar]
- 35.Hodis E, Schreiber G, Rother K, Sussman JL. eMovie: a storyboard-based tool for making molecular movies. Trends Biochem. Sci. 2007;32:199–204. doi: 10.1016/j.tibs.2007.03.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Two movies associated with this article can be found in the online version.
Supplementary Fig. 1. Spectra of constrained residues. Black traces, spin-labeled SecA alone; red traces, spin-labeled SecA in complex with ligand. (a) Binding of polypeptide ligands as indicated. Spectra acquired at 6°C. (b) Binding of SecB. Temperature of acquisition indicated. The insets show the region of the spectra, indicated by vertical lines, magnified by a factor of four for both the intensity and the field.
Supplementary Fig. 2. Spectra of constrained residues. Binding of SecY. Traces and insets are as described in Supplementary Fig. 1.
Movies. Contact sites for SecYEG. The domains are colored as described in the main text. Spin-labeled residues on SecA showing constraints by interaction with SecYEG are colored red. The residues shown in gray are those that showed no change with any binding partner. Movie 1 rotates around the x-axis. Movie 2 rotates around the y-axis.