Abstract
A universal cellular defense mechanism against viral invasion is the elimination of infected cells through apoptotic cell death. To counteract host defenses many viruses have evolved complex apoptosis evasion strategies. The oncogenic human retrovirus HTLV-I is the etiological agent of adult-T-cell leukemia/lymphoma (ATLL) and the neurodegenerative disease known as HTLV-associated myelopathy/tropical spastic paraparesis (HAM/TSP). The poor prognosis in HTLV-I-induced ATLL is linked to the resistance of neoplastic T cells against conventional therapies and the immunocompromised state of patients. Nevertheless, several studies have shown that the apoptotic pathway is largely intact and can be reactivated in ATLL tumor cells to induce specific killing. A better understanding of the molecular mechanisms employed by HTLV-I to counteract cellular death pathways remains an important challenge for future therapies and the treatment of HTLV-I-associated diseases.
Introduction
Apoptosis, or programmed cell death, plays a major role in tissue development, homeostasis, and the immune response [1]. Virus-infected cells are frequently removed from the body through apoptosis, effectively eliminating the infection in the absence of an inflammatory response. Apoptosis is tightly controlled by a group of cysteine proteases known as caspases, as well as the Bcl-2 family of proteins which regulate the release of pro-apoptotic proteins from the mitochondria. Despite multiple levels of regulation, deregulated apoptosis contributes to the development of cancer, while excessive apoptosis is conversely associated with tissue destruction seen in various autoimmune disorders [2]. To regulate apoptosis induced by the host, many viruses have evolved strategies to modulate key checkpoints of the apoptotic pathway. Some viruses, such as members of the γ-herpesvirus family, encode a homologue of cellular anti-apoptotic Bcl-2 [3]. A variety of other novel viral anti-apoptotic mechanisms have been characterized, including: caspase inhibitors (i.e. poxviruses, murine herpes virus-68, and African swine fever virus); soluble cytokine receptors (EBV); the inhibition of cellular stress responses (Papillomaviridae, Polyomaviridae, and Adenoviridae); and the inhibition of death receptor-mediated apoptosis (γ-herpesviruses and poxviruses) [4-7]. A number of DNA viruses, such as poxviruses, adenoviruses, and human cytomegalovirus (CMV), also encode mitochondrial-localized inhibitors of apoptosis which function to regulate cytochrome c release [5].
In stark contrast to these anti-apoptotic mechanisms, other viruses appear to sensitize cells to apoptosis to the benefit of virus replication and egress. Human immunodeficiency virus (HIV) and hepatitis B (HBV) virus encode pro-apoptotic alpha-helical proteins Vpr and HBX that form pores in the mitochondrial membrane [5], thereby sensitizing the mitochondria to cytochrome c release. Other viral proteins, including E1A from adenovirus, the envelope protein from HIV, human papilloma virus (HPV) protein E1E4, the fusion protein from respiratory syncytial virus (RSV), and the reovirus protein mu1, also induce apoptosis through various mechanisms, including the disruption of the mitochondrial network and p53 activation [8, 9]. While some of these viral proteins, such as Vpr and HBX, specifically induce programmed cell death to the benefit of the virus, other viral proteins such as E1A appear to induce apoptosis as a consequence of detection by innate cellular defense mechanisms.
HTLV-1: Human T-cell leukemia virus type I
The retrovirus human T-cell leukemia virus (HTLV)-1 is the etiological agent of adult T-cell leukemia/lymphoma (ATLL), a fatal lymphoproliferative disease [10]. While the majority of HTLV-1-infected individuals remain asymptomatic, upwards of 5% of patients ultimately develop ATLL. ATLL is characterized by the rapid and uncontrolled clonal proliferation of mature transformed CD25+CD4+ T cells, and the mean survival of patients in the acute phase of the disease is approximately 6 months [11]. HTLV-1-infection is also associated with a neurodegenerative disease known as HTLV-associated myelopathy/tropical spastic paraparesis (HAM/TSP)[12, 13]. Other autoimmune diseases, including uveitis, arthritis, polymyositis, Sjögren syndrome, atopic dermatitis, and alveolitis, have been reported in HTLV-I infected individuals [13]. Altogether, the treatment of HTLV-I-infected patients is generally difficult as infected cells are refractory to conventional chemotherapy and radiation-based cancer treatments.
HTLV-1-infected cells and ATLL cells from patients are highly resistant to multiple pro-apoptotic stimuli, including death receptor-mediated, DNA damage-induced, and γ-irradiation apoptosis compared to uninfected normal cells [14-18]. HTLV-1-infected ATLL cells removed from the in vivo environment, however, die spontaneously by apoptosis when cultured in vitro, thereby complicating investigations into mechanisms employed by patient-derived ATLL cells [19]. As a result, most studies with ATLL and HTLV-1-infected cells rely on HTLV-1-transformed cells in vitro or short term culture of ATLL derived cells.
In contrast to ATLL, TSP/HAM is associated with chronic and progressive inflammation of the spinal cord [12]. TSP/HAM derived cell lines, like ATLL [20, 21], also exhibit resistance to FasL- and etoposide-induced apoptosis [22] [23], and FasL and the Fas-associated phosphatase are upregulated in TSP/HAM cells [24] [22, 25]. While TSP/HAM cell lines exhibit a general resistance to apoptosis, expression of the viral protein Tax sensitizes astrocytomas to programmed cell death, and HTLV-1-infection induces the expression of IL-1β, IL-1α, IL-6, TNF-α, TNF-β[26]. A rat model for HTLV-1 infection demonstrated a role for apoptosis in the destruction of oligodendricytes and Schwann cells associated with the down-regulation of Bcl-2 and the up-regulation of Bax and p53 [27, 28]. Future work is needed to fully elucidate the roles of programmed cell death and the induction of a pro-inflammatory response in this chronic inflammatory disease.
HTLV-I exhibits several unique properties not seen in other animal onco-retroviruses. The end of the proviral genome contains several open reading frames encoding for the regulatory proteins p12, p30, p13 and HBZ (Figure 1), which are involved in virus infectivity, immune escape, and the establishment of a latent state [29]. The viral protein Rex binds an RNA element (RxRE) present in the 3’ region of the viral mRNA and stimulates the transport of unspliced or singly spliced viral RNA to the cytoplasm to express structural proteins. Perhaps the most studied viral protein is the viral transcriptional transactivator Tax, which is involved in cellular transformation and specifically interacts with CREB, coactivators CBP/p300, and PCAF to stimulate transcription from the viral long terminal repeat (LTR)[30-34]. Tax plays an important role in the initiation of cellular transformation and also stimulates cellular proliferation by inactivating several cell cycle checkpoints [35-37]. In addition, several studies have shown that Tax inhibits the nucleotide excision repair (NER) pathway, beta-polymerase and topoisomerase [37-39]. While these events may facilitate cellular transformation, it is likely that cells need to acquire a pre-tumoral genotype and tolerance to Tax expression before transformation takes place.
Figure 1.
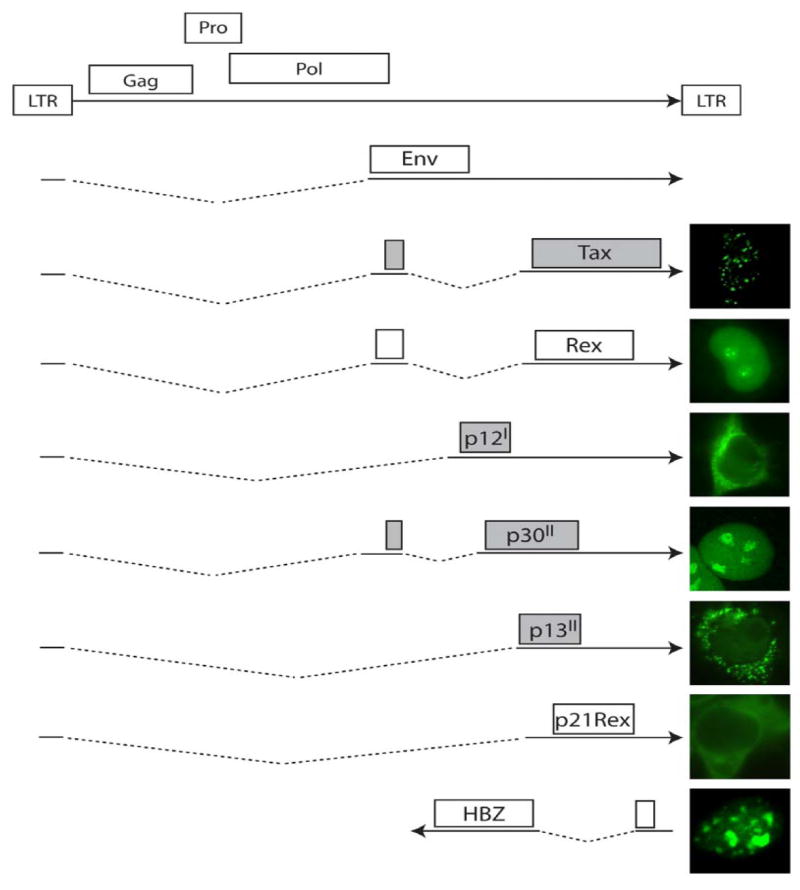
Proteins encoded by HTLV type I. Multiple differentially spliced mRNA molecules transcribed from the genome of HTLV-1 encode for a dozen known proteins, with transcription initiated via the long terminal repeat (LTR). Homologues of proteins such as Gag, Pol (polymerase), Pro (protease), and Env (envelope protein) are also found in other retroviruses such as HIV, and are responsible for virus replication and virion formation. The remaining non-structural proteins characterized to date, such as Tax, Rex, p13, p12, p30, p21Rex and HBZ, are unique proteins translated from the pX region of the viral genome, and their localization is shown at right. Proteins shaded in grey have been shown to play either a direct or indirect role in modulating the apoptotic cascade in HTLV-1-infected cells.
Recent studies have shown that the apoptotic pathway can be reactivated in HTLV-1-transformed cells, indicating that the apoptotic machinery is likely intact. It is the focus of this review to examine the underlying mechanisms HTLV-1 uses to repress apoptosis, and to highlight the therapies being evaluated to reactivate and trigger the apoptotic pathway in HTLV-1-transformed cells and infected patients.
HTLV-I Tax: Regulation of NF-κB, Akt, and gene expression
Tax is a potent trans-activator of transcription, and induces the constitutive activation of the major cellular pro-survival pathways NF-κB and Akt. Twenty years ago, it was first documented that Tax could induce transcription from the interleukin-2 gene via NF-κB related factors, indicating that Tax could activate NF-κB-regulated genes [40, 41]. It has since been demonstrated that Tax activates NF-κB through several different mechanisms. Tax can directly interact with IKKγ, ultimately triggering the continual phosphorylation and ubiquitin-mediated degradation of IκB to allow NF-κB translocation to the nucleus (Figure 1)[42-45]. The direct activation of IKK by Tax has recently been demonstrated using an in vitro assay [46], and this activation step requires the phosphorylation of IKK. Alternatively, Tax can form a complex with the p100 NF-κB precursor protein along with IKKα/IKKγ to facilitate the cleavage of p100 into the active p52 NF-κB subunit [47]. Thirdly, Tax can interact directly with NF-κB subunits to facilitate NF-κB transcriptional activation [48-50], and has also been shown to directly recruit transcriptional co-activators CBP/p300 to NF-κB complexes in the nucleus [32, 51, 52].
The nuclear translocation and activation of NF-κB can lead to the transcriptional up-regulation of a number of anti-apoptotic proteins (Fig. 1). One potent anti-apoptotic protein up-regulated by Tax-mediated NF-κB and CREB activation is Bcl-xL [53, 54], and T-cells from HTLV-1-infected patients correspondingly display up-regulated levels of Bcl-xL [55]. In support of the role that NF-κB plays in the inhibition of cell death in HTLV-I infected cells, drugs which inhibit NF-κB are potent inducers of tumor cell death in vitro [56](Discussed below, see Table 1). The induction of NF-κB activation by Tax also increases expression of the inhibitor of apoptosis (IAP) family (Fig. 1) [57, 58]. IAPs are capable of directly binding to caspases, and can induce caspase degradation. Indeed, siRNA directed against one IAP, HIAP, greatly sensitized cells to apoptosis, suggesting HIAP expression may be important for Tax-mediated survival [58]. The cell regulatory protein p21 is also transactivated by Tax, and contributes to an anti-apoptotic phenotype of Tax-immortalized cells via the transactivation of NF-κB/CREB leading to the activation of anti-apoptotic genes [59]. The T-cell co-stimulatory molecule 4-1BB (TNFRSF9/CD137/ILA), which is involved in cell proliferation and survival, is also up-regulated by Tax, likely through NF-κB [60].
Table 1.
Drugs which induce apoptosis in HTLV-1-infected cells
| Drug Target | Drug Name | Class | Predicted Mechanism of Action | References |
|---|---|---|---|---|
| NF-κB | Bortezomib/PS-341 | Proteasome inhibitor | Stabilizes IκB, p21, p53 and Tax; ceramide induction | [123-125, 156, 178] |
| ACHP | Inhibits IKK activity | [122] | ||
| Bay 11-7082 | Inhibits IKK activity | [121] | ||
| Fludarabine | Purine analogue | Inhibits NF-κB nuclear translocation | [128] | |
| NIK-333 | Synthetic retinoid | Cell cycle arrest, NF-κB inhibition, IAP down-regulation | [179] | |
| Ritonavir | Protease inhibitor | Inhibits NF-κB | [129] | |
| DHMEQ | Epoxyquinomycin derivative | Inhibits NF-κB, p65 nuclear translocation | [180, 181] | |
| Galectin-9 (modified protease resistant) | Lectin | Inhibits IκB phosphorylation | [182] | |
| FR901228/depsipeptide | Histone deacetylase inhibitors | Inhibits NF-κB and AP-1 DNA binding | [183] | |
| Capsaicin | Capsaicinoid | Upregulation of IκBα, Tax degradation | [184] | |
| L-lysine | Inhibits p65 NF-κB subunit | [185] | ||
| As2O3 + IFNα | NF-κB inhibition, stabilization of IκBα, cell cycle arrest, Tax down-regulation | [132-134, 136-138, 186] | ||
| Adenosine-2,3-dialdehyde (Adox) | adenosine analog, methyltransferase inhibitor | IKK degradation, p53 reactivation, cell cycle arrest | [187] | |
| Reactive Oxygen Species | DHA | Polyunsaturated fatty acid | ROS production, in combination with As2O3 and emodin | [139] |
| Emodin | Anthraquinone | ROS production, in conjunction with As2O3 and DHA | [139] | |
| Cell cycle | ATRA, 9-cis-RA, 13-cis-RA, | Retinoic acids | Cell cycle arrest, ceramide accumulation | [160, 161, 163] |
| Ascorbic acid | Inhibition of proliferation, alterations in gene expression | [188] | ||
| LY294002 | PI3 kinase inhibitor | PI3 kinase inhibitor, inhibits AKT activation | [189] | |
| Epigallocatechin-3-gallate | Antioxidant | Induces cell cycle arrest | [190] | |
| EAPB0203 | imidazo[1,2-a]quinoxalines | Induces cell cycle arrest, p53 stabilization | [191] | |
| Gene Expression | Fucoidan | Polysaccharide | Inactivates NF-κB and AP-1 | [192] |
| Resveratrol | Polyphenol | Downregulation of survivin | [193] | |
| DCQ | Heterocylic aromatic antimicrobial | Upregulation of TGF-β1, p53, p21 | [194] | |
| Roscovitine | Purine analogue | Inhibits STAT5 activity | [115] | |
| 17-AAG | Geldanamycin derivative | Inhibitor of Hsp-90 | [145] | |
| Curcumin | Polyphenol | Inhibits AP-1, NF-κB, AKT, induces cell cycle arrest | [142, 143] | |
| Valproate | Histone deacetylase inhibitor | Transcriptional activation | [195, 196] | |
| MS-275, SAHA, LBH589 | Histone deacetylase inhibitors | Inhibits NF-κB nuclear translocation | [197] | |
| Dihyrdoflavanol BB-1 | Increase in TRAIL-R2 expression | [198] | ||
| Celecoxib | COX-II inhibitor | Inhibits Akt activation | [199] | |
| Epican Forte | Nutrient formula | Induces p53, p21, Bax; Downregulates Bcl-2 | [200] | |
| AG490 | Tyrosine kinase inhibitor | Inhibits Jak/STAT pathway | [141] | |
| Cell surface proteins | Anti-Tf receptor | Binds to overexpressed transferrin receptor | [152, 158] | |
| Anti-IL-2Rα (CD25) | Interacts with overexpressed IL-2 receptor | [150, 151, 155, 201] | ||
| Anti-CD52 | Interacts with overexpressed CD52 | [157] |
17-AAG, geldanaymcin derivative; ACHP, 2-amino-6-[2-(cyclopropylmethoxy)-6-hydroxyphenyl]-4-piperidin-4-yl nicotinonitrile; ATRA, all-trans-retinoic acid; COX-II, cyclooxygenase II; DHA, docosahexaenoic acid; DHQ, 2-benzoyl-3-phenyl-6,7-dichloroquinoxaline 1,4-dioxide; DHMEQ, dehydroxymethyleopxyquinomicin; Hsp, heat shock protein; IAP, inhibitor of apoptosis; IFN, interferon; IKK, inhibitor of κB kinase; IL, interleukin; RA, retinoic acid; ROS, reactive oxygen species; STAT, signal transducer and activator of transcription; Tf, transferrin; TGF, transforming growth factor; TRAIL, TNF-related apoptosis-inducing ligand.
Another cell signaling pathway modulated by Tax is Akt, a pro-survival serine/threonine kinase that is constitutively activated in most ATLL patients [61]. Akt is phosphorylated on Serine473 in most ATLL patients, and Tax promotes this by interacting with and activating the upstream phosphatidylinositol-3-kinase (PI3K) [62, 63]. Activated Akt induces the downstream activation of additional transcription factors such as AP-1 and β-catenin [64] (Fig. 1), leading to Bcl-xL expression, p53 repression, and cell survival. Indeed, under specific conditions treatment of HTLV-1-infected cells with LY294002, an inhibitor of the PI3K pathway, induces cell death [61, 65], supporting the role that Akt plays in Tax-mediated cell survival. As well, certain reports have suggested that there is a cross-talk between Akt and NF-κB [61].
In addition to the activation of the NF-κB and Akt pathways, Tax also alters the transcription factor AP-1 [66, 67], although the specific effects of Tax-mediated AP-1 activation remain to be characterized. Tax also modulates a number of apoptotic genes via unknown mechanisms. Tax induces the production of cellular FLICE-inhibitory proteins (c-FLIPs) [68], which can inhibit CD95-induced cell death. HTLV-1 infection also induces the expression of the telomerase gene hTERT to protect transformed cells from replicative senescence [69]. Interestingly, hTERT also has the ability to inhibit mitochondrial cell death induced by specific pro-apoptotic stimuli [70]. Whether the induction of hTERT expression by Tax also has pro-survival effects at the mitochondria has yet to be explored. Recent microarray data demonstrated the general down-regulation of anti-apoptotic genes in HTLV-1-transformed cells [71] and that the induction of Akt/PI3K and the inactivation and phosphorylation of the pro-apoptotic Bcl-2 family member Bad might be critical to the regulation of apoptosis.
While Tax constitutively activates Akt and NF-κB, Tax also negatively regulates the cell cycle checkpoint tumor suppressor p53, which normally triggers cell cycle arrest and apoptosis in response to DNA damage [72]. p53 is functionally inactivated by Tax and is mutated in approximately 30% of all ATLL patients [73-75], thereby abrogating p53-mediated G1 cell cycle arrest and p53-mediated apoptosis in ATLL tumour cells [76]. Even in absence of genetic mutation, p53 appears to be inactivated in ATLL cells in vivo [77]. Tax-mediated inactivation of p53 is believed to occur through p53 phosphorylation on specific residues [74, 78]. As well, a recent study also suggests that the transcriptional repressor of p53, MdmX, is up-regulated in HTLV-I infected cells in vitro and in vivo, and may play an important role in the inactivation of p53 in the absence of Tax expression [79]. The ability of Tax to repress the non-transcriptional functions of p53 is intriguing. Tax-mediated repression of p53 transactivation has a profound effect on G1 arrest and apoptosis induced by p53 overexpression [80]. In that study, the CREB/ATF, but not the NF-κB activation by Tax was essential for p53-inhibition [81]. The amount of protection from apoptosis obtained upon expression of Tax correlated with the decreased transcriptional activation of p53 observed in the various cell lines, indicating that the Tax-mediated protection from apoptosis may in part be related to the suppression of p53 transcriptional activity. Interestingly, p53 has also been shown to have a direct pro-apoptotic role at the mitochondria [82]. Whether this particular pro-apoptotic function is altered in ATLL or HTLV-1-infected cells is unknown.
In addition to p53, Tax has been shown to affect virtually every other cell cycle phase and checkpoint, including G1phase, G1/S checkpoint, S phase, G2/M checkpoint, and mitosis. Tax directly interacts with the cell cycle checkpoint kinase 2 (Chk2), and inhibits gamma-irradiation-induced apoptosis [17]. Additional effects of Tax on the cell cycle have been recently reviewed [36, 37].
The fact that Tax constitutively activates both NF-kB and Akt, and that Tax simultaneously inactivates p53 should point to a broad anti-apoptotic activity of Tax. Experimental data, however, remain controversial, as numerous studies have reported that the overexpression of Tax induces apoptosis. Over-expression of Tax sensitized cells to DNA-damage-induced apoptosis in a p53-independent manner [83, 84], and induced cell death in Jurkat cells expressing CD95 (Fas) in a caspase-dependent manner [85]. This Tax-induced death can be blocked by Bcl-2 expression [86]. Tax was also observed to induce caspase-dependent cell death that correlated with the ability of Tax to regulate p300/CBP activity, but not NF-κB activity [87]. These observations are similar to those seen with other oncogenic factors such as Myc, Cyclin D and E1A, which also display both proliferative and pro-apoptotic effects. In contrast to most viral proteins which directly inhibit a particular checkpoint of the apoptotic cascade, Tax alters the expression of cellular genes and hijacks cell signaling pathways. Therefore, cells of different origin expressing different proteins may respond in different ways to Tax expression adding confusion to the field. Additional factors that influence cellular fate are the levels and duration of Tax expression. Tax transgenic mice develop numerous tumors and cells isolated from those tumors are highly refractory to various apoptotic stimuli [56]. It is possible that Tax directly protects these tumor cells by inducing NF-kB or Akt activation or other pathways. On the other hand, it is also possible that Tax does exert an initial pro-apoptotic stimulus, and that tumor cells are derived from cells that have subsequently acquired resistance to Tax-induced pro-apoptotic signals. In support of such model, thymus atrophy has been reported in some transgenic models, and was also associated with massive amounts of apoptosis [88].
Although Tax appears to be required for cell transformation and the inhibition of apoptosis, ATLL tumor cells do not express detectable levels of Tax [89-91]. Surprisingly, ATLL tumor cells that lack Tax still retain the characteristics of Tax-expressing cells, and multiple signaling pathways such as NF-κB are constitutively active in ATLL cells. These observations suggest that following cell transformation, cellular signaling molecules remain permanently activated in the absence of Tax. This correlates with the requirement of Tax for the initial transformation event, but not for the maintenance of the transformed state.
ORFII: the mitochondrial p13 and the regulatory protein p30
Many viruses encode proteins that localize to the mitochondria to modulate this important apoptotic checkpoint, and HTLV-1 appears to be no exception. The small HTLV protein p13 is a small, 87 amino acid non-structural protein encoded by the XII open reading frame. p13 targets to mitochondria via an N-terminal mitochondrial targeting motif between amino acids 19 and 31 that allows p13 to insert into the inner mitochondrial membrane (Fig. 1)[92]. While most integral inner membrane proteins of the mitochondria possess classic signal sequences which are cleaved during protein import, p13 does not appear to be cleaved, leaving the mechanism of import unknown. The targeting motif is rich in arginine residues and is predicted to resemble an amphipathic alpha helix, similar to other mitochondrial proteins produced by RNA viruses. One such alpha-helical protein is the viroporin Vpr from HIV [93]. The amphipathic alpha-helical nature of Vpr allows it to form cation-selective channels in the mitochondria membrane. This results in mitochondrial depolarization [93], which is dependent on the mitochondrial permeability transition pore proteins ANT and VDAC that interact with Vpr [93]. Other viroporins include HBX from hepatitis B virus and PB1-F2 from Influenza A virus, which also localize to the mitochondria via short transmembrane domains and induce mitochondrial alterations leading to apoptosis [94-98]. Although Vpr and HBX induce cytochrome c release, there is no evidence to suggest that p13 similarly induces cytochrome c release. p13, however, does appear to sensitize cells to pro-apoptotic stimuli, as p13 expression has a dose-dependent effect on amplifying apoptosis induced by either anti-Fas or ceramide [99]. p13 directly interacts with farnesyl pyrophosphate synthetase, which catalyzes the generation of substrates involved in the Ras pathway [100]. Inclusion of a farnesyl transferase inhibitor that blocks Ras prenylation also blocks FasL- and ceramide-induced apoptosis in p13-expressing T-cells [99]. Exactly how p13 modulates apoptosis at the mitochondria is unknown, although biochemical studies showed that p13 expression induced the loss of the mitochondrial membrane potential and caused a decrease in the calcium retention capacity of mitochondria [101]. These events appear to be independent of the permeability transition (PT) pore, as the PT pore inhibitor cyclosporine A has no effect on p13-mediated PT [101]. This is in contrast with other viral mitochondrial pro-apoptotic proteins such as Vpr, which interact with components of the PT pore to directly induce PT [93]. It has been demonstrated that accumulation of p13 at the mitochondria results in the rounding and fragmentation of the mitochondrial network, and is associated with mitochondrial swelling and cristae fragmentation [101]. Substitution of glutamine for each of the four arginine residues present in the N-terminal alpha-helix has no effect on p13 localization, but prevents p13-dependent mitochondrial rearrangement and fragmentation [101]. This may be important since recent work has implicated the fission and fusion of the mitochondrial network in the regulation of apoptotic cell death [102-105]. How p13 controls mitochondrial morphology remains to be investigated. Future work using p13 mutants which localize to but do not induce mitochondrial rearrangements will help elucidate the mechanism used by p13 to modulate mitochondrial morphology and establish whether these morphological changes are required for the regulation of apoptosis or virus virulence.
Another viral protein synthesized from ORFII is p30, which is a post-transcriptional regulator of translation. p30 expression inhibits the translocation of Tax/Rex mRNA from the nucleus to the cytoplasm, thereby inhibiting Tax and Rex protein production [106]. While it remains to be seen, the expression of p30 may alter the ability of Tax and HTLV-1 to modulate programmed cell death. In cases where high Tax expression is detrimental to the cell, it is possible that the inhibition of Tax synthesis by p30 decreases the likelihood of apoptosis induction, thereby facilitating virus latency. Alternatively, since p30 selectively blocks mRNA nuclear export, p30 expression might somehow inhibit the pro-survival mechanisms that Tax uses, thereby sensitizing the cell to apoptosis. Microarray analysis examining the effect of p30 on cellular gene expression demonstrated that the expression of a number of apoptosis-related genes was altered, including genes encoding Mcl-1, A1, Bik, and caspases 2 and 4 [107]. Whether the regulation of any of these apoptotic genes is involved in the modulation of apoptosis by HTLV-1 remains to be investigated.
ORFI: p12I and IL-2R signaling survival pathway
A hallmark of HTLV-I transformed cells is the constitutive activation of the Jak/STAT (Janus activating kinase/signal transducer and activator of transcription) pathway [108, 109], which rids infected lymphocytes of their dependence on IL-2 for proliferation and activation. Jak/STATs are involved in a number of cell processes, from cytokine signaling to the interferon response. Although various members of the Jak/STAT family have the potential to elicit both pro- and anti-apoptotic effects, one STAT, STAT5, specifically has anti-apoptotic effects [110, 111]. This includes the up-regulation of anti-apoptotic Bcl-2 family members such as Bcl-xL and Bcl-2, as well as the down-regulation of caspases 3 and 9 [110].
The HTLV-I non-structural protein p12 from open reading frame I is critical for establishing viral infection in vivo [112, 113]. p12I enhances STAT5 activation by binding the β and γc chains of the IL-2 receptor, resulting in Jak1/Jak3 activation, STAT5a/b phosphorylation and nuclear translocation of the STAT5 heterodimer (Fig. 1). p12I increases STAT5 phosphorylation and STAT5 DNA binding in the absence of IL-2 [114]. The STAT5 activation induced by p12I appears to up-regulate X-linked IAP (XIAP), as the nucleoside analogue Roscovotine inhibits STAT5 and results in a decrease in XIAP expression in HTLV-1-infected cells [115].
HBZ: New player on the scene?
Recent research has characterized a novel protein transcribed from the negative strand of the HTLV-1 genome, HTLV-1 basic leucine-zipper factor, or HBZ [116]. This protein interacts with transcription factors CREB and those of the Jun family, and impairs the DNA binding ability of c-Jun [117-119]. As a result, HBZ has the ability to repress transcription of factors such as AP-1, Tax, and NF-κB. Although HBZ appears to play a repressive role in expression of certain cellular factors and viral genes, whether HBZ also affects the ability of Tax and other viral proteins to modulate the apoptotic cascade remains to be investigated.
Treatment of HTLV-1: Drug-induced apoptosis
To date, a successful therapy for HTLV-1 has remained elusive in that many broad-range cancer therapies are ineffective. A wide range of combinatorial anti-cancer therapies have been used in clinical trials with limited degrees of success [120]. Recently, a number of new compounds and therapies have been shown to specifically induce apoptosis in HTLV-1 and ATLL cells (see Table 1), and many of these drugs target the aforementioned changes in gene expression and protein function that are essential for ATLL cell survival.
Considering the importance of NF-κB in ATLL cell survival, one group of drugs being examined targets the NF-κB pathway. Bay 11-7082 and ACHP, inhibitors of IκB phosphorylation, and the proteasome inhibitor bortezomib/PS-341 inhibit both HTLV-1 and Tax-mediated NF-κB activation and induce apoptosis in infected cells [121] [122] [123-126]. Notably, cells treated with bortezomib/PS-341 in the presence of the caspase-inhibitor zVAD-fmk appear to undergo necrosis instead of apoptosis, indicating that the mechanism of death is still unclear [123]. The in vivo efficacy of bortezomib, however, remains to be seen, as one particular clinical trial demonstrated that one ATLL patient did not respond to bortezomib treatment [127]. The purine analogue Fludarabine also inhibits NF-kB activation resulting in the induction of apoptosis in HTLV-1-infected cells [128]. An HIV protease inhibitor, ritonavir, induces apoptosis in HTLV-1-leukemic cells by inhibiting NF-κB activity[129]. While ritonavir has not yet been tested for ATLL, it has shown efficacy in the treatment of HIV-related AIDS [130]. Altogether, the inhibition of NF-κB signaling appears to be a very promising target for new and developing HTLV-1-therapies. The role of NF-κB regulation in ATLL is not unique, as multiple lymphomas, such as Hodgkins disease, MALT lymphomas, and Kaposi’s sarcoma are associated with the deregulation of NF-κB activity [131].
Arsenic trioxide, alone or in combination with other pro-apoptotic stimuli, has also been examined as a possible treatment and induces apoptosis in HTLV-1-infected cells lines [132-134]. While arsenic induces the generation of hydrogen peroxide leading to cytochrome c release and caspase activation [135], recent work has also suggested that arsenic trioxide causes cell cycle arrest, NF-κB repression, and the down-regulation of Tax [132-134, 136-138]. Clinical use of arsenic, however, is problematic as there are differences in sensitivity to As2O3, and arsenic itself is toxic at high doses. Inclusion of polyunsaturated fatty acids such as docosahexaenoic acid (DHA), which increases ROS production and lipid peroxidation, and Emodin significantly increases necrotic cell death in HTLV-1-infected cells following treatment with As2O3 [139]. The use of combinatorial therapies with arsenic may allow for lower doses of As2O3 to be used.
Other cell signaling pathways that are deregulated in HTLV-1-infected cells have also been areas for drug development. The purine analogue roscovitine inhibits STAT5 activation and XIAP expression to induce apoptosis in MT-2 HTLV-1-infected cells [115]. Curcumin, a natural pigment of the spice turmeric, has been used extensively as an anticancer drug, and treatment of HTLV-1-infected cells with curcumin induces apoptosis by targeting the Akt-survival pathway or the Jak/STAT pathway [140-143]. Results suggesting that a specific Jak-inhibitor, AG-490, induces cell cycle arrest, however, are controversial [141, 144]. The genldanamycin derivative 17-AAG inhibits the activity of heat shock protein 90 (Hsp90), and is able to induce apoptosis in primary ATLL cells [145]. Although there is no clinical data for the use of 17-AAG in ATLL patients, 17-AAG has been successfully tested in a phase I clinical trial for various other malignancies [146].
ATLL cells are often characterized by the overexpression of specific cell surface markers, and a number of monoclonal antibodies have correspondingly been developed with the intention of inducing cell death. One early antibody therapy attempted was the use of anti-Fas [147-149]. Despite early success, however, the efficacy of anti-Fas in providing long-term remission was inadequate for clinical use. Other monoclonal antibody therapies directed at the overexpressed IL-2 receptor (CD25) have shown a greater degree of promise[150-152]. Early clinical trials with the antibody anti-Tac, which is directed against the IL-2α receptor, demonstrated limited success, although recent developments using a Yttrium-90-radiolabeled antibody has exhibited an increased activity against ATLL cells [150, 151, 153-155]. Anti-IL-2Rα antibody, in conjunction with bortezomib/PS-341 treatment was able to elicit the complete remission in ATLL-tumour-bearing mice [156], again demonstrating the efficacy of a combinatorial therapy. A major positive for anti-Tac therapy is the low level of side-effects, which is in stark contrast to standard chemotherapy reagents. More recently, other monoclonal antibodies have been directed at CD52 and the transferrin receptor, both of which are also overexpressed in HTLV-transformed cells [157-159].
Another class of pro-apoptotic drugs being investigated to treat ATLL targets the cell cycle. Retinoic acids induce apoptosis in HTLV-1-infected cells and ex vivo ATL cells [160-162], primarily by inducing cell cycle arrest. One retinoic acid, N-(4-hydroxyphenyl) retinamide, induced the dramatic death of malignant ATL, and was associated with elevated ceramide levels leading to cell cycle arrest and Bax activation [163]. Although there are specific effects on gene expression, these retinoids ultimately induce cell death through the mitochondrial pathway which is regulated by Bcl-2 [164]. A number of other retinoids have also been documented to induce apoptosis in HTLV-I-infected cells [165-169]. Perhaps the most-studied anti-retroviral drug is zidovudine (AZT), which is used extensively to treat HIV-1-infected individuals. Despite early reports suggesting that zidovudine provided some level of anti-cancer effect in ATL patients [170, 171], ATL cells do not appear to exhibit a high degree of apoptosis in response to zidovudine, even in combination with IFNα [172], and the mechanism of inhibition is likely through telomere attrition and reactivation of a p53-dependent senescent pathway [79, 173, 174].
Considering the extensive work performed in pursuit of new potential therapies, it is of note that certain members of the multi-drug resistance (MDR) protein family are up-regulated in HTLV-1-infected cells and ATLL patients [175-177]. Adaptations such as these may dictate the relative sensitivity to various drug therapies for ATLL patients, and should be noted when promising new emerging therapies are investigated.
Concluding Remarks
Like many viruses, HTLV-1 modulates the apoptotic pathway using multiple tactics, ranging from Tax-mediated modulation of gene expression and the cell cycle, to STAT activation by p12, to the regulation of the mitochondria by p13. Future work will hopefully further expose the specific mechanisms used by HTLV-1 to control cell death, and these investigations will aid in our understanding of the pathology of HTLV-1-infection and ensuing ATLL. Therapeutic strategies aimed at inducing virus-infected cell death must consider the fact that not all deaths are equal. Necrotic cell death results in an inflammatory response, while apoptosis, in contrast, is a tightly controlled process that does not lead to inflammation. The ability of HTLV-1-infected ATLL cells to resist apoptosis likely greatly contributes to the development of ATLL. In contrast, the pathogenesis of TSP/HAM is associated with high inflammation and hyper-immune responses [12]. Taking these findings into considerations, whether it is beneficial to induce either apoptosis in TSP/HAM and necrotic cell death in ATLL patients has not been addressed. The development of new therapies to treat HTLV-1-infected patients diagnosed with ATLL or TSP/HAM may hinge upon the ability of new drugs or combinatorial therapies to specifically induce death in HTLV-1-infected T cells in vivo.
Figure 2.
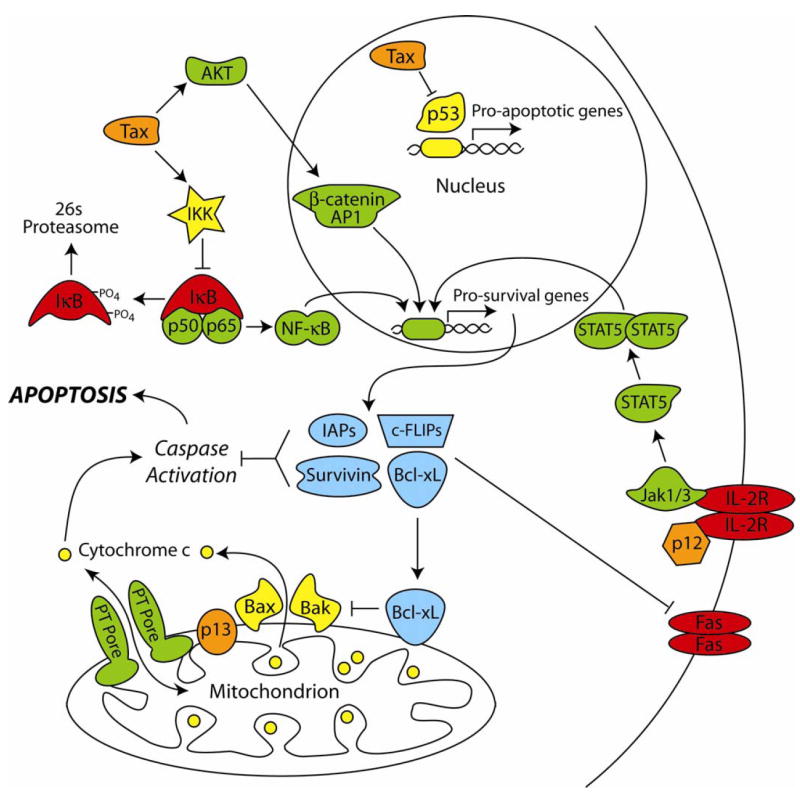
Apoptotic regulatory pathways interrupted by HTLV-1 proteins. The viral oncoprotein Tax inactivates the inhibitors of κB through the activation of IKK, resulting in IκB phosphorylation and degradation, and the release of NF-κB. NF-κB is free to translocate to the nucleus to induce the transcription of pro-survival genes. Tax also stimulates the constitutive activation of Akt, resulting in the activation of β-catenin and AP-1 transcriptional pathways, leading to the up-regulation of additional anti-apoptotic genes. The small viral protein p12 has been shown to interact with the IL-2 receptor (IL-2R), thus stimulating Jak-recruitment. This leads to the phosphorylation, dimerization, and nuclear translocation of STAT5 to facilitate the up-regulation of pro-survival gene products. The HTLV-1 protein p13 localizes to the inner mitochondrial membrane where it may play a role in mitochondrial morphology and regulation of the permeability transition (PT) pore.
Figure 3.
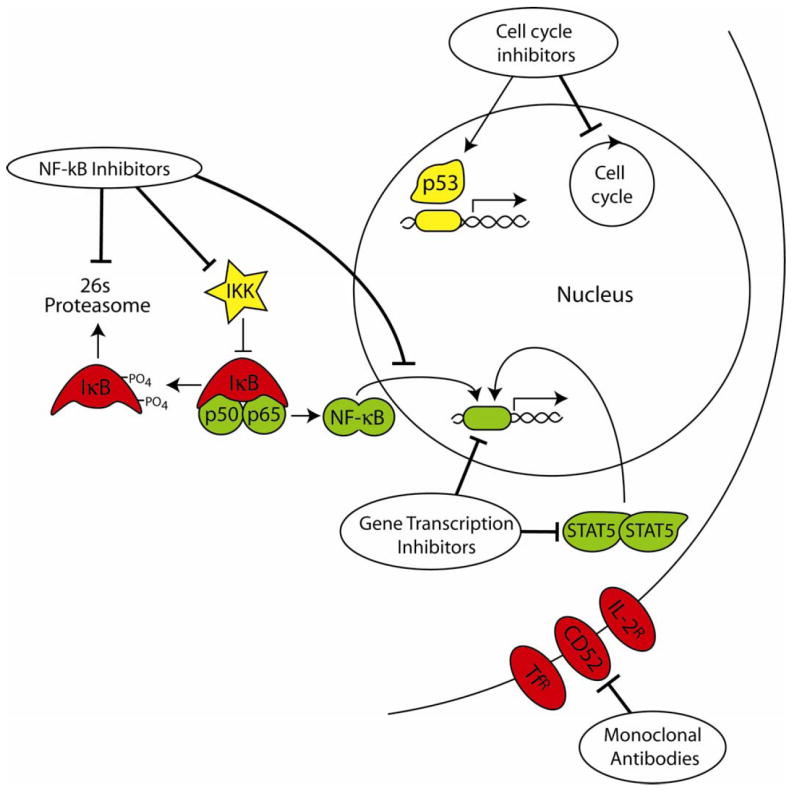
Cellular pathways targeted by drugs which induce apoptosis in HTLV-1-infected cells. Drugs used to induce apoptosis in HTLV-1 and ATLL cells in vitro have targeted various aspects of the NF-κB pathway, such as inhibition of the proteasome, inhibition of the IKK complex, and inhibition of nuclear translocation of NF-κB. Other drugs have been used to target the cell cycle by stabilizing p53 or by inducing cell cycle arrest. Inhibitors of gene transcription have targeted STAT5 and the downregulation of various anti-apoptotic proteins. More recently, a number of monoclonal antibody therapies have targeted cell surface proteins upregulated in HTLV-1-infected cells, such as the IL-2 receptor, transferrin receptor, and CD52.
Acknowledgments
The authors wish to thank Dr. V. Ciminale (Department of Oncology and Surgical Sciences, University of Padua, Italy); Dr. J. Semmes (Department of Microbiology and Molecular Cell Biology, Eastern Virginia Medical School, Norfolk, Virginia 23507, USA); Dr. J.M. Mesnard (Laboratoire Infections Rétrovirales et Signalisation Cellulaire, CNRS/UM I UMR 5121/IFR 122, Institut de Biologie, 34000 Montpellier, France) for kindly providing pictures for cellular localization of p13, Tax and HBZ, respectively. This work was supported by grants CA 106258 and CA115398 from the National cancer Institute to C.Nicot.
References
- 1.Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–57. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thompson CB. Apoptosis in the pathogenesis and treatment of disease. Science. 1995;267:1456–62. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]
- 3.Hardwick JM, Bellows DS. Viral versus cellular BCL-2 proteins. Cell Death Differ. 2003;10(Suppl 1):S68–76. doi: 10.1038/sj.cdd.4401133. [DOI] [PubMed] [Google Scholar]
- 4.Benedict CA, Norris PS, Ware CF. To kill or be killed: viral evasion of apoptosis. Nat Immunol. 2002;3:1013–8. doi: 10.1038/ni1102-1013. [DOI] [PubMed] [Google Scholar]
- 5.Boya P, Pauleau AL, Poncet D, Gonzalez-Polo RA, Zamzami N, Kroemer G. Viral proteins targeting mitochondria: controlling cell death. Biochim Biophys Acta. 2004;1659:178–89. doi: 10.1016/j.bbabio.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 6.Roulston A, Marcellus RC, Branton PE. Viruses and apoptosis. Annu Rev Microbiol. 1999;53:577–628. doi: 10.1146/annurev.micro.53.1.577. [DOI] [PubMed] [Google Scholar]
- 7.Taylor JM, Barry M. Near death experiences: poxvirus regulation of apoptotic death. Virology. 2006;344:139–50. doi: 10.1016/j.virol.2005.09.032. [DOI] [PubMed] [Google Scholar]
- 8.Danthi P, Kobayashi T, Holm GH, Hansberger MW, Abel TW, Dermody TS. Reovirus apoptosis and virulence are regulated by host cell membrane penetration efficiency. J Virol. 2008;82:161–72. doi: 10.1128/JVI.01739-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eckardt-Michel J, Lorek M, Baxmann D, Grunwald T, Keil GM, Zimmer G. The fusion protein of respiratory syncytial virus triggers p53-dependent apoptosis. J Virol. 2008 doi: 10.1128/JVI.01887-07. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Franchini G. Molecular mechanisms of human T-cell leukemia/lymphotropic virus type I infection. Blood. 1995;86:3619–39. [PubMed] [Google Scholar]
- 11.Nicot C. Current views in HTLV-I-associated adult T-cell leukemia/lymphoma. Am J Hematol. 2005;78:232–9. doi: 10.1002/ajh.20307. [DOI] [PubMed] [Google Scholar]
- 12.Kiwaki T, Umehara F, Arimura Y, Izumo S, Arimura K, Itoh K, et al. The clinical and pathological features of peripheral neuropathy accompanied with HTLV-I associated myelopathy. J Neurol Sci. 2003;206:17–21. doi: 10.1016/s0022-510x(02)00279-4. [DOI] [PubMed] [Google Scholar]
- 13.Nakagawa M, Izumo S, Ijichi S, Kubota H, Arimura K, Kawabata M, et al. HTLV-I-associated myelopathy: analysis of 213 patients based on clinical features and laboratory findings. J Neurovirol. 1995;1:50–61. doi: 10.3109/13550289509111010. [DOI] [PubMed] [Google Scholar]
- 14.Yang YC, Hsu TY, Lin RH, Su IJ, Chen JY, Yang CS. Resistance to tumor necrosis factor-alpha-induced apoptosis in human T-lymphotropic virus type I-infected T cell lines. AIDS Res Hum Retroviruses. 2002;18:207–12. doi: 10.1089/08892220252781266. [DOI] [PubMed] [Google Scholar]
- 15.Copeland KF, Haaksma AG, Goudsmit J, Krammer PH, Heeney JL. Inhibition of apoptosis in T cells expressing human T cell leukemia virus type I Tax. AIDS Res Hum Retroviruses. 1994;10:1259–68. doi: 10.1089/aid.1994.10.1259. [DOI] [PubMed] [Google Scholar]
- 16.Kishi S, Saijyo S, Arai M, Karasawa S, Ueda S, Kannagi M, et al. Resistance to fas-mediated apoptosis of peripheral T cells in human T lymphocyte virus type I (HTLV-I) transgenic mice with autoimmune arthropathy. J Exp Med. 1997;186:57–64. doi: 10.1084/jem.186.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park HU, Jeong SJ, Jeong JH, Chung JH, Brady JN. Human T-cell leukemia virus type 1 Tax attenuates gamma-irradiation-induced apoptosis through physical interaction with Chk2. Oncogene. 2006;25:438–47. doi: 10.1038/sj.onc.1209059. [DOI] [PubMed] [Google Scholar]
- 18.Hasegawa H, Yamada Y, Harasawa H, Tsuji T, Murata K, Sugahara K, et al. Sensitivity of adult T-cell leukaemia lymphoma cells to tumour necrosis factor-related apoptosis-inducing ligand. Br J Haematol. 2005;128:253–65. doi: 10.1111/j.1365-2141.2004.05289.x. [DOI] [PubMed] [Google Scholar]
- 19.Tsuda H, Huang RW, Takatsuki K. Interleukin-2 prevents programmed cell death in adult T-cell leukemia cells. Jpn J Cancer Res. 1993;84:431–7. doi: 10.1111/j.1349-7006.1993.tb00154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tamiya S, Etoh K, Suzushima H, Takatsuki K, Matsuoka M. Mutation of CD95 (Fas/Apo-1) gene in adult T-cell leukemia cells. Blood. 1998;91:3935–42. [PubMed] [Google Scholar]
- 21.Maeda T, Yamada Y, Moriuchi R, Sugahara K, Tsuruda K, Joh T, et al. Fas gene mutation in the progression of adult T cell leukemia. J Exp Med. 1999;189:1063–71. doi: 10.1084/jem.189.7.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arai M, Kannagi M, Matsuoka M, Sato T, Yamamoto N, Fujii M. Expression of FAP-1 (Fas-associated phosphatase) and resistance to Fas-mediated apoptosis in T cell lines derived from human T cell leukemia virus type 1-associated myelopathy/tropical spastic paraparesis patients. AIDS Res Hum Retroviruses. 1998;14:261–7. doi: 10.1089/aid.1998.14.261. [DOI] [PubMed] [Google Scholar]
- 23.Hamasaki S, Nakamura T, Furuya T, Kawakami A, Ichinose K, Nakashima T, et al. Resistance of CD4-positive T lymphocytes to etoposide-induced apoptosis mediated by upregulation of Bcl-xL expression in patients with HTLV-I-associated myelopathy. J Neuroimmunol. 2001;117:143–8. doi: 10.1016/s0165-5728(01)00332-0. [DOI] [PubMed] [Google Scholar]
- 24.Kawahigashi N, Furukawa Y, Saito M, Usuku K, Osame M. Predominant expression of Fas ligand mRNA in CD8+ T lymphocytes in patients with HTLV-1 associated myelopathy. J Neuroimmunol. 1998;90:199–206. doi: 10.1016/s0165-5728(98)00147-7. [DOI] [PubMed] [Google Scholar]
- 25.Chen X, Zachar V, Zdravkovic M, Guo M, Ebbesen P, Liu X. Role of the Fas/Fas ligand pathway in apoptotic cell death induced by the human T cell lymphotropic virus type I Tax transactivator. J Gen Virol. 1997;78(Pt 12):3277–85. doi: 10.1099/0022-1317-78-12-3277. [DOI] [PubMed] [Google Scholar]
- 26.Banerjee P, Rochford R, Antel J, Canute G, Wrzesinski S, Sieburg M, et al. Proinflammatory cytokine gene induction by human T-cell leukemia virus type 1 (HTLV-1) and HTLV-2 Tax in primary human glial cells. J Virol. 2007;81:1690–700. doi: 10.1128/JVI.01513-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tomaru U, Ikeda H, Jiang X, Ohya O, Yoshiki T. Provirus expansion and deregulation of apoptosis-related genes in the spinal cord of a rat model for human T-lymphocyte virus type I-associated myeloneuropathy. J Neurovirol. 2003;9:530–8. doi: 10.1080/13550280390241160. [DOI] [PubMed] [Google Scholar]
- 28.Ohya O, Tomaru U, Yamashita I, Kasai T, Morita K, Ikeda H, et al. HTLV-I induced myeloneuropathy in WKAH rats: apoptosis and local activation of the HTLV-I pX and TNF-alpha genes implicated in the pathogenesis. Leukemia. 1997;11(Suppl 3):255–7. [PubMed] [Google Scholar]
- 29.Nicot C, Harrod RL, Ciminale V, Franchini G. Human T-cell leukemia/lymphoma virus type 1 nonstructural genes and their functions. Oncogene. 2005;24:6026–34. doi: 10.1038/sj.onc.1208977. [DOI] [PubMed] [Google Scholar]
- 30.Zhao LJ, Giam CZ. Human T-cell lymphotropic virus type I (HTLV-I) transcriptional activator, Tax, enhances CREB binding to HTLV-I 21-base-pair repeats by protein-protein interaction. Proc Natl Acad Sci U S A. 1992;89:7070–4. doi: 10.1073/pnas.89.15.7070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Suzuki T, Fujisawa JI, Toita M, Yoshida M. The trans-activator tax of human T-cell leukemia virus type 1 (HTLV-1) interacts with cAMP-responsive element (CRE) binding and CRE modulator proteins that bind to the 21-base-pair enhancer of HTLV-1. Proc Natl Acad Sci U S A. 1993;90:610–4. doi: 10.1073/pnas.90.2.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bex F, Yin MJ, Burny A, Gaynor RB. Differential transcriptional activation by human T-cell leukemia virus type 1 Tax mutants is mediated by distinct interactions with CREB binding protein and p300. Mol Cell Biol. 1998;18:2392–405. doi: 10.1128/mcb.18.4.2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jiang H, Lu H, Schiltz RL, Pise-Masison CA, Ogryzko VV, Nakatani Y, et al. PCAF interacts with tax and stimulates tax transactivation in a histone acetyltransferase-independent manner. Mol Cell Biol. 1999;19:8136–45. doi: 10.1128/mcb.19.12.8136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Franklin AA, Kubik MF, Uittenbogaard MN, Brauweiler A, Utaisincharoen P, Matthews MA, et al. Transactivation by the human T-cell leukemia virus Tax protein is mediated through enhanced binding of activating transcription factor-2 (ATF-2) ATF-2 response and cAMP element-binding protein (CREB) J Biol Chem. 1993;268:21225–31. [PubMed] [Google Scholar]
- 35.Hall WW, Fujii M. Deregulation of cell-signaling pathways in HTLV-1 infection. Oncogene. 2005;24:5965–75. doi: 10.1038/sj.onc.1208975. [DOI] [PubMed] [Google Scholar]
- 36.Grassmann R, Aboud M, Jeang KT. Molecular mechanisms of cellular transformation by HTLV-1 Tax. Oncogene. 2005;24:5976–85. doi: 10.1038/sj.onc.1208978. [DOI] [PubMed] [Google Scholar]
- 37.Marriott SJ, Semmes OJ. Impact of HTLV-I Tax on cell cycle progression and the cellular DNA damage repair response. Oncogene. 2005;24:5986–95. doi: 10.1038/sj.onc.1208976. [DOI] [PubMed] [Google Scholar]
- 38.Suzuki T, Uchida-Toita M, Andoh T, Yoshida M. HTLV-1 tax oncoprotein binds to DNA topoisomerase I and inhibits its catalytic activity. Virology. 2000;270:291–8. doi: 10.1006/viro.2000.0266. [DOI] [PubMed] [Google Scholar]
- 39.Jeang KT, Widen SG, Semmes OJ, Wilson SH. HTLV-I trans-activator protein, tax, is a trans-repressor of the human beta-polymerase gene. Science. 1990;247:1082–4. doi: 10.1126/science.2309119. [DOI] [PubMed] [Google Scholar]
- 40.Ballard DW, Bohnlein E, Lowenthal JW, Wano Y, Franza BR, Greene WC. HTLV-I tax induces cellular proteins that activate the kappa B element in the IL-2 receptor alpha gene. Science. 1988;241:1652–5. doi: 10.1126/science.241.4873.1652. [DOI] [PubMed] [Google Scholar]
- 41.Leung K, Nabel GJ. HTLV-1 transactivator induces interleukin-2 receptor expression through an NF-kappa B-like factor. Nature. 1988;333:776–8. doi: 10.1038/333776a0. [DOI] [PubMed] [Google Scholar]
- 42.Iha H, Kibler KV, Yedavalli VR, Peloponese JM, Haller K, Miyazato A, et al. Segregation of NF-kappaB activation through NEMO/IKKgamma by Tax and TNFalpha: implications for stimulus-specific interruption of oncogenic signaling. Oncogene. 2003;22:8912–23. doi: 10.1038/sj.onc.1207058. [DOI] [PubMed] [Google Scholar]
- 43.Lacoste J, Petropoulos L, Pepin N, Hiscott J. Constitutive phosphorylation and turnover of I kappa B alpha in human T-cell leukemia virus type I-infected and Tax-expressing T cells. J Virol. 1995;69:564–9. doi: 10.1128/jvi.69.1.564-569.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sun SC, Elwood J, Beraud C, Greene WC. Human T-cell leukemia virus type I Tax activation of NF-kappa B/Rel involves phosphorylation and degradation of I kappa B alpha and RelA (p65)-mediated induction of the c-rel gene. Mol Cell Biol. 1994;14:7377–84. doi: 10.1128/mcb.14.11.7377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maggirwar SB, Harhaj E, Sun SC. Activation of NF-kappa B/Rel by Tax involves degradation of I kappa B alpha and is blocked by a proteasome inhibitor. Oncogene. 1995;11:993–8. [PubMed] [Google Scholar]
- 46.Mukherjee S, Negi VS, Keitany G, Tanaka Y, Orth K. In Vitro activation of the IKK complex by HTLV-1 Tax. J Biol Chem. 2008 doi: 10.1074/jbc.M704831200. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xiao G, Cvijic ME, Fong A, Harhaj EW, Uhlik MT, Waterfield M, et al. Retroviral oncoprotein Tax induces processing of NF-kappaB2/p100 in T cells: evidence for the involvement of IKKalpha. Embo J. 2001;20:6805–15. doi: 10.1093/emboj/20.23.6805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Beraud C, Sun SC, Ganchi P, Ballard DW, Greene WC. Human T-cell leukemia virus type I Tax associates with and is negatively regulated by the NF-kappa B2 p100 gene product: implications for viral latency. Mol Cell Biol. 1994;14:1374–82. doi: 10.1128/mcb.14.2.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Suzuki T, Hirai H, Fujisawa J, Fujita T, Yoshida M. A trans-activator Tax of human T-cell leukemia virus type 1 binds to NF-kappa B p50 and serum response factor (SRF) and associates with enhancer DNAs of the NF-kappa B site and CArG box. Oncogene. 1993;8:2391–7. [PubMed] [Google Scholar]
- 50.Hirai H, Fujisawa J, Suzuki T, Ueda K, Muramatsu M, Tsuboi A, et al. Transcriptional activator Tax of HTLV-1 binds to the NF-kappa B precursor p105. Oncogene. 1992;7:1737–42. [PubMed] [Google Scholar]
- 51.Suzuki T, Hirai H, Yoshida M. Tax protein of HTLV-1 interacts with the Rel homology domain of NF-kappa B p65 and c-Rel proteins bound to the NF-kappa B binding site and activates transcription. Oncogene. 1994;9:3099–105. [PubMed] [Google Scholar]
- 52.Bex F, McDowall A, Burny A, Gaynor R. The human T-cell leukemia virus type 1 transactivator protein Tax colocalizes in unique nuclear structures with NF-kappaB proteins. J Virol. 1997;71:3484–97. doi: 10.1128/jvi.71.5.3484-3497.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mori N, Fujii M, Cheng G, Ikeda S, Yamasaki Y, Yamada Y, et al. Human T-cell leukemia virus type I tax protein induces the expression of anti-apoptotic gene Bcl-xL in human T-cells through nuclear factor-kappaB and c-AMP responsive element binding protein pathways. Virus Genes. 2001;22:279–87. doi: 10.1023/a:1011158021749. [DOI] [PubMed] [Google Scholar]
- 54.Tsukahara T, Kannagi M, Ohashi T, Kato H, Arai M, Nunez G, et al. Induction of Bcl-x(L) expression by human T-cell leukemia virus type 1 Tax through NF-kappaB in apoptosis-resistant T-cell transfectants with Tax. J Virol. 1999;73:7981–7. doi: 10.1128/jvi.73.10.7981-7987.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nicot C, Mahieux R, Takemoto S, Franchini G. Bcl-X(L) is up-regulated by HTLV-I and HTLV-II in vitro and in ex vivo ATLL samples. Blood. 2000;96:275–81. [PubMed] [Google Scholar]
- 56.Portis T, Harding JC, Ratner L. The contribution of NF-kappa B activity to spontaneous proliferation and resistance to apoptosis in human T-cell leukemia virus type 1 Tax-induced tumors. Blood. 2001;98:1200–8. doi: 10.1182/blood.v98.4.1200. [DOI] [PubMed] [Google Scholar]
- 57.Kawakami A, Nakashima T, Sakai H, Urayama S, Yamasaki S, Hida A, et al. Inhibition of caspase cascade by HTLV-I tax through induction of NF-kappaB nuclear translocation. Blood. 1999;94:3847–54. [PubMed] [Google Scholar]
- 58.Waldele K, Silbermann K, Schneider G, Ruckes T, Cullen BR, Grassmann R. Requirement of the human T-cell leukemia virus (HTLV-1) tax-stimulated HIAP-1 gene for the survival of transformed lymphocytes. Blood. 2006;107:4491–9. doi: 10.1182/blood-2005-08-3138. [DOI] [PubMed] [Google Scholar]
- 59.Kawata S, Ariumi Y, Shimotohno K. p21(Waf1/Cip1/Sdi1) prevents apoptosis as well as stimulates growth in cells transformed or immortalized by human T-cell leukemia virus type 1-encoded tax. J Virol. 2003;77:7291–9. doi: 10.1128/JVI.77.13.7291-7299.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pichler K, Kattan T, Gentzsch J, Kress AK, Taylor GP, Bangham CR, et al. Strong induction of 4-1BB, a growth and survival promoting costimulatory receptor, in HTLV-1-infected cultured and patients’ T-cells by the viral Tax oncoprotein. Blood. 2008 doi: 10.1182/blood-2007-10-115220. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 61.Jeong SJ, Pise-Masison CA, Radonovich MF, Park HU, Brady JN. Activated AKT regulates NF-kappaB activation, p53 inhibition and cell survival in HTLV-1-transformed cells. Oncogene. 2005;24:6719–28. doi: 10.1038/sj.onc.1208825. [DOI] [PubMed] [Google Scholar]
- 62.Liu Y, Wang Y, Yamakuchi M, Masuda S, Tokioka T, Yamaoka S, et al. Phosphoinositide-3 kinase-PKB/Akt pathway activation is involved in fibroblast Rat-1 transformation by human T-cell leukemia virus type I tax. Oncogene. 2001;20:2514–26. doi: 10.1038/sj.onc.1204364. [DOI] [PubMed] [Google Scholar]
- 63.Peloponese JM, Jr, Jeang KT. Role for Akt/protein kinase B and activator protein-1 in cellular proliferation induced by the human T-cell leukemia virus type 1 tax oncoprotein. J Biol Chem. 2006;281:8927–38. doi: 10.1074/jbc.M510598200. [DOI] [PubMed] [Google Scholar]
- 64.Tomita M, Kikuchi A, Akiyama T, Tanaka Y, Mori N. Human T-cell leukemia virus type 1 tax dysregulates beta-catenin signaling. J Virol. 2006;80:10497–505. doi: 10.1128/JVI.00739-06. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 65.Ikezoe T, Nishioka C, Bandobashi K, Yang Y, Kuwayama Y, Adachi Y, et al. Longitudinal inhibition of PI3K/Akt/mTOR signaling by LY294002 and rapamycin induces growth arrest of adult T-cell leukemia cells. Leuk Res. 2007;31:673–82. doi: 10.1016/j.leukres.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 66.Mori N, Fujii M, Iwai K, Ikeda S, Yamasaki Y, Hata T, et al. Constitutive activation of transcription factor AP-1 in primary adult T-cell leukemia cells. Blood. 2000;95:3915–21. [PubMed] [Google Scholar]
- 67.Fujii M, Niki T, Mori T, Matsuda T, Matsui M, Nomura N, et al. HTLV-1 Tax induces expression of various immediate early serum responsive genes. Oncogene. 1991;6:1023–9. [PubMed] [Google Scholar]
- 68.Krueger A, Fas SC, Giaisi M, Bleumink M, Merling A, Stumpf C, et al. HTLV-1 Tax protects against CD95-mediated apoptosis by induction of the cellular FLICE-inhibitory protein (c-FLIP) Blood. 2006;107:3933–9. doi: 10.1182/blood-2005-06-2567. [DOI] [PubMed] [Google Scholar]
- 69.Sinha-Datta U, Horikawa I, Michishita E, Datta A, Sigler-Nicot JC, Brown M, et al. Transcriptional activation of hTERT through the NF-kappaB pathway in HTLV-I-transformed cells. Blood. 2004;104:2523–31. doi: 10.1182/blood-2003-12-4251. [DOI] [PubMed] [Google Scholar]
- 70.Massard C, Zermati Y, Pauleau AL, Larochette N, Metivier D, Sabatier L, et al. hTERT: a novel endogenous inhibitor of the mitochondrial cell death pathway. Oncogene. 2006;25:4505–14. doi: 10.1038/sj.onc.1209487. [DOI] [PubMed] [Google Scholar]
- 71.Akl H, Badran BM, Zein NE, Bex F, Sotiriou C, Willard-Gallo KE, et al. HTLV-I infection of WE17/10 CD4+ cell line leads to progressive alteration of Ca2+ influx that eventually results in loss of CD7 expression and activation of an antiapoptotic pathway involving AKT and BAD which paves the way for malignant transformation. Leukemia. 2007;21:788–96. doi: 10.1038/sj.leu.2404585. [DOI] [PubMed] [Google Scholar]
- 72.Vousden KH, Lane DP. p53 in health and disease. Nat Rev Mol Cell Biol. 2007;8:275–83. doi: 10.1038/nrm2147. [DOI] [PubMed] [Google Scholar]
- 73.Mahieux R, Pise-Masison CA, Nicot C, Green P, Hall WW, Brady JN. Inactivation of p53 by HTLV type 1 and HTLV type 2 Tax trans-activators. AIDS Res Hum Retroviruses. 2000;16:1677–81. doi: 10.1089/08892220050193137. [DOI] [PubMed] [Google Scholar]
- 74.Pise-Masison CA, Mahieux R, Radonovich M, Jiang H, Duvall J, Guillerm C, et al. Insights into the molecular mechanism of p53 inhibition by HTLV type 1 Tax. AIDS Res Hum Retroviruses. 2000;16:1669–75. doi: 10.1089/08892220050193128. [DOI] [PubMed] [Google Scholar]
- 75.Nagai H, Kinoshita T, Imamura J, Murakami Y, Hayashi K, Mukai K, et al. Genetic alteration of p53 in some patients with adult T-cell leukemia. Jpn J Cancer Res. 1991;82:1421–7. doi: 10.1111/j.1349-7006.1991.tb01815.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mesnard JM, Devaux C. Multiple control levels of cell proliferation by human T-cell leukemia virus type 1 Tax protein. Virology. 1999;257:277–84. doi: 10.1006/viro.1999.9685. [DOI] [PubMed] [Google Scholar]
- 77.Takemoto S, Trovato R, Cereseto A, Nicot C, Kislyakova T, Casareto L, et al. p53 stabilization and functional impairment in the absence of genetic mutation or the alteration of the p14(ARF)-MDM2 loop in ex vivo and cultured adult T-cell leukemia/lymphoma cells. Blood. 2000;95:3939–44. [PubMed] [Google Scholar]
- 78.Pise-Masison CA, Brady JN. Setting the stage for transformation: HTLV-1 Tax inhibition of p53 function. Front Biosci. 2005;10:919–30. doi: 10.2741/1586. [DOI] [PubMed] [Google Scholar]
- 79.Datta A, Nicot C. Telomere attrition induces a DNA double-strand break damage signal that reactivates p53 transcription in HTLV-I leukemic cells. Oncogene. 2008;27:1135–41. doi: 10.1038/sj.onc.1210718. [DOI] [PubMed] [Google Scholar]
- 80.Haoudi A, Semmes OJ. The HTLV-1 tax oncoprotein attenuates DNA damage induced G1 arrest and enhances apoptosis in p53 null cells. Virology. 2003;305:229–39. doi: 10.1006/viro.2002.1642. [DOI] [PubMed] [Google Scholar]
- 81.Mulloy JC, Kislyakova T, Cereseto A, Casareto L, LoMonico A, Fullen J, et al. Human T-cell lymphotropic/leukemia virus type 1 Tax abrogates p53-induced cell cycle arrest and apoptosis through its CREB/ATF functional domain. J Virol. 1998;72:8852–60. doi: 10.1128/jvi.72.11.8852-8860.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chipuk JE, Kuwana T, Bouchier-Hayes L, Droin NM, Newmeyer DD, Schuler M, et al. Direct activation of Bax by p53 mediates mitochondrial membrane permeabilization and apoptosis. Science. 2004;303:1010–4. doi: 10.1126/science.1092734. [DOI] [PubMed] [Google Scholar]
- 83.Kao SY, Lemoine FJ, Mariott SJ. HTLV-1 Tax protein sensitizes cells to apoptotic cell death induced by DNA damaging agents. Oncogene. 2000;19:2240–8. doi: 10.1038/sj.onc.1203559. [DOI] [PubMed] [Google Scholar]
- 84.Kao SY, Lemoine FJ, Marriott SJ. p53-independent induction of apoptosis by the HTLV-I tax protein following UV irradiation. Virology. 2001;291:292–8. doi: 10.1006/viro.2001.1200. [DOI] [PubMed] [Google Scholar]
- 85.Chlichlia K, Busslinger M, Peter ME, Walczak H, Krammer PH, Schirrmacher V, et al. ICE-proteases mediate HTLV-I Tax-induced apoptotic T-cell death. Oncogene. 1997;14:2265–72. doi: 10.1038/sj.onc.1201070. [DOI] [PubMed] [Google Scholar]
- 86.Yamada T, Yamaoka S, Goto T, Nakai M, Tsujimoto Y, Hatanaka M. The human T-cell leukemia virus type I Tax protein induces apoptosis which is blocked by the Bcl-2 protein. J Virol. 1994;68:3374–9. doi: 10.1128/jvi.68.5.3374-3379.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nicot C, Harrod R. Distinct p300-responsive mechanisms promote caspase-dependent apoptosis by human T-cell lymphotropic virus type 1 Tax protein. Mol Cell Biol. 2000;20:8580–9. doi: 10.1128/mcb.20.22.8580-8589.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hall AP, Irvine J, Blyth K, Cameron ER, Onions DE, Campbell ME. Tumours derived from HTLV-I tax transgenic mice are characterized by enhanced levels of apoptosis and oncogene expression. J Pathol. 1998;186:209–14. doi: 10.1002/(SICI)1096-9896(1998100)186:2<209::AID-PATH162>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 89.Franchini G, Wong-Staal F, Gallo RC. Human T-cell leukemia virus (HTLV-I) transcripts in fresh and cultured cells of patients with adult T-cell leukemia. Proc Natl Acad Sci U S A. 1984;81:6207–11. doi: 10.1073/pnas.81.19.6207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kinoshita T, Shimoyama M, Tobinai K, Ito M, Ito S, Ikeda S, et al. Detection of mRNA for the tax1/rex1 gene of human T-cell leukemia virus type I in fresh peripheral blood mononuclear cells of adult T-cell leukemia patients and viral carriers by using the polymerase chain reaction. Proc Natl Acad Sci U S A. 1989;86:5620–4. doi: 10.1073/pnas.86.14.5620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tendler CL, Greenberg SJ, Blattner WA, Manns A, Murphy E, Fleisher T, et al. Transactivation of interleukin 2 and its receptor induces immune activation in human T-cell lymphotropic virus type I-associated myelopathy: pathogenic implications and a rationale for immunotherapy. Proc Natl Acad Sci U S A. 1990;87:5218–22. doi: 10.1073/pnas.87.13.5218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ciminale V, Zotti L, D’Agostino DM, Ferro T, Casareto L, Franchini G, et al. Mitochondrial targeting of the p13II protein coded by the x-II ORF of human T-cell leukemia/lymphotropic virus type I (HTLV-I) Oncogene. 1999;18:4505–14. doi: 10.1038/sj.onc.1203047. [DOI] [PubMed] [Google Scholar]
- 93.Jacotot E, Ravagnan L, Loeffler M, Ferri KF, Vieira HL, Zamzami N, et al. The HIV-1 viral protein R induces apoptosis via a direct effect on the mitochondrial permeability transition pore. J Exp Med. 2000;191:33–46. doi: 10.1084/jem.191.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Su F, Schneider RJ. Hepatitis B virus HBx protein sensitizes cells to apoptotic killing by tumor necrosis factor alpha. Proc Natl Acad Sci U S A. 1997;94:8744–9. doi: 10.1073/pnas.94.16.8744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Takada S, Shirakata Y, Kaneniwa N, Koike K. Association of hepatitis B virus X protein with mitochondria causes mitochondrial aggregation at the nuclear periphery, leading to cell death. Oncogene. 1999;18:6965–73. doi: 10.1038/sj.onc.1203188. [DOI] [PubMed] [Google Scholar]
- 96.Shirakata Y, Koike K. Hepatitis B virus X protein induces cell death by causing loss of mitochondrial membrane potential. J Biol Chem. 2003;278:22071–8. doi: 10.1074/jbc.M301606200. [DOI] [PubMed] [Google Scholar]
- 97.Chen W, Calvo PA, Malide D, Gibbs J, Schubert U, Bacik I, et al. A novel influenza A virus mitochondrial protein that induces cell death. Nat Med. 2001;7:1306–12. doi: 10.1038/nm1201-1306. [DOI] [PubMed] [Google Scholar]
- 98.Gibbs JS, Malide D, Hornung F, Bennink JR, Yewdell JW. The influenza A virus PB1-F2 protein targets the inner mitochondrial membrane via a predicted basic amphipathic helix that disrupts mitochondrial function. J Virol. 2003;77:7214–24. doi: 10.1128/JVI.77.13.7214-7224.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hiraragi H, Michael B, Nair A, Silic-Benussi M, Ciminale V, Lairmore M. Human T-lymphotropic virus type 1 mitochondrion-localizing protein p13II sensitizes Jurkat T cells to Ras-mediated apoptosis. J Virol. 2005;79:9449–57. doi: 10.1128/JVI.79.15.9449-9457.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lefebvre L, Vanderplasschen A, Ciminale V, Heremans H, Dangoisse O, Jauniaux JC, et al. Oncoviral bovine leukemia virus G4 and human T-cell leukemia virus type 1 p13(II) accessory proteins interact with farnesyl pyrophosphate synthetase. J Virol. 2002;76:1400–14. doi: 10.1128/JVI.76.3.1400-1414.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.D’Agostino DM, Silic-Benussi M, Hiraragi H, Lairmore MD, Ciminale V. The human T-cell leukemia virus type 1 p13II protein: effects on mitochondrial function and cell growth. Cell Death Differ. 2005;12(Suppl 1):905–15. doi: 10.1038/sj.cdd.4401576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cereghetti GM, Scorrano L. The many shapes of mitochondrial death. Oncogene. 2006;25:4717–24. doi: 10.1038/sj.onc.1209605. [DOI] [PubMed] [Google Scholar]
- 103.Karbowski M, Norris KL, Cleland MM, Jeong SY, Youle RJ. Role of Bax and Bak in mitochondrial morphogenesis. Nature. 2006;443:658–62. doi: 10.1038/nature05111. [DOI] [PubMed] [Google Scholar]
- 104.Scorrano L. Proteins that fuse and fragment mitochondria in apoptosis: con-fissing a deadly con-fusion? J Bioenerg Biomembr. 2005;37:165–70. doi: 10.1007/s10863-005-6572-x. [DOI] [PubMed] [Google Scholar]
- 105.Sugioka R, Shimizu S, Tsujimoto Y. Fzo1, a protein involved in mitochondrial fusion, inhibits apoptosis. J Biol Chem. 2004;279:52726–34. doi: 10.1074/jbc.M408910200. [DOI] [PubMed] [Google Scholar]
- 106.Nicot C, Dundr M, Johnson JM, Fullen JR, Alonzo N, Fukumoto R, et al. HTLV-1-encoded p30II is a post-transcriptional negative regulator of viral replication. Nat Med. 2004;10:197–201. doi: 10.1038/nm984. [DOI] [PubMed] [Google Scholar]
- 107.Michael B, Nair AM, Hiraragi H, Shen L, Feuer G, Boris-Lawrie K, et al. Human T lymphotropic virus type-1 p30II alters cellular gene expression to selectively enhance signaling pathways that activate T lymphocytes. Retrovirology. 2004;1:39. doi: 10.1186/1742-4690-1-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Migone TS, Lin JX, Cereseto A, Mulloy JC, O’Shea JJ, Franchini G, et al. Constitutively activated Jak-STAT pathway in T cells transformed with HTLV-I. Science. 1995;269:79–81. doi: 10.1126/science.7604283. [DOI] [PubMed] [Google Scholar]
- 109.Takemoto S, Mulloy JC, Cereseto A, Migone TS, Patel BK, Matsuoka M, et al. Proliferation of adult T cell leukemia/lymphoma cells is associated with the constitutive activation of JAK/STAT proteins. Proc Natl Acad Sci U S A. 1997;94:13897–902. doi: 10.1073/pnas.94.25.13897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Debierre-Grockiego F. Anti-apoptotic role of STAT5 in haematopoietic cells and in the pathogenesis of malignancies. Apoptosis. 2004;9:717–28. doi: 10.1023/B:APPT.0000045785.65546.a2. [DOI] [PubMed] [Google Scholar]
- 111.Collins ND, D’Souza C, Albrecht B, Robek MD, Ratner L, Ding W, et al. Proliferation response to interleukin-2 and Jak/Stat activation of T cells immortalized by human T-cell lymphotropic virus type 1 is independent of open reading frame I expression. J Virol. 1999;73:9642–9. doi: 10.1128/jvi.73.11.9642-9649.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Albrecht B, Collins ND, Burniston MT, Nisbet JW, Ratner L, Green PL, et al. Human T-lymphotropic virus type 1 open reading frame I p12(I) is required for efficient viral infectivity in primary lymphocytes. J Virol. 2000;74:9828–35. doi: 10.1128/jvi.74.21.9828-9835.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Collins ND, Newbound GC, Albrecht B, Beard JL, Ratner L, Lairmore MD. Selective ablation of human T-cell lymphotropic virus type 1 p12I reduces viral infectivity in vivo. Blood. 1998;91:4701–7. [PubMed] [Google Scholar]
- 114.Nicot C, Mulloy JC, Ferrari MG, Johnson JM, Fu K, Fukumoto R, et al. HTLV-1 p12(I) protein enhances STAT5 activation and decreases the interleukin-2 requirement for proliferation of primary human peripheral blood mononuclear cells. Blood. 2001;98:823–9. doi: 10.1182/blood.v98.3.823. [DOI] [PubMed] [Google Scholar]
- 115.Mohapatra S, Chu B, Wei S, Djeu J, Epling-Burnette PK, Loughran T, et al. Roscovitine inhibits STAT5 activity and induces apoptosis in the human leukemia virus type 1-transformed cell line MT-2. Cancer Res. 2003;63:8523–30. [PubMed] [Google Scholar]
- 116.Gaudray G, Gachon F, Basbous J, Biard-Piechaczyk M, Devaux C, Mesnard JM. The complementary strand of the human T-cell leukemia virus type 1 RNA genome encodes a bZIP transcription factor that down-regulates viral transcription. J Virol. 2002;76:12813–22. doi: 10.1128/JVI.76.24.12813-12822.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Basbous J, Arpin C, Gaudray G, Piechaczyk M, Devaux C, Mesnard JM. The HBZ factor of human T-cell leukemia virus type I dimerizes with transcription factors JunB and c-Jun and modulates their transcriptional activity. J Biol Chem. 2003;278:43620–7. doi: 10.1074/jbc.M307275200. [DOI] [PubMed] [Google Scholar]
- 118.Matsumoto J, Ohshima T, Isono O, Shimotohno K. HTLV-1 HBZ suppresses AP-1 activity by impairing both the DNA-binding ability and the stability of c-Jun protein. Oncogene. 2005;24:1001–10. doi: 10.1038/sj.onc.1208297. [DOI] [PubMed] [Google Scholar]
- 119.Lemasson I, Lewis MR, Polakowski N, Hivin P, Cavanagh MH, Thebault S, et al. Human T-cell leukemia virus type 1 (HTLV-1) bZIP protein interacts with the cellular transcription factor CREB to inhibit HTLV-1 transcription. J Virol. 2007;81:1543–53. doi: 10.1128/JVI.00480-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Taylor GP, Matsuoka M. Natural history of adult T-cell leukemia/lymphoma and approaches to therapy. Oncogene. 2005;24:6047–57. doi: 10.1038/sj.onc.1208979. [DOI] [PubMed] [Google Scholar]
- 121.Mori N, Yamada Y, Ikeda S, Yamasaki Y, Tsukasaki K, Tanaka Y, et al. Bay 11-7082 inhibits transcription factor NF-kappaB and induces apoptosis of HTLV-I-infected T-cell lines and primary adult T-cell leukemia cells. Blood. 2002;100:1828–34. doi: 10.1182/blood-2002-01-0151. [DOI] [PubMed] [Google Scholar]
- 122.Sanda T, Asamitsu K, Ogura H, Iida S, Utsunomiya A, Ueda R, et al. Induction of cell death in adult T-cell leukemia cells by a novel IkappaB kinase inhibitor. Leukemia. 2006;20:590–8. doi: 10.1038/sj.leu.2404129. [DOI] [PubMed] [Google Scholar]
- 123.Satou Y, Nosaka K, Koya Y, Yasunaga JI, Toyokuni S, Matsuoka M. Proteasome inhibitor, bortezomib, potently inhibits the growth of adult T-cell leukemia cells both in vivo and in vitro. Leukemia. 2004;18:1357–63. doi: 10.1038/sj.leu.2403400. [DOI] [PubMed] [Google Scholar]
- 124.Mitra-Kaushik S, Harding JC, Hess JL, Ratner L. Effects of the proteasome inhibitor PS-341 on tumor growth in HTLV-1 Tax transgenic mice and Tax tumor transplants. Blood. 2004;104:802–9. doi: 10.1182/blood-2003-11-3967. [DOI] [PubMed] [Google Scholar]
- 125.Nasr R, El-Sabban ME, Karam JA, Dbaibo G, Kfoury Y, Arnulf B, et al. Efficacy and mechanism of action of the proteasome inhibitor PS-341 in T-cell lymphomas and HTLV-I associated adult T-cell leukemia/lymphoma. Oncogene. 2005;24:419–30. doi: 10.1038/sj.onc.1208212. [DOI] [PubMed] [Google Scholar]
- 126.Palombella VJ, Conner EM, Fuseler JW, Destree A, Davis JM, Laroux FS, et al. Role of the proteasome and NF-kappaB in streptococcal cell wall-induced polyarthritis. Proc Natl Acad Sci U S A. 1998;95:15671–6. doi: 10.1073/pnas.95.26.15671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Strauss SJ, Maharaj L, Hoare S, Johnson PW, Radford JA, Vinnecombe S, et al. Bortezomib therapy in patients with relapsed or refractory lymphoma: potential correlation of in vitro sensitivity and tumor necrosis factor alpha response with clinical activity. J Clin Oncol. 2006;24:2105–12. doi: 10.1200/JCO.2005.04.6789. [DOI] [PubMed] [Google Scholar]
- 128.Nishioka C, Ikezoe T, Yang J, Koeffler HP, Taguchi H. Fludarabine induces apoptosis of human T-cell leukemia virus type 1-infected T cells via inhibition of the nuclear factor-kappaB signal pathway. Leukemia. 2007;21:1044–9. doi: 10.1038/sj.leu.2404622. [DOI] [PubMed] [Google Scholar]
- 129.Dewan MZ, Uchihara JN, Terashima K, Honda M, Sata T, Ito M, et al. Efficient intervention of growth and infiltration of primary adult T-cell leukemia cells by an HIV protease inhibitor, ritonavir. Blood. 2006;107:716–24. doi: 10.1182/blood-2005-02-0735. [DOI] [PubMed] [Google Scholar]
- 130.De Clercq E. Antiviral drugs in current clinical use. J Clin Virol. 2004;30:115–33. doi: 10.1016/j.jcv.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 131.Jost PJ, Ruland J. Aberrant NF-kappaB signaling in lymphoma: mechanisms, consequences, and therapeutic implications. Blood. 2007;109:2700–7. doi: 10.1182/blood-2006-07-025809. [DOI] [PubMed] [Google Scholar]
- 132.Nasr R, Rosenwald A, El-Sabban ME, Arnulf B, Zalloua P, Lepelletier Y, et al. Arsenic/interferon specifically reverses 2 distinct gene networks critical for the survival of HTLV-1-infected leukemic cells. Blood. 2003;101:4576–82. doi: 10.1182/blood-2002-09-2986. [DOI] [PubMed] [Google Scholar]
- 133.Mahieux R, Pise-Masison C, Gessain A, Brady JN, Olivier R, Perret E, et al. Arsenic trioxide induces apoptosis in human T-cell leukemia virus type 1-and type 2-infected cells by a caspase-3-dependent mechanism involving Bcl-2 cleavage. Blood. 2001;98:3762–9. doi: 10.1182/blood.v98.13.3762. [DOI] [PubMed] [Google Scholar]
- 134.Mahieux R, Hermine O. In vivo and in vitro treatment of HTLV-1 and HTLV-2 infected cells with arsenic trioxide and interferon-alpha. Leuk Lymphoma. 2005;46:347–55. doi: 10.1080/10428190400019966. [DOI] [PubMed] [Google Scholar]
- 135.Chen YC, Lin-Shiau SY, Lin JK. Involvement of reactive oxygen species and caspase 3 activation in arsenite-induced apoptosis. J Cell Physiol. 1998;177:324–33. doi: 10.1002/(SICI)1097-4652(199811)177:2<324::AID-JCP14>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 136.El-Sabban ME, Nasr R, Dbaibo G, Hermine O, Abboushi N, Quignon F, et al. Arsenic-interferon-alpha-triggered apoptosis in HTLV-I transformed cells is associated with tax down-regulation and reversal of NF-kappa B activation. Blood. 2000;96:2849–55. [PubMed] [Google Scholar]
- 137.Bazarbachi A, El-Sabban ME, Nasr R, Quignon F, Awaraji C, Kersual J, et al. Arsenic trioxide and interferon-alpha synergize to induce cell cycle arrest and apoptosis in human T-cell lymphotropic virus type I-transformed cells. Blood. 1999;93:278–83. [PubMed] [Google Scholar]
- 138.Ishitsuka K, Hanada S, Uozumi K, Utsunomiya A, Arima T. Arsenic trioxide and the growth of human T-cell leukemia virus type I infected T-cell lines. Leuk Lymphoma. 2000;37:649–55. doi: 10.3109/10428190009058521. [DOI] [PubMed] [Google Scholar]
- 139.Brown M, Bellon M, Nicot C. Emodin and DHA potently increase arsenic trioxide interferon-alpha-induced cell death of HTLV-I-transformed cells by generation of reactive oxygen species and inhibition of Akt and AP-1. Blood. 2007;109:1653–9. doi: 10.1182/blood-2006-04-015537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Rajasingh J, Raikwar HP, Muthian G, Johnson C, Bright JJ. Curcumin induces growth-arrest and apoptosis in association with the inhibition of constitutively active JAK-STAT pathway in T cell leukemia. Biochem Biophys Res Commun. 2006;340:359–68. doi: 10.1016/j.bbrc.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 141.Tomita M, Kawakami H, Uchihara JN, Okudaira T, Masuda M, Matsuda T, et al. Inhibition of constitutively active Jak-Stat pathway suppresses cell growth of human T-cell leukemia virus type 1-infected T-cell lines and primary adult T-cell leukemia cells. Retrovirology. 2006;3:22. doi: 10.1186/1742-4690-3-22. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 142.Tomita M, Kawakami H, Uchihara JN, Okudaira T, Masuda M, Takasu N, et al. Curcumin (diferuloylmethane) inhibits constitutive active NF-kappaB, leading to suppression of cell growth of human T-cell leukemia virus type I-infected T-cell lines and primary adult T-cell leukemia cells. Int J Cancer. 2006;118:765–72. doi: 10.1002/ijc.21389. [DOI] [PubMed] [Google Scholar]
- 143.Tomita M, Matsuda T, Kawakami H, Uchihara JN, Okudaira T, Masuda M, et al. Curcumin targets Akt cell survival signaling pathway in HTLV-I-infected T-cell lines. Cancer Sci. 2006;97:322–7. doi: 10.1111/j.1349-7006.2006.00175.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 144.Kirken RA, Erwin RA, Wang L, Wang Y, Rui H, Farrar WL. Functional uncoupling of the Janus kinase 3-Stat5 pathway in malignant growth of human T cell leukemia virus type 1-transformed human T cells. J Immunol. 2000;165:5097–104. doi: 10.4049/jimmunol.165.9.5097. [DOI] [PubMed] [Google Scholar]
- 145.Kawakami H, Tomita M, Okudaira T, Ishikawa C, Matsuda T, Tanaka Y, et al. Inhibition of heat shock protein-90 modulates multiple functions required for survival of human T-cell leukemia virus type I-infected T-cell lines and adult T-cell leukemia cells. Int J Cancer. 2007;120:1811–20. doi: 10.1002/ijc.22403. [DOI] [PubMed] [Google Scholar]
- 146.Banerji U, O’Donnell A, Scurr M, Pacey S, Stapleton S, Asad Y, et al. Phase I pharmacokinetic and pharmacodynamic study of 17-allylamino, 17-demethoxygeldanamycin in patients with advanced malignancies. J Clin Oncol. 2005;23:4152–61. doi: 10.1200/JCO.2005.00.612. [DOI] [PubMed] [Google Scholar]
- 147.Yonehara S, Ishii A, Yonehara M. A cell-killing monoclonal antibody (anti-Fas) to a cell surface antigen co-downregulated with the receptor of tumor necrosis factor. J Exp Med. 1989;169:1747–56. doi: 10.1084/jem.169.5.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Debatin KM, Goldman CK, Waldmann TA, Krammer PH. APO-1-induced apoptosis of leukemia cells from patients with adult T-cell leukemia. Blood. 1993;81:2972–7. [PubMed] [Google Scholar]
- 149.Debatin KM, Goldmann CK, Bamford R, Waldmann TA, Krammer PH. Monoclonal-antibody-mediated apoptosis in adult T-cell leukaemia. Lancet. 1990;335:497–500. doi: 10.1016/0140-6736(90)90735-n. [DOI] [PubMed] [Google Scholar]
- 150.Waldmann TA, White JD, Goldman CK, Top L, Grant A, Bamford R, et al. The interleukin-2 receptor: a target for monoclonal antibody treatment of human T-cell lymphotrophic virus I-induced adult T-cell leukemia. Blood. 1993;82:1701–12. [PubMed] [Google Scholar]
- 151.Waldmann TA, Goldman CK, Bongiovanni KF, Sharrow SO, Davey MP, Cease KB, et al. Therapy of patients with human T-cell lymphotrophic virus I-induced adult T-cell leukemia with anti-Tac, a monoclonal antibody to the receptor for interleukin-2. Blood. 1988;72:1805–16. [PubMed] [Google Scholar]
- 152.Callens C, Moura IC, Lepelletier Y, Coulon S, Renand A, Dussiot M, et al. Recent advances in adult T-cell leukemia therapy: focus on a new anti-transferrin receptor monoclonal antibody. Leukemia. 2008;22:42–8. doi: 10.1038/sj.leu.2404958. [DOI] [PubMed] [Google Scholar]
- 153.Waldmann TA, White JD, Carrasquillo JA, Reynolds JC, Paik CH, Gansow OA, et al. Radioimmunotherapy of interleukin-2R alpha-expressing adult T-cell leukemia with Yttrium-90-labeled anti-Tac. Blood. 1995;86:4063–75. [PubMed] [Google Scholar]
- 154.Waldmann TA. Daclizumab (anti-Tac, Zenapax) in the treatment of leukemia/lymphoma. Oncogene. 2007;26:3699–703. doi: 10.1038/sj.onc.1210368. [DOI] [PubMed] [Google Scholar]
- 155.Phillips KE, Herring B, Wilson LA, Rickford MS, Zhang M, Goldman CK, et al. IL-2Ralpha-Directed monoclonal antibodies provide effective therapy in a murine model of adult T-cell leukemia by a mechanism other than blockade of IL-2/IL-2Ralpha interaction. Cancer Res. 2000;60:6977–84. [PubMed] [Google Scholar]
- 156.Tan C, Waldmann TA. Proteasome inhibitor PS-341, a potential therapeutic agent for adult T-cell leukemia. Cancer Res. 2002;62:1083–6. [PubMed] [Google Scholar]
- 157.Zhang Z, Zhang M, Goldman CK, Ravetch JV, Waldmann TA. Effective therapy for a murine model of adult T-cell leukemia with the humanized anti-CD52 monoclonal antibody, Campath-1H. Cancer Res. 2003;63:6453–7. [PubMed] [Google Scholar]
- 158.Moura IC, Lepelletier Y, Arnulf B, England P, Baude C, Beaumont C, et al. A neutralizing monoclonal antibody (mAb A24) directed against the transferrin receptor induces apoptosis of tumor T lymphocytes from ATL patients. Blood. 2004;103:1838–45. doi: 10.1182/blood-2003-07-2440. [DOI] [PubMed] [Google Scholar]
- 159.Vidal C, Matsushita S, Colamonici OR, Trepel JB, Mitsuya H, Neckers LM. Human T lymphotropic virus I infection deregulates surface expression of the transferrin receptor. J Immunol. 1988;141:984–8. [PubMed] [Google Scholar]
- 160.Maeda Y, Miyatake J, Sono H, Matsuda M, Tatsumi Y, Horiuchi F, et al. 13-cis retinoic acid inhibits growth of adult T cell leukemia cells and causes apoptosis; possible new indication for retinoid therapy. Intern Med. 1996;35:180–4. doi: 10.2169/internalmedicine.35.180. [DOI] [PubMed] [Google Scholar]
- 161.Fujimura S, Suzumiya J, Anzai K, Ohkubo K, Hata T, Yamada Y, et al. Retinoic acids induce growth inhibition and apoptosis in adult T-cell leukemia (ATL) cell lines. Leuk Res. 1998;22:611–8. doi: 10.1016/s0145-2126(98)00049-6. [DOI] [PubMed] [Google Scholar]
- 162.Furuke K, Sasada T, Ueda-Taniguchi Y, Yamauchi A, Inamoto T, Yamaoka Y, et al. Role of intracellular redox status in apoptosis induction of human T-cell leukemia virus type I-infected lymphocytes by 13-cis-retinoic acid. Cancer Res. 1997;57:4916–23. [PubMed] [Google Scholar]
- 163.Darwiche N, Hatoum A, Dbaibo G, Kadara H, Nasr R, Abou-Lteif G, et al. N-(4-hydroxyphenyl)retinamide induces growth arrest and apoptosis in HTLV-I-transformed cells. Leukemia. 2004;18:607–15. doi: 10.1038/sj.leu.2403245. [DOI] [PubMed] [Google Scholar]
- 164.Boya P, Morales MC, Gonzalez-Polo RA, Andreau K, Gourdier I, Perfettini JL, et al. The chemopreventive agent N-(4-hydroxyphenyl)retinamide induces apoptosis through a mitochondrial pathway regulated by proteins from the Bcl-2 family. Oncogene. 2003;22:6220–30. doi: 10.1038/sj.onc.1206827. [DOI] [PubMed] [Google Scholar]
- 165.Darwiche N, Abou-Lteif G, Bazarbachi A. Reactive oxygen species mediate N-(4-hydroxyphenyl)retinamide-induced cell death in malignant T cells and are inhibited by the HTLV-I oncoprotein Tax. Leukemia. 2007;21:261–9. doi: 10.1038/sj.leu.2404472. [DOI] [PubMed] [Google Scholar]
- 166.Maeda Y, Yamaguchi T, Ueda S, Miyazato H, Matsuda M, Kanamaru A. All-trans retinoic acid reduced skin involvement of adult T-cell leukemia. Leukemia. 2004;18:1159–60. doi: 10.1038/sj.leu.2403356. [DOI] [PubMed] [Google Scholar]
- 167.Inozume T, Matsue H, Furuhashi M, Nakamura Y, Mitsui H, Ando N, et al. Successful use of etretinate for long-term management of a patient with cutaneous-type adult T-cell leukaemia/lymphoma. Br J Dermatol. 2005;153:1239–41. doi: 10.1111/j.1365-2133.2005.06974.x. [DOI] [PubMed] [Google Scholar]
- 168.Nawata H, Maeda Y, Sumimoto Y, Miyatake J, Kanamaru A. A mechanism of apoptosis induced by all-trans retinoic acid on adult T-cell leukemia cells: a possible involvement of the Tax/NF-kappaB signaling pathway. Leuk Res. 2001;25:323–31. doi: 10.1016/s0145-2126(00)00126-0. [DOI] [PubMed] [Google Scholar]
- 169.Darwiche N, El-Sabban M, Bazzi R, Nasr R, Al-Hashimi S, Hermine O, et al. Retinoic acid dramatically enhances the arsenic trioxide-induced cell cycle arrest and apoptosis in retinoic acid receptor alpha-positive human T-cell lymphotropic virus type-I-transformed cells. Hematol J. 2001;2:127–35. doi: 10.1038/sj/thj/6200098. [DOI] [PubMed] [Google Scholar]
- 170.Gill PS, Harrington W, Jr, Kaplan MH, Ribeiro RC, Bennett JM, Liebman HA, et al. Treatment of adult T-cell leukemia-lymphoma with a combination of interferon alfa and zidovudine. N Engl J Med. 1995;332:1744–8. doi: 10.1056/NEJM199506293322603. [DOI] [PubMed] [Google Scholar]
- 171.Hermine O, Bouscary D, Gessain A, Turlure P, Leblond V, Franck N, et al. Brief report: treatment of adult T-cell leukemia-lymphoma with zidovudine and interferon alfa. N Engl J Med. 1995;332:1749–51. doi: 10.1056/NEJM199506293322604. [DOI] [PubMed] [Google Scholar]
- 172.Bazarbachi A, Nasr R, El-Sabban ME, Mahe A, Mahieux R, Gessain A, et al. Evidence against a direct cytotoxic effect of alpha interferon and zidovudine in HTLV-I associated adult T cell leukemia/lymphoma. Leukemia. 2000;14:716–21. doi: 10.1038/sj.leu.2401742. [DOI] [PubMed] [Google Scholar]
- 173.Datta A, Nicot C. Telomere attrition induces a DNA double-strand break damage signal that reactivates p53 transcription in HTLV-I leukemic cells. Oncogene. 2007 doi: 10.1038/sj.onc.1210718. [DOI] [PubMed] [Google Scholar]
- 174.Datta A, Bellon M, Sinha-Datta U, Bazarbachi A, Lepelletier Y, Canioni D, et al. Persistent inhibition of telomerase reprograms adult T-cell leukemia to p53-dependent senescence. Blood. 2006;108:1021–9. doi: 10.1182/blood-2006-01-0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Ikeda K, Oka M, Yamada Y, Soda H, Fukuda M, Kinoshita A, et al. Adult T-cell leukemia cells over-express the multidrug-resistance-protein (MRP) and lung-resistance-protein (LRP) genes. Int J Cancer. 1999;82:599–604. doi: 10.1002/(sici)1097-0215(19990812)82:4<599::aid-ijc21>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 176.Yasunami T, Wang YH, Tsuji K, Takanashi M, Yamada Y, Motoji T. Multidrug resistance protein expression of adult T-cell leukemia/lymphoma. Leuk Res. 2007;31:465–70. doi: 10.1016/j.leukres.2006.10.012. [DOI] [PubMed] [Google Scholar]
- 177.Lau A, Nightingale S, Taylor GP, Gant TW, Cann AJ. Enhanced MDR1 gene expression in human T-cell leukemia virus-I-infected patients offers new prospects for therapy. Blood. 1998;91:2467–74. [PubMed] [Google Scholar]
- 178.Hamamura RS, Ohyashiki JH, Kurashina R, Kobayashi C, Zhang Y, Takaku T, et al. Induction of heme oxygenase-1 by cobalt protoporphyrin enhances the antitumour effect of bortezomib in adult T-cell leukaemia cells. Br J Cancer. 2007;97:1099–105. doi: 10.1038/sj.bjc.6604003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Okudaira T, Tomita M, Uchihara JN, Matsuda T, Ishikawa C, Kawakami H, et al. NIK-333 inhibits growth of human T-cell leukemia virus type I-infected T-cell lines and adult T-cell leukemia cells in association with blockade of nuclear factor-kappaB signal pathway. Mol Cancer Ther. 2006;5:704–12. doi: 10.1158/1535-7163.MCT-05-0434. [DOI] [PubMed] [Google Scholar]
- 180.Watanabe M, Ohsugi T, Shoda M, Ishida T, Aizawa S, Maruyama-Nagai M, et al. Dual targeting of transformed and untransformed HTLV-1-infected T cells by DHMEQ, a potent and selective inhibitor of NF-kappaB, as a strategy for chemoprevention and therapy of adult T-cell leukemia. Blood. 2005;106:2462–71. doi: 10.1182/blood-2004-09-3646. [DOI] [PubMed] [Google Scholar]
- 181.Ariga A, Namekawa J, Matsumoto N, Inoue J, Umezawa K. Inhibition of tumor necrosis factor-alpha -induced nuclear translocation and activation of NF-kappa B by dehydroxymethylepoxyquinomicin. J Biol Chem. 2002;277:24625–30. doi: 10.1074/jbc.M112063200. [DOI] [PubMed] [Google Scholar]
- 182.Okudaira T, Hirashima M, Ishikawa C, Makishi S, Tomita M, Matsuda T, et al. A modified version of galectin-9 suppresses cell growth and induces apoptosis of human T-cell leukemia virus type I-infected T-cell lines. Int J Cancer. 2007;120:2251–61. doi: 10.1002/ijc.22534. [DOI] [PubMed] [Google Scholar]
- 183.Mori N, Matsuda T, Tadano M, Kinjo T, Yamada Y, Tsukasaki K, et al. Apoptosis induced by the histone deacetylase inhibitor FR901228 in human T-cell leukemia virus type 1-infected T-cell lines and primary adult T-cell leukemia cells. J Virol. 2004;78:4582–90. doi: 10.1128/JVI.78.9.4582-4590.2004. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 184.Zhang J, Nagasaki M, Tanaka Y, Morikawa S. Capsaicin inhibits growth of adult T-cell leukemia cells. Leuk Res. 2003;27:275–83. doi: 10.1016/s0145-2126(02)00164-9. [DOI] [PubMed] [Google Scholar]
- 185.Harakeh S, Diab-Assaf M, Abu-El-Ardat K, Niedzwiecki A, Rath M. Mechanistic aspects of apoptosis induction by l-lysine in both HTLV-1-positive and -negative cell lines. Chem Biol Interact. 2006;164:102–14. doi: 10.1016/j.cbi.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 186.Ishitsuka K, Ikeda R, Utsunomiya A, Uozumi K, Hanada S, Suzuki S, et al. Arsenic trioxide induces apoptosis in HTLV-I infected T-cell lines and fresh adult T-cell leukemia cells through CD95 or tumor necrosis factor alpha receptor independent caspase activation. Leuk Lymphoma. 2002;43:1107–14. doi: 10.1080/10428190290021461. [DOI] [PubMed] [Google Scholar]
- 187.Dasgupta A, Jung KJ, Jeong SJ, Brady JN. Inhibition of methyltransferases results in induction of g2/m checkpoint and programmed cell death in human T-lymphotropic virus type 1-transformed cells. J Virol. 2008;82:49–59. doi: 10.1128/JVI.01497-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Harakeh S, Diab-Assaf M, Khalife JC, Abu-el-Ardat KA, Baydoun E, Niedzwiecki A, et al. Ascorbic acid induces apoptosis in adult T-cell leukemia. Anticancer Res. 2007;27:289–98. [PubMed] [Google Scholar]
- 189.Jeong SJ, Dasgupta A, Jung KJ, Um JH, Burke A, Park HU, et al. PI3K/AKT inhibition induces caspase-dependent apoptosis in HTLV-1-transformed cells. Virology. 2008;370:264–72. doi: 10.1016/j.virol.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Harakeh S, Abu-El-Ardat K, Diab-Assaf M, Niedzwiecki A, El-Sabban M, Rath M. Epigallocatechin-3-gallate induces apoptosis and cell cycle arrest in HTLV-1-positive and -negative leukemia cells. Med Oncol. 2008;25:30–9. doi: 10.1007/s12032-007-0036-6. [DOI] [PubMed] [Google Scholar]
- 191.Moarbess G, El-Hajj H, Kfoury Y, El-Sabban ME, Lepelletier Y, Hermine O, et al. EAPB0203, a member of the imidazoquinoxaline family, inhibits growth and induces caspase dependent apoptosis in T cell lymphomas and HTLV-I associated adult T-cell leukemia/lymphoma. Blood. 2008 doi: 10.1182/blood-2007-11-121913. [DOI] [PubMed] [Google Scholar]
- 192.Haneji K, Matsuda T, Tomita M, Kawakami H, Ohshiro K, Uchihara JN, et al. Fucoidan extracted from Cladosiphon okamuranus Tokida induces apoptosis of human T-cell leukemia virus type 1-infected T-cell lines and primary adult T-cell leukemia cells. Nutr Cancer. 2005;52:189–201. doi: 10.1207/s15327914nc5202_9. [DOI] [PubMed] [Google Scholar]
- 193.Hayashibara T, Yamada Y, Nakayama S, Harasawa H, Tsuruda K, Sugahara K, et al. Resveratrol induces downregulation in survivin expression and apoptosis in HTLV-1-infected cell lines: a prospective agent for adult T cell leukemia chemotherapy. Nutr Cancer. 2002;44:193–201. doi: 10.1207/S15327914NC4402_12. [DOI] [PubMed] [Google Scholar]
- 194.Harakeh S, Diab-Assef M, El-Sabban M, Haddadin M, Gali-Muhtasib H. Inhibition of proliferation and induction of apoptosis by 2-benzoyl-3-phenyl-6,7-dichloroquinoxaline 1,4-dioxide in adult T-cell leukemia cells. Chem Biol Interact. 2004;148:101–13. doi: 10.1016/j.cbi.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 195.Achachi A, Florins A, Gillet N, Debacq C, Urbain P, Foutsop GM, et al. Valproate activates bovine leukemia virus gene expression, triggers apoptosis, and induces leukemia/lymphoma regression in vivo. Proc Natl Acad Sci U S A. 2005;102:10309–14. doi: 10.1073/pnas.0504248102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Lezin A, Gillet N, Olindo S, Signate A, Grandvaux N, Verlaeten O, et al. Histone deacetylase mediated transcriptional activation reduces proviral loads in HTLV-1 associated myelopathy/tropical spastic paraparesis patients. Blood. 2007;110:3722–8. doi: 10.1182/blood-2007-04-085076. [DOI] [PubMed] [Google Scholar]
- 197.Nishioka C, Ikezoe T, Yang J, Komatsu N, Bandobashi K, Taniguchi A, et al. Histone deacetylase inhibitors induce growth arrest and apoptosis of HTLV-1-infected T-cells via blockade of signaling by nuclear factor kappaB. Leuk Res. 2008;32:287–96. doi: 10.1016/j.leukres.2007.05.026. [DOI] [PubMed] [Google Scholar]
- 198.Hasegawa H, Yamada Y, Komiyama K, Hayashi M, Ishibashi M, Yoshida T, et al. Dihydroflavonol BB-1, an extract of natural plant Blumea balsamifera, abrogates TRAIL resistance in leukemia cells. Blood. 2006;107:679–88. doi: 10.1182/blood-2005-05-1982. [DOI] [PubMed] [Google Scholar]
- 199.Sinha-Datta U, Taylor JM, Brown M, Nicot C. Celecoxib disrupts the canonical apoptotic network in HTLV-I cells through activation of Bax and inhibition of PKB/Akt. Apoptosis. 2008;13:33–40. doi: 10.1007/s10495-007-0148-7. [DOI] [PubMed] [Google Scholar]
- 200.Harakeh S, Diab-Assaf M, Niedzwiecki A, Khalife J, Abu-El-Ardat K, Rath M. Apoptosis induction by Epican Forte in HTLV-1 positive and negative malignant T-cells. Leuk Res. 2006;30:869–81. doi: 10.1016/j.leukres.2005.11.027. [DOI] [PubMed] [Google Scholar]
- 201.Waldmann TA, Longo DL, Leonard WJ, Depper JM, Thompson CB, Kronke M, et al. Interleukin 2 receptor (Tac antigen) expression in HTLV-I-associated adult T-cell leukemia. Cancer Res. 1985;45:4559s–4562s. [PubMed] [Google Scholar]


