Abstract
Background
The presence and extent of contraction within the pulmonary veins (PV) have not been defined clearly.
Objective
To determine if PV contraction exists, and can be visualized using multislice CT scanning (MSCT) as this may indicate that this modality may be useful for monitoring patients after pulmonary vein isolation procedures.
Methods
Analysis was performed on 29 patients (mean age 57.5± 12 years) undergoing MSCT for suspected coronary artery disease without structural heart disease or left atrial anatomical variants. Multiplane reconstructions were used to measure PV diameters at 0, 5, 10, and 15 mm from the ostium in two phases (maximum and minimum size). The ejection fractions of three 5mm segments were calculated for each pulmonary vein.
Results
Right-sided and left-sided pulmonary vein contraction and maximal atrial contraction occurred at a median of 85% and 95% of the cardiac cycle, respectively. The temporal concordance of minimal PV volume during peak atrial contraction indicated that the PV volume changes are secondary to active contraction rather than passive reflux and PV distension. The ejection fractions were highest in the superior veins: right superior PV (36.7%, 27.8% and 16%, respectively for the three segments from proximal to distal) and left superior PV (26.9%, 21.3% and 12.1%), in comparison to the right inferior PV (21.1%, 6.6%, - 0.7%) and left inferior PV (15%, 9.3%, 7.6%).
Conclusion
Volume changes related to active pulmonary vein contraction occur extending up to 15mm into the veins, and this effect is most pronounced in the superior veins.
Keywords: Atrium, Imaging, CT scanning, Myocardial Contraction/*physiology
Introduction
The expanding role of catheter ablation1 for the treatment of atrial fibrillation has increased the significance of establishing a uniform end-point for these procedures. Pulmonary vein (PV) isolation is now accepted as the best candidate end-point; however, it remains unclear whether PV isolation is sufficient, or even necessary, for clinical success. Furthermore, confirming long-term pulmonary vein isolation requires a repeat mapping and ablation study, along with its associated risks. It would be preferable to have a non-invasive strategy during long-term follow-up to establish a correlation between clinical outcome and PV isolation and reconnection.
Pathological examination has demonstrated the existence of muscle fibers arranged in a circular pattern around the pulmonary vein ostia, and of layers of atrial muscle extending up to several centimeters into the pulmonary veins.2 It has been postulated that the circumferential layer of atrial musculature around the pulmonary veins may work as a sphincter-like mechanism, preventing backflow of blood during atrial contraction3. It has also been postulated that the layers of myocardium inside the pulmonary veins are the triggers of atrial fibrillation in most patients with paroxysmal atrial fibrillation.4 However, it is possible that significant contraction occurs not only at the ostia, but also more distally within the pulmonary veins. Although dynamic motion of the PV ostia has been studied using direct angiography,5 intracardiac echocardiography,6 magnetic resonance imaging7-10 and multisclice CT scanning 11, 12, the presence of pulmonary vein contraction distal to the ostia has not been studied systematically.
We hypothesized that 64-slice MSCT scanning would allow noninvasive evaluation of pulmonary vein contraction patterns. This would be useful for the evaluation of patients post pulmonary vein ablation, wherein the absence of PV contraction could act as a surrogate marker for successful pulmonary vein isolation.
Methods
This study was approved by the Massachusetts General Hospital Human Research Committee and was performed according to institutional guidelines. Imaging studies from patients in sinus rhythm undergoing 64- MSCT imaging for suspected coronary disease were screened for inclusion. Patients were excluded from the study if the pulmonary veins were not within the reconstructed field of the multiphase study or if the imaging quality was insufficient to allow accurate measurement of pulmonary vein dimensions. Patients with structural heart disease, known atrial arrhythmias, or anomalous atrial anatomy were also excluded, as shown in Table 1.
Table 1.
Study Inclusion and Exclusion Criteria
| Inclusion criteria |
| Age > 18 years |
| Sinus rhythm during CT scan |
| Multiphase reconstruction available |
| All four pulmonary veins visible on multiphase reconstruction |
| Multiphase image quality sufficient to allow measurement of pulmonary vein dimensions |
| Exclusion criteria |
| Structural heart disease |
| Anomalies of pulmonary vein anatomy (e.g. common PV ostia or accessory PVs) |
| Previous radiofrequency ablation of the atria |
| Past history of atrial or ventricular arrhythmia |
Multislice CT imaging details
Multislice CT images were acquired using a 64-slice CT scanner (Sensation 64, Siemens, Germany). Intravenous metoprolol (maximum dose 20 mg) was administered as needed to reduce the heart rate to approximately 60 bpm, unless contraindicated. As per coronary MSCT protocol, a single dose of sublingual nitroglycerin was also administered unless contraindicated. Imaging was performed during a single inspiratory breath hold. A test bolus of 15 ml of iodinated contrast agent (Iodhexodol 320 g/cm3, Visipaque, Nycomed Amersham, Princeton, NJ) was used to determine the optimal imaging time. During imaging, the following parameters were used: 64 × 0.6-mm slice collimation, tube voltage of 120 kV, gantry rotation time of 330 ms, and 850 mA tube current.
Axial multiphase images were reconstructed (slice thickness and increment of 1.5 mm) using retrospective ECG-gating. Images were reconstructed at 10 phases of the cardiac cycle (5% to 95% of the R-R interval).
Image analysis
Image analysis was performed using the Osirix Dicom imaging system (Version 2.7.5, Osirix) on a Macintosh computer (Apple Inc, Cupertino, CA). Measurements of pulmonary vein size were performed by orienting a viewing plane perpendicular to the direction of the vein, using the double oblique technique (Figure 1).11
Figure 1.
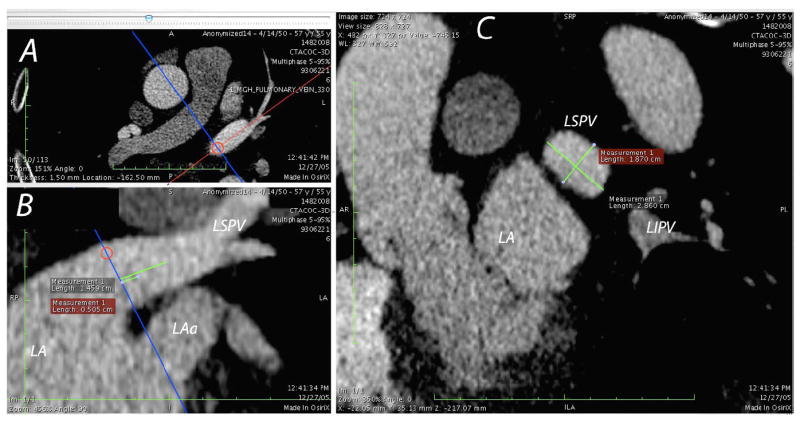
Multiplane reconstruction allows measurement of the pulmonary vein dimensions using a double oblique technique. In the upper left panel, the left superior vein (LSPV) is shown in an axial view. The orientation of a second plane (panel B) perpendicular to the axial plane is orientated along the long axis of the LSPV (red line in Panel A). Marker lines are drawn on the second panel to indicate the location of the PV ostia, 5 mm, 10 mm and 15 mm locations. The orientation of the measurement plane (panel C) is then adjusted on the second plane to ensure that measurement of the pulmonary vein dimensions are performed perpendicular to the long axis of the vein. The location of the left atrium body (LA), left atrial appendage (LAa) and left inferior pulmonary vein (LIPV) are also marked.
The double oblique technique was performed by orienting two other planes along the center-line of the pulmonary vein to ensure that the measurement plane was truly perpendicular to the pulmonary vein. A measurement line was then drawn along the centre of the vein, indicating the location of the PV ostia and 5, 10 and 15 mm distal to the ostia. The pulmonary vein dimensions (long and short axis) were measured at each of these four locations for two phases (maximal size and minimal size). The circularity index was then calculated by dividing the short axis measurement by the long axis.
To ensure that movement of the atrial wall did not affect measurement accuracy, the location of the pulmonary vein ostia was corrected between the two cardiac phases by noting the movement of a distinctive point within the vein, such as the first branching point. Note that the term ‘contraction’ is used to refer to changes in volume in the pulmonary vein – the term is used in the sense of ‘a diminishment in size’, but not to imply that this volume reduction is caused entirely by myocardial contraction within the pulmonary vein. The volume of each pulmonary vein segment was calculated using Simpson’s disc method to allow calculation of the ejection fraction.
Statistical analysis
Statistical analysis was performed using R for Mac OS X (Version 2.5.1: The R Foundation for Statistical Computing, http://www.R-project.org.). Except where stated otherwise, data are reported in the tables as means ± standard deviations. Student’s T-test was used to test the difference between means.
Results
Patient demographic data
A total of 29 patients were analyzed. The mean age was 57.5 ± 12 years and 16 patients were female. The mean heart rate during scanning was 66 beats per minute.
Volume rendered images – Qualitative analysis
Volume rendered images allowed for the recognition of pulmonary vein anomalies. 4D volume rendered images were also useful for recognizing the spatial and temporal pattern of atrial and pulmonary vein motion (Figures 2&3). Maximal atrial contraction predominantly occurred at the 85% phase. The earliest visible atrial contraction was usually located in the atrial wall, at the base of the RSPV. The minimum volume of the right-sided pulmonary veins occurred earlier than that of the left sided pulmonary veins (median 85% phase versus 95% for the LSPV and LIPV). In the majority of patients, contraction of the atrial wall was visible at the ostium of all four veins. The right and left inferior veins usually showed early branching and lacked prominent distal contraction, whereas the right and left superior veins largely showed visible contraction, predominantly in an anterior-posterior direction.
Figure 2.
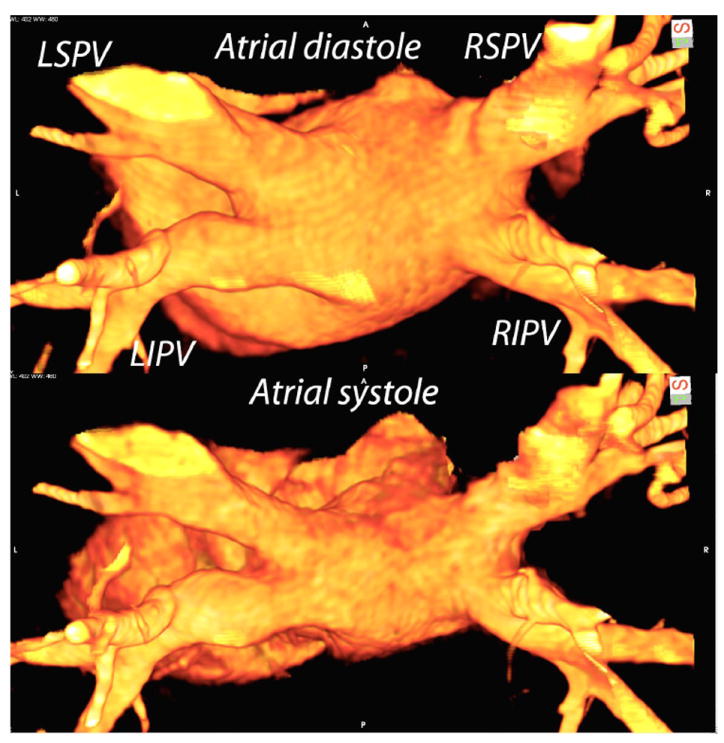
Volume rendered MSCT images from two cardiac phases (35% and 95% corresponding PV maximal and minimal volumes). The images are viewed from a posterosuperior angle and the location of all four pulmonary veins is marked in the upper panel. In the lower panel, atrial contraction is visible and there is significant contraction in left superior pulmonary with a lesser degree of contraction of the right superior pulmonary vein. The inferior pulmonary veins appear to be the same volume between the two phases in this case.
Figure 3.
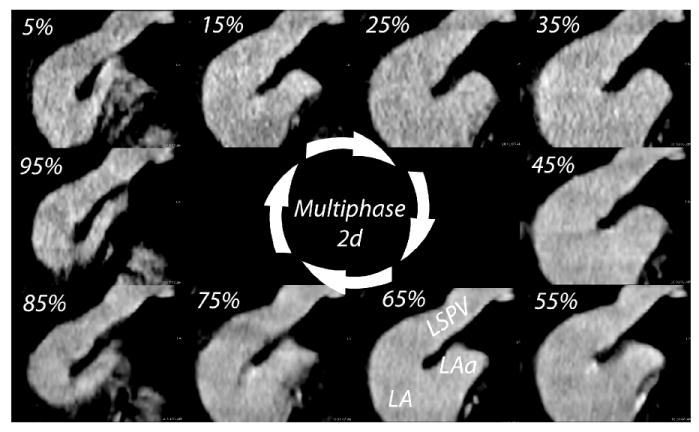
Multiphase MSCT images from the same patient shown in Figure 2. The 10 phases used for analysis (5% - 95%) are shown as 2d images oriented on the left superior pulmonary vein (LSPV). The body of the left atrium (LA) and the left atrial appendage (LAa) are also visible. The maximal LSPV size occurs at 35% and the minimum at 95%.
Quantitative analysis
The PV ostia were displaced by a median of 1 mm between the two cardiac phases for the LSPV (range 0 to 5 mm), 1 mm for right superior (range 0 to 2 mm), 1 mm for the left inferior (range 0 to 3 mm) and 0 mm for the right inferior (range 0 to 2 mm). The ostial dimensions for the four pulmonary veins varied markedly over the cardiac cycle (Table 2). The shape of the left-sided pulmonary vein ostia was significantly more elliptical than the right-sided PV ostia (p=0.016 and p < 0.001 for comparison of the RSPV against the RIPV and the LSPV against the LIPV respectively).
Table 2. Ostial measurements.
Pulmonary vein ostial measurements were all significantly different (marked with *) between maximal and minimal phases The superior pulmonary veins were also significantly more circular during the maximal volume phase than the minimal volume phase.
| Long axis | Short Axis | Circularity index | p | |
|---|---|---|---|---|
| RPSV Maximum | 22.3 ± 4.9 mm* | 17.8 ± 3.8 mm* | 0.8 ± 0.11 | }p=0.02 |
| RSPV Minimum | 17.6 ± 4.1 mm* | 13.1 ± 3.9 mm* | 0.75 ± 0.13 | |
| RIPV Maximum | 19.2 ± 3.0 mm* | 16.0 ± 2.2 mm* | 0.84 ± 0.11 | }p=0.08 |
| RIPV Minimum | 16.5 ± 3.1* | 13.3 ± 2.9 mm* | 0.81 ± 0.11 | |
| LSPV Maximum | 18.9 ± 3.4 mm* | 13.4 ± 3.2 mm* | 0.71 ± 0.14 | }p=0.04 |
| LSPV Minimum | 16.3 ± 2.4 mm* | 11.0 ± 2.7 mm* | 0.67 ± 0.13 | |
| LIPV Maximum | 17.4 ± 2.6* | 11.3 ± 2.9* | 0.66 ± 0.16 | }p=0.3 |
| LIPV Minimum | 15.6 ± 2.1* | 9.8 ± 2.3* | 0.64 ± 0.14 |
Changes in pulmonary vein volume during the cardiac cycle (‘ejection fraction’) were seen in the proximal 5 mm segments of all of the pulmonary veins (ejection fraction of 36.7 ± 16.2 % for RSPV, 26.8 ± 14.3% for LSPV, 21.1 ± 10.2% for the RIPV and 15.0 ± 14.2% for the LIPV). Volume changes decreased as measurements were taken further inside the pulmonary veins (Figure 4). The ejection fraction for the entire first 15 mm of the pulmonary veins was 29.0 ± 15.1% for the RSPV, 20.5 ±13.4% for the LSPV, 11.1 ± 7.3% for the RIPV and 10.7 ± 9.3% for the LIPV. The superior veins had a significantly higher ejection fraction in comparison with the corresponding inferior veins (p = 0.0001 and p=0.001 for comparisons of right and left sided pulmonary veins respectively).
Figure 4.
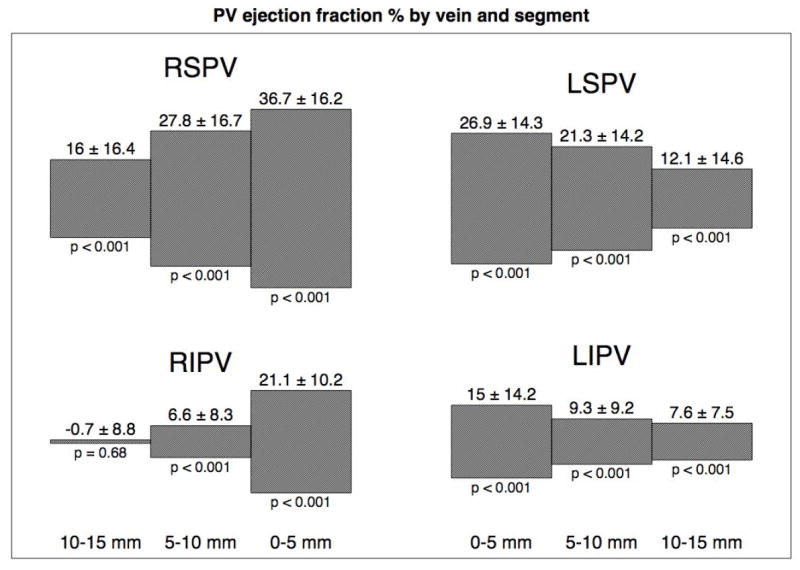
The contraction of each 5 mm pulmonary vein segment (0 to 5 mm, 5 to 10 mm and 10 to 15 mm) is shown graphically for the four pulmonary veins. The significance value is given below the plot (Student’s T-test ejection fraction is 0). There is more contraction in the superior veins, which appears to extend further into the pulmonary veins. The right inferior pulmonary vein appears to have some significant ostial contraction with minimal extension deeper into the vein.
Discussion
Pulmonary vein motion was analyzed for the first 15 mm of each pulmonary vein. Maximal contraction of the pulmonary veins was found to occur approximately the same time as atrial contraction, with the most prominent contraction occurring within the proximal 5 mm segments. Pulmonary vein contraction was limited to the proximal/ostial region in the inferior pulmonary veins, but extended more distally in the superior veins. These findings demonstrate that mechanical pulmonary vein contraction can be assessed using 64-MSCT scanning.
Measuring pulmonary vein contraction
There is extensive evidence establishing changes in pulmonary vein ostial size during the cardiac cycle.5-10, 12 Choi and colleagues11 analyzed MSCT images from 19 patients without atrial disease and measured the size of the right inferior pulmonary vein at 20 phases of the cardiac cycle. They found that the maximal size occurred at 35% of the R-R interval (i.e. near the time of mitral valve opening) and the minimal size at approximately 85% (i.e. during atrial contraction). In the present study we examined all four pulmonary veins over 10 phases and found that the minimal size of the right-sided pulmonary veins occurred at 85% and the left sided pulmonary veins at 95%. This finding is consistent with the spread of activation within the left atrium, originating from the atrial septum. Lickfett and coworkers7 analyzed pulmonary vein ostial dimensions and movement using MRI scans from 25 healthy volunteers. They found two distinct phases in which the pulmonary vein ostial dimensions decreased: (i) after opening of the mitral valve (approximately 45% of the cardiac cycle), and (ii) during atrial contraction (i.e. at ~85% of the cardiac cycle). They also found that the PV ostia moved a mean of 4-6 mm during the cardiac cycle when measured in the coronal and sagittal planes, in contrast to a median of 1 mm in the current study. Some important methodological differences are likely to explain these divergent results: (i) in the current study, the movement of the vein was determined by measuring the movement of a distinct point within the vein, such as a vein branch, whereas Lickfett and colleagues measured the location of the apparent PV ostium and (ii) in the current study, only movement of the vein perpendicular to left atrial wall (i.e. movement towards or away from the center of the left atrium) was measured, whereas Lickfett and colleagues measured all movements, including movement secondary to atrial motion.
Is pulmonary vein contraction active or passive?
The apparent pulmonary vein contraction observed in this study could have been artifactual due to (i) movement of the pulmonary vein during the cardiac cycle, leading to erroneous measurements at different locations within the vein or (ii) inaccurate identification of the PV ostia (i.e. resulting in measurement of contraction within atrial antral regions), particularly in the superior pulmonary veins. However, movement of the pulmonary veins was specifically corrected for by measuring the location of distinct points within the pulmonary vein (such as small branch veins), though these branch points were usually located > 1 cm within the vein. Significant stretching of the vein would underestimate movement of the ostia. Regardless of this, we felt this approach was more accurate, as vigorous ostial contraction will deform the shape of the atria-PV junction region and give the appearance that the ostia have moved. This is likely problematic for the superior pulmonary veins, as the antral regions surrounding the superior vein ostia are conical and therefore more difficult to define than the inferior pulmonary veins. Measurement of the pulmonary vein ostia is also dependent on the technique used; MSCT offers the advantage of true 3d imaging, which is likely more accurate than 2D modalities such as intracardiac echocardiography.13 Nonetheless, the ostial dimensions measured in this study are similar to other studies in which MSCT imaging was used to define the PV ostia (eg. LSPV dimensions of 18.9 × 13.4 mm in the current study compared with 19.0 × 14.6 mm reported by Jongbloed13 and 21.6 × 14.1 mm by Choi11).
If the observed pulmonary vein contraction is not due to artifactual factors, it may be caused by (i) contraction of myocardium within the pulmonary vein, (ii) passive emptying, (iii) compression by adjacent structures or (iv) contraction of myocardium in the antral region surrounding the pulmonary vein acting as a ‘purse-string’ to constrict the proximal portion of the pulmonary vein. The contraction that occurs after the peak in PV size at ~35% of R-R interval (i.e. the drop in volume following opening of the mitral valve) is consistent with being mainly a passive phenomena. However, the second phase of pulmonary vein contraction that occurs concurrently with atrial contraction is most consistent with being an active process, as unopposed atrial contraction would lead to reflux of blood and an increase in pulmonary vein volume. Similarly, the pulmonary veins are in close apposition to other cardiac structures such as the pulmonary artery and aorta; but these structures usually expand much earlier in the cardiac cycle than the time at which minimum pulmonary vein volume is observed.
Pathological studies have established the presence of myocardial sleeves within the pulmonary veins with more prominent extension into the superior veins, in comparison with the inferior veins. Nathan and Eliakim3 analyzed 16 hearts obtained from autopsies, and found that myocardial fibers were often arranged in a circular or ring-like pattern around the pulmonary vein ostia. These myocardial sleeves extended a variable distance within the pulmonary veins with a greater degree of extension in the superior veins (RSPV 5-25 mm, LSPV 8-24mm, RIPV 1-17 mm and LIPV 1-19 mm). Ho and colleagues2 examined 20 structurally-normal hearts from patients who had died of non-cardiac causes and sectioned nine of the pulmonary veins longitudinally. They also found that the greatest extension of myocardium into the pulmonary veins occurred in the superior veins with a maximum length of 25 mm.
The physiological significance of pulmonary vein contraction has been postulated to include: (i) prevention of backflow (ii) active expulsion of blood into the left atrium and (iii) regulation of pulmonary venous pressure and blood flow.3 There is speculation that the presence of increased back flow of blood into the pulmonary veins may be associated with an increased chance of progression to permanent AF in patients with paroxysmal AF and hypertension.14
Clinical Implications
The ability to quantify pulmonary venous contraction using 64-slice MSCT scanning may have important clinical implications; the absence of PV contraction, or a significant change in the pattern of PV contraction, could represent an alternative, non-invasive means of assessing for persistent pulmonary vein isolation after catheter ablation. However, it is important to note that in this study, there were no analyses of the effect of PV isolation procedures on PV mechanical function. Additional investigation is required before on can conclude on the efficacy of pre-/post- CT scanning to non-invasively follow for PV isolation.
But if this bears out, it is fortunate that multislice CT scanning (MSCT) is often performed before pulmonary vein isolation procedures to provide detailed information on left atrial and pulmonary vein anatomy.15, 16 One advantage of MSCT is that 4D data (i.e. motion or cine cardiac images) are concomitantly acquired during the imaging process; this eliminates the need to subject the patient to additional or lengthier scans for assessment of left atrial and pulmonary vein motion. MSCT scans are performed in many patients after pulmonary vein isolation to exclude the presence of pulmonary vein stenosis.17 These findings suggest that further analysis of pulmonary vein motion in these data sets may help identify successful isolation of pulmonary veins.
Limitations
This study was performed in patients with structurally normal hearts – patients with a history of atrial fibrillation may have altered pulmonary vein and left atrial anatomy18, and therefore, extrapolation of these results to different patient groups is speculative. The applicability of these findings is limited to patients in sinus rhythm when the MSCT scan is performed.
CONCLUSION
Pulmonary vein anatomy and motion examined using 4D multislice CT scanning shows significant volume changes in the pulmonary veins. These changes were most pronounced in the superior veins and were present up to 15 mm from the ostium. The minimum pulmonary vein volume occurred at the same time as atrial contraction, indicating that they are caused by active contraction of the pulmonary veins. MSCT scanning may have important clinical implications for the non-invasive evaluation of pulmonary vein isolation procedural success and follow-up.
Acknowledgments
The authors would like to Drs Ray Chan and Robert Manzke for their advice and assistance in designing the pulmonary vein measurement protocol.
Dr Thiagalingam is supported by a NHF/NHMRC Neil Hamilton Fairley Fellowship NHMRC Grant ID: 408106
Footnotes
Disclosures: None of the authors have disclosures relevant to this topic
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chugh A, Morady F. Atrial fibrillation: catheter ablation. J Interv Card Electrophysiol. 2006;16(1):15–26. doi: 10.1007/s10840-006-9018-4. [DOI] [PubMed] [Google Scholar]
- 2.Ho SY, Cabrera JA, Tran VH, et al. Architecture of the pulmonary veins: relevance to radiofrequency ablation. Heart (British Cardiac Society) 2001;86(3):265–270. doi: 10.1136/heart.86.3.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nathan H, Eliakim M. The junction between the left atrium and the pulmonary veins. An anatomic study of human hearts. Circulation. 1966;34(3):412–422. doi: 10.1161/01.cir.34.3.412. [DOI] [PubMed] [Google Scholar]
- 4.Haissaguerre M, Jais P, Shah DC, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. The New England Journal of Medicine. 1998;339(10):659–666. doi: 10.1056/NEJM199809033391003. [DOI] [PubMed] [Google Scholar]
- 5.Lin WS, Prakash VS, Tai CT, et al. Pulmonary vein morphology in patients with paroxysmal atrial fibrillation initiated by ectopic beats originating from the pulmonary veins: implications for catheter ablation. Circulation. 2000;10(111):1274–1281. doi: 10.1161/01.cir.101.11.1274. [DOI] [PubMed] [Google Scholar]
- 6.Schwartzman D, Kanzaki H, Bazaz R, et al. Impact of catheter ablation on pulmonary vein morphology and mechanical function. J Cardiovasc Electrophysiol. 2004;15(2):161–167. doi: 10.1046/j.1540-8167.2004.03451.x. [DOI] [PubMed] [Google Scholar]
- 7.Lickfett L, Dickfeld T, Kato R, et al. Changes of pulmonary vein orifice size and location throughout the cardiac cycle: dynamic analysis using magnetic resonance cine imaging. J Cardiovasc Electrophysiol. 2005;16(6):582–588. doi: 10.1046/j.1540-8167.2005.40724.x. [DOI] [PubMed] [Google Scholar]
- 8.Bowman AW, Kovacs SJ. Prediction and assessment of the time-varying effective pulmonary vein area via cardiac MRI and Doppler echocardiography. American Journal of Physiology. 2005;288(1):H280–286. doi: 10.1152/ajpheart.00713.2004. [DOI] [PubMed] [Google Scholar]
- 9.Hauser TH, Yeon SB, Kissinger KV, et al. Variation in pulmonary vein size during the cardiac cycle: implications for non-electrocardiogram-gated imaging. American Heart Journal. 2006;152(5):974, e971–976. doi: 10.1016/j.ahj.2006.05.018. [DOI] [PubMed] [Google Scholar]
- 10.Syed MA, Peters DC, Rashid H, et al. Pulmonary vein imaging: comparison of 3D magnetic resonance angiography with 2D cine MRI for characterizing anatomy and size. J Cardiovasc Magn Reson. 2005;7(2):355–360. doi: 10.1081/jcmr-200053458. [DOI] [PubMed] [Google Scholar]
- 11.Choi SI, Seo JB, Choi SH, et al. Variation of the size of pulmonary venous ostia during the cardiac cycle: optimal reconstruction window at ECG-gated multi-detector row CT. Eur Radiol. 2005;15(7):1441–1445. doi: 10.1007/s00330-005-2694-0. [DOI] [PubMed] [Google Scholar]
- 12.Ueda M, Tada H, Kurosaki K, et al. Pulmonary vein morphology before and after segmental isolation in patients with atrial fibrillation. Pacing Clin Electrophysiol. 2005;28(9):944–953. doi: 10.1111/j.1540-8159.2005.00214.x. [DOI] [PubMed] [Google Scholar]
- 13.Jongbloed MR, Bax JJ, Lamb HJ, et al. Multislice computed tomography versus intracardiac echocardiography to evaluate the pulmonary veins before radiofrequency catheter ablation of atrial fibrillation: a head-to-head comparison. Journal of the American College of Cardiology. 2005;45(3):343–350. doi: 10.1016/j.jacc.2004.10.040. [DOI] [PubMed] [Google Scholar]
- 14.Maruyama T, Kishikawa T, Ito H, et al. Augmentation of Pulmonary Vein Backflow Velocity during Left Atrial Contraction: A Novel Phenomenon Responsible for Progression of Atrial Fibrillation in Hypertensive Patients. Cardiology. 2008;109:33–40. doi: 10.1159/000105324. [DOI] [PubMed] [Google Scholar]
- 15.Lacomis JM, Goitein O, Deible C, et al. CT of the pulmonary veins. Journal of thoracic imaging. 2007;22(1):63–76. doi: 10.1097/RTI.0b013e3180317aaf. [DOI] [PubMed] [Google Scholar]
- 16.Cronin P, Sneider MB, Kazerooni EA, et al. MDCT of the left atrium and pulmonary veins in planning radiofrequency ablation for atrial fibrillation: a how-to guide. Ajr. 2004;183(3):767–778. doi: 10.2214/ajr.183.3.1830767. [DOI] [PubMed] [Google Scholar]
- 17.Scharf C, Sneider M, Case I, et al. Anatomy of the pulmonary veins in patients with atrial fibrillation and effects of segmental ostial ablation analyzed by computed tomography. J Cardiovasc Electrophysiol. 2003;14(2):150–155. doi: 10.1046/j.1540-8167.2003.02444.x. [DOI] [PubMed] [Google Scholar]
- 18.Jongbloed MR, Dirksen MS, Bax JJ, et al. Atrial fibrillation: multi-detector row CT of pulmonary vein anatomy prior to radiofrequency catheter ablation--initial experience. Radiology. 2005;234(3):702–709. doi: 10.1148/radiol.2343031047. [DOI] [PubMed] [Google Scholar]


