Abstract
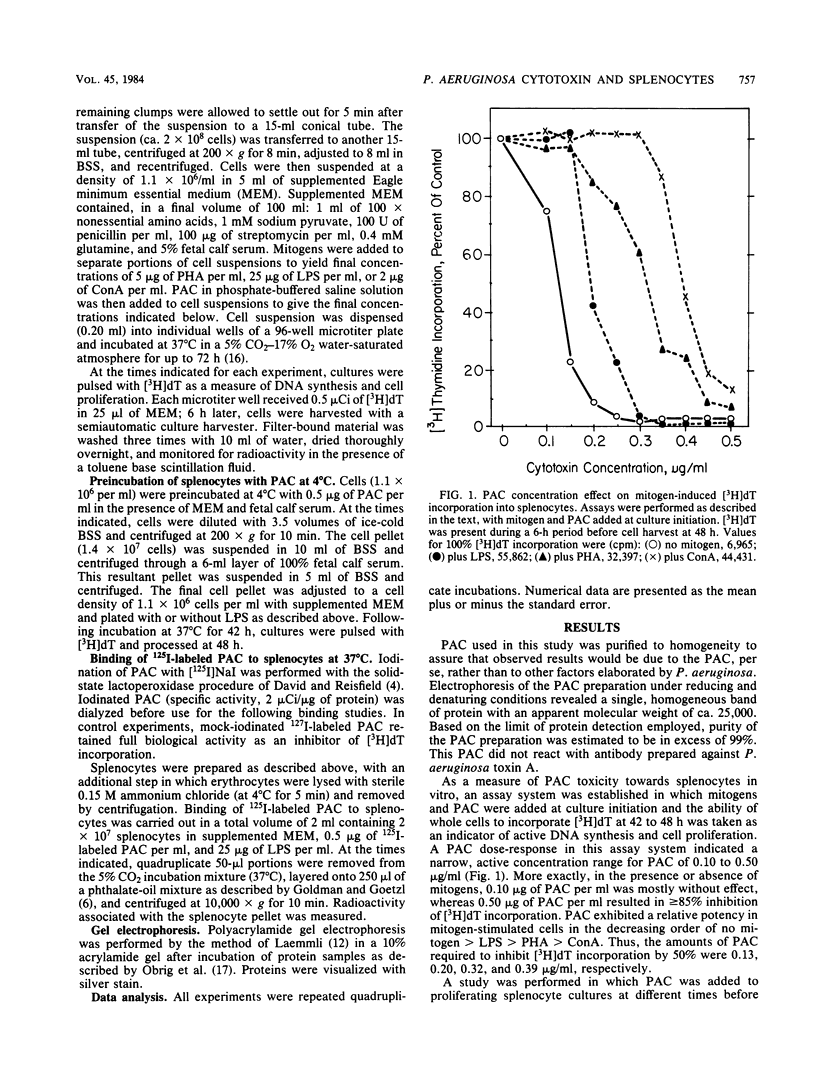
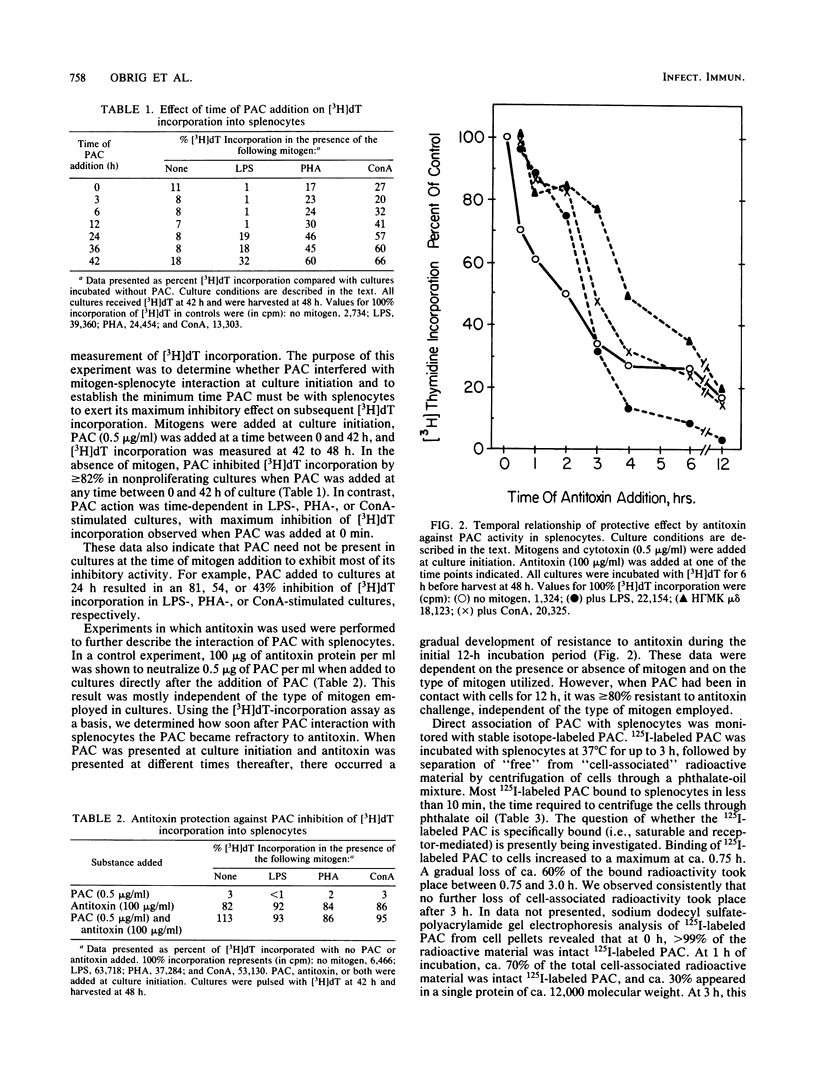
The interaction of highly purified Pseudomonas aeruginosa cytotoxin (PAC) with murine splenocytes was examined. Added at culture initiation, PAC (0.1 to 0.5 microgram/ml) inhibited subsequent [3H]deoxythymidine incorporation measured between 42 to 48 h. Incorporation of [3H]deoxythymidine was inhibited 50% in lipopolysaccharide-, phytohemagglutinin-, and concanavalin A-stimulated cultures by 0.20, 0.32, and 0.39 microgram of PAC per ml, respectively. It is concluded that PAC exhibits a narrow inhibitory concentration response range of 0.1 to 0.5 microgram/ml which, secondarily, is affected by the presence of mitogens. Antitoxin added at splenocyte culture initiation, directly after PAC, yielded greater than or equal to 86% protection against PAC inhibition of [3H]deoxythymidine incorporation. Addition of antitoxin to cultures at different times after PAC demonstrated a time-dependent loss of antitoxin protective effect over a 12-h period, indicating that PAC became cell associated and refractory to antitoxin within this time period. PAC preincubated with splenocytes at 4 degrees C for less than or equal to 1 h could not be removed by washing of cells and was fully inhibitory to [3H]deoxythymidine incorporation when these cells were cultured at 37 degrees C. This finding was confirmed by demonstrating that 125I-labeled PAC bound immediately to cells. It is concluded that PAC action on splenocytes is dose- and time-dependent and consists of a two-phase process: (i) a very rapid binding of PAC to the cell surface available to antitoxin, and (ii) a slower toxicity development phase of ca. 12 h, during which PAC becomes refractory to antitoxin.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baltch A. L., Hammer M., Smith R. P., Sutphen N. Pseudomonas aeruginosa bacteremia: susceptibility of 100 blood culture isolates to seven antimicrobial agents and its clinical significance. J Lab Clin Med. 1979 Aug;94(2):201–214. [PubMed] [Google Scholar]
- Chung D. W., Collier R. J. Enzymatically active peptide from the adenosine diphosphate-ribosylating toxin of Pseudomonas aeruginosa. Infect Immun. 1977 Jun;16(3):832–841. doi: 10.1128/iai.16.3.832-841.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David G. S., Reisfeld R. A. Protein iodination with solid state lactoperoxidase. Biochemistry. 1974 Feb 26;13(5):1014–1021. doi: 10.1021/bi00702a028. [DOI] [PubMed] [Google Scholar]
- Frimmer M., Homann J., Petzinger E., Rufeger U., Scharmann W. Comparative studies on isolated rat hepatocytes and AS-30D hepatoma cells with leucocidin from Pseudomonas aeruginosa. Naunyn Schmiedebergs Arch Pharmacol. 1976 Oct;295(1):63–69. doi: 10.1007/BF00509774. [DOI] [PubMed] [Google Scholar]
- Goldman D. W., Goetzl E. J. Specific binding of leukotriene B4 to receptors on human polymorphonuclear leukocytes. J Immunol. 1982 Oct;129(4):1600–1604. [PubMed] [Google Scholar]
- Harboe N., Ingild A. Immunization, isolation of immunoglobulins, estimation of antibody titre. Scand J Immunol Suppl. 1973;1:161–164. doi: 10.1111/j.1365-3083.1973.tb03798.x. [DOI] [PubMed] [Google Scholar]
- Hirayama T., Kato I. Mode of cytotoxic action of pseudomonal leukocidin on phosphatidylinositol metabolism and activation of lysosomal enzyme in rabbit leukocytes. Infect Immun. 1984 Jan;43(1):21–27. doi: 10.1128/iai.43.1.21-27.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglewski B. H., Kabat D. NAD-dependent inhibition of protein synthesis by Pseudomonas aeruginosa toxin,. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2284–2288. doi: 10.1073/pnas.72.6.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreger A. S., Griffin O. K. Physicochemical fractionation of extracellular cornea-damaging proteases of Pseudomonas aeruginosa. Infect Immun. 1974 May;9(5):828–834. doi: 10.1128/iai.9.5.828-834.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Liu P. V. The roles of various fractions of Pseudomonas aeruginosa in its pathogenesis. 3. Identity of the lethal toxins produced in vitro and in vivo. J Infect Dis. 1966 Oct;116(4):481–489. doi: 10.1093/infdis/116.4.481. [DOI] [PubMed] [Google Scholar]
- Lutz F. Purification of a cytotoxic protein from Pseudomonas aeruginosa. Toxicon. 1979;17(5):467–475. doi: 10.1016/0041-0101(79)90280-0. [DOI] [PubMed] [Google Scholar]
- Middlebrook J. L., Dorland R. B. Response of cultured mammalian cells to the exotoxins of Pseudomonas aeruginosa and Corynebacterium diphtheriae: differential cytotoxicity. Can J Microbiol. 1977 Feb;23(2):183–189. doi: 10.1139/m77-026. [DOI] [PubMed] [Google Scholar]
- Obrig T., Shen A., Kwoka S., Chudyk M. A. Protein composition of L-cell messenger ribonucleoproteins. Biochem Biophys Res Commun. 1979 Dec 14;91(3):1062–1067. doi: 10.1016/0006-291x(79)91988-0. [DOI] [PubMed] [Google Scholar]
- Pavlovskis O. R., Voelker F. A., Shackelford A. H. Pseudomonas aeruginosa exotoxin in mice: histopathology and serum enzyme changes. J Infect Dis. 1976 Mar;133(3):253–259. doi: 10.1093/infdis/133.3.253. [DOI] [PubMed] [Google Scholar]
- Pollack M., Anderson S. E., Jr Toxicity of Pseudomonas aeruginosa exotoxin A for human macrophages. Infect Immun. 1978 Mar;19(3):1092–1096. doi: 10.1128/iai.19.3.1092-1096.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack M., Young L. S. Protective activity of antibodies to exotoxin A and lipopolysaccharide at the onset of Pseudomonas aeruginosa septicemia in man. J Clin Invest. 1979 Feb;63(2):276–286. doi: 10.1172/JCI109300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozzan T., Corps A. N., Hesketh T. R., Metcalfe J. C. Mitogenic stimulation and the redistribution of concanavalin A receptors on lymphocytes. Exp Cell Res. 1981 Aug;134(2):399–408. doi: 10.1016/0014-4827(81)90439-0. [DOI] [PubMed] [Google Scholar]
- Scharmann W. Cytotoxic effects of leukocidin from Pseudomonas aeruginosa on polymorphonuclear leukocytes from cattle. Infect Immun. 1976 Mar;13(3):836–843. doi: 10.1128/iai.13.3.836-843.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharmann W. Formation and isolation of leucocidin from Pseudomonas aeruginosa. J Gen Microbiol. 1976 Apr;93(2):283–291. doi: 10.1099/00221287-93-2-283. [DOI] [PubMed] [Google Scholar]
- Scharmann W. Interaction of purified leukocidin from Pseudomonas aeruginosa with Bovine polymorphonuclear leukocytes. Infect Immun. 1976 Apr;13(4):1046–1053. doi: 10.1128/iai.13.4.1046-1053.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharmann W., Jacob F., Porstendörfer J. The cytotoxic action of leucocidan from Pseudomonas aeruginosa on human polymorphonuclear leucocytes. J Gen Microbiol. 1976 Apr;93(2):303–308. doi: 10.1099/00221287-93-2-303. [DOI] [PubMed] [Google Scholar]
- Scharmann W. Purification and characterization of leucocidin from Pseudomonas aeruginosa. J Gen Microbiol. 1976 Apr;93(2):292–302. doi: 10.1099/00221287-93-2-292. [DOI] [PubMed] [Google Scholar]
- Schultz D. R., Miller K. D. Elastase of Pseudomonas aeruginosa: inactivation of complement components and complement-derived chemotactic and phagocytic factors. Infect Immun. 1974 Jul;10(1):128–135. doi: 10.1128/iai.10.1.128-135.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snell K., Holder I. A., Leppla S. A., Saelinger C. B. Role of exotoxin and protease as possible virulence factors in experimental infections with Pseudomonas aeruginosa. Infect Immun. 1978 Mar;19(3):839–845. doi: 10.1128/iai.19.3.839-845.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart R. K., Pollack M. Pseudomonas aeruginosa exotoxin A inhibits proliferation of human bone marrow progenitor cells in vitro. Infect Immun. 1982 Oct;38(1):206–211. doi: 10.1128/iai.38.1.206-211.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]


