Abstract
We have previously presented a MEMS viscometric sensor for continuous glucose monitoring using protein Concanavalin A (Con A). To address its drawbacks including immunotoxicity and instability issues, we have synthesized stable, biocompatible copolymers poly(acrylamide-ran-3- acrylamidophenylboronic acid) (PAA-ran-PAAPBA) for viscosity based glucose sensing. We found that PAA-ran-PAAPBA showed very high binding specificity to glucose. Several key factors such as polymer compositions, polymer molecular weights and polymer concentrations have been investigated to optimize viscometric responses. This polymer is able to detect glucose under physiological conditions in a reversible manner. Therefore, it has the potential to enable the highly reliable, continuous monitoring of glucose in subcutaneous tissue using the MEMS device.
Keywords: MEMS, boronic acid, glucose sensing, copolymer, viscosity, affinity biosensors
Introduction
About 21 million people in the U.S. and 171 million worldwide suffer from diabetes mellitus. A continuous glucose monitoring (CGM) device allows timely detection of abnormal glucose levels, and enables active intervention by taking carbohydrates or injecting insulin, which has been shown to reduce the risk of diabetes-related complications.1 Currently, the affinity glucose sensing is most commonly based on the reversible binding of glucose to concanavalin A (Con A), a glucose-specific lectin. The system has excellent specificity, as there are no other sugars at significant concentration levels in blood serum that might interfere with Con A binding.2–4 For example, Con A based affinity glucose sensors have been fabricated using a solution of Con A and fluorescently labeled dextran. Glucose binding to Con A was detected via the resulting fluorescence change. This approach has been further investigated in vitro and in vivo, and there are active efforts to develop optimized fluorescence-based sensors.2, 5–12
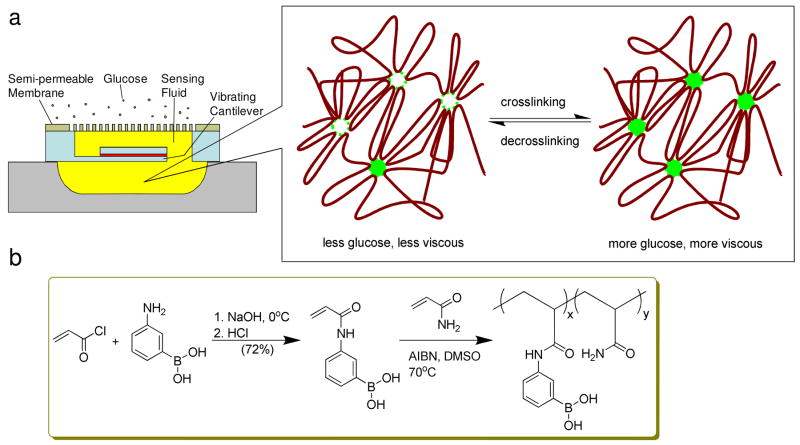
To enhance the reliability of affinity based sensors, sensors with fully electronic readout via viscosity measurement have been reported.13–17 We recently developed a microelectromechanical (MEMS) viscometric sensor which was aimed for the continuous monitoring of glucose levels in diabetes patients.18 The device shown in Figure 1a featured a magnetically driven vibrating microcantilever, which was situated in a microchamber and separated from the environment by a semi-permeable membrane. Glucose sensing was based on affinity binding principles using a solution of dextran and Con A as the sensing fluid. The glucose concentration was determined by detecting viscosity changes induced by the binding of glucose to Con A through measurement of the cantilever’s vibration parameters. While our device represented a first step toward an implantable MEMS sensor that was miniaturized and afforded the excellent stability, there were significant safety concerns with the toxicological properties of Con A. For instance, Con A is known to stimulate enhanced immunogenicity,19,20 and to induce antigen-specific cellular cytotoxicity.21,22 The stability of Con A is another problem. Although native dimeric Con A is fairly stable, removal of metal ions (Ca2+ and Mn2+) will lower its stability considerably.23 To address these concerns, new biocompatible sensing liquids for viscometric glucose sensing are highly desired.
Figure 1.
a) Schematic illustration of the MEMS viscometric device and its sensing mechanism design. b) Synthesis route of poly(acrylamide-ran-3-acrylamidophenylboronic acid) (PAA-ran-PAAPBA).
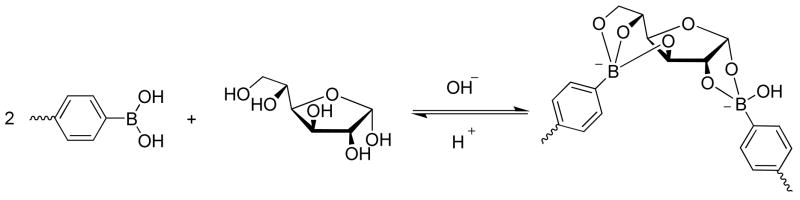
It is well-known that boronic acid binds reversibly to diols to form a cyclic boronic ester in aqueous media,24 while a tridentate complex was formed when a second aromatic boronic acid was bound to glucose (Scheme 1).25 It was also found that 3-pyridinylboronic acid were able to form such kind of 1:2 complex with glucose by 11B NMR.26 In general, the boronic acid is a biocompatible functional group with low cytotoxicity and low immunogenicity.27 Therefore, considerable research interests have been attracted to develop a variety of glucose sensors through different sensing mechanisms in polymers. For example, fluorescent changes because of photoelectron transfer, fluorescence resonance energy transfer, or internal charge change have been used to build fluorescent sensors.25,28 Asher et al. introduced the use of polymerized crystalline colloidal array for colorimetric detection of glucose.29 Lei et al. reported a thin-film wireless pressure sensor using a phenylborate based hydrogel which could bind with glucose resulting in swelling.30 Arnold et al. reported preliminary data from a conductimetric sensor with a boronic acid immobilized in a hydrogel, whose binding to permeated glucose changed the ionic concentration conductivity, resulting in the change of conductivity.31 Recently, the Zhou and Hoare groups developed a poly(N-isopropylacrylamide) copolymer microgel sensor for glucose based on the volume change caused by the repulsion of the boronate groups.32,33 Kumar et al. showed improved potentiometric sensing capability using enzymatic polymerized self-doped copolymer of poly(aniline-co- 3-aminobenzeneboronic acid).34
Scheme 1.
Interaction of boronic acid and D-glucose.
In this paper, we report the development of a stable, biocompatible boronic acid based polymeric sensing fluid that can be used in MEMS affinity glucose sensors18. In our design, the polymeric sensing fluid consists of PAA-ran-PAAPBA and physiological phosphate buffer saline (PBS) at pH7.4. Introduction of hydrophilic PAA segments can improve the water solubility of the copolymer,35 as well as possibly provide the additional neighbor coordinating effect via carbonyl oxygen and boron chelating to enhance the binding of boronic acid to carbohydrates.36,37
Experimental Section
Materials
3-Aminophenylboronic acid (PBA) was purchased from Oakwood Products, Inc. SnakeSkin™ Pleated Dialysis Tubing (MWCO 3500) was purchased from Pierce Biotechnology, Inc. Ubbelohde viscometer was obtained from CANNON® Instrument Company. All other reagents D-(−)-fructose, D-(+)-glucose, D-(+)-lactose, D-(+)-galactose D-(+)-sucrose, D-(+)-cellobiose, D-(+)-mannose, PEG8000, sodium azide, sodium chloride, potassium phosphate monobasic and potassium phosphate dibasic were purchased from Sigma-Aldrich, Inc. Nanopure water was purified by Milli-Q Ultrapure system purchased from Millipore Corporation.
Preparation of monomer N-3-acrylamidophenylboronic acid (AAPBA)
Monomer AAPBA was synthesized adopting similar conditions to the reference.38 3- Aminophenylboronic acid (5 g, 36.5 mmol) was dissolved in NaOH solution (2 M, 73 mL, 146 mmol) at 0 °C. Cold acryloyl chloride (5.9 mL, 73 mmol) was added dropwisely to the vigorously stirred mixture over 15 min. HCl solution (1 M) was slowly added to the reaction mixture till the pH reached 1.0. A lot of white solids precipitated, which were filtered, washed by cold water. The filtrate was extracted with EtOAc three times. The organic phase was washed with brine and evaporated to give off-white solids which were combined with the above precipitates. Recrystallization in H2O afforded 5.0 g off-white AAPBA crystals (yield: 72%). The 1H NMR and 13C NMR spectra were recorded on a Mercury VX-300 spectrometer (Varian, USA). 1H NMR (300 MHz, DMSO-d6): δ = 10.06 (s, 1H, O=CNH), 8.01 (s, 2H, B-OH), 7.87 (s, 2H, Ar-H), 7.81 (d, J = 8.1 Hz, 1H; Ar-H), 7.49 (d, J = 7.2 Hz, 1H; Ar-H), 7.27 (t, J1 = 7.5 Hz, J2 = 7.8 Hz, 1H; Ar-H), 6.44 ( dd, J1 = 16.8 Hz, J2 = 9.9 Hz, 1H; C=CHC=O), 6.23 (dd, J1 = 17.1 Hz, J2 = 2.1 Hz, 1H, C=CH2), 5.72 (dd, J1 = 9.9 Hz, J2 = 2.1 Hz, 1H; C=CH2). 13C NMR (75.5 MHz, DMSO-d6): δ = 163.8, 138.8, 135.6, 132.7, 130.0, 128.4, 127.3, 126.0, 122.0. Control monomer N-phenylacrylamide (NPAA) was prepared as reported with a similar yield.39 1H NMR (300 MHz, CDCl3): δ = 7.58 (d, J1 = 8.1 Hz, 2H; ArH), 7.51 (s, 1H, O=CNH), 7.37 (d, J = 1.8 Hz, 1H, Ar-H), 7.32 (t, J1 = 6.6 Hz, J2 = 1.8 Hz, 1H, Ar-H), 7.13 (t, J1 = 7.5 Hz, J2 = 7.2 Hz, 1H; Ar-H), 6.44 (dd, J1 = 16.8 Hz, J2 = 1.5 Hz, 1H; C=CH2), 6.24 (dd, J1 = 16.8 Hz, J2 = 10.2 Hz, 1H; C=CHC=O), 5.78 (dd, J1 = 10.5 Hz, J2 = 1.5 Hz, 1H; C=CH2). 13C NMR (75.5 MHz, CDCl3): δ = 164.4, 138.1, 131.6, 129.2, 127.9, 124.8, 120.6.
Preparation of PAA-ran-PAAPBA
A typical free radical polymerization was conducted as following: acrylamide (3.72 g, 52.4 mmol), AAPBA (0.20 g, 2 mmol) and 2,2′-azodiisobutyronitrile (AIBN, 21.5 mg, 0.13 mmol) were dissolved in DMSO. The mixture was bubbled by nitrogen for half an hour, and subjected to 70 °C oil bath for 24 h. After cooling down to room temperature, the gel was subjected to dialysis against nanopure water for 24 h. The aqueous phase was precipitated by acetone and dried in vacuum oven to give 3.07 g white solids (Yield: 78%). A series of polymer with different percent compositions were prepared and characterized by 1H NMR in D2O, 11B NMR and viscometry.40 1H NMR (300 MHz, D2O) for a typical polymer: δ = 7.41 (bm, 4H; ArH), 2.06 (bm, 1H, O=CCH-), 1.50 (bm, 2H, -CH2-). The 11B NMR spectrum of solid-state polymer was recorded on a Varian Inova 500 spectrometer at 160.5 MHz (Varian, USA) using Doty XC-4mm MAS probe. Bloch decays were collected using 1H dipolar decoupling and a spinning rate of 10 kHz. 11B NMR (160.5 MHz, solid) for a typical polymer: δ = 25 ppm (a broad peak). Control polymer polyacrylamide-ran-N-phenylacrylamide (PAA-ran-PNPAA) was prepared and characterized in the similar way. 1H NMR (300 MHz, D2O): δ = 7.3 (bm, 5H; ArH), 2.07 (bm, 1H, O=CCH-), 1.50 (bm, 2H, -CH2-). The viscosity of the copolymers was measured by Ubbelohde viscometer in 0.12 M NaCl at pH 6.0 at 25°C.40 According to the formula for polyacrylamide, the weight-average molecular weights (Mw) of PAA-ran-PAAPBA polymers were calculated from their intrinsic viscosities:
| (1) |
The experimental results were summarized in Table 1.
Table 1.
Characteristics of polymers prepared in DMSO at 70 °C.
| Polymera | Component Molar Ratio AM/monomerb/AIBN | Yield | Mw*10−4c | AAPBA%d |
|---|---|---|---|---|
| 1 | 100/2/0.5 | 29% | 8.3 | 2.3% |
| 2 | 100/2/0.25 | 78% | 10.8 | 2.9% |
| 3 | 100/5/0.25 | 43% | 13.0 | 4.7% |
| 4 | 100/8/0.25 | 55% | 5.7 | 8.7% |
| 5 | 100/5/0.25 | 44% | 16.2 | 0% |
Polymers 1–4 are polymers PAA-ran-PAAPBA; 5 is the control polymer PAA-ran-PPAA.
Polymerization co-monomer is AAPBA, except for polymer 5, which is NPAA.
The weight-average molecular weight was measured by viscometry.
The percent composition was calculated by the integration ratio of the aromatic protons to methylene and methine protons using 1H NMR spectroscopy.
A typical sensing reversibility experiment
Polymer 3 (124 mg) was dissolved in PBS (4 mL) to give the blank polymer solution viscosity. Glucose (20 mg) was added to measure the crosslinked mixture viscosity. Then the mixture was subjected to dialysis against copious fresh PBS buffer (400 mL) through a SnakeSkin™ Pleated Dialysis membrane (MWCO 3500) overnight with PEG8000 (18.0 g) to prevent volume increase due to osmotic effect. More PBS buffer was added to the viscometer to keep the solution volume same as 4 mL.
Results and Discussions
We started to prepare the glucose sensing fluid with the monomer synthesis (Figure 1b). Monomer AAPBA was prepared in a yield of 72%, much higher than those found in the references,38,41–46 using our modified method where more product was recovered using ethyl acetate to extract the acidic aqueous filtrate. The control monomer NPAA was prepared in a similar yield as in the reference.39 Copolymers PAA-ran-PAAPBA with various PAAPBA contents were synthesized through classic free radical solution polymerization of acrylamide and AAPBA.40 we confirmed the presence of trigonal boron in polymer using solid state 11B NMR technique by the presence of a broad peak centered at δ 25 ppm. Monomers like acrylamide are known highly toxic. For the sake of biosafety concerns, at the end of reaction the radical polymerization mixture was scheduled to dialysis through a semi-permeable membrane of MWCO 3500 Dalton against pure water to remove water soluble unreacted acrylamide and other small oligomers. The possible residue and less water soluble AAPBA were dissolved in acetone and filtered away during the polymer precipitation process.
Due to the possible binding between boronic acid and polar stationary phase like silica of aqueous gel permeation chromatography, their weight-average molecular weight were calculated based on their intrinsic viscosities obtained under similar conditions used by Galaev et al.40 Because the polymers were polyacrylamide analogs, the Mark-Houwink parameters for polyacrylamide were used in the calculation. A variety of free radical polymerization conditions were tested, among which we discovered that the polymer molecular weight was not under direct control by the ratio of initiator to monomers (Table 1). It was observed that using 0.25 molar ratio of the initiator to acrylamide gave the best results with reproducible copolymer composition and higher molecular weight. The final percent composition of PAAPBA segment could be determined by 1H NMR through the integration ratio of the aromatic protons to methylene and methine protons, which was fairly consistent with the initial molar ratio before polymerization (Table 1). However, when the molar ratio of AAPBA to acrylamide was more than 8:100 in the monomer mixture, it was very difficult to generate polymers with high molecular weights, likely due to the low solubility of the final polymers.
Conventional Ubbelohde capillary viscometer was employed to measure the kinematic viscosity property of our polymer solutions at room temperature 25 °C, which was converted to viscosity because the polymer solution density was approximately the same as water. In order to make sure of the accuracy of fluid viscosity response, multiple measurements were taken for each data point, where the errors were all within 2% range that may be due to possible temperature fluctuations and human errors (Figure 2a). After the polymer was dissolved in phosphate buffer saline (PBS, pH 7.4, 150 mM NaCl, 0.05% NaN3) that is mimic the physiological pH conditions, the polymer solution was loaded into the viscometer, followed by addition of different amounts of glucose for varying glucose concentrations. The viscosity values became steady within minutes (data not shown), which showed little variations even after hours, suggesting that the system quickly reached an equilibrium state. This rapid response made the polymer a desirable alternative to Con A for detection of glucose.
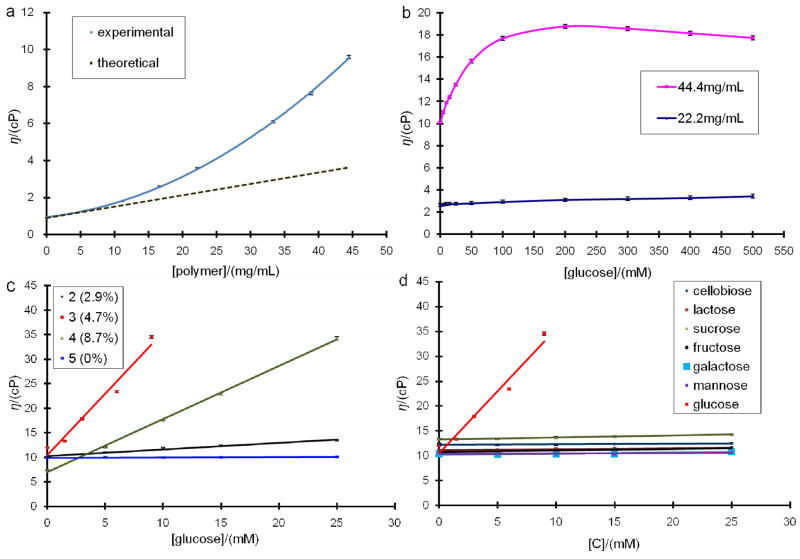
Figure 2.
a) Viscosity responses of 15 mM glucose PBS solution to polymer 1 (2.3% of PAAPBA, from 0 to 45 mg/mL). b) Viscosity profile of polymer 2 (2.9% of PAAPBA) solutions to glucose (from 0 to 500 mM). c) Viscosity responses of different polymers (2–5 with the percentage of the PAAPBA varied from 2.9%, 4.7%, 8.7% and 0%, respectively) to various glucose concentrations. For polymer 3, the viscosity was out of the detection limit when the glucose concentration is higher than 9 mM. d) Viscosity responses of polymer 3 (44.4 mg/mL, 4.7% of PAAPBA) solutions to monosaccharides: glucose, fructose, galactose and mannose; and disaccharides: cellobiose, lactose and sucrose.
In order to find an ideal polymer concentration for glucose sensing, polymer 1 with a variety of concentrations was added to a solution of 15 mM glucose concentration (Figure 2a). The viscosity increased parabolically when polymer concentration increased as shown by the experimental curve. Normally, at low polymer concentration range, the solution viscosity should have increased linearly as the theoretical dash line. The deviation shown here could be attributed to the increasing crosslinking of polymer by glucose. When the concentration of polymer was 44.4 mg/mL, the increment of viscosity upon addition of glucose reached 9.6 cP that has fallen into the detecting range from 8.7 to 43.4 cp of our MEMS device.18 Due to the limited solubility of these polymers in PBS buffer, it was difficult to get the solution with a concentration higher than 44.4 mg/mL. Polymer 2, prepared using 100:2:0.25 molar ratio of acrylamide to AAPBA to initiator, was used to study the viscosity response with different amount of glucose. As shown in Figure 2b, at the polymer concentration of 44.4 mg/mL, the solution viscosity increased gradually from 10.1 cP (without glucose) to 18.8 cP at which the glucose concentration was 200 mM, and then slowly declined to 17.8 cP at 500 mM glucose. When the polymer concentration was 22.2 mg/mL, the viscosity change over glucose concentration from 0 to 500 mM was almost negligible. This result suggested that only at a high concentration the polymer would have significant response to glucose. Therefore, in all the following studies except the reversibility experiment, the concentration of polymers was fixed at 44.4 mg/mL.
The glucose binding in response to the composition of the polymer was further tested using polymers 2–4, which were synthesized using 2%, 5% and 8% molar ratios of AAPBA, respectively. As shown in Figure 2c, the viscosity of all boronic acid grafted polymers (2–4) increased gradually along with the increment of the glucose concentration. In particular, the viscosity of polymer 3 increased sharply from 12.0 to 34.5 cP as the glucose concentration increased from 0 to 9 mM, which was comparable to that of Con A system used in our previous MEMS device.18 Polymer 4 also showed significant response to glucose additions, however it turned a blurry when the concentration of glucose was higher than 10 mM, suggesting that glucose caused significant amount of crosslinking and agglomeration. In the same glucose range, the control polymer 5 (using NPAA instead of AAPBA as the monomer) without phenylboronic acid group showed no obvious change. It was noticed that the molecular weights of the polymers had a big impact on the viscosity response. Though polymer 3 PAAPBA percentage 4.7% was almost half that of polymer than polymer 4 (8.7%), with its higher molecular weight 130K Da that is almost doubled that of 4 (57K Da), it boosted the viscosity more significantly than its counterpart. Comparing 2 and 3, which shared similar molecular weight, we found that the percentage of the boronic acid components in the polymers also had direct correlations with the viscosity responses when mixed with glucose, confirming that the ability of the crosslinking between boronic acid and glucose also play an important role on the viscosity increase. In other words, the higher percentage of PAAPBA in the polymer, the more viscous the polymer and glucose mixture was, and the more sensitive the polymer was to recognize glucose. However, there was a limitation of percentage of PAAPBA that could be incorporated in the copolymer. If the molar ratio of AAPBA and acrylamide used in the initial polymerization was over 8:100, no water soluble polymer could be synthesized. It was observed that, with ~5% of PAAPBA segment in the polymer, it gave a clear solution as well as the best reproducibility in the viscosity measurement. Therefore, polymer 3 was used in the following experiments.
Compared to the enzymatically polymerized poly(aniline-co-3-aminobenzeneboronic acid) by Kumar et al. that showed high sensitivity but poor selectivity over saccharides,34 this sensing fluid showed an unexpected high specificity towards glucose. Figure 2d shows that when the concentration of various monosaccharides increased from 0 to 25 mM, little viscosity increases were observed for polymer 3 with fructose, galactose and mannose, i.e. ~1 cP differences were observed, which were much less than the viscosity response to glucose, ~22.5 cP. It also shows the interactions of different disaccharides including cellobiose, lactose and sucrose with polymer: 3:0 cP for cellobiose, 1.2 cP for lactose and 1.0 cP for sucrose. Again, no apparent increments of viscosities were observed, indicating that the polymers could not be crosslinked with those disaccharides.
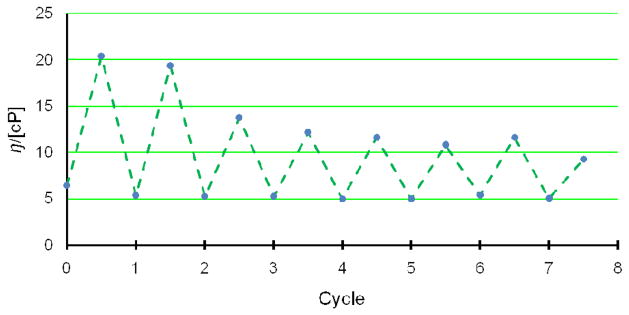
Moreover, reversibility experiments showed that the response of the fluid to glucose was reversible. The concentration of polymer 3 was lowered to 31 mg/mL in order to detect the viscosity response with 25 mM glucose. The blank polymer solution showed a viscosity of 6.4 cP. After addition of glucose, the viscosity was bumped up to 20.4 cP. Upon dialysis against PBS buffer, its viscosity significantly dropped to 5.4 cP, suggesting that removal of glucose led to the dissociation of the crosslinking network and resulted in lower viscosity. Such kind of response was reversible as shown in Figure 3. Although the increment amplitudes were slightly different, which was presumably due to the loss of polymer on the dialysis device, the reproducibility of glucose responses were fully validated overall.
Figure 3.
Viscosity response of polymer 3 (31 mg/mL) to glucose. (high points) Treatment with 28 mM of glucose. (low points) Dialysis against buffer for 12 h except the first run which was in PBS.
These experiments revealed a completely different sensing mechanism than Con A system. Previous Con A viscometric affinity glucose sensor was based on the competitive binding of dextran and free glucose to Con A, where the viscosity was provided by the crosslinked Con A and dextran mixture.18 In this study, the copolymer PAA-ran-PAAPBA showed extremely high specificity towards glucose. When other monosaccharides or disaccharides were tested in the experiments, no viscosity responses were observed. Thus, there must be some sort of crosslinking or structural change among the polymer chains upon contact with glucose. It is well known that interactions of phenylboronic acid moieties with amino functionalities in ortho-position of the same phenyl ring enhance the binding of sugars to the boronic acid because of a chelating effect between B and N atoms.25,37 However, similar interaction seems weaker between weak Lewis base of amide nitrogen and boron, likely due to the weaker electron donating effect of amide nitrogen.47,48 Therefore, it is more likely that sugar binding was augmented by the interaction involving the carbonyl oxygen coordination to the boron open shell as in ortho-carbonyl oxygen and boron,36,37 in addition to hydrogen bond formation between the N-H group and the oxygen on the boronic acid moiety, which were observed in aliphatic amidoboronic acids.49
Therefore, the introduction of polyacrylamide can potentially enhance glucose binding via a B-O chelating. In addition, it helped increasing the water solubility of hydrophobic PAAPBA segments. Statistically, there are about twenty hydrophilic acrylamide units per every hydrophobic AAPBA unit on the polymer backbone. The amphiphilic polymer is dissolved in PBS solution and would behave like a surfactant. The detail glucose sensing mechanism was still under investigation. Nevertheless, from mechanistic standpoint, the sensing would most likely proceed via the synergistic interaction between the phenylboronic acid moieties on the polymer backbone and glucose at the ratio of two to one (Scheme 1),25,26 which induce the crosslinking and an increase in the solution viscosity. This whole crosslinking process is completely reversible because of the reversibility of the formation of borate esters. When the environmental glucose concentration decreases, the equilibrium would shift to left according to Le Chatelier law. Dissociation of the glucose would break the crosslinked network and reduce the viscosity.
It is well known that temperature have strong impact on the fluid viscosity. We have observed that the sensing fluid viscosity decreased when temperature increased because of the high molecule mobility, however still maintained sufficient sensing capability. At physiological temperature, lower fluid viscosity will be expected. This temperature effect will be further studied and published elsewhere. In our future studies with MEMS, we will also give strict control on temperature to mimic physiological conditions and study the sensing performance of this fluid.
Conclusions
A novel glucose selective polymeric sensing fluid based on direct binding was successfully developed. The polymer was easy to be prepared through free radical polymerization. This sensing fluid eliminated the usage of Dextran, simplifying the sensing system and lowering the cost. Its sensitivity to glucose was strongly dependant on the polymer molecular weight and percent composition of boronic acid monomer in copolymer and the polymer concentration. Through proper adjustment of the molecular weight and percent composition of the boronic acid segment in the polymer and the polymer concentration, the sensing fluid was able to detect and differentiate glucose from other monosaccharides and disaccharides. Furthermore, the binding of the polymer with glucose showed good reversibility. Unlike proteins, synthetic polymers are more stable for applications under physiological conditions. They do not require any activation metal ions (unlike Con A), therefore can be used under different physiological environments. Currently we are trying to apply this fluid to MEMS affinity sensors that will potentially enable highly reliable, continuous monitoring of glucose in subcutaneous interstitial fluid. Though polyacrylamide and boronic acid were biocompatible and safe to use, toxicity effects of this new polymer are unclear at this point, and toxicity studies will be performed in future to ensure biosafety in the future application in continuous glucose monitor in vivo. Moreover, further control of the polymer structures can be achieved by controlled free radical polymerization of acrylamide in water such as reversible addition–fragmentation chain transfer (RAFT) reaction as well as other living polymerization methods 51–52 to optimize the response sensitivity.
Acknowledgments
This work was supported by NSF-ECCS-0702101 and the Columbia Diabetes and Endocrinology Research Center (NIH grant # DK63068-05). We are grateful to the reviewers of this paper for many helpful suggestions.
References
- 1.Centers for Disease Control and Prevention. National Diabetes Fact Sheet. 2005. [Google Scholar]
- 2.Ballerstadt R, Schultz JS. Anal Chim Acta. 1997;345:203–212. [Google Scholar]
- 3.Schultz JS, Sims G. Biotechnol Bioeng Symp. 1979;9:65–71. [PubMed] [Google Scholar]
- 4.Schultz JS, Mansouri S, Goldstein IJ. Diabetes Care. 1982;5:245–253. doi: 10.2337/diacare.5.3.245. [DOI] [PubMed] [Google Scholar]
- 5.Mansouri S, Schultz JS. Biotechnol. 1984;2:885–890. [Google Scholar]
- 6.Meadows DL, Schultz JS. Anal Chim Acta. 1993;280:21–30. [Google Scholar]
- 7.Russell RJ, Pishko MV, Gefrides CC, McShane MJ, Cote GL. Anal Chem. 1999;71:3126–3132. doi: 10.1021/ac990060r. [DOI] [PubMed] [Google Scholar]
- 8.Ballerstadt R, Schultz JS. Anal Chem. 2000;72:4185–4192. doi: 10.1021/ac000215r. [DOI] [PubMed] [Google Scholar]
- 9.Liang F, Pan T, Sevick-Muraca E. Photochem Photobiol. 2005;81:1386–1394. doi: 10.1562/2005-02-14-RA-440. [DOI] [PubMed] [Google Scholar]
- 10.Ballerstadt R, Gowda A, McNichols R. Diabetes Technol Ther. 2004;6:191–200. doi: 10.1089/152091504773731375. [DOI] [PubMed] [Google Scholar]
- 11.Ballerstadt R, Polak A, Beuhler A, Frye J. Biosens Bioelectron. 2004;19:905–914. doi: 10.1016/j.bios.2003.08.019. [DOI] [PubMed] [Google Scholar]
- 12.Friedl KE. Diabetes Tech Therapeut. 2004;6:201–202. doi: 10.1089/152091504773731384. [DOI] [PubMed] [Google Scholar]
- 13.Ballerstadt R, Ehwald R. Biosens Bioelectron. 1994;9:557–567. [Google Scholar]
- 14.Ehwald R, Ballerstadt R, Dautzenberg H. Anal Biochem. 1996;234:1–8. doi: 10.1006/abio.1996.0040. [DOI] [PubMed] [Google Scholar]
- 15.Beyer U, Ehwald R, Fleischer LG. Biotechnol Prog. 1997;13:722–726. [Google Scholar]
- 16.Beyer U, Ehwald R. Biotechnol Prog. 2000;16:1119–1123. doi: 10.1021/bp000119b. [DOI] [PubMed] [Google Scholar]
- 17.Beyer U, Schafer D, Thomas A, Aulich H, Haueter U, Reihl B, Ehwald R. Diabetologia. 2001;44:416–423. doi: 10.1007/s001250051637. [DOI] [PubMed] [Google Scholar]
- 18.Zhao Y, Li S, Davidson A, Yang B, Wang Q, Lin Q. J Micromech Microeng. 2007;17:2528–2537. [Google Scholar]
- 19.Kataoka T, Oh-Hashi F, Sakurai Y. Gann. 1982;73:193–205. [PubMed] [Google Scholar]
- 20.Enker WE, Craft K, Wissler RW. J Surg Res. 1974;16:66–68. doi: 10.1016/0022-4804(74)90013-4. [DOI] [PubMed] [Google Scholar]
- 21.Möller E. Science. 1964;147:873–879. doi: 10.1126/science.147.3660.873. [DOI] [PubMed] [Google Scholar]
- 22.Phillips JH, Lanier LL. J Immunol. 1986;136:1579–1585. [PubMed] [Google Scholar]
- 23.Zahnley C. J Inorg Biochem. 1981;15:67–78. doi: 10.1016/s0162-0134(00)80136-1. [DOI] [PubMed] [Google Scholar]
- 24.Kuivila HG, Keough AH, Soboczenzki EJ. J Org Chem. 1954;19:780–783. [Google Scholar]
- 25.Yan J, Fang H, Wang B. Medic Res Rev. 2005;25:490–520. doi: 10.1002/med.20038. [DOI] [PubMed] [Google Scholar]
- 26.Boduroglu S, El Khoury JM, Venkat RD, Rinaldi PL, Hu J Bioorg Medic Chem Lett. 2005;15:3974–3977. doi: 10.1016/j.bmcl.2005.05.075. [DOI] [PubMed] [Google Scholar]
- 27.Yang W, Gao X, Wang B. Med Res Rev. 2003;23:346–368. doi: 10.1002/med.10043. [DOI] [PubMed] [Google Scholar]
- 28.Fang H, Kaur G, Wang B. J Fluoresc. 2004;14:481–489. doi: 10.1023/b:jofl.0000039336.51399.3b. [DOI] [PubMed] [Google Scholar]
- 29.Asher SA, Alexeev VL, Goponenko AV, Sharma AC, Lednev IK, Wilcox CS, Finegold DN. J Am Chem Soc. 2003;125:3322–3329. doi: 10.1021/ja021037h. [DOI] [PubMed] [Google Scholar]
- 30.Lei M, Baldi A, Nuxoll E, Siegel RA, Ziaie B. Diabetes Technol Therap. 2006;8:112–122. doi: 10.1089/dia.2006.8.112. [DOI] [PubMed] [Google Scholar]
- 31.Arnold FH, Zheng WG, Michaels AS. J Membrane Sci. 2000;167:227–239. [Google Scholar]
- 32.Zhang Y, Guan Y, Zhou S. Biomacromolecules. 2006;7:3196–3201. doi: 10.1021/bm060557s. [DOI] [PubMed] [Google Scholar]
- 33.Hoare T, Pelton R. Macromolecules. 2007;40:670–678. [Google Scholar]
- 34.Huh P, Kim SC, Kim Y, Wang Y, Singh J, Kumar J, Samuelson LA, Kim BS, Jo NJ, Lee JO. Biomacromolecules. 2007;8:3602–3607. doi: 10.1021/bm070421+. [DOI] [PubMed] [Google Scholar]
- 35.Kitano H, Kuwayama M, Kanayama N, Ohno K. Langmuir. 1998;14:165–170. [Google Scholar]
- 36.Hall DG. Boronic Acids, VCH, Weinheim. 2005;Ch.1:1–100. [Google Scholar]
- 37.Yang X, Lee MC, Sartain F, Pan X, Lowe CR. Chem Eur J. 2006;12:8491–8497. doi: 10.1002/chem.200600442. [DOI] [PubMed] [Google Scholar]
- 38.Ivanov AE, Larsson H, Galaev IY, Mattiasson B. Polymer. 2004;45:2495–2505. [Google Scholar]
- 39.Hegedus LS, Allen GF, Olsen DJ. J Am Chem Soc. 1980;102:3583–3587. [Google Scholar]
- 40.Kuzimenkova MV, Ivanov AE, Galaev IY. Macromol Biosci. 2006;6:170–178. doi: 10.1002/mabi.200500185. [DOI] [PubMed] [Google Scholar]
- 41.Shiomori K, Ivanov AE, Larsson H, Galaev IY, Kawano Y, Mattiasson B. Macromol Chem Phys. 2004;205:27–34. [Google Scholar]
- 42.Zhang D, Tanaka H, Pelton R. Langmuir. 2007;23:8806–8809. doi: 10.1021/la700711a. [DOI] [PubMed] [Google Scholar]
- 43.Lapeyre V, Gosse I, Chevreux S, Ravaine V. Biomacromolecules. 2006;7:3356–3363. doi: 10.1021/bm060588n. [DOI] [PubMed] [Google Scholar]
- 44.De Geest BG, Jonas AM, Demeester J, De Smedt SC. Langmuir. 2006;22:5070–5074. doi: 10.1021/la053368o. [DOI] [PubMed] [Google Scholar]
- 45.Lee YJ, Pruzinsky SA, Braun PV. Langmuir. 2004;20:3096–3106. [PubMed] [Google Scholar]
- 46.Kanekiyo Y, Sano M, Iguchi R, Shinkai S. J Polym Sci, Part A: Polym Chem. 2000;38:1302–1310. [Google Scholar]
- 47.Franzen S, Ni W, Wang B. J Phys Chem B. 2003;107:12942–12948. [Google Scholar]
- 48.Ni W, Kaur G, Springsteen G, Wang B, Franzen S. Bioorg Chem. 2004;32:571–581. doi: 10.1016/j.bioorg.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 49.Lai JH, Liu Y, Wu W, Zhou Y, Maw HH, Bachovchin WW, Bhat KL, Bock CW. J Org Chem. 2006;71:512–519. doi: 10.1021/jo051757h. [DOI] [PubMed] [Google Scholar]
- 50.Thomas DB, Sumerlin BS, Lowe AB, McCormick CL. Macromolecules. 2003;36:1436–1439. [Google Scholar]
- 51.Kitano H, Morokoshi S, Ohhori K, Gemmei-Ide M, Yokoyama Y, Ohno K. J Colloid Interface Sci. 2004;273:106–114. doi: 10.1016/j.jcis.2004.01.027. [DOI] [PubMed] [Google Scholar]
- 52.Roy D, Cambre JN, Sumerlin BS. Chem Commun. 2008:2477–2479. doi: 10.1039/b802293c. [DOI] [PubMed] [Google Scholar]