Abstract
Occlusive vascular disease is a widespread abnormality leading to lethal or debilitating outcomes such as myocardial infarction and stroke. It is part of atherosclerosis and is evoked by clinical procedures including angioplasty and grafting of saphenous vein in bypass surgery. A causative factor is the switch in smooth muscle cells to an invasive and proliferative mode, leading to neointimal hyperplasia. Here we reveal the importance to this process of TRPC1, a homolog of Drosophila transient receptor potential. Using 2 different in vivo models of vascular injury in rodents we show hyperplasic smooth muscle cells have upregulated TRPC1 associated with enhanced calcium entry and cell cycle activity. Neointimal smooth muscle cells after balloon angioplasty of pig coronary artery also express TRPC1. Furthermore, human vein samples obtained during coronary artery bypass graft surgery commonly exhibit an intimal structure containing smooth muscle cells that expressed more TRPC1 than the medial layer cells. Veins were organ cultured to allow growth of neointimal smooth muscle cells over a 2-week period. To explore the functional relevance of TRPC1, we used a specific E3-targeted antibody to TRPC1 and chemical blocker 2-aminoethoxydiphenyl borate. Both agents significantly reduced neointimal growth in human vein, as well as calcium entry and proliferation of smooth muscle cells in culture. The data suggest upregulated TRPC1 is a general feature of smooth muscle cells in occlusive vascular disease and that TRPC1 inhibitors have potential as protective agents against human vascular failure.
Keywords: neointimal hyperplasia, vascular smooth muscle cells, calcium channel, transient receptor potential
Atherosclerosis is a common medical problem in industrialized countries and has major impact on survival, quality of life, and health services.1,2 Primary treatments include bypass surgery and angioplasty, usually with stenting. The procedures are successful but hampered by the marked tendency of injured vascular smooth muscle cells to undergo phenotypic modulation: a shift from their stable quiescent contractile state to an invasive proliferative state that includes increased secretion of extracellular matrix components.2 Such modulation is important physiologically for vascular development and adaptation, but it is also part of occlusive vascular disease including atherosclerosis itself, as well as adverse responses to invasive vascular procedures resulting in neointimal hyperplasia or restenosis, which is the growth of new cells and matrix in the inner structure of the vessel wall, leading to progressive reduction of blood flow and increased risk of thrombosis. Occlusions often develop in the arterial side of the circulation but are also striking in the saphenous vein when it is used as a graft in coronary artery bypass surgery.3 Although use of the vein is routine, it has a marked tendency to rapidly develop neointimal hyperplasia, with ultimate failure of approximately half of the grafts over a period of months to years following the operation.3 Potential therapeutic measures to prevent this venous graft occlusion have been subject to intense investigation, but understanding of the underlying molecular and cellular mechanisms is rudimentary and available pharmacological interventions limited.3,4
To delineate basic mechanisms of human neointimal hyperplasia and identify promising drug targets, we addressed membrane proteins because they have generally provided good sites for drug action, often presenting specific extracellular sites accessible to small molecule and immunological drugs. Of particular interest in cell proliferation are calcium channels because of the profound antiproliferative effect of removing extracellular calcium and evidence from studies of many cell types that calcium entry mechanisms have an essential role.5,6 Specifically in relation to vascular smooth muscle, it has been recognized that phenotypic modulation is associated with downregulation of L-type voltage-gated calcium channels,7 channels that provide calcium entry for contraction when the cells are in the contractile phenotype of the physiological blood vessel and that are the target for the antihypertensive calcium antagonist drugs. What are the calcium channels of the proliferating vascular smooth muscle cell? Could they be targeted specifically by drugs, avoiding other effects on the vasculature including hypotension and headache? One hypothesis is that L-type channels are replaced, at least quantitatively, by T-type calcium channels or another type of calcium channel: one that is not voltage-gated. This latter type is linked to the widely studied store-operated calcium entry mechanism, shown to be up-regulated in proliferating pulmonary artery smooth muscle cells maintained in cell culture.8 We have found that such calcium entry involves the TRPC1 calcium channel subunit9,10 and is depressed by cholesterol depletion,11 ie, under conditions protecting against vascular disease. Experiments in cell culture previously linked TRPC1 to pulmonary arterial smooth muscle proliferation, although a link to pulmonary hypertension was not found.12,13
TRPC1 was the first-identified9 mammalian homolog of Drosophila transient receptor potential (TRP), a protein with an essential role in the visual response of the fly. Since the discovery of TRPC1, an extensive family of mammalian TRP channels has been found with intriguing sensing capabilities for a range of factors including redox status, arachidonic acid metabolites, and growth factors.14 Nevertheless, there has been relatively little association of TRP channels with human disease, little direct evidence that blocking TRP channels could be an effective therapeutic strategy, and no link between TRPC1 and organ function. We have focused on the idea that TRP channels might have a role in vascular disease. In this article, we show evidence from in vivo studies that TRPC1 is a common upregulated calcium channel in the adaptive injury response of blood vessels and then focus on the relevance to occlusive vascular disease directly in humans, measuring neointimal hyperplasia in saphenous vein.
Materials and Methods
In Vivo Vascular Injury
Male 12-week-old C57Bl/6 mice were anesthetized with IP injection of Avertin (0.015 mL/g of 2.5% solution) and the left carotid artery isolated under a dissection microscope. A 2- to 3 mm-long plastic tube (cuff) was placed around the artery and secured with suture and the skin incision closed. After 21 days, the animal was anesthetized with Avertin (0.030 mL/g of 2.5% solution) and carotid arteries were dissected out for Ca2+ measurements (see below). The cuff and loose connective tissue were removed and arteries placed in nominally Ca2+-free HEPES buffered solution. For immunohistochemistry experiments, animals were perfused at physiological pressure with 4% paraformaldehyde in PBS before dissection of the arteries. The cuff model of vascular injury is notable for the minimal contribution of bone marrow–derived cells to the intimal plaque.
Male 12-week-old Wistar Kyoto rats (300 to 310 g) (Charles River, L'Arbresele, France) were submitted to left common carotid arteriotomy and c-Myc antisense treatment as described.15,16 Briefly, a plastic Scanlon clamp for coronary artery bypass grafting was placed on the carotid artery for 10 sec to cause a crushing lesion to the vessel. At the same point where the clamp was applied, a 0.5-mm longitudinal incision was made through the full thickness of the carotid artery. Two hundred micrograms of phosphorothioate c-Myc antisense (5′-CACGTTGAGGGGCAT-3′) or control sense (5′-ATGCCCCTCAACGTG-3′) oligonucleotides (Genset Oligos, Paris, France) were locally applied soon after arteriotomy on the periadventitial side of the injured carotid through 100 mL of F127 pluronic gel (Sigma).15 Antisense DNA was targeted to rat c-Myc mRNA (GenBank accession no. Y00396). Five days after arteriotomy, rats were anesthetized and carotid arteries were carefully dissected free from surrounding tissue. Animals were then perfused at physiological pressure with 4% paraformaldehyde in PBS for fixation.
Large white pigs (20 to 25 kg) underwent percutaneous transluminal coronary angioplasty (PTCA). Animals received 150 mg of aspirin 24 hours before and 48 hours post-PTCA. Anesthesia was induced by inhalation of 4% isoflurane. An endotracheal tube was inserted and anesthesia maintained using 2% isoflurane. The left carotid artery was exposed and an 8F guide catheter inserted. Heparin (2500 IU) was injected before coronary artery cannulation. Two hundred micrograms of glyceryl trinitrate were injected into each coronary artery and angiography performed using a digital image intensifier. Vessel segments in the left anterior descending and/or right coronary arteries (diameter, 2.0 to 2.5 mm) were selected for angioplasty. A 3-mm angioplasty balloon was used for injury (2x30s at 8 atmospheres). The balloon was removed, 200 μg of glyceryl trinitrate injected and a postinjury angiogram performed. The carotid artery was ligated, the neck incision closed, and the animal allowed to recover. Arteries were collected 3 days after PTCA and pressure fixed in situ with 4% formaldehyde.
Human Saphenous Vein Preparation, Culture, and Treatment
Undistended, nonvaricosed long saphenous vein (2 to 5 cm) was harvested using a no-touch technique from patients undergoing surgery for ischemic heart disease and immediately placed in DMEM (Gibco BRL, Paisley, Scotland) supplemented with 30% FCS (Bio-Whittaker Europe, Verviers, Belgium), penicillin and streptomycin (100 U/mL and 100 μg/mL, respectively), and L-glutamine. Extreme care was taken to ensure aseptic conditions throughout. In some instances, vein was used immediately for isolation of RNA or protein. Planar culture of smooth muscle cells from vein was achieved by explant technique in DMEM supplemented with 10% FCS, and cells were used at passage 3 or 4. For organ culture, vein with its transport medium was transferred to a sterile 90-mm Petri dish. Excess perivenous tissue was carefully dissected from the vein using fine scissors. Potentially damaged ends of vein were discarded. The vein was divided into segments resulting in at least 3 rings approximately 0.5 cm in length each. One segment was used to check cellular viability by opening the vein longitudinally and immersing it in 0.2% trypan blue for 1 minute. All experiments were paired, ie, at least 2 segments of adjacent vein were compared from the same patient: 1 under control conditions, the other in the presence of a test agent. T1E3 antibody (1:1000 dilution of antiserum) or 2-aminoethoxydiphenyl borate (2-APB) (75 μmol/L; Sigma) was present throughout the culture period; matched controls contained 0.0001% sodium azide or 0.1% dimethylsulphoxide, respectively. For the antibody-peptide control experiment (Figure 3f), T1E3 was preadsorbed to its antigenic peptide (10 μmol/L) and the matching control group was peptide alone.
Figure 3.

TRPC1 blocking antibody protects against neointimal growth in human saphenous vein. a and b, Red autofluorescence from actin/Miller-stained vein from the same patient after 14 days in organ culture with (b) or without (a) anti-TRPC1 T1E3 antibody. c and d, The same images but with superimposed sketches to illustrate the boundary limit of the preexisting intima (i) and the neointima (ii) grown in culture and showing the area of the neointima (blue) and residual lumenal space (black). The calibration bar in a is 200 μm and applies to a through d. e, For experiments such as that shown in a and b, summary of data from 8 patients. In each case, the neointimal growth of the T1E3-treated vein (black) is normalized to that in the paired control vein (white) from the same patient. f, T1E3 (blocking antibody, n=8), 2-APB (n=4), and T1E3+peptide (n=3; 1 result was omitted from the mean because it was extreme, showing large growth after T1E3+peptide but little after peptide alone). For all experiments, the mean size of the neointimal growth as a percentage of the respective matched control is shown and n is the number of patients. **P<0.01.
For organ culture, the medium was changed every 2 days without touching the vessel, and, after 2 weeks, veins were fixed in 10% formalin and embedded in paraffin wax. Vein sections were labeled with antibody to smooth muscle α-actin and Miller's stain of the elastic laminae. Neointimal hyperplasia was the new cellular layer that developed on the lumenal aspect of the preexisting intima (eg, see Figure 2h). Although this stain was sufficient for analysis we discovered that autofluorescence (excitation wavelength 530 nm) gave a better definition of the neointimal growth and so these images have been used for presentation in the figure. To confirm each result, neointimal growth was analyzed using ImageJ software and 3 different measurements: thickness at north, south, east, and west orientations; the neointimal area (eg, the blue area in Figure 3c); and the density of the structure delineated by line (i) in Figure 3c relative to a reference point of “no occlusion.” Measurements were made independently by at least 2 investigators, yielding similar results.
Figure 2.
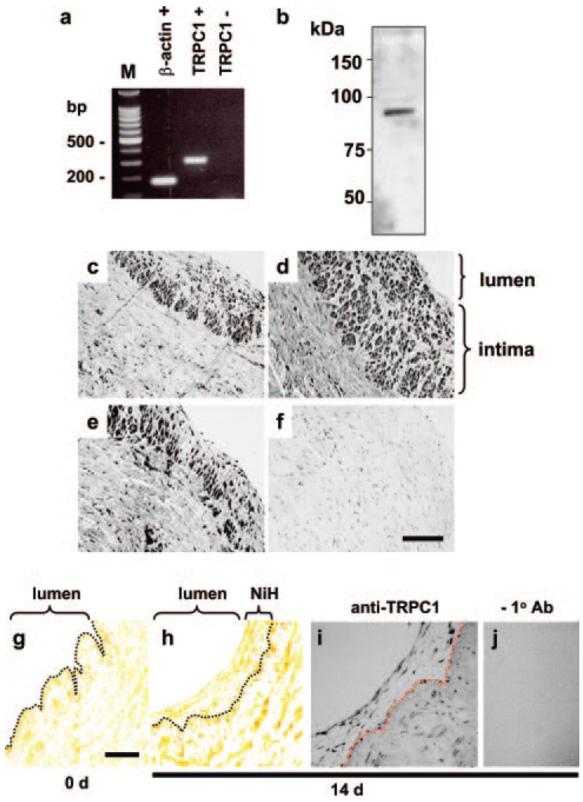
Intimal and neointimal TRPC1 in human saphenous vein. a, Gel electrophoresis of PCR products from vein RNA with (+RT) and without (−RT) prior reverse transcriptase reaction. The TRPC1 product size is 303 bp. M indicates 100-bp DNA ladder. b, Western blot for vein proteins probed with anti-TRPC1 T1E3 antibody (1:500 dilution), showing a single labeled protein at the expected molecular mass of TRPC1 (≈90 kDa). Protein was not labeled by preimmune serum from rabbit used to generate T1E3 (data not shown, but see Xu and Beech 10 for antibody characterization). c through f, Sections of vein stained with anti-TRPC1 antibody: T1E3 (c), affinity-purified T1E3 (d), T1-A antibody (e), or T1-A antibody preadsorbed to its antigenic peptide (f) (control experiment). Staining intensity is on gray-scale, where black is maximum intensity. Sections were counter stained with hematoxylin, labeling nuclei (evident in isolation in f). The calibration bar (100 μm) applies to c through f. g and h, Anti-smooth muscle α-actin (brown) and Miller's stain of vein from the same patient before (g) and after (h) organ culture. The dotted line is superimposed to mark the inner limit of the preexisting intima. The calibration bar (30 μm) applies to g through j. i and j, Organ-cultured vein labeled with (i) or without (j) anti-TRPC1 T1E3 antibody.
Ethics
Freshly discarded human blood vessels were obtained anonymously and with informed consent from adult patients undergoing coronary artery bypass graft surgery and with ethical approval from Leeds Teaching Hospitals Local Research Ethics Committee. All experimental protocols for the use of animals were approved by the local Animal Ethics Committee, and all guidelines were subsequently followed. Porcine PTCA procedures conformed to the UK Animals (Scientific Procedures) Act 1986 and were authorized by the Home Office.
cDNA Clones and Cell Transfection
HEK-293 (tsA 201) cells used for transient transfection were grown in DMEM-F12 (Gibco) medium containing 10% FCS, 100 U/mL penicillin, and 100 μg/mL streptomycin. Cells were maintained at 37°C under 95% air and 5% CO2. Fifty % confluent cells in 10-cm dishes were cotransfected with 5 μg FLAG epitope tagged TRPC1 (accession no. X89066, a gift from C. Montell, Johns Hopkins University School of Medicine, Baltimore, Md) and 5 μg TRPC5 (accession no. AF029983, a gift from Y. Mori, National Institute for Physiological Sciences, Aichi, Japan) cDNA using a standard calcium phosphate protocol. Cells were harvested for experiments 36 hours after transfection.
RNA Isolation and RT-PCR
Total RNA was isolated using a standard TriReagent protocol, reverse transcribed, and subjected to PCR essentially as described previously.11 For human TRPC1, PCR primers were (5′-3′; forward and reverse): TTAGCGCATGTGGCAA and CCACTTACTGAGGCTACTAAT; and the predicted amplicon was 303 bp. For mouse TRPC1, PCR primers were: TTTGGCAGAATCATTCACAC and CTTCCAACCCTTCATACCAC; and the predicted amplicon was 220 bp. Products were sequenced to confirm identity.
Western Blotting
General protocols have been described.10 In brief, tissues or cells were placed in phosphate-buffered saline (PBS) containing protease inhibitor cocktail (Sigma) and lysed in Laemmli buffer containing 320 μmol/L dithiothreitol at 80 to 100°C for 15 minutes. Proteins were separated on 8% SDS-PAGE gels and transferred to nitrocellulose membrane (Millipore, Bedford, Mass), and probed with appropriate primary antibodies (1:500 dilution) and secondary antibodies conjugated with horseradish peroxidase. Membranes were washed with PBS and labeling detected by ECLplus (Amersham Pharmacia Biotech).
Immunocytochemistry
Human and pig tissues were fixed and embedded in paraffin wax. 4-μm sections were cut, hot-plated, dried overnight and stored at 4°C until use. Labeling occurred according to standard protocols: Briefly, sections were quenched for endogenous peroxidase and blocked in 10% horse serum. Primary antibodies were incubated with sections overnight at 4°C, labeled using Envision (Dako Ltd) or ABC kit (Vector Labs, Burlingame, Calif) and visualized using diaminobenzidine as substrate. Parallel control experiments were performed using adjacent sections and omission of the primary antibody or following pre-adsorption of antibody to its antigenic peptide. Counterstaining was achieved with Ehlrich's hematoxylin.
Mice and rat samples were fixed in 4% formaldehyde/PBS for 5 to 12 hour, embedded in Tissue-Tek (Sakura) and frozen. Sections (10 μm) were permeabilized with 0.2% Triton X-100 in PBS for 10 minutes and blocked with 2% bovine serum albumin (BSA) in PBS for 2 hour. Rabbit anti-TRPC1 (T1-A, 1:100) was applied overnight at 4°C and Cy5-anti-rabbit IgG (1:500) for 1 hour at room temperature. Cy5 fluorescence was monitored using a Zeiss LSM 510 laser scanning confocal microscope and 633-nm excitation wavelength. For quantification, multiple fields for each vessel were imaged and analyzed under blind conditions. Three to five boxes of defined dimensions (500 μm2) were randomly positioned within the vessel media layer and mean pixel intensity (range 0 to 255 grayscale values) after background subtraction was calculated using the Zeiss LSM 510 Pascal Analysis software. For comparisons, control and treated carotid arteries were studied in parallel. Specificity of immune staining was confirmed by the absence of staining when primary antibody was omitted from the protocol or antibody was preadsorbed to its antigenic peptide.
Antibodies
Primary antibodies targeted to TRPC1 were the rabbit polyclonal T1E310,11 or T1-A (Alomone Labs, Jerusalem, Israel). Where specified, T1E3 was affinity purified on columns containing immobilized peptides according to standard protocols. Successful transfection of tsA201 cells with TRPC5 was determined using the rabbit polyclonal anti-TRPC5 antibody T5C2, which is targeted to the C-terminus (a gift from D.E. Clapham, Children's Hospital, Howard Hughes Medical Institute, Boston, Mass). Mouse monoclonal anti-FLAG and anti-smooth muscle α-actin antibodies were from Sigma; anti-smooth muscle myosin embryonic antibody was from Covance Labs; Cy5-conjugated anti-rabbit IgG was from Jackson ImmunoResearch Laboratories; Alexa Fluor 488 anti-goat IgG was from Molecular Probes.
Intracellular Calcium Measurement
Intracellular calcium was monitored in mouse carotid artery using fura-2 and an IonOptix imaging system. Endothelium was eliminated by sliding arterial segments ≈20× over a needle. Segments were inverted, threaded on a glass capillary, and incubated for 90 minutes with 13.3 μmol/L fura-2 acetoxymethyl ester (Molecular Probes), 2% DMSO, and 0.05% pluronic F-127 in HEPES-buffered solution containing (in mmol/L): NaCl 135.5, KCl 5.9, CaCl2 2.5, MgCl2 1.2, HEPES 11.6, and glucose 11.5. The capillary was mounted in a superfusion chamber on a Nikon TMD inverted microscope. Calibration using high (10 mmol/L Ca2+) and low (2 mmol/L EGTA) extracellular Ca2+ after ionomycin permeabilization (50 μmol/L, 75 sec) was performed. For saphenous vein smooth muscle cell Ca2+ measurement, fura-2 acetoxymethyl ester was used on a real-time fluorescence 96-well plate reader (Flexstation II, Molecular Devices). The recording medium contained (in mmol/L): 130 NaCl, 5 KCl, 8 d-glucose, 10 HEPES, 1.2 MgCl2, titrated to pH 7.4 with NaOH. When indicated, 1.5 mmol/L CaCl2 was added.
Cell Proliferation Assay
The general protocol has been described.24 Briefly, A7r5 cells (smooth muscle cells derived from rat aorta) were grown in DMEMF12 medium containing 10% FCS, 100 U/mL penicillin, and 100 μg/mL streptomycin, and a 5-bromo-2′ deoxyuridine (BrdUrd) labeling kit (Roche) was used to measure proliferation. Cells were split onto a 24-well plate and cultured for 24 to 48 hours before incubation with BrdUrd for 2 hours. After removal of labeling medium, cells were washed, fixed, and incubated with anti-BrdUrd antibody for 2 hours at room temperature. After washing (3 times), substrate was added for color development, and absorbance was measured.
Data Analysis
Averaged data are mean±SEM. Statistical differences were tested using paired or unpaired Student's t test or 1-way ANOVA followed by Bonferroni or Tukey–Kramer tests (for comparisons between ≤5 groups and ≥6, respectively); in the Figure 3, significance is indicated by P<0.01 (**). Human tissue staining was repeated independently on samples from at least 3 patients, yielding similar results.
Results
In Vivo Hyperplasia Associated With Upregulated TRPC1 and Calcium Entry
To determine whether enhanced store-operated calcium entry and TRPC1 are features of vascular hyperplasia in response to injury in vivo, we studied animal models. Periadventitial cuff injury of mouse carotid artery led to enhanced calcium entry following store depletion (Figure 1a; increased [Ca2+]i above basal 184±30 nmol/L [cuff], cf, 76±8 nmol/L, P<0.05, 4 mice) but significantly suppressed calcium entry in response to high potassium-evoked depolarization (increased [Ca2+]i above basal 52±3 nmol/L [cuff], cf, 72±3 nmol/L, P<0.05, 4 mice), consistent with there being upregulated store-operated channels and downregulated voltage-gated calcium channels. Furthermore, there was increased mRNA encoding TRPC1 (Figure I in the online data supplement available at http://circres.ahajournals.org). TRPC1 protein was explored using antibodies that bind TRPC1 and distinguish it from the closely-related protein TRPC5 (supplemental Figure II). Injury caused an increase in TRPC1 staining (Figure 1b and 1c; 74±6 [cuff], cf, 65±6 arbitrary intensity units, P<0.05, 3 mice, 2 sections each). Similarly, a longitudinal arteriotomy and crushing injury of the rat carotid artery led to upregulated TRPC1 in vivo (Figure 1d and 1e; supplemental Figure III). In this system, antisense DNA targeted to c-Myc inhibits G1–S phase cell cycle transition, reducing the injury response and preserving smooth muscle differentiation.15,16 Inhibiting the cell cycle also prevented upregulation of TRPC1 (Figure 1e and 1f; supplemental Figure III), suggesting TRPC1 expression is linked to cell cycle activity, although the mechanism of such coupling is unknown. Furthermore, we found that TRPC1 was almost undetectable in normal pig coronary arteries, whereas there was clear expression in cells of neointimal plaques after percutaneous transluminal coronary angioplasty (Figure 1g through 1i). These cells stained positively with antibody to smooth muscle myosin embryonic, suggesting they were smooth muscle cells (data not shown). We conclude from these data that in vivo hyperplasia is accompanied by upregulation of TRPC1 expression. We therefore sought to determine whether this effect translates to function in human vascular disease.
Figure 1.
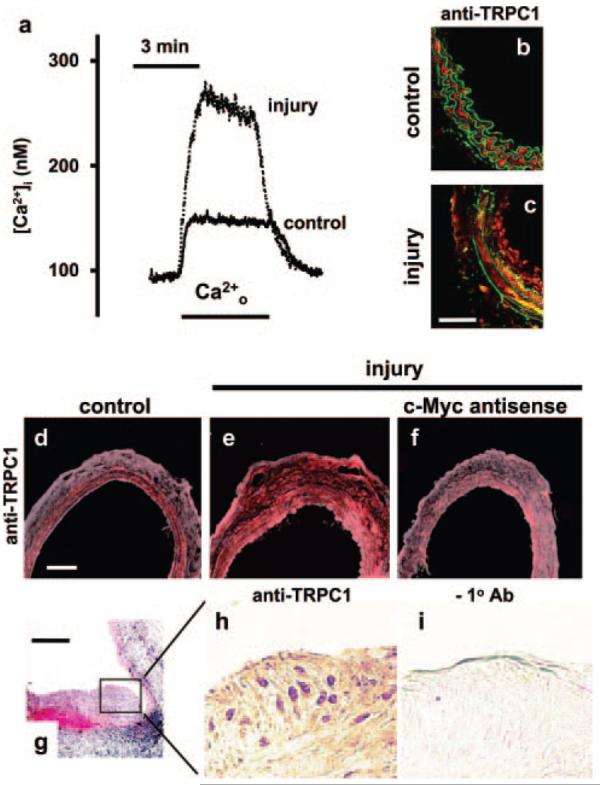
Upregulated TRPC1 in vivo. a, Intracellular calcium concentration ([Ca2+]i) in control and cuff-injured mouse carotid artery. Ca2+o indicates reapplication of extracellular 2.5-mmol/L Ca2+ in the presence of 10 μmol/L verapamil after a 10-minute period in calcium-free solution with 10 μmol/L thapsigargin to deplete intracellular stores. b and c, Immunofluorescence images showing labeling by anti-TRPC1 T1-A antibody (red) in control and parallel cuff-injured mouse carotid artery. The green is autofluorescence from the elastic laminae. The arterial lumen is to the right. The calibration bar in c is 50 μm and applies to b and c. d through f, Immunofluorescence labeling of rat carotid artery with anti-TRPC1 T1-A antibody (red) showing the control contralateral (d) and injured (e) arteries from the same rat and an injured artery from a different rat treated with antisense DNA targeting c-Myc (f). The calibration bar in d is 100 μm and applies to d through f. g, Section of balloon-injured porcine coronary artery stained with hematoxylin and eosin. The calibration bar is 200 μm. h and i, Magnified images of the neointimal plaque shown in g but labeled with (h) or without (i) anti-TRPC1 T1E3 antibody.
TRPC1 in Intimal and Neointimal Smooth Muscle Cells of Human Saphenous Vein
Messenger RNA species were explored by RT-PCR applied to saphenous vein freshly discarded during coronary artery bypass graft surgery. RNA encoding TRPC1 was notable (Figure 2a). Antibody that specifically binds TRPC1 (supplemental Figure II) showed that TRPC1 mRNA was translated and that TRPC1 was the sole protein labeled by T1E3 in saphenous vein (Figure 2b). Most of the veins had existing, extensive intima-containing cells labeled by antibody targeted to the smooth muscle lineage marker smooth muscle α-actin. Such intimal thickening is a feature of ageing and has been described as soil for atherogenesis and restenosis.17,18 Notably, although TRPC1 was detected in the contractile (medial layer) smooth muscle cells, it was most obvious in intimal smooth muscle cells (Figure 2c through 2f). The intimal cells were likely, however, to have been long established, not reflecting recent growth. We therefore initiated a study involving growth of new intimal smooth muscle in situ within human veins. Previous reports have shown the potential of organ culture for such studies19 and modification of these methods enabled growth of neointimal smooth muscle cells within the normal architecture of human saphenous vein (Figure 2g and 2h). TRPC1 was expressed in these neointimal cells (Figure 2i and 2j), encouraging us to pursue functional studies.
TRPC1 Inhibitors Suppress Vascular Smooth Muscle Proliferation and Human Neointimal Hyperplasia
To test the functional role of TRPC1, we first explored the rat vascular smooth muscle cell line (A7r5), which expresses TRPC1.20 Cell proliferation was quantified by measuring BrdUrd incorporation, thus measuring new DNA synthesis. To inhibit TRPC1, we used 2 types of pharmacological agents, an antibody and a chemical. The most powerful is the E3-targeted antibody, recently reported by us as a general approach for making isoform-specific ion channel tools.21 For TRPC1, such an antibody (referred to as T1E3) has already been made and shown to inhibit store-operated Ca2+ entry.10,11,22 T1E3 also binds specifically to protein of the correct mass in human saphenous vein (Figure 2b), and we provide further evidence of its isoform specificity, showing absence of binding to the related TRPC5 protein (supplemental Figure II). Highly selective small molecule inhibitors of TRPC1 are not known; therefore, we turned to 2-APB, which blocks TRPC1 at 75 μmol/L, although it lacks specificity for TRPC1.23,24 The data show T1E3 and 2-APB inhibit A7r5 cell proliferation (supplemental Figure IV), reinforcing the hypothesis that TRPC1 is functionally relevant for vascular smooth muscle growth in cell culture.12 However, of primary interest is the determination of the functional relevance to human neointimal hyperplasia because mechanisms may vary in importance between cell culture as well as between different blood vessels and species.
Neointimal hyperplasia was grown in situ (Figure 2g and 2h) in 2 adjacent segments of vein from each patient: one incubated in test agent, the other serving as a matched control. An adjacent segment of vein was used to test for cellular viability by trypan blue exclusion, and only vein pairs developing neointimal hyperplasia were included in the analysis because new growth would be validation of cell viability. The new growth is composed almost exclusively of smooth muscle α-actin positive cells (Figure 2h), and there is little deposition of extracellular matrix at this stage, giving the hyperplasia a fine mesh-like appearance relative to the adjacent dense structure of the pre-existing vein (Figure 3a). We tested whether inclusion of TRPC1 inhibitor would reduce the hyperplasia and found that T1E3 was effective, reducing neointimal cell growth and protecting the residual lumenal area (Figure 3a through 3d). Two sets of experiments were completed independently by different investigators, each on veins from 4 patients. In veins from all 8 patients, inclusion of T1E3 reduced hyperplasia (Figure 3e). The effect was statistically significant and reproduced by 2-APB (Figure 3f), approximately matching the result obtained with the A7r5 smooth muscle cell-line (supplemental Figure IV). Preadsorption of T1E3 to its antigenic peptide prevented its effect (Figure 3f), showing that inhibition of hyperplasia depends on binding to the antigenic site. Ca2+ entry in proliferating human saphenous vein smooth muscle cells was inhibited similarly (supplemental Figure V). Collectively the data suggest a role for TRPC1 in human neointimal growth and that pharmacological agents targeted to TRPC1 and the associated Ca2+ entry are effective inhibitors.
Discussion
Here we show a previously unrecognized important role of the Drosophila TRP homolog TRPC1, demonstrating direct relevance to vascular occlusion in human saphenous vein, a graft used in many thousands of coronary artery bypass operations every year. TRPC1 has a unique extracellular binding site that can be targeted with antibody, leading to reduction in new smooth muscle cell growth within the preexisting lumen. The increasing success of antibody therapies21,25 indicates this strategy has clinical potential. Thus, longer term studies and in vivo trials in pigs and other large mammals would now seem justified. Importantly, anti-TRPC1 treatments reduce but do not abolish vascular adaptation, which is presumably a necessary phenomenon if the venous graft is to adapt successfully to life on the arterial side of the circulation. TRPC1 is present in some cultured and animal endothelial cells and thus compromise to endothelial function might be a concern. However, we failed to detect significant TRPC1 in the native endothelial layer of human saphenous vein and left internal mammary artery (S.S.S. and D.J.B., unpublished data, 2005), suggesting it would not be a significant issue in the human.
Our findings are consistent with a hypothesis whereby the switch of vascular smooth muscle cells to an invasive mode depends on the cells shifting emphasis from depolarization activated to passive calcium entry.7,26 Mechanisms regulating the passive calcium entry in situ remain unclear,27 but identification of TRPC1 as a functional component should aid elucidation of relevant signaling pathways. Additional elements of the TRPC1 system may be other TRP channels and proteins already known to associate with TRPC1 and which show expression in vascular smooth muscle.27
For decades, voltage-gated calcium channels have proved valuable therapeutic drug targets as exemplified by the cardiovascular benefits of the calcium antagonists, including nifedipine, nimodipine, verapamil, and diltiazem. The TRP calcium channels are structurally related to voltage-gated calcium channels but lack dependence on depolarization as a trigger for activation and show resistance to classical calcium antagonists. The TRPs are a discrete and novel family of calcium channels14 that have emerging functions in disease13,28-30 (and this study). There is thus potential for TRP channels as targets for a new generation of calcium antagonists, perhaps with particular effectiveness against diseases involving cellular adaptive responses depending on proliferation and migration.
Supplementary Material
Acknowledgments
This work was supported by the Wellcome Trust and British Heart Foundation (to D.J.B.); Biotechnology and Biological Sciences Research Council PhD Studentship (to J.N.); Nuffield Hospital Leeds (to B.K. and S.S.); the Swedish Research Council (to P.H. and A.H.-N.); the Swedish Heart-Lung Foundation, the Torsten and Ragnar Söderberg Foundations, and the Vascular Wall Program, Lund University (to P.H., A.H.-N., and M.F.G.); the Magnus Bergvall, Thelma Zoéga, and Crafoord Foundations and the Royal Physiographic Society (to M.F.G.); a graduate fellowship from the National Network for Cardiovascular Research (to A.B.); and Progetto Finalizzato Sanità 2003 “Patologie infettive e insulto chirurgico: studi di genomica e proteomica nel remodeling vascolare” (to A.C.).
References
- 1.Faxon DP, Fuster V, Libby P, Beckman JA, Hiatt WR, Thompson RW, Topper JN, Annex BH, Rundback JH, Fabunmi RP, Robertson RM, Loscalzo J. Atherosclerotic Vascular Disease Conference: Writing Group III: pathophysiology. Circulation. 2004;109:2617–2625. doi: 10.1161/01.CIR.0000128520.37674.EF. [DOI] [PubMed] [Google Scholar]
- 2.Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev. 2004;84:767–801. doi: 10.1152/physrev.00041.2003. [DOI] [PubMed] [Google Scholar]
- 3.Angelini GD, Jeremy JY. Towards the treatment of saphenous vein bypass graft failure—a perspective of the Bristol Heart Institute. Biorheology. 2002;39:491–499. [PubMed] [Google Scholar]
- 4.Mann MJ. Novel strategies for the prevention of bypass graft failure. BioDrugs. 2004;18:1–8. doi: 10.2165/00063030-200418010-00001. [DOI] [PubMed] [Google Scholar]
- 5.Magnier-Gaubil C, Herbert JM, Quarck R, Papp B, Corvazier E, Wuytack F, Levy-Toledano S, Enouf J. Smooth muscle cell cycle and proliferation. Relationship between calcium influx and sarco-endoplasmic reticulum Ca2+ ATPase regulation. J Biol Chem. 1996;271:27788–27794. doi: 10.1074/jbc.271.44.27788. [DOI] [PubMed] [Google Scholar]
- 6.Munaron L, Antoniotti S, Fiorio Pla A, Lovisolo D. Blocking Ca2+ entry: a way to control cell proliferation. Curr Med Chem. 2004;11:1533–1543. doi: 10.2174/0929867043365008. [DOI] [PubMed] [Google Scholar]
- 7.Quignard JF, Harricane MC, Menard C, Lory P, Nargeot J, Capron L, Mornet D, Richard S. Transient down-regulation of L-type Ca2+ channel and dystrophin expression after balloon injury in rat aortic cells. Cardiovasc Res. 2001;49:177–188. doi: 10.1016/s0008-6363(00)00210-8. [DOI] [PubMed] [Google Scholar]
- 8.Golovina VA, Platoshyn O, Bailey CL, Wang J, Limsuwan A, Sweeney M, Rubin LJ, Yuan JX. Upregulated TRP and enhanced capacitative Ca2+ entry in human pulmonary artery myocytes during proliferation. Am J Physiol Heart Circ Physiol. 2001;280:H746–H755. doi: 10.1152/ajpheart.2001.280.2.H746. [DOI] [PubMed] [Google Scholar]
- 9.Wes PD, Chevesich J, Jeromin A, Rosenberg C, Stetten G, Montell C. TRPC1, a human homolog of a Drosophila store-operated channel. Proc Natl Acad Sci U S A. 1995;92:9652–9656. doi: 10.1073/pnas.92.21.9652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu SZ, Beech DJ. TrpC1 is a membrane-spanning subunit of store-operated Ca2+ channels in native vascular smooth muscle cells. Circ Res. 2001;88:84–87. doi: 10.1161/01.res.88.1.84. [DOI] [PubMed] [Google Scholar]
- 11.Bergdahl A, Gomez MF, Dreja K, Xu SZ, Adner M, Beech DJ, Broman J, Hellstrand P, Sward K. Cholesterol depletion impairs vascular reactivity to endothelin-1 by reducing store-operated Ca2+ entry dependent on TRPC1. Circ Res. 2003;93:839–847. doi: 10.1161/01.RES.0000100367.45446.A3. [DOI] [PubMed] [Google Scholar]
- 12.Sweeney M, Yu Y, Platoshyn O, Zhang S, McDaniel SS, Yuan JX. Inhibition of endogenous TRP1 decreases capacitative Ca2+ entry and attenuates pulmonary artery smooth muscle cell proliferation. Am J Physiol Lung Cell Mol Physiol. 2002;283:L144–L155. doi: 10.1152/ajplung.00412.2001. [DOI] [PubMed] [Google Scholar]
- 13.Yu Y, Fantozzi I, Remillard CV, Landsberg JW, Kunichika N, Platoshyn O, Tigno DD, Thistlethwaite PA, Rubin LJ, Yuan JX. Enhanced expression of transient receptor potential channels in idiopathic pulmonary arterial hypertension. Proc Natl Acad Sci U S A. 2004;101:13861–13866. doi: 10.1073/pnas.0405908101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clapham DE. TRP channels as cellular sensors. Nature. 2003;426:517–524. doi: 10.1038/nature02196. [DOI] [PubMed] [Google Scholar]
- 15.Di Micco G, Forte A, Cipollaro M, Renzulli A, De Feo M, Rossi F, Cascino A, Cotrufo M. Surgical injury of rat arteries: genetic control of the remodelling process. Eur J Cardiothorac Surg. 2002;22:266–270. doi: 10.1016/s1010-7940(02)00274-9. [DOI] [PubMed] [Google Scholar]
- 16.Forte A, Galderisi U, De Feo M, Gomez MF, Esposito S, Sante P, Renzulli A, Agozzino L, Hellstrand P, Berrino L, Cipollaro M, Cotrufo M, Rossi F, Cascino A. c-Myc antisense oligonucleotides preserve smooth muscle differentiation and reduce negative remodelling following rat carotid arteriotomy. J Vasc Res. 2005;42:214–225. doi: 10.1159/000085379. [DOI] [PubMed] [Google Scholar]
- 17.O'Leary DH, Polak JF, Kronmal RA, Manolio TA, Burke GL, Wolfson SK., Jr. Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. Cardiovascular Health Study Collaborative Research Group. N Engl J Med. 1999;340:14–22. doi: 10.1056/NEJM199901073400103. [DOI] [PubMed] [Google Scholar]
- 18.Schwartz SM, deBlois D, O'Brien ER. The intima. Soil for atherosclerosis and restenosis. Circ Res. 1995;77:445–465. doi: 10.1161/01.res.77.3.445. [DOI] [PubMed] [Google Scholar]
- 19.Porter KE, Varty K, Jones L, Bell PR, London NJ. Human saphenous vein organ culture: a useful model of intimal hyperplasia? Eur J Vasc Endovasc Surg. 1996;11:48–58. doi: 10.1016/s1078-5884(96)80134-1. [DOI] [PubMed] [Google Scholar]
- 20.Jung S, Strotmann R, Schultz G, Plant TD. TRPC6 is a candidate channel involved in receptor-stimulated cation currents in A7r5 smooth muscle cells. Am J Physiol Cell Physiol. 2002;282:C347–C359. doi: 10.1152/ajpcell.00283.2001. [DOI] [PubMed] [Google Scholar]
- 21.Xu SZ, Zeng F, Lei M, Li J, Gao B, Xiong C, Sivaprasadarao A, Beech DJ. Generation of functional ion-channel tools by E3 targeting. Nat Biotechnol. 2005;23:1289–1293. doi: 10.1038/nbt1148. [DOI] [PubMed] [Google Scholar]
- 22.Bergdahl A, Gomez MF, Wihlborg AK, Erlinge D, Eyjolfson A, Xu SZ, Beech DJ, Dreja K, Hellstrand P. Plasticity of TRPC expression in arterial smooth muscle: correlation with store-operated Ca2+ entry. Am J Physiol Cell Physiol. 2005;288:C872–C880. doi: 10.1152/ajpcell.00334.2004. [DOI] [PubMed] [Google Scholar]
- 23.Delmas P, Wanaverbecq N, Abogadie FC, Mistry M, Brown DA. Signaling microdomains define the specificity of receptor-mediated InsP3 pathways in neurons. Neuron. 2002;34:209–220. doi: 10.1016/s0896-6273(02)00641-4. [DOI] [PubMed] [Google Scholar]
- 24.Xu SZ, Zeng F, Boulay G, Grimm C, Harteneck C, Beech DJ. Block of TRPC5 channels by 2-aminoethoxydiphenyl borate: a differential, extracellular and voltage-dependent effect. Br J Pharmacol. 2005;145:405–414. doi: 10.1038/sj.bjp.0706197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferrara N, Hillan KJ, Gerber HP, Novotny W. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat Rev Drug Discov. 2004;3:391–400. doi: 10.1038/nrd1381. [DOI] [PubMed] [Google Scholar]
- 26.Cheong A, Bingham AJ, Li J, Kumar B, Sukumar P, Munsch C, Buckley NJ, Neylon CB, Porter KE, Beech DJ, Wood IC. Down-regulated REST transcription factor is a switch enabling critical potassium channel expression and cell proliferation. Mol Cell. 2005;20:45–52. doi: 10.1016/j.molcel.2005.08.030. [DOI] [PubMed] [Google Scholar]
- 27.Beech DJ, Muraki K, Flemming R. Non-selective cationic channels of smooth muscle and the mammalian homologues of Drosophila TRP. J Physiol. 2004;559:685–706. doi: 10.1113/jphysiol.2004.068734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim K, Drummond I, Ibraghimov-Beskrovnaya O, Klinger K, Arnaout MA. Polycystin 1 is required for the structural integrity of blood vessels. Proc Natl Acad Sci U S A. 2000;97:1731–1736. doi: 10.1073/pnas.040550097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schlingmann KP, Weber S, Peters M, Niemann Nejsum L, Vitzthum H, Klingel K, Kratz M, Haddad E, Ristoff E, Dinour D, Syrrou M, Nielsen S, Sassen M, Waldegger S, Seyberth HW, Konrad M. Hypomagnesemia with secondary hypocalcemia is caused by mutations in TRPM6, a new member of the TRPM gene family. Nat Genet. 2002;31:166–170. doi: 10.1038/ng889. [DOI] [PubMed] [Google Scholar]
- 30.Reiser J, Polu KR, Moller CC, Kenlan P, Altintas MM, Wei C, Faul C, Herbert S, Villegas I, Avila-Casado C, McGee M, Sugimoto H, Brown D, Kalluri R, Mundel P, Smith PL, Clapham DE, Pollak MR. TRPC6 is a glomerular slit diaphragm-associated channel required for normal renal function. Nat Genet. 2005;37:739–744. doi: 10.1038/ng1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


