Abstract
The skin not only plays an important role as a physical barrier between the host and the environment, but also plays a key immunologic role in sensing and responding to invading pathogens from the environment. Toll-like receptors (TLRs), which are expressed by many different types of cells in human skin, have been found to be important pattern recognition receptors that are involved in recognizing components of microbial pathogens and initiating and instructing cutaneous immune responses. This review examines the similarities and differences among the ten known TLRs in humans. In addition, the role of TLRs in cutaneous host defense mechanisms against a variety of microorganisms, including bacteria, fungi, and viruses, as well as the involvement of TLRs in the pathogenesis of certain skin diseases will be discussed.
Keywords: Toll-like receptors, TLR, Skin, Cutaneous, Innate immunity, Immunology
Introduction
In addition to its role as a physical barrier between the host and the environment, the skin also has an important immunologic role in detecting invading pathogens (1–3). The skin immune system can be divided into early innate immune responses, which promote cutaneous inflammation and adaptive immune responses that promote memory responses against foreign antigens (4;5). Toll-like receptors (TLRs) are a recently identified group of pattern recognition receptors (PRRs) that are involved in mechanisms of host defense against a wide range of pathogenic microorganisms (6–8). TLRs are thought to function by detecting the presence of components of microorganisms and subsequently activating different gene programs, which promote various innate and adaptive immune responses (6–8). There are many different types of cells in the skin that express TLRs, including keratinocytes and Langerhans cells (LCs) in the epidermis, resident and trafficking immune system cells such as monocytes/macrophages, dendritic cells (DCs), T and B lymphocytes, and mast cells in the dermis, endothelial cells of the skin microvasculature, and stromal cells such as fibroblasts and adipocytes(3). All of the cells through their distinct expression of TLRs can recognize different components of microorganisms and initiate host defense mechanisms(3). Thus, TLRs expressed by cells in skin enable these cells to play an integral role in cutaneous immune responses against microbial pathogens. In addition, TLRs have been implicated in the pathogenesis of certain skin diseases (1–3).
Innate and adaptive immunity
Innate immunity was once considered to be an early nonspecific proinflammatory response whose primary function was to recruit and activate phagocytes such as neutrophils and monocytes/macrophages to phagocytize microorganisms (9;10). It is now known that the innate immune response has considerable specificity that is directed against conserved molecular patterns of components of microorganisms, which are called pathogen-associated molecular patterns (PAMPs) (9;10). The receptors on immune system cells that recognize PAMPs are called pattern recognition receptors (PRRs) (9;10). TLRs are a major class of PRRs and each TLR recognizes a different PAMP (see below) (6–10). In contrast, adaptive immunity is mediated by T and B lymphocytes, which have somatically generated receptors on their cell surface (4;6). The receptors of the adaptive immune system cells are not PRRs and do not recognize PAMPs, but instead recognize a diverse array of foreign antigenic components (4;6). These adaptive immunity receptors are generated by somatic hypermutation and genomic DNA recombination of antigen receptor gene segments (4;6). This process allows for a small number of genes to produce a vast array of different antigen receptors on each T and B lymphocyte with each receptor having a unique affinity for a given antigen (4;6). In addition, since the gene rearrangement is permanent, all of the progeny of a given T or B lymphocyte will express the same antigen receptor (4;6). This results in the production of “memory” T and B lymphocytes, which are responsible for mediating long-lived immunologic memory responses (4;6). Interestingly, T and B cells, in addition to expressing these adaptive immune receptors, also express TLRs, which can modify the immune responses generated by these cells (3). The early immune response to an invading pathogen is largely mediated by innate immunity whereas the subsequent cell-mediated and humoral immune responses and ensuing memory responses are mediated by T and B lymphocytes of the of the adaptive immune response (4;6).
Toll-like receptors
TLRs are transmembrane glycoproteins that contain an ectodomain of leucine-rich motifs, which is involved in recognition of components of microbes (6–8). TLRs also contain a transmembrane domain and a cytoplasmic tail domain that is homologous to the interleukin-1 receptor and is responsible for initiating various intracellular signaling cascades (Fig. 1) (6–8). These signaling cascades include activation of nuclear factor-κB (NF-κB), which is a key transcription factor that promotes expression of genes involved in immune responses such as cytokines, chemokines, and co-stimulatory and adhesion molecules (6–8). Thus far, ten TLRs have been identified in humans (Fig. 2) (6–8). Interestingly, each TLR recognizes a distinct PAMP (6–8). TLR2 can form a heterodimer with either TLR1 or TLR6 to recognize tri- or diacyl lipopeptides of bacteria, respectively (6–8). TLR2/6, along with CD36, has also been shown to recognize lipoteichoic acid (which is diacylated) of Gram-positive bacteria (6–8). TLR2 can also recognize peptidoglycan of most bacterial species and can also components of fungi (6–8). TLR3 recognizes double-stranded RNA (dsRNA), which is found during the replication cycle of most viruses (6–8). TLR4 along with CD14 recognizes lipopolysaccharide (LPS) of Gram-negative bacteria (6–8). TLR5 recognizes bacterial flagellin (6–8). TLR7 and TLR8 recognize single-stranded RNA (ssRNA) found in certain viruses and also recognizes the imidazoquinoline compounds, imiquimod and resiquimod (R-848) (6–8;11). Imiquimod (Aldara®) is the first TLR ligand that has been used to treat human disease. Imiquimod is FDA-approved to treat genital warts, actinic keratoses, and superficial basal cell carcinomas (11;12). TLR9 recognizes hypomethylated CpG motifs of bacterial double-stranded DNA (dsDNA) and DNA generated during the replication process of dsDNA viruses such as herpes simplex virus(6–8). The PAMP recognized by TLR10 is unknown.
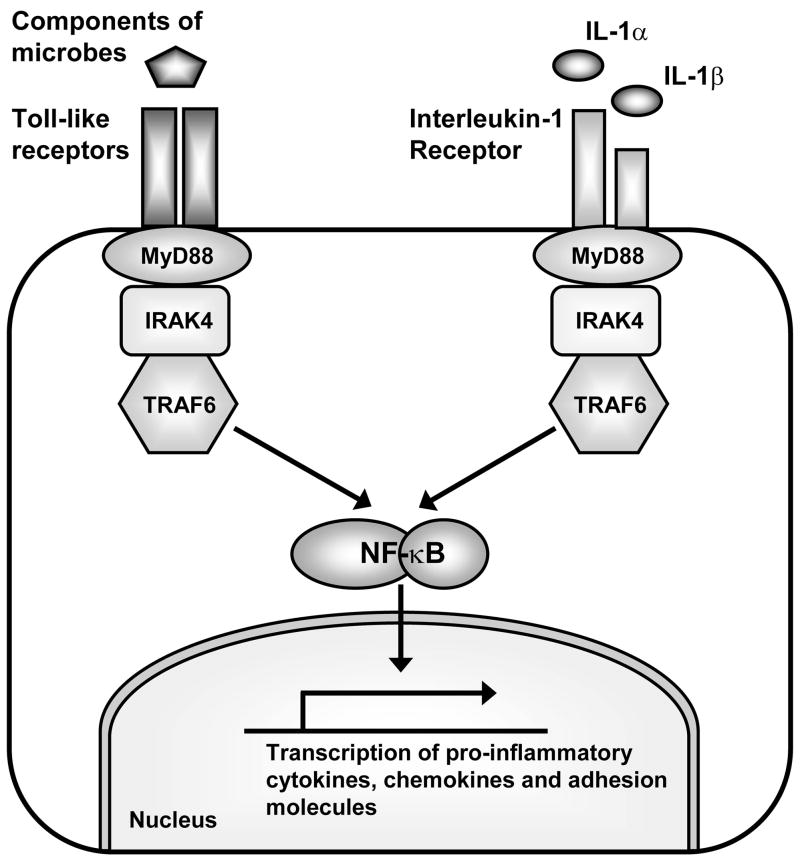
Figure 1. TLRs and the interleukin-1 receptor share a similar signaling cascade to initiate immune responses.
TLRs and the interleukin 1 receptor (IL-1R) share a similar signaling cascade, which involve activation of the adapter molecule MyD88. MyD88 forms an initial signaling complex with IRAK4 and TRAF6. Formation of this complex results in activation of a signaling cascade that eventually leads to activation of NF-κB (and other pathways) to promote transcription of pro-inflammatory cytokines, chemokines and co-stimulatory and adhesion molecules involved in innate and adaptive immune responses.
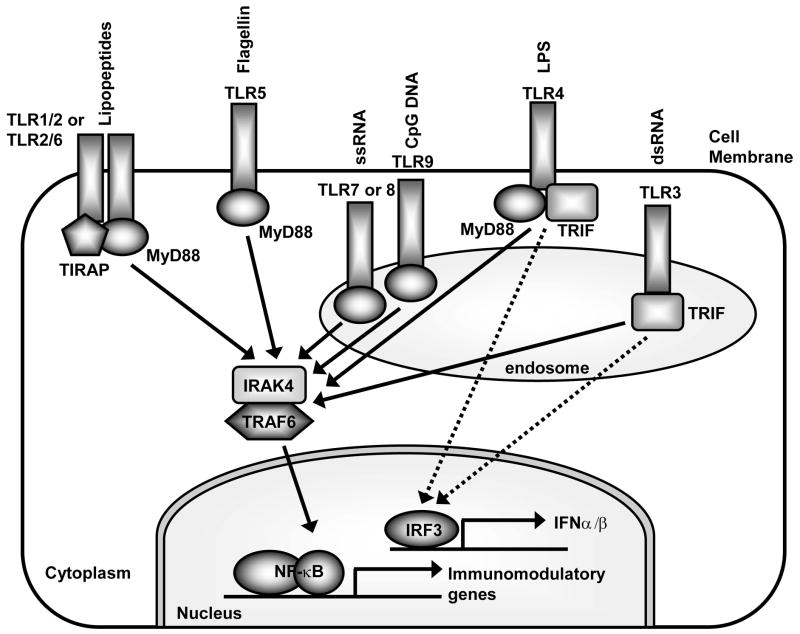
Figure 2. Pathogen-associated molecular patterns (PAMPs) recognized by TLRs, the cellular location of TLRs and the different MyD88 adapters used by TLRs that promote distinct immune responses.
Each TLR recognizes a different microbial component. TLR2 forms a heterodimer with TLR1 or TLR6 to recognize tri- and di-acyl lipopeptides, respectively. TLR4 recognizes LPS and TLR5 recognizes flagellin. These TLRs are located on the cell membrane and become internalized into phagosomes after interaction with their ligands. In contrast, TLR3 recognizes viral dsRNA, TLR7 and TLR8 recognize viral ssRNA, and TLR9 recognizes hypomethylated DNA (CpG motifs) of both bacteria and viruses and are located in intracellular membranes of endosomes and lysosomes. TLRs utilize MyD88 and TRIF adapters to initiate signaling. All TLRs except TLR3 can signal via MyD88. TLR2 and TLR4 also require the presence of TIRAP. MyD88 initiates a signaling cascade that eventually results in activation of NF-κB (and other pathways) to promote transcription of immunomodulatory genes. In contrast, TLR3 and TLR4 can also signal via TRIF in a MyD88-independent pathway. The TRIF pathway is critical in activating IRF3 (and IRF7), which promotes production of type I interferon (i.e. IFNα and IFNβ) and anti-viral immune responses.
TLRs can also be classified into two groups based upon cellular location (Fig. 2) (6–8). TLRs 1, 2, 4, 5 and 6 are found on the cell membrane and can be activated by extracellular PAMPs. In contrast, TLRs 3, 7, 8 and 9 are found in membranes of intracellular compartments, such as endosomes and lysosomes (6–8). The intracellular location of TLRs 3, 7, 8 and 9 enable them to detect nucleic acids (i.e. DNA or RNA) that have been released from viruses or bacteria that are degraded within endosomes and lysosomes inside the cell (6–8).
Toll-like receptor signaling and immune responses
Activation of TLRs by their ligands results in initiation of several signaling cascades, which eventually result in expression of cytokines (e.g. TNFα, IL-1β, IL-6, IL-12), chemokines (IL-8, GRO-α, MCP-1, -2, -3, -4, MIP1α/β, and RANTES), anti-microbial peptides, (beta-defensins and cathelicidin), co-stimulatory molecules (CD40, CD80 and CD86), and adhesion molecules (ICAM-1) that are involved in innate and adaptive immune responses (Fig. 3) (6–8;13;14). In certain instances, TLRs can cause tissue injury in conditions such as sepsis, autoimmunity, and apoptosis of cells (7;13;14). The early signaling events initiated by TLR activation is mediated by members of the myeloid differentiation factor 88 (MyD88) family of adapter proteins (7;13;14). Activation of MyD88 adapters results in recruitment of interleukin-1 receptor-associated kinases (IRAKs) and tumor necrosis factor receptor-associated factors (TRAFs) (especially IRAK4 and TRAF6), which form the initial signaling complex (7;13;14). Formation of this complex eventually leads to activation of downstream signaling pathways, including activation of members of the MAP kinases (mitogen-activated protein kinases) such as ERK (extracellular-signal-regulated kinase), JNK (c-Jun-NH2-terminal kinase), and p38, and also activates transcription factors such as NF-κB and AP-1 (activator protein-1) (7;13;14). Activation of these pathways leads to expression of inflammatory cytokine genes and subsequent immune responses (6–8;13).
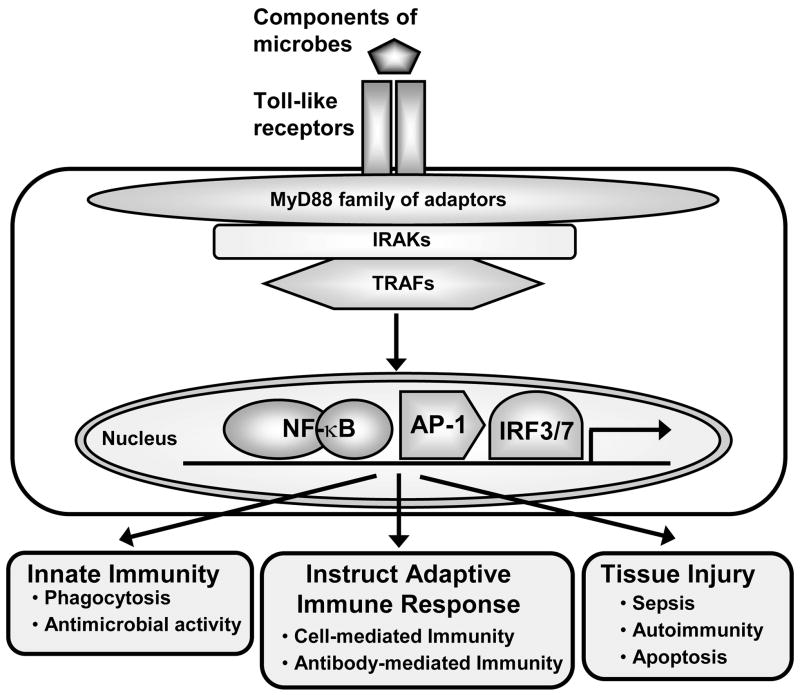
Figure 3. Immune responses generated by activation of TLRs.
TLRs recognize various microbial components and transduce signals via the family of MyD88 adapter molecules. MyD88 adapters recruit interleukin-1 receptor-associated kinases (IRAKs) and tumor necrosis factor receptor-associated factors (TRAFs) to form the initial signaling complexes that lead to activation of downstream signaling pathways, including activation of transcription factors such as NF-κB, AP-1 (activator protein-1), and IRF3/7 (interferon regulatory factors 3 and 7). These signaling pathways are responsible for distinct gene programs involved in different innate and acquired immune responses. TLRs have also been implicated in tissue injury in conditions such as sepsis, autoimmunity, and apoptosis.
Interestingly, despite the common use of several signaling molecules, the different MyD88 adapter proteins are only utilized by certain TLRs (7;13;14). This difference in adapters leads to activation of distinct signaling pathways and gene programs that contribute to different cellular responses (Fig. 2). These different adapters include MyD88, TIRAP (Toll-interleukin 1 receptor domain-containing adapter protein), TRIF (Toll-interleukin 1 receptor domain-containing adapter-inducing interferon-β) and TRAM (TRIF-related adapter molecule) (7;13;14). All of the TLRs, with the exception of TLR3, utilize MyD88 to initiate signaling (Fig. 2). In addition, TLRs 2 and 4 also require the presence of TIRAP (along with MyD88) to initiate signaling (7;13;14). Interestingly, TLR3 exclusively utilizes TRIF in a MyD88-independent pathway to initiate signaling (7;13;14). TLR4, in addition to utilizing MyD88, can also utilize TRIF (which in the case of TLR4 also requires the presence of TRAM) to initiate signaling (7;13;14). Utilization of the TRIF pathway by TLR3 or TLR4 results in the activation both NFκB and MAP kinases in a similar manner as the MyD88 pathway (7;13;14). However, TRIF, but not MyD88, specifically activates interferon (IFN)-regulatory factors 3 and 7 (IRF3/7), which promote production of type I interferon (i.e. IFNα and IFNβ) (7;13;14). These type I interferon responses are critical in the immune response against viruses (6–8;13;14).
Even though TLRs are PRRs and are thought to be involved in the early sensing of an infection during the innate immune response, TLRs also can instruct subsequent adaptive immune responses (6). For example, activation of TLRs on dendritic cells (DCs) can promote upregulation of co-stimulatory molecules such as CD40, CD80, and CD86 and production of IL-12 (6). CD80 and CD86 are important co-stimulatory molecules that help promote interaction and stimulation of antigen specific T cells of the adaptive immune response (6). In addition, IL-12 produced by TLR-stimulated DCs specifically promotes the induction of T helper 1 (Th-1) cell-mediated immune responses (6). Thus, in certain instances, TLR activation can instruct adaptive immune responses by inducing a Th-1 type immune response (6).
Toll-like receptors can induce a vitamin D-dependent antimicrobial pathway
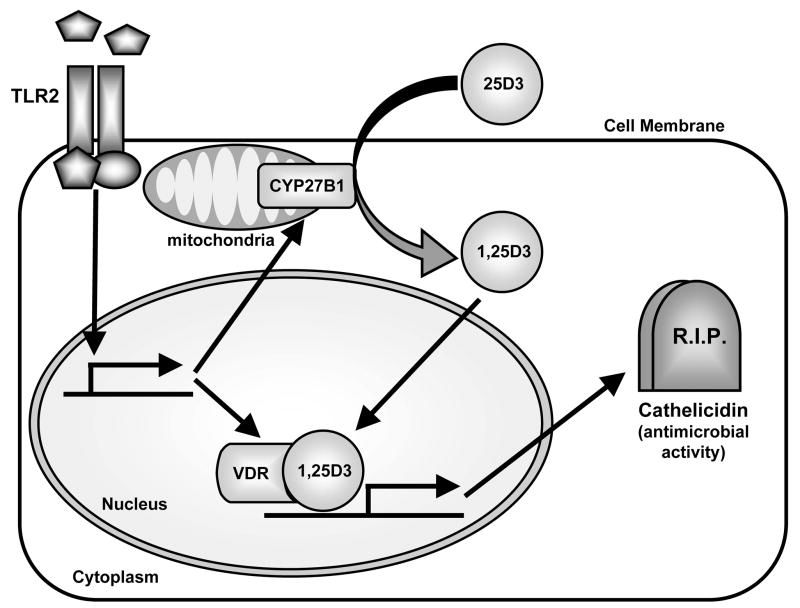
Recently, Liu et al. demonstrated that activation of TLR2/1 on human monocytes/macrophages upregulated a vitamin D-1-hydroxylase (CYP27B1) and the vitamin D receptor (VDR) (15;16) (Fig. 4). This activity of TLR2/1 resulted in the conversion of the inactive form of vitamin D (25D3) to its active form (1,25D3), which subsequently activated the VDR and led to the production of the antimicrobial peptide cathelicidin (15;16). Since cathelicidin has microbicidal activity against a variety of pathogenic microorganisms, TLR2 induction of a vitamin D-dependent antimicrobial pathway may be an important mechanism for host defense. Similarly, Schauber et al. demonstrated that wounding of skin or stimulation of keratinocytes with TGF-β resulted in increased expression of TLR2 and CYP27B1 expression by keratinocytes (Fig. 4) (17). This resulted in increased cathelicidin expression via a vitamin D-dependent pathway (17). Taken together, these studies demonstrate that TLRs can increase cellular antimicrobial responses via a vitamin D-dependant pathway and represent another way that TLRs can protect the skin against infection.
Figure 4. TLR2 activates a vitamin D-dependent antimicrobial pathway.
Activation of TLR2 on human monocytes/macrophages or keratinocytes from healing wounds (or keratinocytes stimulated with TGF-β) in increased expression of the vitamin D-1-hydroxylase CYP27B1 and the vitamin D receptor (VDR). CYP27B1 converts the inactive form of vitamin D (25D3) to its active form (1,25D3). 1,25D3 binds to and activates the VDR, which induces production of the antimicrobial peptide cathelicidin. Since cathelicidin has microbicidal activity against a variety of pathogenic microorganisms, TLR2 induction of a vitamin D-dependent antimicrobial pathway may be an important mechanism for cutaneous host defense.
Toll-like receptor expression and function of skin-specific cells
There are many different cell types in human skin that express TLRs (1–3). In the epidermis, keratinocytes have been shown to express functional TLRs. In addition, there are resident and trafficking immune system cells in the skin that express TLRs, including Langerhans cells (LCs), monocytes/macrophages, dendritic cells (DCs), T and B lymphocytes, and mast cells (1–3). Lastly, endothelial cells of the microvasculature and stromal cells such as fibroblasts and adipocytes also express TLRs (1–3). Each of these cell types have distinct TLR expression patterns and likely contribute to cutaneous immune responses (1–3). This section will discuss TLR expression and function on keratinocytes and Langerhans cells.
Keratinocytes
Keratinocytes of the epidermis not only play an important role in maintaining the physical barrier between the host and the environment, but also participate in cutaneous immune responses (5;18). In particular, keratinocytes have been shown to express TLRs 1–6 and 9, which can help keratinocytes act as first-responders against pathogenic microorganisms (17;19–31). For example, activation of TLR2 on keratinocytes by S. aureus or its components, peptidoglycan and lipoteichoic acid, results in activation of NF-κB and subsequent production of the neutrophil chemotactic factor IL-8 and iNOS (26). Other studies have demonstrated that activation of TLR3 by its ligand, dsRNA (poly I:C), on human keratinocytes induced production of IL-8, TNFα, IL-18, and type I interferon (IFNα/β) and the development of Th-1 type immune responses (20;24;25;30;32). Since TLR3 is thought to play an important role in recognizing viral infections, keratinocytes via TLR3 activation may play an important role in anti-viral immune responses in skin. Other studies have also demonstrated that activation of TLR5 on human keratinocytes by its ligand, flagellin, resulted in production of TNFα, IL-8, and the antimicrobial peptides, human β-defensins 2 and 3 (hBD2 and hBD3) (25;32;33). Lebre et al. demonstrated that activation of TLR3 and TLR9 on keratinocytes (by poly I:C and CpG DNA, respectively) leads to selective production of the chemokines, CXCL9 and CXCL10, which promote memory T cell responses and production of type I interferon (25). Another study by Miller found that the keratinocyte growth and differentiation factor, TGFα, which is found at high levels in healing wounds, upregulated expression and function of TLR5 and TLR9 (27). Thus, TGFα may not only stimulate keratinocytes to repair the barrier after wounding, but may also increase the ability of keratinocytes to sense an infection via increasing their functional responsiveness to TLR ligands (27).
Langerhans cells
Langerhans cells (LCs) are a subset of dendritic cells (DCs) that are found in the epidermis. Langerhans cells express the protein, langerin, which is found in birbeck granules, the intracellular organelle of LCs. Several studies have demonstrated that LCs express TLRs and may participate in mediating TLR responses (34–39). A previous study by Renn et al. demonstrated that LC-like DCs that were derived from human cord blood express mRNA for TLRs 1–10 (40). LC-like DCs were most responsive to TLR2 ligands and TLR7/8 ligands (40). Furthermore, activation of LC-like DCs by TLR3 stimulation resulted in production of type I interferon (IFNα/β), suggesting a role for LC-like DCs in anti-viral immunity (34;40). However, recently, a study by Flacher et al. demonstrated that freshly isolated LCs that were purified from human skin express only TLRs 1, 2, 3, 5, 6 and 10 (41). These skin LCs responded to ligands to TLR2, TLR3 and produced IL-6, IL-8, and TNFα, but not IL-12 or IFNα/β (41). Interestingly, in response to peptidoglycan stimulation, the skin-derived LCs produced the Th2 cytokine, IL-10, suggesting that LCs may play a role in tolerance against commensal Gram-positive bacteria (41). Taken together, there are significant differences among TLR expression and function of LC-like DCs and LCs purified from human skin, and the LCs purified from human skin appear to play an important role in immunologic tolerance.
Toll-like receptors in the pathophysiology of skin disease
Atopic dermatitis
Atopic dermatitis or eczema is an inflammatory skin disease that is associated with a hereditary predisposition to atopic conditions, which include allergic rhinitis, allergic keratoconjuntivitis, asthma, and eczema (5). Clinically, atopic dermatitis is characterized by the presence of inflammatory skin lesions that are extremely pruritic (5). Like other allergic diseases, the pathophysiology of atopic dermatitis involves a Th-2 type immune response in the skin. The role of TLRs in the pathophysiology of atopic dermatitis is not entirely understood. However, the skin lesions of atopic dermatitis are highly susceptible to superinfection by bacterial and viral pathogens, such as S. aureus and herpes simplex virus (42–45). Several studies have demonstrated that the skin lesions of atopic dermatitis have decreased levels of various antimicrobial peptides (beta-defensins, cathelicidin, and dermcidin) as compared with normal skin or psoriatic skin lesions and these lower levels of antimicrobial peptides may contribute to the increased susceptibility to infection (42–45). Recent studies have identified the presence of certain polymorphisms in TLRs or TLR signaling molecules in patients with atopic dermatitis. One study found that a specific polymorphism in TLR2 (R753Q), which was previously associated with a subset of patients with severe S. aureus infections (46), defined a group of atopic dermatitis patients with a severe phenotype (47). However, another study did not show an association with TLR2 polymorphisms among patients with atopic dermatitis (48). Other studies have demonstrated that polymorphism in the TLR9 promoter or in TOLLIP, an inhibitory adapter protein within the TLR pathway, were also associated with atopic dermatitis (49;50). Lastly, monocytes from patients with atopic dermatitis have been shown to have a significant impairment in TLR2-mediated production of proinflammatory cytokines (51). Taken together, polymorphisms in TLRs or TLR signaling molecules may impair the functional responsiveness of TLRs (especially TLR2 and TLR9) in patients with atopic dermatitis. This impairment in TLR function may contribute the increased susceptibility of lesions of atopic dermatitis to bacterial and viral superinfection and may also contribute to the pathophysiology of the disease.
Psoriasis
Psoriasis is an inflammatory skin disease that is characterized clinically by cutaneous erythematous plaques with thick slivery scale (5). Histologic examination of psoriasis lesions reveals epidermal hyperplasia (acanthosis), dilation of papillary dermal blood vessels, and a dermal inflammatory infiltrate composed of predominantly of T cells and histiocytes (5;52). In contrast to atopic dermatitis, psoriasis has been associated with a Th-1 cytokine profile and more recently a Th-17 cytokine profile (5;52). Also, it is well known that psoriatic plaques are highly resistant to superinfection by pathogenic bacteria such as S. aureus (53). This increased resistance to infection may be partly explained by the high levels of antimicrobial peptides found in psoriatic scales (42;43;54;55). In addition, several studies have found that keratinocytes in psoriatic lesions have increased levels of TLRs 1, 2, 4, 5 and 9 compared with normal skin (19–21;27;56). As mentioned above, Miller et al., demonstrated that the keratinocyte growth factor TGFα, which is found at high levels in healing wounds and in psoriatic lesions, increased expression of TLRs 5 and 9 and increased TLR-dependent production of pro-inflammatory cytokines (e.g. IL-8) and beta-defensins (27). Therefore, TLRs may contribute to the increased levels of antimicrobial peptides and cutaneous immune responses in psoriatic lesions. Interestingly, a previous report demonstrated that topical application of the TLR7 agonist imiquimod induced the spreading of a psoriatic plaque (57). Thus, TLR activation may also play a role in the pathophysiology of psoriasis by exacerbating the disease process (57). Lastly, a recent study demonstrated that the antimicrobial peptide cathelicidin (LL-37), which is found at high levels in psoriatic skin, can convert otherwise non-stimulatory self-DNA into a potent activator of TLR9 on plasmacytoid DCs resulting in production of IFNα (58). This may be one important mechanism of how TLRs can promote autoimmunity in psoriasis (58).
Acne vulgaris
Acne vulgaris is an inflammatory skin disease that occurs mostly during adolescence and involves inflammation of the pilosebaceous unit. The anerobic bacterium Propionibacterium acnes has been associated with the inflammation in acne lesions. Kim et al. demonstrated that TLR2 on human monocytes can be activated by P. acnes in vitro, resulting in increased production of IL-12 and IL-8 (59). Furthermore, macrophages expressing TLR2 were found surrounding pilosebaceous units of histologic sections of acne lesions from patients (59). Interestingly, topical retinoids such as all-trans retinoic acid and adapalene, which are used clinically to treat acne, have been shown to decrease TLR2 expression (60;61). Liu et al. demonstrated that all-trans retinoic acid can decrease TLR2 expression and function on cultured human monocytes (60). Tenaud et al. demonstrated that adapalene can decrease TLR2 expression on epidermal keratinocytes of explants of normal human skin and explants of acne lesions (61). Thus, TLR2 has been implicated in the inflammatory process in acne vulgaris and topical retinoids may help decrease the inflammation in acne lesions by decreasing expression and function of TLR2.
Toll-like receptors in skin infections
Staphylococcus aureus
S. aureus is a Gram-positive bacterium that is the most common cause of bacterial skin infections in humans such as impetigo, folliculitis/furunculosis, and cellulitis. TLR2 has been shown to recognize various components of S. aureus, including peptidoglycan and lipopeptides (62–64). In addition, the TLR2/6 heterodimer along with CD36 has been shown to recognize S. aureus lipoteichoic acid (65). A recent study demonstrated that TLR2 on primary human keratinocytes contributed to upregulation of human beta-defensin 3 (hBD3), which has potent microbicidal activity against S. aureus (66). Other studies in mice have shown that mice deficient in TLR2 developed larger skin lesions in response to S. aureus skin infection (65;67). However, mice deficient in MyD88-developed much larger lesions than TLR2-deficient mice, suggesting that other receptors that signal via MyD88 may be important in cutaneous host defense against S. aureus (67). Miller et al. determined that mice deficient in IL-1R, which also signals via MyD88, developed large lesions that closely resembled those of MyD88-deficient mice, suggesting that IL-1R may play a more prominent role in cutaneous host defense against S. aureus than TLR2 (67).
Mycobacterium leprae
M. leprae is an intracellular bacterium that causes the clinical disease leprosy (68). Clinically, leprosy has a broad clinical spectrum. The tuberculoid form has few skin lesions, rare bacteria seen by histology, and is associated with a Th-1 type cell-mediated immune response (68). In contrast, the lepromatous form has numerous skin lesions, readily detectable bacteria by histology and is associated with a Th-2 type antibody-mediated immune response (68). There is considerable evidence that TLRs are involved in the immune response against leprosy. In vitro studies by Krutzik et al. have found that lipoproteins from M. leprae mediate cellular activation via TLR2/1 (69). Furthermore, another study demonstrated that TLR2 induced apoptosis of Schwann cells, which may contribute to the nerve damage seen in leprosy patients (70). In addition, individuals with polymorphisms in TLR2, which impair TLR2 function, have been shown to have an increased susceptibility to leprosy and the development of lepromatous form (71–73). In particular, peripheral blood leukocytes isolated from patients with these polymorphisms produced less TNFα and Th-1 cytokines (e.g. IL-2, IL-12, and IFNγ) and increased IL-10 levels compared with individuals without the polymorphism (71;72). Thus, TLR2 has shown to be important in host defense against M. leprae, but may also increase nerve damage seen in leprosy lesions by increasing apoptosis of Schwann cells.
Candida albicans
C. albicans is a fungal pathogen that causes mucocutaneous infections and even life-threatening infections, especially in immunocompromised individuals (74). TLR2 recognizes C. albicans phospholipomannan (75). In contrast, TLR4 recognizes C. albicans O-bound mannan (76). In human keratinocyte cultures, Pivarcsi et al. demonstrated that keratinocyte-induced killing of C. albicans was dependent upon TLR2 and TLR4 activation(28). These studies suggest that TLR2 and TLR4 not only can recognize components of C. albicans, but also play a role in keratinocyte antimicrobial activity against C. albicans.
Herpes simplex and varicella-zoster virus
Herpes simplex virus (HSV) and varicella-zoster virus (VZV) are viral pathogens of the Herpesviridae family of dsDNA viruses that commonly infect human skin and mucosa (77;78). Infections by either HSV and VZV typically result in grouped vesicles that ulcerate and then heal (77;78). VZV is responsible for the clinical manifestations of varicella (chicken pox) during the first exposure and zoster (shingles) during re-activation of latent viral infection (78). Several TLRs have been implicated in the immune response to HSV. Individuals with genital HSV infections who had polymorphisms in TLR2, which caused impairment of TLR2 activity, had increased viral shedding and more recurrent infections than patients without TLR2 polymorphisms (79). Furthermore, individuals deficient in TLR3 had increased spreading of HSV infection from keratinocytes to cranial nerves, resulting in an increased susceptibility to HSV encephalitis (80). In cultures systems, TLR2 and TLR9 have been shown to recognize HSV glycoproteins and HSV dsDNA, respectively, and promote production of inflammatory cytokines(81–84). Similarly, TLR2 can be activated by VZV to induce production of proinflammatory cytokines (78). Taken together, there is evidence that TLRs 2, 3, and 9 are involved in the cutaneous innate immune response against HSV and VZV infections.
Toll-like receptor-based treatments of skin disease
Since activation of TLRs promote immune responses, there has been a growing interest in pharmacologic targeting of TLRs in the treatment of various medical conditions, including certain skin diseases and skin cancer. In fact, imiquimod 5% topical cream (Aldara®), which is a nucleoside analog and a TLR7 agonist, has already been FDA approved to treat genital warts, actinic keratoses and superficial basal cell carcinomas (85;86). Through activation of TLR7, imiquimod induces expression of proinflammatory cytokines such as IFNα, TNFα, IL-6, IL-8, and IL-12 that promote a Th-1 type immune response (85;86). Furthermore, imiquimod has shown to have pro-apoptotic activity against tumors, which may explain its activity against skin cancers such as basal cell carcinomas (86). In addition, other studies have demonstrated that TLR9 agonists (i.e. CpG oligodeoxynucleotides) can be used as adjuvants in anti-cancer vaccines, which may be important in promoting specific anti-cancer immune responses against malignant melanoma and perhaps other cancers (87–89). Thus, pharmacologic targeting of the immunomodulatory effects of TLRs may provide a basis for future therapies or vaccine development against certain skin diseases, skin cancer, and infections.
Conclusions
TLRs have emerged as a major class of PRRs that are involved in detecting invading pathogens in the skin and initiating cutaneous immune responses. TLRs are expressed on many different cell types in the skin, including keratinocytes and Langerhans cells in the epidermis. Each TLR can recognize a different microbial component and there are differences among the TLR signaling pathways, which lead to distinct immune responses against a given pathogen. Certain TLRs have been implicated in the pathogenesis of skin diseases, such as atopic dermatitis, psoriasis, and acne vulgaris. In addition, TLRs have been shown to be important in cutaneous host defense mechanisms against common bacterial, fungal, and viral pathogens in the skin, such as S. aureus, C. albicans, and HSV. Since the discovery that topical TLR agonists promote anti-viral and anti-tumor immune responses, there has been considerable interest in the development of TLR-based therapies for skin diseases, skin cancer and infections. Future research involving TLRs in skin will hopefully provide new insights into host defense against skin pathogens and novel therapeutic targets aimed at treating skin disease and skin cancer.
Footnotes
Dr. Miller and his colleagues at the University of California - Los Angeles have been national leaders in elucidating the roles of a family of proteins called Toll-like receptors (TLRs) in processes as diverse as Staphylococcus aureus skin infections, acne vulgaris, and leprosy infection. TLRs are a highly conserved family of pattern recognition receptors that have emerged as critical sensors of bacterial, fungal and viral pathogens by recognizing conserved components of these microorganisms, such as bacterial lipopeptides and virally-derived single-stranded and double-stranded RNA. The mechanism of action of current FDA-approved drugs, such as imiquimod, are based on the ability of these agents to activate TLRs. Agents that activate TLRs may also be very helpful in cancer therapy as vaccine adjuvants; conversely, inhibition of TLR-mediated signaling may help to down-regulate unwanted inflammation. Interestingly, TLRs have been implicated in cutaneous immune responses against a wide range of skin infections, including infections caused by Staphylococcus aureus, Mycobacterium leprae, Candida albicans, and viruses such as herpes simplex and varicella-zoster, and may contribute to the pathophysiology of common skin diseases such as atopic dermatitis, psoriasis, and acne vulgaris. Therefore, understanding the biology of the TLRs may give us new insights and, potentially, treatments for a variety of skin conditions.
Sam Hwang
Financial Disclosures
The author has no financial conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Kang SS, Kauls LS, Gaspari AA. Toll-like receptors: applications to dermatologic disease. J Am Acad Dermatol. 2006;54(6):951–983. doi: 10.1016/j.jaad.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 2.McInturff JE, Modlin RL, Kim J. The role of toll-like receptors in the pathogenesis and treatment of dermatological disease. J Invest Dermatol. 2005;125(1):1–8. doi: 10.1111/j.0022-202X.2004.23459.x. [DOI] [PubMed] [Google Scholar]
- 3.Miller LS, Modlin RL. Toll-like receptors in the skin. Semin Immunopathol. 2007;29(1):15–26. doi: 10.1007/s00281-007-0061-8. [DOI] [PubMed] [Google Scholar]
- 4.Clark R, Kupper T. Old meets new: the interaction between innate and adaptive immunity. J Invest Dermatol. 2005;125(4):629–637. doi: 10.1111/j.0022-202X.2005.23856.x. [DOI] [PubMed] [Google Scholar]
- 5.Kupper TS, Fuhlbrigge RC. Immune surveillance in the skin: mechanisms and clinical consequences. Nat Rev Immunol. 2004;4(3):211–222. doi: 10.1038/nri1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 2004;5(10):987–995. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- 7.Kawai T, Akira S. TLR signaling. Semin Immunol. 2007;19(1):24–32. doi: 10.1016/j.smim.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 8.Trinchieri G, Sher A. Cooperation of Toll-like receptor signals in innate immune defence. Nat Rev Immunol. 2007;7(3):179–190. doi: 10.1038/nri2038. [DOI] [PubMed] [Google Scholar]
- 9.Medzhitov R. Toll-like receptors and innate immunity. Nat Rev Immunol. 2001;1:135–145. doi: 10.1038/35100529. [DOI] [PubMed] [Google Scholar]
- 10.Medzhitov R, Janeway C., Jr Innate immunity. N Engl J Med. 2000;343:338–344. doi: 10.1056/NEJM200008033430506. [DOI] [PubMed] [Google Scholar]
- 11.Schiller M, Metze D, Luger TA, Grabbe S, Gunzer M. Immune response modifiers--mode of action. Exp Dermatol. 2006;15(5):331–341. doi: 10.1111/j.0906-6705.2006.00414.x. [DOI] [PubMed] [Google Scholar]
- 12.Gupta AK, Cherman AM, Tyring SK. Viral and nonviral uses of imiquimod: a review. J Cutan Med Surg. 2004;8(5):338–352. doi: 10.1007/s10227-005-0023-5. [DOI] [PubMed] [Google Scholar]
- 13.Modlin RL, Cheng G. From plankton to pathogen recognition. Nat Med. 2004;10(11):1173–1174. doi: 10.1038/nm1104-1173. [DOI] [PubMed] [Google Scholar]
- 14.O’Neill LA, Bowie AG. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat Rev Immunol. 2007;7(5):353–364. doi: 10.1038/nri2079. [DOI] [PubMed] [Google Scholar]
- 15.Liu PT, Stenger S, Tang DH, Modlin RL. Cutting edge: vitamin D-mediated human antimicrobial activity against Mycobacterium tuberculosis is dependent on the induction of cathelicidin. J Immunol. 2007;179(4):2060–2063. doi: 10.4049/jimmunol.179.4.2060. [DOI] [PubMed] [Google Scholar]
- 16.Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311(5768):1770–1773. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 17.Schauber J, Dorschner RA, Coda AB, Buchau AS, Liu PT, Kiken D, et al. Injury enhances TLR2 function and antimicrobial peptide expression through a vitamin D-dependent mechanism. J Clin Invest. 2007;117(3):803–811. doi: 10.1172/JCI30142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robert C, Kupper TS. Inflammatory skin diseases, T cells, and immune surveillance. N Engl J Med. 1999;341:1817–1828. doi: 10.1056/NEJM199912093412407. [DOI] [PubMed] [Google Scholar]
- 19.Baker BS, Ovigne JM, Powles AV, Corcoran S, Fry L. Normal keratinocytes express Toll-like receptors (TLRs) 1, 2 and 5: modulation of TLR expression in chronic plaque psoriasis. Br J Dermatol. 2003;148:670–679. doi: 10.1046/j.1365-2133.2003.05287.x. [DOI] [PubMed] [Google Scholar]
- 20.Begon E, Michel L, Flageul B, Beaudoin I, Jean-Louis F, Bachelez H, et al. Expression, subcellular localization and cytokinic modulation of Toll-like receptors (TLRs) in normal human keratinocytes: TLR2 up-regulation in psoriatic skin. Eur J Dermatol. 2007;17(6):497–506. doi: 10.1684/ejd.2007.0264. [DOI] [PubMed] [Google Scholar]
- 21.Curry JL, Qin JZ, Bonish B, Carrick R, Bacon P, Panella J, et al. Innate immune-related receptors in normal and psoriatic skin. Arch Pathol Lab Med. 2003;127:178–186. doi: 10.5858/2003-127-178-IIRRIN. [DOI] [PubMed] [Google Scholar]
- 22.Kawai K, Shimura H, Minagawa M, Ito A, Tomiyama K, Ito M. Expression of functional Toll-like receptor 2 on human epidermal keratinocytes. J Dermatol Sci. 2002;30(3):185–194. doi: 10.1016/s0923-1811(02)00105-6. [DOI] [PubMed] [Google Scholar]
- 23.Kawai K. Expression of functional toll-like receptors on cultured human epidermal keratinocytes. J Invest Dermatol. 2003;121:217–218. doi: 10.1046/j.1523-1747.2003.12305.x. [DOI] [PubMed] [Google Scholar]
- 24.Lebre MC, Antons JC, Kalinski P, Schuitemaker JH, van Capel TM, Kapsenberg ML, et al. Double-stranded RNA-exposed human keratinocytes promote Th1 responses by inducing a Type-1 polarized phenotype in dendritic cells: role of keratinocyte-derived tumor necrosis factor alpha, type I interferons, and interleukin-18. J Invest Dermatol. 2003;120(6):990–997. doi: 10.1046/j.1523-1747.2003.12245.x. [DOI] [PubMed] [Google Scholar]
- 25.Lebre MC, van der Aar AM, van BL, van Capel TM, Schuitemaker JH, Kapsenberg ML, et al. Human Keratinocytes Express Functional Toll-Like Receptor 3, 4, 5, and 9. J Invest Dermatol. 2006 doi: 10.1038/sj.jid.5700530. [DOI] [PubMed] [Google Scholar]
- 26.Mempel M, Voelcker V, Kollisch G, Plank C, Rad R, Gerhard M, et al. Toll-like receptor expression in human keratinocytes: nuclear factor kappaB controlled gene activation by Staphylococcus aureus is toll-like receptor 2 but not toll-like receptor 4 or platelet activating factor receptor dependent. J Invest Dermatol. 2003;121(6):1389–1396. doi: 10.1111/j.1523-1747.2003.12630.x. [DOI] [PubMed] [Google Scholar]
- 27.Miller LS, Sorensen OE, Liu PT, Jalian HR, Eshtiaghpour D, Behmanesh BE, et al. TGF-alpha regulates TLR expression and function on epidermal keratinocytes. J Immunol. 2005;174(10):6137–6143. doi: 10.4049/jimmunol.174.10.6137. [DOI] [PubMed] [Google Scholar]
- 28.Pivarcsi A, Bodai L, Rethi B, Kenderessy-Szabo A, Koreck A, Szell M, et al. Expression and function of Toll-like receptors 2 and 4 in human keratinocytes. Int Immunol. 2003;15(6):721–730. doi: 10.1093/intimm/dxg068. [DOI] [PubMed] [Google Scholar]
- 29.Pivarcsi A, Koreck A, Bodai L, Szell M, Szeg C, Belso N, et al. Differentiation-regulated expression of Toll-like receptors 2 and 4 in HaCaT keratinocytes. Arch Dermatol Res. 2004;296:120–124. doi: 10.1007/s00403-004-0475-2. [DOI] [PubMed] [Google Scholar]
- 30.Prens EP, Kant M, van DG, van der Wel LI, Mourits S, van der FL. IFN-alpha Enhances Poly-IC Responses in Human Keratinocytes by Inducing Expression of Cytosolic Innate RNA Receptors: Relevance for Psoriasis. J Invest Dermatol. 2007 doi: 10.1038/sj.jid.5701087. [DOI] [PubMed] [Google Scholar]
- 31.Song PI, Park YM, Abraham T, Harten B, Zivony A, Neparidze N, et al. Human keratinocytes express functional CD14 and toll-like receptor 4. J Invest Dermatol. 2002;119(2):424–432. doi: 10.1046/j.1523-1747.2002.01847.x. [DOI] [PubMed] [Google Scholar]
- 32.Kollisch G, Kalali BN, Voelcker V, Wallich R, Behrendt H, Ring J, et al. Various members of the Toll-like receptor family contribute to the innate immune response of human epidermal keratinocytes. Immunology. 2005;114(4):531–541. doi: 10.1111/j.1365-2567.2005.02122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miller LS, Modlin RL. Human keratinocyte Toll-like receptors promote distinct immune responses. J Invest Dermatol. 2007;127(2):262–263. doi: 10.1038/sj.jid.5700559. [DOI] [PubMed] [Google Scholar]
- 34.Fujita H, Asahina A, Mitsui H, Tamaki K. Langerhans cells exhibit low responsiveness to double-stranded RNA. Biochem Biophys Res Commun. 2004;319(3):832–839. doi: 10.1016/j.bbrc.2004.05.059. [DOI] [PubMed] [Google Scholar]
- 35.Takeuchi J, Watari E, Shinya E, Norose Y, Matsumoto M, Seya T, et al. Down-regulation of Toll-like receptor expression in monocyte-derived Langerhans cell-like cells: implications of low-responsiveness to bacterial components in the epidermal Langerhans cells. Biochem Biophys Res Commun. 2003;306(3):674–679. doi: 10.1016/s0006-291x(03)01022-2. [DOI] [PubMed] [Google Scholar]
- 36.Burns RP, Jr, Ferbel B, Tomai M, Miller R, Gaspari AA. The imidazoquinolines, imiquimod and R-848, induce functional, but not phenotypic, maturation of human epidermal Langerhans’ cells. Clin Immunol. 2000;94(1):13–23. doi: 10.1006/clim.1999.4804. [DOI] [PubMed] [Google Scholar]
- 37.Gatti E, Velleca MA, Biedermann BC, Ma W, Unternaehrer J, Ebersold MW, et al. Large-scale culture and selective maturation of human Langerhans cells from granulocyte colony-stimulating factor-mobilized CD34+ progenitors. J Immunol. 2000;164(7):3600–3607. doi: 10.4049/jimmunol.164.7.3600. [DOI] [PubMed] [Google Scholar]
- 38.van der Aar AM, Sylva-Steenland RM, Bos JD, Kapsenberg ML, de Jong EC, Teunissen MB. Loss of TLR2, TLR4, and TLR5 on Langerhans cells abolishes bacterial recognition. J Immunol. 2007;178(4):1986–1990. doi: 10.4049/jimmunol.178.4.1986. [DOI] [PubMed] [Google Scholar]
- 39.Sugita K, Kabashima K, Atarashi K, Shimauchi T, Kobayashi M, Tokura Y. Innate immunity mediated by epidermal keratinocytes promotes acquired immunity involving Langerhans cells and T cells in the skin. Clin Exp Immunol. 2007;147(1):176–183. doi: 10.1111/j.1365-2249.2006.03258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Renn CN, Sanchez DJ, Ochoa MT, Legaspi AJ, Oh CK, Liu PT, et al. TLR activation of Langerhans cell-like dendritic cells triggers an antiviral immune response. J Immunol. 2006;177(1):298–305. doi: 10.4049/jimmunol.177.1.298. [DOI] [PubMed] [Google Scholar]
- 41.Flacher V, Bouschbacher M, Verronese E, Massacrier C, Sisirak V, Berthier-Vergnes O, et al. Human Langerhans cells express a specific TLR profile and differentially respond to viruses and Gram-positive bacteria. J Immunol. 2006;177(11):7959–7967. doi: 10.4049/jimmunol.177.11.7959. [DOI] [PubMed] [Google Scholar]
- 42.Nomura I, Goleva E, Howell MD, Hamid QA, Ong PY, Hall CF, et al. Cytokine milieu of atopic dermatitis, as compared to psoriasis, skin prevents induction of innate immune response genes. J Immunol. 2003;171:3262–3269. doi: 10.4049/jimmunol.171.6.3262. [DOI] [PubMed] [Google Scholar]
- 43.Ong PY, Ohtake T, Brandt C, Strickland I, Boguniewicz M, Ganz T, et al. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N Engl J Med. 2002;347:1151–1160. doi: 10.1056/NEJMoa021481. [DOI] [PubMed] [Google Scholar]
- 44.Howell MD, Gallo RL, Boguniewicz M, Jones JF, Wong C, Streib JE, et al. Cytokine milieu of atopic dermatitis skin subverts the innate immune response to vaccinia virus. Immunity. 2006;24(3):341–348. doi: 10.1016/j.immuni.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 45.Rieg S, Steffen H, Seeber S, Humeny A, Kalbacher H, Dietz K, et al. Deficiency of dermcidin-derived antimicrobial peptides in sweat of patients with atopic dermatitis correlates with an impaired innate defense of human skin in vivo. J Immunol. 2005;174(12):8003–8010. doi: 10.4049/jimmunol.174.12.8003. [DOI] [PubMed] [Google Scholar]
- 46.Lorenz E, Mira JP, Cornish KL, Arbour NC, Schwartz DA. A novel polymorphism in the toll-like receptor 2 gene and its potential association with staphylococcal infection. Infect Immun. 2000;68(11):6398–6401. doi: 10.1128/iai.68.11.6398-6401.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ahmad-Nejad P, Mrabet-Dahbi S, Breuer K, Klotz M, Werfel T, Herz U, et al. The toll-like receptor 2 R753Q polymorphism defines a subgroup of patients with atopic dermatitis having severe phenotype. J Allergy Clin Immunol. 2004;113(3):565–567. doi: 10.1016/j.jaci.2003.12.583. [DOI] [PubMed] [Google Scholar]
- 48.Weidinger S, Novak N, Klopp N, Baurecht H, Wagenpfeil S, Rummler L, et al. Lack of association between Toll-like receptor 2 and Toll-like receptor 4 polymorphisms and atopic eczema. J Allergy Clin Immunol. 2006;118(1):277–279. doi: 10.1016/j.jaci.2006.04.034. [DOI] [PubMed] [Google Scholar]
- 49.Novak N, Yu CF, Bussmann C, Maintz L, Peng WM, Hart J, et al. Putative association of a TLR9 promoter polymorphism with atopic eczema. Allergy. 2007;62(7):766–772. doi: 10.1111/j.1398-9995.2007.01358.x. [DOI] [PubMed] [Google Scholar]
- 50.Schimming TT, Parwez Q, Petrasch-Parwez E, Nothnagel M, Epplen JT, Hoffjan S. Association of toll-interacting protein gene polymorphisms with atopic dermatitis. BMC Dermatol. 2007;7:3. doi: 10.1186/1471-5945-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hasannejad H, Takahashi R, Kimishima M, Hayakawa K, Shiohara T. Selective impairment of Toll-like receptor 2-mediated proinflammatory cytokine production by monocytes from patients with atopic dermatitis. J Allergy Clin Immunol. 2007;120(1):69–75. doi: 10.1016/j.jaci.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 52.van Beelen AJ, Teunissen MB, Kapsenberg ML, de Jong EC. Interleukin-17 in inflammatory skin disorders. Curr Opin Allergy Clin Immunol. 2007;7(5):374–381. doi: 10.1097/ACI.0b013e3282ef869e. [DOI] [PubMed] [Google Scholar]
- 53.Henseler T, Christophers E. Disease concomitance in psoriasis. J Am Acad Dermatol. 1995;32:982–986. doi: 10.1016/0190-9622(95)91336-x. [DOI] [PubMed] [Google Scholar]
- 54.Harder J, Bartels J, Christophers E, Schroder JM. Isolation and characterization of human beta -defensin-3, a novel human inducible peptide antibiotic. J Biol Chem. 2001;276:5707–5713. doi: 10.1074/jbc.M008557200. [DOI] [PubMed] [Google Scholar]
- 55.Harder J, Bartels J, Christophers E, Schroder JM. A peptide antibiotic from human skin. Nature. 1997;387:861. doi: 10.1038/43088. [DOI] [PubMed] [Google Scholar]
- 56.Seung NR, Park EJ, Kim CW, Kim KH, Kim KJ, Cho HJ, et al. Comparison of expression of heat-shock protein 60, Toll-like receptors 2 and 4, and T-cell receptor gammadelta in plaque and guttate psoriasis. J Cutan Pathol. 2007;34(12):903–911. doi: 10.1111/j.1600-0560.2007.00756.x. [DOI] [PubMed] [Google Scholar]
- 57.Fitzgerald KA, O’Neill LA. The role of the interleukin-1/Toll-like receptor superfamily in inflammation and host defence. Microbes Infect. 2000;2(8):933–943. doi: 10.1016/s1286-4579(00)00396-8. [DOI] [PubMed] [Google Scholar]
- 58.Lande R, Gregorio J, Facchinetti V, Chatterjee B, Wang YH, Homey B, et al. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature. 2007;449(7162):564–569. doi: 10.1038/nature06116. [DOI] [PubMed] [Google Scholar]
- 59.Kim J, Ochoa MT, Krutzik SR, Takeuchi O, Uematsu S, Legaspi AJ, et al. Activation of toll-like receptor 2 in acne triggers inflammatory cytokine responses. J Immunol. 2002;169(3):1535–1541. doi: 10.4049/jimmunol.169.3.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu PT, Krutzik SR, Kim J, Modlin RL. Cutting edge: all-trans retinoic acid down-regulates TLR2 expression and function. J Immunol. 2005;174(5):2467–2470. doi: 10.4049/jimmunol.174.5.2467. [DOI] [PubMed] [Google Scholar]
- 61.Tenaud I, Khammari A, Dreno B. In vitro modulation of TLR-2, CD1d and IL-10 by adapalene on normal human skin and acne inflammatory lesions. Exp Dermatol. 2007;16(6):500–506. doi: 10.1111/j.1600-0625.2007.00552.x. [DOI] [PubMed] [Google Scholar]
- 62.Takeuchi O, Hoshino K, Kawai T, Sanjo H, Takada H, Ogawa T, et al. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity. 1999;11(4):443–451. doi: 10.1016/s1074-7613(00)80119-3. [DOI] [PubMed] [Google Scholar]
- 63.Schroder NW, Morath S, Alexander C, Hamann L, Hartung T, Zahringer U, et al. Lipoteichoic acid (LTA) of Streptococcus pneumoniae and Staphylococcus aureus activates immune cells via Toll-like receptor (TLR)-2, lipopolysaccharide-binding protein (LBP), and CD14, whereas TLR-4 and MD-2 are not involved. J Biol Chem. 2003;278(18):15587–15594. doi: 10.1074/jbc.M212829200. [DOI] [PubMed] [Google Scholar]
- 64.Hashimoto M, Tawaratsumida K, Kariya H, Kiyohara A, Suda Y, Krikae F, et al. Not lipoteichoic acid but lipoproteins appear to be the dominant immunobiologically active compounds in Staphylococcus aureus. J Immunol. 2006;177(5):3162–3169. doi: 10.4049/jimmunol.177.5.3162. [DOI] [PubMed] [Google Scholar]
- 65.Hoebe K, Georgel P, Rutschmann S, Du X, Mudd S, Crozat K, et al. CD36 is a sensor of diacylglycerides. Nature. 2005;433(7025):523–527. doi: 10.1038/nature03253. [DOI] [PubMed] [Google Scholar]
- 66.Menzies BE, Kenoyer A. Signal transduction and nuclear responses in Staphylococcus aureus-induced expression of human beta-defensin 3 in skin keratinocytes. Infect Immun. 2006;74(12):6847–6854. doi: 10.1128/IAI.00389-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Miller LS, O’Connell RM, Gutierrez MA, Pietras EM, Shahangian A, Gross CE, et al. MyD88 mediates neutrophil recruitment initiated by IL-1R but not TLR2 activation in immunity against Staphylococcus aureus. Immunity. 2006;24(1):79–91. doi: 10.1016/j.immuni.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 68.Walker SL, Lockwood DN. Leprosy. Clin Dermatol. 2007;25(2):165–172. doi: 10.1016/j.clindermatol.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 69.Krutzik SR, Ochoa MT, Sieling PA, Uematsu S, Ng YW, Legaspi A, et al. Activation and regulation of Toll-like receptors 2 and 1 in human leprosy. Nat Med. 2003;9:525–532. doi: 10.1038/nm864. [DOI] [PubMed] [Google Scholar]
- 70.Oliveira RB, Ochoa MT, Sieling PA, Rea TH, Rambukkana A, Sarno EN, et al. Expression of Toll-like receptor 2 on human Schwann cells: a mechanism of nerve damage in leprosy. Infect Immun. 2003;71(3):1427–1433. doi: 10.1128/IAI.71.3.1427-1433.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bochud PY, Hawn TR, Aderem A. Cutting edge: a Toll-like receptor 2 polymorphism that is associated with lepromatous leprosy is unable to mediate mycobacterial signaling. J Immunol. 2003;170(7):3451–3454. doi: 10.4049/jimmunol.170.7.3451. [DOI] [PubMed] [Google Scholar]
- 72.Kang TJ, Lee SB, Chae GT. A polymorphism in the toll-like receptor 2 is associated with IL-12 production from monocyte in lepromatous leprosy. Cytokine. 2002;20(2):56–62. doi: 10.1006/cyto.2002.1982. [DOI] [PubMed] [Google Scholar]
- 73.Kang TJ, Chae GT. Detection of Toll-like receptor 2 (TLR2) mutation in the lepromatous leprosy patients. FEMS Immunol Med Microbiol. 2001;31(1):53–58. doi: 10.1111/j.1574-695X.2001.tb01586.x. [DOI] [PubMed] [Google Scholar]
- 74.Netea MG, Brown GD, Kullberg BJ, Gow NA. An integrated model of the recognition of Candida albicans by the innate immune system. Nat Rev Microbiol. 2008;6(1):67–78. doi: 10.1038/nrmicro1815. [DOI] [PubMed] [Google Scholar]
- 75.Jouault T, Ibata-Ombetta S, Takeuchi O, Trinel PA, Sacchetti P, Lefebvre P, et al. Candida albicans phospholipomannan is sensed through toll-like receptors. J Infect Dis. 2003;188(1):165–172. doi: 10.1086/375784. [DOI] [PubMed] [Google Scholar]
- 76.Netea MG, Gow NA, Munro CA, Bates S, Collins C, Ferwerda G, et al. Immune sensing of Candida albicans requires cooperative recognition of mannans and glucans by lectin and Toll-like receptors. J Clin Invest. 2006;116(6):1642–1650. doi: 10.1172/JCI27114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Barton GM. Viral recognition by Toll-like receptors. Semin Immunol. 2007;19(1):33–40. doi: 10.1016/j.smim.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 78.Wang JP, Kurt-Jones EA, Shin OS, Manchak MD, Levin MJ, Finberg RW. Varicella-zoster virus activates inflammatory cytokines in human monocytes and macrophages via Toll-like receptor 2. J Virol. 2005;79(20):12658–12666. doi: 10.1128/JVI.79.20.12658-12666.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bochud PY, Magaret AS, Koelle DM, Aderem A, Wald A. Polymorphisms in TLR2 are associated with increased viral shedding and lesional rate in patients with genital herpes simplex virus Type 2 infection. J Infect Dis. 2007;196(4):505–509. doi: 10.1086/519693. [DOI] [PubMed] [Google Scholar]
- 80.Zhang SY, Jouanguy E, Ugolini S, Smahi A, Elain G, Romero P, et al. TLR3 deficiency in patients with herpes simplex encephalitis. Science. 2007;317(5844):1522–1527. doi: 10.1126/science.1139522. [DOI] [PubMed] [Google Scholar]
- 81.Sato A, Linehan MM, Iwasaki A. Dual recognition of herpes simplex viruses by TLR2 and TLR9 in dendritic cells. Proc Natl Acad Sci U S A. 2006;103(46):17343–17348. doi: 10.1073/pnas.0605102103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Aravalli RN, Hu S, Rowen TN, Palmquist JM, Lokensgard JR. Cutting edge: TLR2-mediated proinflammatory cytokine and chemokine production by microglial cells in response to herpes simplex virus. J Immunol. 2005;175(7):4189–4193. doi: 10.4049/jimmunol.175.7.4189. [DOI] [PubMed] [Google Scholar]
- 83.Lund JM, Linehan MM, Iijima N, Iwasaki A. Cutting Edge: Plasmacytoid dendritic cells provide innate immune protection against mucosal viral infection in situ. J Immunol. 2006;177(11):7510–7514. doi: 10.4049/jimmunol.177.11.7510. [DOI] [PubMed] [Google Scholar]
- 84.Kurt-Jones EA, Chan M, Zhou S, Wang J, Reed G, Bronson R, et al. Herpes simplex virus 1 interaction with Toll-like receptor 2 contributes to lethal encephalitis. Proc Natl Acad Sci U S A. 2004;101(5):1315–1320. doi: 10.1073/pnas.0308057100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hemmi H, Kaisho T, Takeuchi O, Sato S, Sanjo H, Hoshino K, et al. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat Immunol. 2002;3(2):196–200. doi: 10.1038/ni758. [DOI] [PubMed] [Google Scholar]
- 86.Schon MP, Schon M. Imiquimod: mode of action. Br J Dermatol. 2007;157(Suppl 2):8–13. doi: 10.1111/j.1365-2133.2007.08265.x. [DOI] [PubMed] [Google Scholar]
- 87.Krieg AM. Development of TLR9 agonists for cancer therapy. J Clin Invest. 2007;117(5):1184–1194. doi: 10.1172/JCI31414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pashenkov M, Goess G, Wagner C, Hormann M, Jandl T, Moser A, et al. Phase II trial of a toll-like receptor 9-activating oligonucleotide in patients with metastatic melanoma. J Clin Oncol. 2006;24(36):5716–5724. doi: 10.1200/JCO.2006.07.9129. [DOI] [PubMed] [Google Scholar]
- 89.Speiser DE, Lienard D, Rufer N, Rubio-Godoy V, Rimoldi D, Lejeune F, et al. Rapid and strong human CD8+ T cell responses to vaccination with peptide, IFA, and CpG oligodeoxynucleotide 7909. J Clin Invest. 2005;115(3):739–746. doi: 10.1172/JCI23373. [DOI] [PMC free article] [PubMed] [Google Scholar]