Abstract
Introduction
Fibrinolytics such as recombinant tissue plasminogen activator (rt-PA) are used to treat thrombotic disease such as acute myocardial infarction (AMI) and ischemic stroke. Interest in increasing efficacy and reducing side effects has led to the study of adjuncts such as GP IIb-IIIa inhibitors and ultrasound (US) enhanced thrombolysis. Currently, GP IIb-IIIa inhibitor and fibrinolytic treatment are often used in AMI, and are under investigation for stroke treatment. However, little is known of the efficacy of combined GP IIb-IIIa inhibitor, fibrinolytic and ultrasound treatment. We measure the lytic efficacy of rt-PA, eptifibatide (Epf) and 120 kHz ultrasound treatment in an in-vitro human clot model.
Materials and Methods
Blood was drawn from 15 subjects after IRB approval. Clots were made in 20 μL pipettes, and placed in a water tank for microscopic visualization during lytic treatment. Clots were exposed to control, rt-PA (rt-PA), eptifibatide (Epf), or rt-PA+eptifibatide (rt-PA+Epf), with or without ultrasound for 30 minutes at 37°C in human plasma. Clot lysis was measured over time, using a microscopic imaging technique. The fractional clot loss (FCL) and initial lytic rate (LR) were used to quantify lytic efficacy.
Results and Conclusions
LR values for (−US) treated clots were 0.8±0.1(control), 1.8±0.3 (Epf), 1.5±0.2 (rt-PA), and 1.3±0.4 (rt-PA+Epf) (% clot width/minute) respectively. In comparison, the (+US) group exhibited LR values of 1.6±0.2 (control), 4.3±0.4 (Epf), 6.3±0.4 (rt-PA), and 4.6±0.6 (rt-PA+Epf). For (−US) treated clots, FCL was 6.0±0.8 (control), 9.2±2.5 (Epf), 15.6±1.7 (rt-PA), and 28.0±2.2% (rt-PA+Epf) respectively. FCL for (+US) clots was 13.5±2.4 (control), 20.7±6.4 (Epf), 44.4±3.6 (rt-PA) and 30.3±3.6% (rt-PA+Epf) respectively. Although the addition of eptifibatide enhances the in-vitro lytic efficacy of rt-PA in the absence of ultrasound, the efficacy of ultrasound and rt-PA is greater than that of combined ultrasound, rt-PA and eptifibatide exposure.
Keywords: ultrasound, recombinant tissue plasminogen activator, eptifibatide, thrombosis
Introduction
Lytic therapy utilizing recombinant tissue plasminogen activator (rt-PA) has been widely used to treat various thrombotic diseases such as myocardial infarction [1], acute ischemic stroke [2], and peripheral vascular occlusions [3, 4]. The desire to improve the lytic efficacy and reduce the bleeding complication of rt-PA thrombolysis has led to substantial interest in potential adjunctive therapies such as ultrasound enhanced thrombolysis (UET) [5–8] and medications such as GP IIb/IIIa inhibitors [9, 10]. However, there is little data on the lytic efficacy of combining such adjunctive therapies.
The ultrasonic enhancement of thrombolytic medications has been under investigation for some time [11, 12]. Suggested mechanisms have included thermal effects [11], microstreaming [13], and cavitation [14, 15]. Cavitation is the creation of small microbubbles by the ultrasound acoustic field. There are two types of cavitation, inertial and stable. In inertial cavitation, the bubbles are created and destroyed very quickly; typically over the time scale of a single ultrasound pulse. Large amounts of energy can be deposited over local length scales by inertial cavitation, and this is the phenomenon responsible for the efficacy of lithotripsy in the treatment of kidney stones, as an example. Recently Datta et al [15] demonstrated a correlation between the presence of stable cavitation and UET in an in-vitro porcine clot. Similar results were obtained by Prokop et al [16]. These results suggest that stable cavitation is the mechanism likely responsible for UET.
The ideal acoustic parameters for UET are unknown at this time. For some applications such as UET treatment of stroke, higher frequencies (~MHz) may be problematic. Approximately 10% of the population exhibits temporal window insufficiency thus preventing transcranial Doppler ultrasound penetration of the skull [17, 18]. Lower ultrasound frequencies (~kHz) have been demonstrated to penetrate the skull and chest wall [19, 20] with less attenuation than at higher frequencies.
GP IIb-IIIa inhibitors are antagonists of platelet GP IIb-IIIa surface receptors, resulting in the inhibition of platelet aggregation and fibrinogen cross-linking. These drugs are used to facilitate intervention in acute coronary syndromes, and to prevent vessel re-occlusion [21]. GP IIb-IIIa inhibitors such as eptifibatide (Epf) and abciximab have been shown to increase arterial recanalization rates when combined with fibrinolytics in patients with myocardial infarction [10, 22]. Currently, ongoing clinical trials such as CLEAR (P50 NS4 4283-01) and ROSIE-2 (NCT00039832) are investigating the efficacy of combining eptifibatide with rt-PA in acute ischemic stroke treatment. However, the lytic efficacy of this treatment regime combined with ultrasound has not been quantified.
The objective of this study was to determine the thrombolytic efficacy of combined rt-PA, eptifibatide and ultrasound treatment in a well-defined in-vitro human clot model. This model uses a novel microscopic imaging technique that allows the quantification of lytic efficacy, and comparison between various treatment regimens. Such data will be useful in planning further in-vitro, in-vivo and clinical trials of such combination therapy.
Methods and Methods
Preparation of rt-PA, Epf, and human plasma
The rt-PA was obtained from the manufacturer (rt-PA, Activase®, Genentech, San Francisco, CA) as a lyophilized powder. Each vial was mixed with sterile water to a concentration of 1 mg/ml as per manufacturer’s instructions, aliquoted into 1.0 ml centrifuge tubes (Fisher Scientific), and stored at −80°C. The enzymatic activity of rt-PA is stable for at least 1 year when stored in this fashion [23]. Eptifibatide (Epf) was obtained (Integrilin®, Millennium Pharmaceuticals, Inc., Cambridge, MA) as a solution at a concentration of 2 mg/ml. The drug was stored at 4–5°C to prevent degradation. Human fresh-frozen plasma (hFFP) was procured from a blood bank in 250–300 ml units. Each unit was briefly thawed, aliquoted into 50 ml centrifuge tubes (Fisher Scientific), and stored at −80°C. Aliquots of rt-PA and plasma were allowed to thaw for experiments, and the remaining amounts discarded following completion of each experiment.
Production of blood clots
Human whole blood was drawn from fifteen volunteers by sterile venipuncture following local Institutional Review Board approval and written informed consent. Samples of 1–2 ml were placed in sterile glass tubes (Vacutainer) and allowed to form clots in and around a small diameter (~600 μm) micropipette (Becton, Dickinson and Company, Franklin Lakes, NJ; 20λ) through which a segment of 7-0 silk suture (Ethicon Industries, Cornelia, GA) had been threaded. The suture diameter ranges from 50 to 69 μm, as per the manufacturer. This is similar to clot production methods used in imaging studies by Winter and Yu [24, 25]. The clots were incubated for three hours at 37°C, and refrigerated at 4–5°C for 3 days ensuring maximal clot retraction, lytic resistance and stability [26–28]. Platelet aggregation is preserved in platelets stored at this temperature for up to 14 days [29]. Before each experiment, the micropipette was removed to produce a cylindrical clot adherent to the suture. The clot was typically 5–8 μl in volume on the order of 300 μm in width (see Figure 1). For all clots used in the work here, the average initial clot diameter was 245 ± 35 μm (N=108 clots). At this size the clots were similar in diameter to the intracerebral segments of the middle cerebral arteries (80 to 840 μm in diameter) or other cerebral vessels such as the recurrent artery of Heubner and its perforators (643 ± 237 μm in diameter) [30, 31]. These small clots were used in this work as it enables measurement of changes in clot size on the order of ~10 μm, which is a typical length scale for in-vitro clot lysis measurements as determined by others [32, 33].
Figure 1.
Picture of apparatus. The inverting microscope is in the center of the image, and the ultrasound transducer is to the left. Note that the apparatus is on a vibration isolation table.
Apparatus
For each experiment, the clot attached to the suture was placed in a clean micropipette (Drummond Scientific Company, Broomall, PA), and inserted into a U-shaped sample holder composed of hollow luer lock connectors and silicone tubing (Cole Parmer, Vernon Hills, IL; outer diameter 0.125″). The sample holder was placed in an acrylic water tank with a microscope slide at the bottom. Water in the tank was maintained at a temperature of 37±1°C during all experiments using two heating elements (Hagen A721, Mansfield, MA; 25 W). The tank was placed over the objective of an inverting microscope (Olympus, Melville, NY) to visualize the clot. The field of view in the image was 340 μm × 260 μm (640 pixels × 480 pixels). The entire apparatus was placed on top of a vibration isolation table (Newport, Irvine, CA; XL-G) for mechanical isolation. Images were recorded at 6 frames/minute using a CCD camera (Hitachi, Woodbury, NY; KP-M1A), and data was stored for later analysis on a computer (Dell, Round Rock, TX; Intel Pentium). A complete description of the imaging apparatus has been previously provided [8, 34].
Ultrasound Treatment
The 120 kHz ultrasound unfocused transducer (Sonic Concepts, Inc., Woodburn, WA) was mounted at one end of the tank at a 30° angle to the tank bottom thus allowing ultrasound exposure of the sample clot. Sound absorbing material (rho-c rubber) was placed at the end of the tank opposite the transducer to inhibit acoustic standing wave formation. The transducer was previously calibrated with a PVDF hydrophone (Reson, Goleta, CA; TC4038), and the −6 dB beam width was measured to be 1.0 cm with a focal length of 2.4 cm. The 120 kHz ultrasound exposures utilized a pressure amplitude of 0.18 MPa, a pulse repetition frequency (PRF) of 1667 Hz, and a duty cycle of 80%. These ultrasound parameters were chosen as substantial clot lysis was observed at these settings in a previous work [8]. In addition, the pressure amplitude is comparable to the stable cavitation threshold value found by Datta et al [15], and is well below the 0.4 MPa threshold value measured by Hynynen et al [35] in a murine model as resulting in blood-brain barrier disruption.
Experimental protocol
Clots were exposed to one of four treatment regimens, with (+US) or without (−US) 120 kHz ultrasound: (1) hFFP alone (control); (2) rt-PA in hFFP (rt-PA) (3) eptifibatide in hFFP (Epf) and (4) eptifibatide and rt-PA in hFFP (rt-PA+Epf). The rt-PA concentration was 3.15 μg/ml for all exposures; this value is well-within the therapeutic concentration range in humans [36–39] The eptifibatide concentration was 2.31 μg/ml which comparable to those used in the CLEAR trial [10] and in the treatment of acute coronary syndromes [40, 41].
Individual trials began by slowly injecting 1 ml of hFFP (control), or 1 ml of hFFP containing rt-PA, Epf, or both (all other trials) into the sample holder. At time t equal to zero, the solution was in contact with the clot. Removing the syringe exposed the ends of the sample holder to atmospheric pressure, and the clot surface to a static fluid column. Clots were exposed to a specific treatment regimen for 30 minutes; previous studies have shown that the majority of thrombolysis occurs within a 30 minute window [8]. Each regimen used n≥6 clots, from at least two donors.
Determination of lytic efficacy
Light intensity transmitted through the sample clot is reduced with increased clot thickness or clot density. The CCD camera records image light intensity I(x,z) at each pixel (x,z). By analyzing the light intensity in each pixel, the clot edges can be identified, thus enabling measurement of clot width.
An image from the CCD camera was stored on a desktop computer for each frame as a function of time. The average clot width (CW) was then calculated, using a computer program written in Matlab 6.5 R13 (Mathworks, Inc., Natwick, MA). First, the spatial gradient of the light intensities ∂I(x,z)/∂x was calculated for each row (fixed z) of pixels. The positions of the two clot-plasma interfaces were determined via an edge-detection routine that finds the values of x = (x1, x2) for each z such that
| (1) |
where Γ is a constant. A Γ of 2.5 was previously found to be sufficient to detect well-defined clot edges [8, 34]. The width W(z) of the clot at each z was then
| (2) |
The average clot width for each image was calculated by averaging the width over all z values. The clot width values for each trial were normalized using the average clot width during the first minute ( , six frames);
| (3) |
The normalized clot width CWNORM values as a function of time were then averaged for each treatment group. The normalized average clot width at 30 minutes was then used for comparing the effects of thrombolysis between treatment regimens. This analysis is similar to that of Meunier et al [8].
It should be noted that the finite silk suture diameter (50–69 μm) contributes to the measured clot width, and the average fractional contribution (average initial clot width 243 μm) is approximately 0.25. For example, if the final clot width in a given experiment is 0.3, very little clot actually remains. Therefore, the above measurement technique will underestimate the actual amount of clot lysis resulting from a given lytic treatment.
Statistical analysis
The effects of the treatment protocol on lytic rate and lytic efficacy at 30 minutes were evaluated using a mixed-model analysis of variance. An estimate of the fixed effects of rt-PA, eptifibatide, and ultrasound was determined and the covariance structure was modeled arising from the within-subject design. Data are presented as mean values with standard errors at each point in time. Parameter estimates and 95% confidence limits of the estimates are used to report the effects of ultrasound, eptifibatide, and rt-PA and their combinations. These calculations were performed using SAS v8.02 (SAS Institute, Cary, NC) and a p value less than 0.05 was considered significant.
Results
Figure 1 shows a photograph of the imaging apparatus. The inverting microscope, transducer and water tank are all mounted on a vibration isolation table. The driving electronics and computer are to the left of this image (not shown). A complete description of the apparatus is discussed in the work of Meunier et al [8].
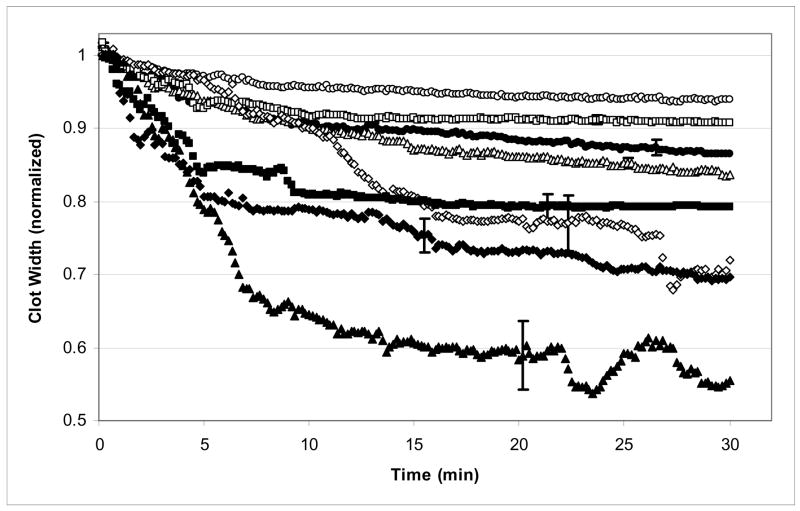
Figure 2 shows the normalized average clot width versus time for the tested treatment protocols. It is interesting to note that the largest reduction in clot width at 30 minutes occurs for clots treated with rt-PA and 120 kHz ultrasound rt-PA (+US). Most of the other treatment groups exhibit clot lysis that ranges between that of the control (−US) group and these two groups.
Figure 2.
Normalized average clot width as a function of time for clots exposed to rt-PA, eptifibatide, and ultrasound. Normalized average clot width is shown for control (−US ○, +US ●), rt-PA (−US △, +US ▲), Epf (−US □, +US ■), and rt-PA+Epf (−US ◇, +US ◆) treatment groups. Overall, there is an initial rapid decline in clot width, followed by a more gradual decline. Vertical bars are representative standard errors for the data. Note that the greatest clot lysis occurs for the rt-PA(+US) group.
The normalized average clot width as a function of time t was found to be well-described by the empirical 2-parameter expression;
| (4) |
for all treatment groups (R2≥0.93). Here B is a parameter of the fit (dimensionless), and k is a rate constant (min−1). Following the treatment of Meunier et al [8], equation (4) can be approximated as
| (5) |
for small values of time t. Note that this expression is linear in time, thus allowing one to define the initial lytic rate LR as
| (6) |
This parameter can then be calculated from the obtained values for B and k, and used to compare the speed of clot lysis for early time t between the various treatment groups.
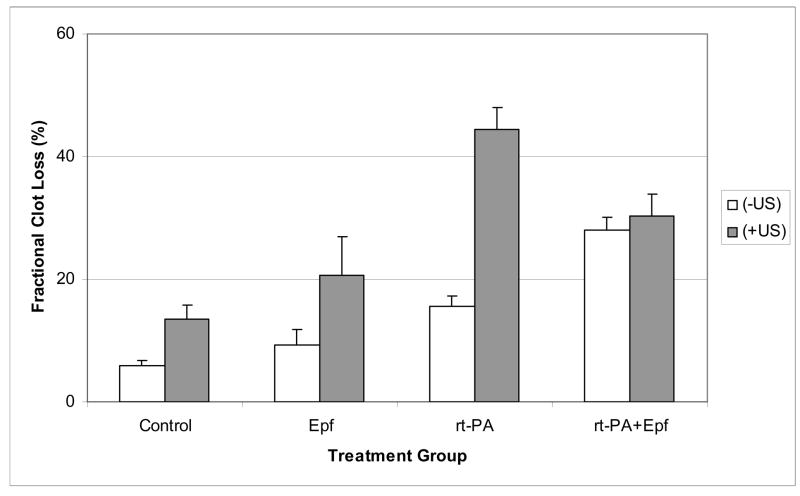
An additional parameter that is useful to compare between treatment groups is the total decrease of the normalized average clot width following thirty minutes of treatment. This quantity is denoted the as the fractional clot loss FCL, and is shown in Figure 3 for each treatment group. For the (−US) treated clots, the FCL values were 6% (95% Confidence Limits: 4.4–7.6%), 9.2% (4.2–14.3%), 15.6% (12.2–19%) and 28.8% (23.7–32.4%) for control, Epf, rt-PA and rt-PA+Epf treated clots respectively. For the (+US) group, the values were 13.5 % (8.8–18.2%), 20.7% (8.0–33.5%), 44.4% (37.3–51.5%) and 30.3% (23.1–37.5%) for control, Epf, rt-PA and rt-PA+Epf treated clots respectively. Overall, the greatest degree of clot lysis in the (−US) clots was the combination rt-PA and eptifibatide treated group. The average FCL for this group was significantly greater than those treated with rt-PA alone. Interestingly, the largest FCL in (+US) treated clots was exhibited in the rt-PA group, and is larger than that achieved in the combined rt-PA+Epf treated clots. Note that the confidence interval for the rt-PA(+US) group barely overlaps that of the rt-PA+Epf (+US) group.
Figure 3.
Percent decrease in normalized average clot width for each treatment group after 30 minutes of lytic exposure. Note that the addition of 120 kHz ultrasound significantly increases clot lysis for control, Epf, and rt-PA treated clots compared with drug treatment alone. The error bars are the standard errors for each treatment group.
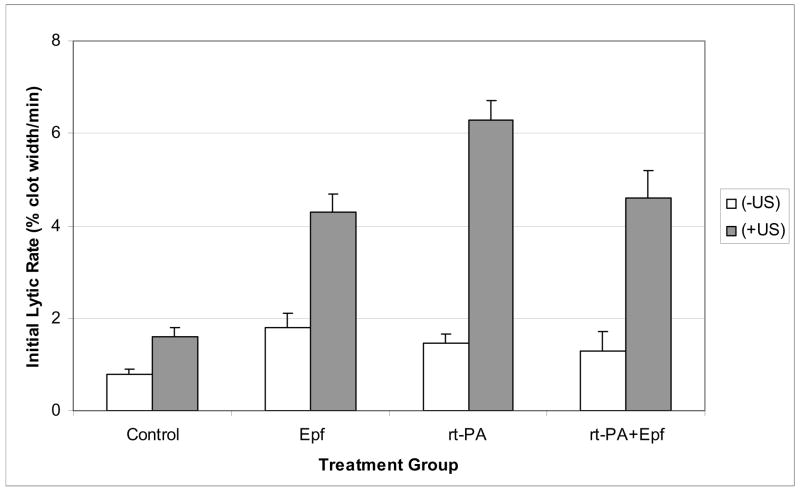
Figure 4 exhibits values for the initial lytic rate for each treatment group. Note that the LR values for the (−US) clots are similar in magnitude. These values were 0.8 (0.6–1), 1.8 (1.2–2.4), 1.5 (1.1–1.8), and 1.3 (0.5–2.1) %/min for control, Epf, rt-PA and rt-PA+Epf treated clots respectively. Ultrasound exposure significantly increased the lytic rate for all treatment groups. The LR values for (+US) treated clots were 1.6 (1.2–2), 4.3 (3.5–5.1), 6.3(5.5–7.1), and 4.6 (3.4–5.8) % clot width/min for control, Epf, rt-PA, and rt-PA+Epf treated clots respectively. As in the discussion of the FCL values, the quantities in parentheses are the 95% confidence limits. Similar to the FCL data, the largest initial lytic rate is achieved in the rt-PA (+US) treated clots.
Figure 4.
Initial lytic rate for each treatment group, as determined from Equation (5) by fitting the data to Equation (4). Values for the lytic rate are given as mean ± standard error, in units of % of normalized clot width per minute. The addition of ultrasound substantially increases the initial lytic rate for all treatment groups over that of drug treatment alone.
Discussion
In summary, we measured the lytic efficacy of the fibrinolytic rt-PA combined with the platelet inhibitory agent eptifibatide, with or without 120 kHz ultrasound exposure, in an in-vitro human clot model. A microscopic imaging technique was used to determine clot diameter as a function of time during lytic treatment (Figure 2). The total decrease in clot width after 30 minutes (Figure 3), and the initial lytic rate (Figure 4), were used as parameters quantifying clot lysis in these experiments.
In summary, in the absence of ultrasound, rt-PA+Epf (−US) treatment yielded a greater fractional clot loss than control (−US), rt-PA (−US) or Epf (−US) treatment (Figure 3). For ultrasound exposed clots, the observed fractional clot loss for control (+US), rt-PA (+US) and Epf (+US) exposed clots was substantially increased compared with control (−US), Epf (−US) and rt-PA (−US) treated clots respectively (Figure 3). However, ultrasound exposure did not increase the fractional clot loss in rt-PA+Epf treated clots. Ultrasound did significantly increase the initial lytic rates for all treatment groups, but the largest LR value was found for rt-PA(+US) treated clots and was substantially greater than that of the rt-PA+Epf (+US) treated group.
Several studies have examined the efficacy of combined thrombolytic, GP IIb-IIIa inhibitor treatment, and ultrasound on clot lysis. In a recent work, Atar et al [9] exposed fresh human-whole blood clots for 30 minutes to combinations of saline, the GP IIb-IIIa inhibitor tirofiban, the ultrasound contrast agent Optison, heparin, and rt-PA. This group measured lytic efficacy by determining the percent mass loss as a result of treatment; this parameter is a measure of total lytic efficacy similar to the FCL values in this study. In addition, some clots were exposed to very low frequency (27 kHz) ultrasound at an intensity of 0.9 W/cm2. For purposes of comparison, the 120 kHz ultrasound parameters used in this work result in an average acoustic intensity of 0.8 W/cm2 [42]. This group found that tirofiban and rt-PA exposure (without ultrasound) resulted in a percent mass loss of 28±4%, whereas rt-PA and tirofiban exposure alone resulted in a mass loss of 33±9% and 19±8% respectively. For purposes of comparison, the fractional clot loss in this work was 28.0±2.2% (rt-PA+Epf, −US), 9.2±2.5 (Epf, −US), 15.6±1.7 (rt-PA, −US) respectively. For ultrasound treated clots, they found that combined rt-PA, tirofiban and ultrasound exposure yielded a percent mass loss of 56 ± 6% compared with values of 72 ± 1% and 37 ± 4% for rt-PA (+US) and tirofiban (+US) exposed clots respectively. These values are quite comparable to the values obtained here of 30.3±3.6%, 20.7±6.4%, and 44.4±3.6%, for the fractional clot loss of rt-PA+Epf (+US), Epf (+US), and rt-PA (+US) treated clots respectively (Figure 3). It is interesting to note that Atar et al [9] also observed the greatest clot lysis for rt-PA treated clots exposed to ultrasound (72 ± 1%), and this value was greater than that measured for clots exposed to combined rt-PA, tirofiban, and ultrasound treatment (56 ± 6%). We observe a similar result here in that FCL (Figure 3) for combined rt-PA+Epf (+US) treatment (30.3 ± 3.6%) is less than that for rt-PA (+US) treated clots (44 ± 3.6%). It must be pointed out that comparisons with Atar et al [9] are approximate at best; their group used fresh clots which can be less resistant to thrombolysis [27, 43], and a different measurement technique was utilized to determine lytic efficacy.
In a similar work, Collet et al [33] studied the effects of combined rt-PA and GP IIb-IIIa inhibitor treatment on in-vitro human clots. In this study, human plasma clots were prepared and exposed to rt-PA (control) or rt-PA plus one of the GP IIb-IIIa inhibitors abciximab or eptifibatide (treated). The progression of the lytic front was measured using an elegant confocal microscopic imaging technique. Qualitatively they found that platelet aggregates within the clot decreased the progression of clot lysis in control clots. However, the addition of either abciximab or eptifibatide substantially increased rt-PA lytic front progression and clot lysis in treated clots. In addition, they found that lysis from treated clots yielded clot fragments with an average diameter of 8–10 μm. In contrast, lysis fragments from untreated clots averaged 60 μm in diameter. Quantitatively, they observed that after 30 minutes of exposure, the lytic front penetrated into the sample clot surface to about 290 μm in treated clots as compared with 160 μm in control clots. These results are similar to those presented here in that the decrease in clot width was 28.0 ± 2.2 % and 15.6 ± 1.7 % in rt-PA+Epf (−US) and rt-PA(−US) treated clots respectively.
The effect of rt-PA, eptifibatide and 120 kHz ultrasound on clot lysis is clearly complex, but one can speculate as to the mechanisms that influence this interaction. In an attempt to delineate a potential mechanism, we note that there are several interesting observations in this work that require explanation; (1) the approximate equivalence of the initial lytic rates for rt-PA, Epf and rt-PA+Epf treated clots in the absence of ultrasound, (2) LR(rt-PA, +US) is greater than LR(rt-PA+Epf, +US), (3) FCL(rt-PA+Epf, −US) is comparable to FCL(rt-PA+Epf,+US), and (4) FCL(rt-PA, +US) is greater than FCL(rt-PA+Epf, +US).
In the absence of ultrasound, exogenous eptifibatide and/or rt-PA can only enter the clot via diffusion [32], which is a slow process. In addition, the pharmacologic mechanism for rt-PA and eptifibatide are quite different. The fibrinolytic rt-PA acts by converting the plasminogen both within and outside of the clot into plasmin. The plasmin then cleaves the fibrin mesh within the clot into fragments, thus lysing the clot. Eptifibatide acts by blocking the GP IIb-IIIa receptor on target platelets, thus interfering with platelet-platelet binding and interaction with the fibrin mesh of a thrombus [44]. Therefore, in clots not exposed to ultrasound, the rt-PA and/or eptifibatide diffuse into the clot volume from the surrounding plasma and act on their respective pharmacologic targets. However, since diffusion is slow, it is likely that both the eptifibatide and rt-PA are initially substrate limited since the clot volume available for these drugs to act is quite small. This likely limits clot lysis for small values of time t and could result in small values of the initial lytic rate. This qualitatively explains the data of Figure (4) in the (−US) treated groups in that the initial lytic rate values for rt-PA (−US), Epf (−US) and rt-PA+Epf (−US) treated clots are quite low and similar in magnitude. For longer exposure times, rt-PA and eptifibatide can act synergistically in achieving clot lysis over a larger clot volume, as discussed previously. Therefore, one can expect a greater degree of clot lysis from combined rt-PA-Epf treatment than would result from either drug alone. The data of Figure (3) provide support for this speculation as the decrease in clot width for the rt-PA+Epf (−US) group is larger than those in the rt-PA (US), Epf(−US) or control (−US) groups.
The addition of 120 kHz ultrasound was shown to increase the initial lytic rate (Figure 4) for all treatment groups, and increased the fractional clot loss (Figure 6) for control, rt-PA, and Epf treated clots. However, ultrasound did not increase the fractional clot loss for rt-PA+Epf (+US) treated clots. The explanation for this result may lie in the combined effects of rt-PA, eptifibatide, and ultrasound on the clot. It is known that rt-PA mediated clot lysis can be limited by the availability of the substrate plasminogen both within the clot and in the plasma surrounding the clot [45, 46]. As previously discussed, Collet et al [33] noted that fragments from clots treated with both rt-PA and a GP IIb-IIIa inhibitor were much smaller than those treated with rt-PA alone. It has been demonstrated in the work of others that ultrasound increases the penetration of rt-PA into the clot [47], likely as a result of the induction of stable cavitation [16, 48]. Therefore, we speculate that treating clots with rt-PA and eptifibatide yields many smaller clot fragments resulting from clot lysis. The surface-to-volume ratio of these clot fragments would be greater than those resulting from rt-PA lysis alone, and could represent a substantial “sink” for plasmin and plasminogen since there are more sites available for plasminogen and plasmin binding in these smaller fragments. For clots not treated with ultrasound, the lytic process is predominantly limited by diffusion of eptifibatide and rt-PA. However the eptifibatide and rt-PA likely penetrate farther into the clot in ultrasound treated clots than in the non ultrasound treated clots. This initially results in faster clot lysis for non ultrasound treated clots, as the rt-PA and eptifibatide are not substrate limited, thus explaining the observation that LR(rt-PA+Epf, +US) is much greater than LR(rt-PA+Epf, −US). However, a later reduction in substrate availability could “soak up” the available plasmin and plasminogen as the combined ultrasound, rt-PA and Epf treatment creates many small clot fragments, as compared with ultrasound and rt-PA treatment. This could reduce fractional clot lysis in the rt-PA+Epf (+US) treated group as compared with rt-PA(+US) treated clots (Figure 3), resulting in total fractional clot loss similar to the rt-PA+Epf(−US) group. Such a mechanism could also reduce LR for clots treated with rt-PA+Epf and ultrasound as compared to LR (rt-PA, +US) since less of the rt-PA is available to lyse the bulk clot in the rt-PA+Epf treated group.
The results presented here are limited by several factors. First, this is an in-vitro model, and certainly does not replicate the complex in-vivo or clinical scenario of lytic therapy. Second, the clots are subject to static pressure; in the in-vivo scenario of arterial obstructing thrombus, the clot is subject to a time-varying blood pressure. The external blood pressure would increase permeation of the clot with lytic drug and increase thrombolysis [32]; therefore the lytic efficacy of the rt-PA and eptifibatide are likely underestimated in this work. In addition, the clots were manufactured from the blood of healthy donors thus reducing the applicability of these results to individuals on anticoagulants or other medications, or those suffering from significant medical problems.
It has been shown in an in-vitro human clot model that the lytic efficacy of combined rt-PA and eptifibatide treatment is greater than that of either medication alone. The addition of 120 kHz ultrasound substantially increases the initial lytic rate of all treatment groups, but does not increase the overall clot lysis resulting from combined rt-PA and eptifibatide treatment. The effects on lytic efficacy of combined ultrasound, rt-PA and eptifibatide treatment are complex, and are likely a result of the different pharmacologic mechanisms of the two drugs. The results overall are promising, and further work is needed to delineate the potential clinical efficacy of such combination therapy.
Acknowledgments
The authors gratefully acknowledge support from NIH/NINDS (K02-NSO56253-01), the Whitaker Foundation (Biomedical Engineering Research Grant RG-01-0218), and the Dean’s Discovery Fund of the University of Cincinnati, College of Medicine. Useful conversations with Dr. Saurrabh Datta are most appreciated.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sura AC, Kelemen MD. Early management of ST-segment elevation myocardial infarction. Cardiol Clin. 2006;24(1):37–51. doi: 10.1016/j.ccl.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 2.Tanne D, V, Bates E, Verro P, Kasner SE, Binder JR, Patel SC, Mansbac HH, Daley S, Schultz LR, Scott P, Dayno JM, Verecskey-Porter K, Benesch C, Book D, Coplin WM, Dulli D, Levine SR. Initial clinical experience with IV tissue plasminogen activator for acute ischemic stroke: a multicenter survey. The t-PA Stroke Survey Group. Neurology. 1999;53(2):424–427. doi: 10.1212/wnl.53.2.424. [DOI] [PubMed] [Google Scholar]
- 3.Protack CD, Bakken AM, Patel N, Saad WE, Waldman DL, Davies MG. Long-term outcomes of catheter directed thrombolysis for lower extremity deep venous thrombosis without prophylactic inferior vena cava filter placement. J Vasc Surg. 2007;45(5):992–7. doi: 10.1016/j.jvs.2007.01.012. discussion 997. [DOI] [PubMed] [Google Scholar]
- 4.Sugimoto K, Hoffman LV, Razavi MK, Kee ST, Sze DY, Dake MD, Semba CP. The safety, efficacy, and pharmacoeconomics of low-dose alteplase compared with urokinase for catheter-directed thrombolysis of arterial and venous occlusions. Journal of Vascular Surgery. 2003;37(3):512–517. doi: 10.1067/mva.2003.41. [DOI] [PubMed] [Google Scholar]
- 5.Alexandrov AV, Molina CA, Grotta JC, Garami Z, Ford SR, Alvarez-Sabin J, Montaner J, Saqqur M, Demchuk AM, Moye LA, Hill MD, Wojner AW. Ultrasound-enhanced systemic thrombolysis for acute ischemic stroke. New England Journal of Medicine. 2004;351(21):2170–2178. doi: 10.1056/NEJMoa041175. [DOI] [PubMed] [Google Scholar]
- 6.Basta G, Lupi C, Lazzerini G, Chiarelli P, L’Abbate A, Rovai D. Therapeutic effect of diagnostic ultrasound on enzymatic thrombolysis. A in vitro study on blood of normal subjects and patients with coronary artery disease. Thrombosis and Haemostasis. 2004;91(6):1078–1083. doi: 10.1160/TH03-11-0684. [DOI] [PubMed] [Google Scholar]
- 7.Suchkova VN, Baggs RB, Francis CW. Effect of 40 kHz ultrasound on acute thrombotic ischemia in a rabbit femoral artery thrombosis model: Enhancement of thrombolysis and improvement in capillary muscle perfusion. Circulation. 2000;101:2296–2301. doi: 10.1161/01.cir.101.19.2296. [DOI] [PubMed] [Google Scholar]
- 8.Meunier JM, Holland CK, Lindsell CJ, Shaw GJ. Duty cycle dependence of 120 kHz ultrasound enhanced thrombolysis in human clot. Ultrasound in Medicine and Biology. 2007;33(4):576–783. doi: 10.1016/j.ultrasmedbio.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Atar S, Luo H, Birnbaum Y, Nagai T, Siegel RJ. Augmentation of in-vitro clot dissolution by low frequency high-intensity ultrasound combined with antiplatelet and antithrombotic drugs. J Thromb Thrombolysis. 2001;11(3):223–8. doi: 10.1023/a:1011912920777. [DOI] [PubMed] [Google Scholar]
- 10.Pancioli AM, Brott TG. Therapeutic potential of platelet glycoprotein IIb/IIIa receptor antagonists in acute ischaemic stroke: scientific rationale and available evidence. CNS Drugs. 2004;18(14):981–8. doi: 10.2165/00023210-200418140-00003. [DOI] [PubMed] [Google Scholar]
- 11.Sakharov DV, Hekkenberg RT, Rijken DC. Acceleration of fibrinolysis by high-frequency ultrasound: The contribution of acoustic streaming and temperature rise. Thrombosis and Haemostasis. 2000;100:333–340. doi: 10.1016/s0049-3848(00)00319-4. [DOI] [PubMed] [Google Scholar]
- 12.Akiyama M, Ishibashi T, Yamada T, Furuhata H. Low frequency ultrasound penetrates the cranium and enhances thrombolysis in vitro. Neurosurgery. 1998;43(4):828–833. doi: 10.1097/00006123-199810000-00062. [DOI] [PubMed] [Google Scholar]
- 13.Francis C, Blinc A, Lee S, Cox C. Ultrasound accelerates transport of recombinant tissue plasminogen activator into clots. Ultrasound in Medicine and Biology. 1995;21(3):419–424. doi: 10.1016/0301-5629(94)00119-x. [DOI] [PubMed] [Google Scholar]
- 14.Everbach C, Francis CD. Cavitational mechanisms in ultrasound accelerated thrombolysis at 1 MHz. Ultrasound in Medicine and Biology. 2000;26(7):1153–1160. doi: 10.1016/s0301-5629(00)00250-7. [DOI] [PubMed] [Google Scholar]
- 15.Datta S, Coussios CC, McAdory LE, Tan J, Porter T, Courten-Meyers G, Holland CK. Correlation of cavitation with ultrasound enhancement of thrombolysis. Ultrasound in Medicine and Biology. 2006;32(8):1257–1267. doi: 10.1016/j.ultrasmedbio.2006.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prokop AF, Soltani A, Roy RA. Cavitational mechanisms in ultrasound-accelerated fibrinolysis. Ultrasound in Medicine and Biology. 2007;33(6):924–933. doi: 10.1016/j.ultrasmedbio.2006.11.022. [DOI] [PubMed] [Google Scholar]
- 17.Marinoni M, Ginanneschi A, Forleo P, Amaducci L. Technical limits in transcranial Doppler recording: Inadequate acoustic windows. Ultrasound in Medicine and Biology. 1997;23:1275–1277. doi: 10.1016/s0301-5629(97)00077-x. [DOI] [PubMed] [Google Scholar]
- 18.Poster T, Federlein J, Przuntek H, Bütner T. Insufficient and absent acoustic temporal bone window: Potential and limitations of transcranial contrast-enhanced color-coded sonography and contrast-enhanced power-based sonography. Ultrasound in Medicine and Biology. 1997;23(6):857–862. doi: 10.1016/s0301-5629(97)00047-1. [DOI] [PubMed] [Google Scholar]
- 19.Siegel RJ, Atar S, Fishbein MC, Brasch AV, Peterson TM, Nagai T, Pal D, Nishioka T, Chae JS, Birnbaum Y, Zanelli C, Luo H. Noninvasive transcutaneous low frequency ultrasound enhances thrombolysis in peripheral and coronary arteries. Echocardiography. 2001;18(3):247–257. doi: 10.1046/j.1540-8175.2001.00247.x. [DOI] [PubMed] [Google Scholar]
- 20.Pfaffenberger S, Devcic-Kuhar B, Kollman C, Kastl SP, Kaun C, Spiedl WS, Weiss TW, Demyanets S, Ullrich R, Sochor H, Wöber C, Zeitlhofer J, Huber K, Gröschl M, Benes E, Maurer G, Wojta MG-WJ. Can a commercial diagnostic ultrasound device accelerate thrombolysis? An in vitro skull model Stroke. 2005;36:124–128. doi: 10.1161/01.STR.0000150503.10480.a7. [DOI] [PubMed] [Google Scholar]
- 21.Tricoci P, Newby LK, Kandzari DE, Harrington RA. Present and evolving role of eptifibatide in the treatment of acute coronary syndromes. Expert Rev Cardiovasc Ther. 2007;5(3):401–12. doi: 10.1586/14779072.5.3.401. [DOI] [PubMed] [Google Scholar]
- 22.Rebeiz AG, Johanson P, Green CL, Crater SW, Roe MT, Langer A, Giugliano RP, Lincoff AM, Newby LK, Harrington RA, Topol EJ, Califf RM, Wagner GS, Krucoff MW. Comparison of ST-segment resolution with combined fibrinolytic and glycoprotein IIb/IIIa inhibitor therapy versus fibrinolytic alone (data from four clinical trials) Am J Cardiol. 2005;95(5):611–4. doi: 10.1016/j.amjcard.2004.10.038. [DOI] [PubMed] [Google Scholar]
- 23.Jaffe GJ, Green GD, Abrams GH. Stability of recombinant tissue plasminogen activator. American Journal of Ophthalmology. 1989;108(1):90–91. doi: 10.1016/s0002-9394(14)73272-6. [DOI] [PubMed] [Google Scholar]
- 24.Winter PM, Shukla HP, Caruthers SD, Scott MJ, Fuhrhop RW, Robertson JD, Gaffney PJ, Wickline SA, Lanzà GM. Molecular imaging of human thrombus with computed tomography. Academic Radiology. 2005;12(Suppl 1):S9–S13. doi: 10.1016/j.acra.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 25.Yu X, Song SK, Chen J, Scott MJ, Fuhrhop RJ, Hall CS, Gaffney PJ, Wickline SA, Lanza GM. High-resolution MRI characterization of human thrombus using a novel fibrin-targeted paramagnetic nanoparticle contrast agent. Magnetic Resonance in Medicine. 2000;44:867–872. doi: 10.1002/1522-2594(200012)44:6<867::aid-mrm7>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 26.Francis CW, Totterman S. Magnetic resonance imaging of deep vein thrombi correlates with response to thrombolytic therapy. Thromb Haemost. 1995;73(3):386–91. [PubMed] [Google Scholar]
- 27.Loren M, Fade-Garcia LJ, Toorado MC, Navarro JL. Thrombus age and tissue plasminogen activator mediated thrombolysis in rats. Thrombosis Research. 1989;56:67–76. doi: 10.1016/0049-3848(89)90009-1. [DOI] [PubMed] [Google Scholar]
- 28.Shaw GJ, Dhamija A, Bavani N, Lindsell CJ. Effect of mild hypothermia on the thrombolytic efficacy of 120 kHz ultrasound enhanced thrombolysis in an in-vitro human clot model. Thrombosis Research. 2006;117:603–608. doi: 10.1016/j.thromres.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 29.Kaufman RM. Uncommon cold: could 4 degrees C storage improve platelet function? Transfusion. 2005;45(9):1407–12. doi: 10.1111/j.1537-2995.2005.00544.x. [DOI] [PubMed] [Google Scholar]
- 30.Marinkovic SV, Miliasavljevic MM, Kovacevic MS, Stevia ZD. Perforating branches of the middle cerebral artery: Microanatomy and clinical significance of their intracerebral segments. Stroke. 1958;15(6):1022–1029. doi: 10.1161/01.str.16.6.1022. [DOI] [PubMed] [Google Scholar]
- 31.Tao X, Yu XJ, Bhattarai B, Li TH, Jin H, Wei GW, Ming JS, Ren W, Jiong C. Microsurgical anatomy of the anterior communicating artery complex in adult Chinese heads. Surgical Neurology. 2006;65:151–161. doi: 10.1016/j.surneu.2005.05.027. [DOI] [PubMed] [Google Scholar]
- 32.Diamond SL, Anand S. Inner clot diffusion and permeating during fibrinolysis. Biophysical Journal. 1993;65:2622–2643. doi: 10.1016/S0006-3495(93)81314-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Collet JP, Montalescot G, Lesty C, Weisel JW. A structural and dynamic investigation of the facilitating effect of glycoprotein IIb/IIIa inhibitors in dissolving platelet-rich clots. Circ Res. 2002;90(4):428–34. doi: 10.1161/hh0402.105095. [DOI] [PubMed] [Google Scholar]
- 34.Cheng JY, Shaw GJ, Holland CK. In vitro microscopic imaging of enhanced thrombolysis with 120 kHz ultrasound in a human clot model. Acoustic Research Letters Online. 2005;6(1):25–29. doi: 10.1121/1.1815039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hynynen K, McDannold N, Sheikov NA, Jolesz FA, Vykhodtseva N. Local and reversible blood-brain barrier disruption by noninvasive focused ultrasound at frequencies suitable for trans-skull sonications. Neuroimaging. 2005;24:12–20. doi: 10.1016/j.neuroimage.2004.06.046. [DOI] [PubMed] [Google Scholar]
- 36.Brott TG, Haley EC, Jr, Levy DE, Barsan W, Broderick J, Sheppard GL, Spilker J, Kongable GL, Massey S, Reed R, et al. Urgent therapy for stroke. Part I. Pilot study of tissue plasminogen activator administered within 90 minutes. Stroke. 1992;23(5):632–40. doi: 10.1161/01.str.23.5.632. [DOI] [PubMed] [Google Scholar]
- 37.Tanswell P, Seifried E, Stang E, Krause J. Pharmacokinetics and hepatic catabolism of tissue-type plasminogen activator. Arzneimittelforschung. 1991;41(12):1310–1319. [PubMed] [Google Scholar]
- 38.Seifried E, Tanswell P, Ellbruck D, Haerer W, Schmidt A. Pharmacokinetics and haemostatic status during consecutive infusion of recombinant tissue-type plasminogen activator. Thrombosis and Haemostasis. 1989;61(3):497–501. [PubMed] [Google Scholar]
- 39.Boden WE, Eagle K, Granger CB. Reperfusion strategies in acute ST-segment elevation myocardial infarction: a comprehensive review of contemporary management options. J Am Coll Cardiol. 2007;50(10):917–29. doi: 10.1016/j.jacc.2007.04.084. [DOI] [PubMed] [Google Scholar]
- 40.Tardiff BE, Jennings LK, Harrington RA, Gretler D, Potthoff RF, Vorchheimer DA, Eisenberg PR, Lincoff AM, Labinaz M, Joseph DM, McDougal MF, Kleiman NS. Pharmacodynamics and pharmacokinetics of eptifibatide in patients with acute coronary syndromes: prospective analysis from PURSUIT. Circulation. 2001;104(4):399–405. doi: 10.1161/hc2901.093500. [DOI] [PubMed] [Google Scholar]
- 41.Gilchrist IC, O’Shea JC, Kosoglou T, Jennings LK, Lorenz TJ, Kitt MM, Kleiman NS, Talley D, Aguirre F, Davidson C, Runyon J, Tcheng JE. Pharmacodynamics and pharmacokinetics of higher-dose, double-bolus eptifibatide in percutaneous coronary intervention. Circulation. 2001;104(4):406–11. doi: 10.1161/hc2901.093504. [DOI] [PubMed] [Google Scholar]
- 42.Kinsler LE, Frey AR, Coppens AB, Sanders JV. Fundamentals of Acoustics. 4. New York: John Wiley & Sons, Inc; 2000. [Google Scholar]
- 43.Wyshelesky A, Iakobishvili Z, Matz I, Golovchiner G, Vaturi M, Siegel RJ, Birnbaum Y. There is synergism between high-intensity, low-frequency ultrasound and streptokinase but not with eptifibatide, heparin, and aspirin. Differential effects on fresh and aged blood clots. An in vitro study. Thromb Res. 2001;103(4):337–44. doi: 10.1016/s0049-3848(01)00323-1. [DOI] [PubMed] [Google Scholar]
- 44.Hankey GJ, Eikelboom JW. Antiplatelet drugs. Med J Aust. 2003;178(11):568–74. doi: 10.5694/j.1326-5377.2003.tb05361.x. [DOI] [PubMed] [Google Scholar]
- 45.Devcic-Kuhar B, Pfaffenberger S, Gherardini L, Mayer C, Gro?schl M, Kaun C, Benes E, Tschachler E, Huber K, Maurer G, Wojta J, Gottsauner-Wolf M. Ultrasound affects distribution of plasminogen and tissue-type plasminogen activator in whole blood clots in vitro. Thrombosis and Haemostasis. 2004;92(5):980–985. doi: 10.1160/TH04-02-0119. [DOI] [PubMed] [Google Scholar]
- 46.Onundarsen PT, Francis CW, Marder VJ. Depletion of plasminogen in vitro or during thrombolytic therapy limits fibrinolytic potential. Journal of Laboratory and Clinical Medicine. 1992;120:120–128. [PubMed] [Google Scholar]
- 47.Francis CW, Blinc A, Lee S, Cox C. Ultrasound accelerates transport of recombinant tissue plasminogen activator into clots. Ultrasound in Medicine and Biology. 1995;21(3):419–424. doi: 10.1016/0301-5629(94)00119-x. [DOI] [PubMed] [Google Scholar]
- 48.Datta S, Coussios CC, McAdory LE, Tan J, Porter T, De Courten-Myers G, Holland CK. Correlation of cavitation with ultrasound enhancement of thrombolysis. Ultrasound in Medicine and Biology. 2006;32(8):1257–1267. doi: 10.1016/j.ultrasmedbio.2006.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]