Abstract
The kinetics of Bacillus anthracis toxin production in culture and its lethal activity in rats, mice, and guinea pigs were investigated. Lethal toxin activity was produced in vitro throughout exponential growth at essentially identical rates in both encapsulated virulent and nonencapsulated avirulent strains. The two toxin proteins which produce lethality when in combination, lethal factor (LF) and protective antigen (PA), could be quantitated directly from culture fluids by rocket immunoelectrophoresis. Using purified preparations of these proteins, we determined that a combination of 8 micrograms of LF and 40 micrograms of PA was required for a maximal rate of killing (39 to 40 min) in Fischer 344 rats (250 to 300 g). Conversely, a minimum of 0.6 microgram of LF and 3 micrograms of PA was required for lethality. The 50% lethal dose for Hartley guinea pigs was 50 micrograms of LF and 250 micrograms of PA, and for Swiss mice it was 2.5 micrograms of LF and 12.5 micrograms of PA. Analyses classically reserved for enzyme kinetic studies were used to study the kinetics of lethal activity in the rat model after intravenous injection of LF-PA mixtures. The amounts of LF and PA which were required to give half the rate of killing (i.e., double the minimum time to death) were 1.2 and 5.8 micrograms, respectively. A theoretical minimum time to death was determined to be 38 min. A third anthrax toxin component, edema factor, was shown to inhibit lethal toxin activity. Edema factor could not be quantitated by rocket immunoelectrophoresis because the protein did not form distinct precipitin bands with available antisera.
Full text
PDF




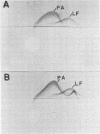
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BEALL F. A., TAYLOR M. J., THORNE C. B. Rapid lethal effect in rats of a third component found upon fractionating the toxin of Bacillus anthracis. J Bacteriol. 1962 Jun;83:1274–1280. doi: 10.1128/jb.83.6.1274-1280.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BELTON F. C., HENDERSON D. W. A method for assaying anthrax immunising antigen and antibody. Br J Exp Pathol. 1956 Apr;37(2):156–160. [PMC free article] [PubMed] [Google Scholar]
- BELTON F. C., STRANGE R. E. Studies on a protective antigen produced in vitro from Bacillus anthracis: medium and methods of production. Br J Exp Pathol. 1954 Apr;35(2):144–152. [PMC free article] [PubMed] [Google Scholar]
- Beall F. A., Dalldorf F. G. The pathogenesis of the lethal effect of anthrax toxin in the rat. J Infect Dis. 1966 Jun;116(3):377–389. doi: 10.1093/infdis/116.3.377. [DOI] [PubMed] [Google Scholar]
- DULBECCO R., VOGT M. Plaque formation and isolation of pure lines with poliomyelitis viruses. J Exp Med. 1954 Feb;99(2):167–182. doi: 10.1084/jem.99.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fish D. C., Lincoln R. E. Biochemical and biophysical characterization of anthrax toxin. Fed Proc. 1967 Sep;26(5):1534–1538. [PubMed] [Google Scholar]
- Fish D. C., Lincoln R. E. In vivo-produced anthrax toxin. J Bacteriol. 1968 Mar;95(3):919–924. doi: 10.1128/jb.95.3.919-924.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fish D. C., Mahlandt B. G., Dobbs J. P., Lincoln R. E. Purification and properties of in vitro-produced anthrax toxin components. J Bacteriol. 1968 Mar;95(3):907–918. doi: 10.1128/jb.95.3.907-918.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill D. M. Bacterial toxins: a table of lethal amounts. Microbiol Rev. 1982 Mar;46(1):86–94. doi: 10.1128/mr.46.1.86-94.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAINES B. W., KLEIN F., LINCOLN R. E. QUANTITATIVE ASSAY FOR CRUDE ANTHRAX TOXINS. J Bacteriol. 1965 Jan;89:74–83. doi: 10.1128/jb.89.1.74-83.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARRIS-SMITH P. W., SMITH H., KEPPIE J. Production in vitro of the toxin of Bacillus anthracis previously recognized in vivo. J Gen Microbiol. 1958 Aug;19(1):91–103. doi: 10.1099/00221287-19-1-91. [DOI] [PubMed] [Google Scholar]
- Johnson A. D., Spero L. Comparison of growth and toxin production in two vaccine strains of Bacillus anthracis. Appl Environ Microbiol. 1981 Jun;41(6):1479–1481. doi: 10.1128/aem.41.6.1479-1481.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppla S. H. Anthrax toxin edema factor: a bacterial adenylate cyclase that increases cyclic AMP concentrations of eukaryotic cells. Proc Natl Acad Sci U S A. 1982 May;79(10):3162–3166. doi: 10.1073/pnas.79.10.3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikesell P., Ivins B. E., Ristroph J. D., Dreier T. M. Evidence for plasmid-mediated toxin production in Bacillus anthracis. Infect Immun. 1983 Jan;39(1):371–376. doi: 10.1128/iai.39.1.371-376.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ristroph J. D., Ivins B. E. Elaboration of Bacillus anthracis antigens in a new, defined culture medium. Infect Immun. 1983 Jan;39(1):483–486. doi: 10.1128/iai.39.1.483-486.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STANLEY J. L., SMITH H. Purification of factor I and recognition of a third factor of the anthrax toxin. J Gen Microbiol. 1961 Sep;26:49–63. doi: 10.1099/00221287-26-1-49. [DOI] [PubMed] [Google Scholar]
- STANLEY J. L., SMITH H. The three factors of anthrax toxin: their immunogenicity and lack of demonstrable enzymic activity. J Gen Microbiol. 1963 May;31:329–337. doi: 10.1099/00221287-31-2-329. [DOI] [PubMed] [Google Scholar]
- Smith H., Stoner H. B. Anthrax toxic complex. Fed Proc. 1967 Sep;26(5):1554–1557. [PubMed] [Google Scholar]
- THORNE C. B., BELTON F. C. An agar-diffusion method for titrating Bacillus anthracis immunizing antigen and its application to a study of antigen production. J Gen Microbiol. 1957 Oct;17(2):505–516. doi: 10.1099/00221287-17-2-505. [DOI] [PubMed] [Google Scholar]
- THORNE C. B. Biochemical properties of virulent and avirulent strains of Bacillus anthracis. Ann N Y Acad Sci. 1960 Nov 21;88:1024–1033. doi: 10.1111/j.1749-6632.1960.tb20094.x. [DOI] [PubMed] [Google Scholar]
- THORNE C. B., MOLNAR D. M., STRANGE R. E. Production of toxin in vitro by Bacillus anthracis and its spearation into two components. J Bacteriol. 1960 Mar;79:450–455. doi: 10.1128/jb.79.3.450-455.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEINBERG E. D. MANGANESE REQUIREMENT FOR SPORULATION AND OTHER SECONDARY BIOSYNTHETIC PROCESSES OF BACILLUS. Appl Microbiol. 1964 Sep;12:436–441. doi: 10.1128/am.12.5.436-441.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg E. D. Secondary metabolism: raison d'être. Perspect Biol Med. 1971;14(4):565–577. doi: 10.1353/pbm.1971.0033. [DOI] [PubMed] [Google Scholar]
- Wilkie M. H., Ward M. K. Characterization of anthrax toxin. Fed Proc. 1967 Sep;26(5):1527–1531. [PubMed] [Google Scholar]
- ZWARTOUW H. T., SMITH H. Polyglutamic acid from Bacillus anthracis grown in vivo; structure and aggressin activity. Biochem J. 1956 Jul;63(3):437–442. doi: 10.1042/bj0630437. [DOI] [PMC free article] [PubMed] [Google Scholar]