Abstract
Triacylglycerol (TAG) in adipose tissue serves as the major energy storage form in higher eukaryotes. Obesity, resulting from excess white adipose tissue, has increased dramatically in recent years resulting in a serious public health problem. Understanding of adipocyte-specific TAG synthesis and hydrolysis is critical to the development of strategies to treat and prevent obesity and its closely associated diseases, for example, Type 2 diabetes, hypertension and atherosclerosis. In this review, we present an overview of the major enzymes in TAG synthesis and lipolysis, including the recent discovery of a novel adipocyte TAG hydrolase.
Keywords: 1-acylglycerol-3-phosphate acyltransferase, desnutrin/adipose triglyceride lipase, diacylglycerol acyltransferase, glycerol-3-phosphate acyltransferase, hormone-sensitive lipase, lipolysis, phosphatidic acid phosphatase, triacylglycerol
White adipose tissue (WAT) is the major energy reserve in higher eukaryotes. The primary purposes of WAT are synthesis and storage of triacylglycerol (TAG) in periods of energy excess, and hydrolysis of TAG to generate fatty acids for use by other organs during periods of energy deprivation [1]. Adipose tissue also secretes adipokines that regulate energy intake and metabolism.
The prevalence of obesity resulting from excess WAT has increased dramatically in recent years resulting in a serious public health problem, since obesity is closely associated with a number of disorders including Type 2 diabetes, hypertension and atherosclerosis [2]. While adipocyte number has been shown to increase (hyperplasia) in morbid obesity, obesity is primarily attributed to adipocyte hypertrophy that occurs when TAG synthesis (esterification) exceeds TAG breakdown (lipolysis), resulting in elevated TAG storage [1]. Although it has been postulated that large adipocyte size may contribute to insulin resistance, the molecular mechanism is not clear.
Paradoxically, the metabolic abnormalities typically found in obesity are also associated with lipodystrophies that are characterized by selective loss of adipose tissue from particular regions of the body [3-6]. Although the underlying molecular mechanisms are not clear, metabolic complications may result from ectopic storage of TAG in tissues such as the liver and muscle [7-9]. A proper capacity for TAG storage in adipocytes is clearly important for normal metabolic regulation. In view of the wide range of health problems associated with improper fat storage and release, it is critical to understand adipocyte-specific regulation of TAG synthesis and hydrolysis.
Biosynthesis of triacylglycerol
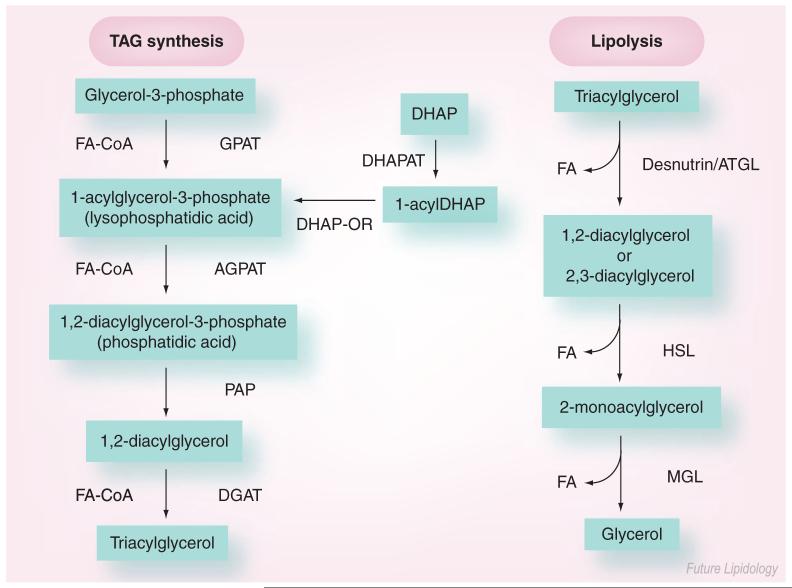
The synthesis of phosphatidic acid and its subsequent conversion to TAG was described more than 40 years ago by Kennedy and coworkers [10], and is summarized in Figure 1. The initial committal step in this pathway is the formation of lysophosphatidic acid (1-acylglycerol-3-phosphate) catalyzed by glycerol-3-phosphate acyltransferase (GPAT), which occurs in both the endoplasmic reticulum (ER) and mitochondria [11,12]. GPAT is believed to be the rate-limiting factor in glycerophospholipid synthesis. Lysophosphatidic acid can also be formed by the acylation of dihydroxyacetone-phosphate (DHAP) by acylcoenzyme A (CoA):DHAP acyltransferase and the subsequent reduction of 1-acyl-DHAP by DHAP oxido-reductase. However, the contribution of the DHAP pathway to TAG synthesis remains unclear [13]. Lysophosphatidic acid is further esterified and converted into phosphatidic acid (1,2-diacylglycerol-3-phosphate) in the reaction catalyzed by 1-acylglycerol-3-phosphate acyltransferase (AGPAT), also named lysophosphatidate acyltransferase, an enzyme mainly present in the ER. Phosphatidic acid is then shunted into the synthesis of various phospholipids or into the synthesis of TAG, two separate branches of glycerolipid synthesis. Entry into TAG synthesis involves the conversion of phosphatidic acid to the intermediate 1,2-diacylglycerol (1,2-DAG) by the phosphatidic acid phosphatase (PAP) reaction. Diacylglycerol acyltransferase (DGAT) catalyzes the acylation of 1,2-DAG to form TAG.
Figure 1. The enzymes of triacylglycerol synthesis and hydrolysis.
AGPAT: 1-acylglycerol-3-phosphate acyltransferase; ATGL: Adipose triglyceride lipase; CoA: Coenzyme A; DGAT: Diacylglycerol acyltransferase; DHAP: Dihydroxyacetone-phosphate; DHAPAT: Acyl-CoA:dihydroxyacetone-phosphate acyltransferase; DHAP-OR: Dihydroxyacetone-phosphate oxido-reductase; FA: Fatty acid; GPAT: Glycerol-3-phosphate acyltransferase; HSL: Hormone-sensitive lipase; MGL: Monoglyceride lipase; PAP: Phosphatidic acid phosphatase; TAG: Triacylglycerol.
In naturally occurring glycerophospholipids, saturated fatty acids are predominantly esterified at the sn-1 position and unsaturated fatty acids at the sn-2 position [14]. It is believed that the substrate selectivities of mitochondrial GPAT (mtGPAT) and AGPAT play key roles in this non-random distribution of fatty acids, but the mechanism(s) have not yet been elucidated [12]. Most enzymes in TAG synthesis are intrinsic membrane proteins and their substrates and products are hydrophobic, making purification and kinetic analysis difficult. Cloning and characterization of the enzymes mentioned previously has only been achieved during the last decade following advances in molecular biological techniques.
Our laboratory originally cloned mtGPAT, which was the first mammalian enzyme in TAG and phospholipid biosynthesis to be cloned [15-17]. While mammalian cells are known to have two GPATs, one in the ER (microsomal GPAT) and another in the mitochondria (mtGPAT), microsomal GPAT has not been cloned [13,18]. mtGPAT expression is regulated in a manner that is consistent with its role in energy storage. mtGPAT is not detectable in fasted animals but is transcriptionally induced, especially in liver and adipose tissue, when fasted animals are re-fed a high carbohydrate diet, or when diabetic animals are treated with insulin [19]. On the other hand, microsomal GPAT plays a housekeeping role, as it is not regulated by nutritional and hormonal signals. While mtGPAT preferentially uses saturated fatty acyl-CoA as a substrate, microsomal GPAT shows no preference [19]. Studies in mtGPAT-null mice have confirmed the importance of this enzyme in the synthesis of TAG. The absence of mtGPAT resulted in lower body weights, decreased fat-pad mass, lower liver and plasma triacylglycerol and a lower rate of very low-density lipoprotein (VLDL) secretion compared with wild-type controls [20]. Furthermore, examination of mtGPAT-null mice showed the potential presence of another mtGPAT isoform [17]. Additionally, a murine GPAT-like protein (xGPAT1) was identified [21]. It is unclear whether xGPAT1 is microsomal GPAT, the potential mtGPAT isoform mentioned above or whether it represents an entirely new isoform. It is also unclear how the product of mtGPAT (1-acylglycerol-3-phosphate) is transported out of the mitochondria to the ER where the final enzymes of TAG synthesis are located. In vitro studies have revealed that liver fatty-acid binding protein can transport 1-acyl glycerol-3-phosphate from the mitochondria to target microsomes [13]. However, whether fatty-acid binding protein serves as a transporter in vivo remains elusive.
Based on their known homology to mtGPAT, other acyltransferases for TAG synthesis in the Kennedy pathway were subsequently identified and studied, including AGPAT, DGAT and their isoforms [18,22-27]. To date, eight AGPAT genes have been identified, although the physiological functions of each are not well established [28,29]. Mutations in AGPAT2, the major isoform in adipose tissue, have been reported in patients with congenital generalized lipodystrophy, a rare autosomal recessive disorder characterized by a marked lack of body fat since birth [3]. The discovery of these AGPAT2 mutations suggested an important physiological function for these enzymes in TAG synthesis. Recent findings in AGPAT6-null mice further support this role [29]. AGPAT6 is normally expressed at high levels in WAT, brown adipose tissue and liver. AGPAT6-null mice exhibited a 25% reduction in body weight and resistance to both diet- and genetically-induced obesity. These results indicate that AGPAT6 may play a role in determining triglyceride content in adipose tissue, and its loss is not fully compensated by other members of the AGPAT family.
As described previously, the PAP reaction generates 1,2-DAG, which is utilized as an acyl-acceptor by DGAT during the synthesis of TAG. There are two types of PAPs, Mg2+-dependent (PAP1) and Mg2+-independent (PAP2) [30]. Until recently, only genes encoding PAP2s have been identified in yeast and mammalian systems. However, recent analysis has shown that phophatidic acid phosphohydrolase (PAH)1 (previously known as SMP2) from Saccharomyces cerevisiae is a PAP1 that functions in de novo lipid synthesis [31]. PAH1 has homology with lipin-1 (lipin) and, when expressed in Escherichia coli, lipin displays Mg2+-dependent PAP activity [31]. The Lpin1 gene is mutated in fatty liver dystrophy mice that display lipodystrophy with impaired TAG storage in adipose tissue [32]. Over-expression of lipin in adipose tissue results in increased adiposity and improved insulin sensitivity [32]. Recently, phosphorylation of lipin has been demonstrated to be affected by insulin or epinephrine, resulting in redistribution between the cytosol and ER [33]. Similar to the other enzymes in TAG synthesis, there are multiple isoforms of lipin. The synthesis of TAG catalyzed by DGAT is the final and unique step in TAG formation. It is believed that DGAT diverts 1,2-DAG from membrane phospholipid synthesis into TAG synthesis. Two mammalian DGATs have been identified, DGAT 1 and 2 [13]. Both enzymes are membrane-associated and share similar biochemical functions and tissue expression patterns. Both enzymes are also ubiquitously expressed with the highest expression in tissues involved in TAG metabolism, such as the liver, WAT, mammary gland and small intestine [22,25]. Despite these similarities, DGAT1 and 2 have significant differences. DGAT1 belongs to a family of enzymes that catalyze cholesterol ester biosynthesis and include the acyl-CoA:cholesterol acyltransferase enzymes, ACAT1 and 2 [22]. DGAT2 family members include wax synthases and acyl-CoA:monoacylglycerol acyltransferase [34]. Furthermore, in vitro studies have revealed that DGAT1, but not DGAT2, is a multifunctional acyltransferase able to catalyze the synthesis of DAG, retinyl esters and waxes, in addition to TAG [35]. The functional differences between these two enzymes are further emphasized by studies in mice lacking DGAT1 and 2 [34,36-39]. While mice lacking DGAT2 have a drastic reduction in whole body TAG and die shortly after birth, mice lacking DGAT1 are viable, have improved insulin sensitivity, demonstrate modest reductions in tissue TAG and are resistant to diet-induced obesity. The phenotypic differences between these two mouse models indicate DGAT1 cannot compensate for the loss of DGAT2.
In addition to the previosuly described acyl-CoA-dependent reactions, acyl-CoA-independent pathways of TAG synthesis from DAG exist, including reactions catalyzed by phospholipid:diacylglycerol acyltransferase (PDAT) [40,41] and sn-1,2(2,3) diacylglycerol transacylase (DGTA) [42]. PDAT has been cloned and characterized in both yeast and plants but not in mammals [40,41]. The PDAT reaction is proposed to contribute to fatty-acid specificity in seed oils by transacylation reactions that enrich the oils in polyunsaturated fatty acids [40]. The DGTA reaction is believed to be important in TAG resynthesis during the lipolysis/re-esterification cycle [42]. Only partial purification and characterization of rodent microsomal DGTA has been reported [42]. Recently, in vitro acylglycerol transacylase activity has been reported for three mammalian enzymes, desnutrin (also named adipose triglyceride lipase [ATGL], iPLA2ζ, TTS2.2), adiponutrin (iPLA2ζ) and GS2 (iPLA2η) [43]. While desnutrin/ATGL has clearly been demonstrated to function as a lipase in vivo [44], further work will be required to understand the function of the other enzymes in acyl-CoA-independent TAG synthesis in mammals, particularly those enzymes that are specific to adipose tissue. In this regard, adiponutrin is highly and specifically expressed in adipose tissue where its expression is almost undetectable in fasted animals, but is dramatically upregulated upon refeeding [45].
Hydrolysis of triacylglycerol
While TAG synthesis occurs in multiple tissues (e.g., in the liver for VLDL formation) lipolysis is quantitatively the most predominant in adipose tissue. During periods of energy demand, TAG synthesized in WAT is rapidly mobilized by the hydrolytic action of lipases, resulting in the release of free fatty acids that are taken up by other organs to meet the energy requirements of the organism [46,47]. Figure 1 contains an overview of this process. Until recently, the model of adipocyte lipolysis has been that hormone-sensitive lipase (HSL) catalyzes the hydrolysis of fatty acids from the sn-1 and sn-3 positions, generating 2-monoglycerol that is subsequently hydrolyzed by monoglyceride lipase to yield glycerol and fatty acids [48,49]. In times of energy need, such as fasting and exercise, adipocyte lipolysis is markedly increased by catecholamines that activate Gs-coupled receptors. These receptors, in turn, activate adenylyl cyclase to generate cyclic (c)AMP. Rising cellular concentrations of cAMP activate protein kinase A (PKA; cAMP-dependent protein kinase) that phosphorylates HSL at three serine residues (563, 659 and 660) in a 150 amino acid stretch termed the regulatory module. This regulatory module is found within the C-terminal domain of HSL, which also contains the catalytic triad [50]. Phosphorylation results in increased hydrolytic activity [51], translocation of HSL from the cytosol to the lipid-droplet surface and enhanced TAG breakdown in the cell [49]. HSL can also be regulated by other kinases [49,52], including AMP-activated protein kinase [53]. The hydrolytic action of HSL, and possibly other non-HSL TAG lipases, is regulated by perilipin, a lipid droplet-associated protein [54]. Association of perilipin with lipid droplets may keep lipolysis at a basal level [55]. Under stimulated conditions, however, perilipin increases lipolysis [56-58]. Perilipin may translocate HSL to the lipid droplet and, presumably, allow access of this lipase to its substrate [56,59]. Perilipin is phosphorylated at multiple sites (Ser-81, Ser-223, Ser-277, Ser-434, Ser-492 and Ser-517) [57,60], and recently phosphorylation of Ser-517 was found to play a global role in adipocytes for PKA-stimulated lipolysis [61]. While recent evidence suggests that perilipin phosphorylation, per se, may not be necessary for HSL translocation, it is required for increased activity by HSL under stimulated conditions [56]. In addition to perilipin, a number of PAT (perilipin/adipophilin/TIP47) family proteins have been identified on the lipid droplet and may contribute to the regulation of lipolysis [62]. Comparative gene identification (CGI)-58, another lipid droplet-associated protein, has been shown to colocalize with perilipin [63,64] and increase lipolysis by activating desnutrin/ATGL (discussed in the next section) [65].
HSL has broad substrate specificity, with hydrolytic activity against TAG, diacylglycerol [DAG] and monoacylglycerol, as well as cholesteryl and retinyl esters [51]. In vitro, HSL is 10-20-fold more active against DAG than TAG, and it may be the only, or at least the major, neutral cholesteryl ester (CE) hydrolase in vivo [51,66,67]. Until recently, HSL was believed to be the major regulatory enzyme in TAG hydrolysis. Studies in HSL-null mice, however, demonstrated normal WAT mass [68] and retained approximately 40% of TAG lipase activity [69-71]. While, HSL deficiency caused an accumulation of DAG in adipose tissue, no significant increase in TAG levels in WAT was observed [69]. Taken together, these findings suggested that HSL may be rate-limiting in DAG, but not TAG, hydrolysis and strongly suggested the presence in adipose tissue of additional distinct neutral lipase(s). Interestingly, absence of HSL in WAT causes a shift of the fatty-acid composition in TAG to higher levels of long-chain unsaturated fatty acids, an indication that in vivo HSL may prefer long-chain unsaturated fatty acids as a substrate [69] and, thereby, contribute to the observed preferential mobilization of some highly unsaturated fatty acids [72].
Recently, we identified and characterized a novel adipocyte TAG lipase that we called desnutrin [45]. Two other laboratories subsequently identified this same enzyme from database searches of proteins containing the conserved GXSXG pentapeptide motif and α/β-hydrolase fold, and named it ATGL or iPLA2ζ [43,73]. Murine desnutrin is a 54 kDa protein found predominantly in adipose tissue, but also at much lower levels in other tissues, notably cardiac and skeletal muscle and testis. In cells that contain fat stores, such as differentiated 3T3-L1 cells [73] and HeLa cells grown in oleic acid-rich medium [74], desnutrin/ATGL is found tightly associated with the lipid droplet, as well as throughout the cytoplasm [45,73,74]. Desnutrin/ATGL contains an N-terminal patatin-like domain found in many plant acyl hydrolases, which is characterized by a conserved serine in the GXSXG motif, an α/β-hydrolase fold, a conserved aspartate belonging to the DX(G/A) motif and a glycine-rich region [45]. Desnutrin/ATGL is regulated by nutritional and hormonal signals. In mice, it is induced by fasting and suppressed by refeeding [45]. Glucocorticoids, which are elevated during fasting, increase desnutrin/ATGL expression [45], while insulin, which is increased during feeding, downregulates desnutrin/ATGL [75]. We have also found that desnutrin/ATGL is downregulated in ob/ob and db/db mice, further supporting a role for the enzyme in fat breakdown and contribution in the development of obesity [45].
Several lines of evidence indicate that desnutrin/ATGL is a TAG-specific lipase. Overexpression of desnutrin/ATGL in 293HEK cells [76] or COS-7 cells increases free fatty-acid release to the medium, decreasing intracellular stores of TAG, without affecting intracellular phospholipid stores [45]. Desnutrin/ATGL has significantly higher TAG lipase activity (approximately six to tenfold higher) than DAG lipase activity, suggesting a primary role for the enzyme in catalyzing the first, rate-limiting step in lipolysis [73]. Desnutrin/ATGL does not exhibit activity against CEs and retinyl esters [73]. The evolutionary conservation between the Drosophila TAG lipase, Brummer [77], the Saccharomyces cerevisiae TAG lipase, Tgl4 [78] and desnutrin/ATGL provide further support for desnutrin/ATGL’s function as a lipase [73]. Mice lacking desnutrin/ATGL have provided further evidence supporting a role for desnutrin/ATGL in TAG hydrolysis in multiple tissues. Global loss of desnutrin/ATGL in mice resulted in increased body weight [44]. In these mice, WAT mass increased somewhat (twofold), while brown adipose tissue mass increased dramatically (8.5-fold). Compensatory increases in the hydrolytic activity of other adipocyte lipases, or decreases in the activity of enzymes involved in re-esterification, may explain the relatively small increase in WAT. Desnutrin/ATGL also caused substantial ectopic storage of TAG in multiple other organs, which may also have influenced TAG accumulation in WAT. Studies in mice lacking desnutrin/ATGL, specifically in adipose tissue, are required to determine the effects of this enzyme on TAG metabolism in adipocytes.
It has recently been reported that PKA-mediated phosphorylation of Ser-517 of perilipin A is important for desnutrin/ATGL-dependent lipolysis. In addition, CGI-58 has also been shown to stimulate ATGL activity [65] and mutations in CGI-58 in humans are associated with Chanarin-Dorfman Syndrome, a rare genetic disease where TAG accumulates excessively in multiple tissues [65]. Recent studies that utilized in vitro assays and organ cultures of murine WAT have shown that, together, desnutrin/ATGL and HSL are responsible for more than 95% of the TAG hydrolase activity in murine WAT [79]. However, additional TAG lipases, including triacylglycerol hydrolase (TGH) [80], TGH2 [81], as well as the patatin-domain-containing proteins, GS2 and GS2-like [76], have been identified in adipocytes, but their role in adipocyte lipolysis requires further investigation.
Conclusion
Dysregulation of adipocyte TAG synthesis and lipolysis may contribute to the development of obesity, lipodystrophy and associated pathologies. Recently, many exciting advances have been made, including the discovery of a major TAG lipase that contributes to in vivo adipocyte lipolysis, as well as the identification of enzymes involved in TAG synthesis. Continued advances in our understanding of adipocytespecific TAG metabolism are expected as new tools are developed.
Future perspective
Major enzymes in adipocyte TAG metabolism have recently been identified. However, much remains to be elucidated regarding the in vivo function and properties of these enzymes and their isoforms, especially with regards to their relative roles in adipose-tissue metabolism. Studies involving differential regulation of various isoforms of enzymes in TAG synthesis by nutritional and hormonal factors are also lacking. Desnutrin/ATGL has been found to be the major adipocyte TAG lipase and thus plays a critical role in lipolysis. Generation of adipose-specific null and overexpressing mice will be required to clarify the adipose-specific role of desnutrin/ATGL as well as other enzymes in TAG metabolism. Further studies are needed to determine the function of lipid-droplet-associated proteins, such as caveolin-1, and their roles in lipid-droplet formation and lipolysis. Fatty acids are utilized not only as fuels, but also as membrane components, precursors of eicosanoids and mediators of gene expression. Fatty-acid composition of adipose tissue is not strictly correlated with dietary fatty acids, but may be regulated through selective incorporation and mobilization. Therefore, further studies need to be performed to characterize the fatty-acid specificity of enzymes in TAG metabolism and to determine whether different isoforms of these enzymes possess different fatty-acid specificities.
Executive summary.
Introduction
The primary purposes of white adipose tissue are synthesis and storage of triacylglycerol (TAG) in periods of energy excess and hydrolysis of TAG to generate fatty acids for use by other organs during periods of energy deprivation.
There are a wide range of health problems associated with improper fat storage and release. Therefore, it is critical to understand adipocyte-specific regulation of TAG synthesis and hydrolysis.
Biosynthesis of triacylglycerol
Glycerol 3-phosphate acyltransferase (GPAT) is believed to be a rate-limiting step in glycerophospholipid synthesis. GPAT isoforms are found in both the endoplasmic reticulum (ER; microsomal GPAT) and mitochondria ([mt]GPAT) where they catalyze the formation of lysophosphatidic acid (1-acylglycerol-3-phosphate). In mice, mtGPAT is not detectable during fasting, but is transcriptionally induced during refeeding.
Lysophosphatidic acid is further esterified and converted into phosphatidic acid (1,2-diacylglycerol-3-phosphate) in a reaction catalyzed by 1-acylglycerol-3-phosphate acyltransferase (AGPAT), an enzyme mainly present in the ER. There are eight known isoforms of AGPAT. Mutations in AGPAT2, the major isoform in adipose tissue, have been reported in patients with congenital generalized lipodystrophy. Studies from AGPAT6-null mice indicate that this enzyme may play a role in determining the TAG content in adipose tissue.
Phosphatidic acid phosphatase (PAP) converts phosphatidic acid to the intermediate 1,2-diacylglycerol (1,2-DAG). Lipin has been shown to have PAP activity. Phosphorylation of lipin was reported to change localization between the cytoplasm and the ER.
Synthesis of TAG by diacylglycerol acyltransferase (DGAT) is the final and unique step in TAG formation. It is believed that DGAT diverts 1,2-DAG from membrane phospholipid synthesis into TAG synthesis. Two different mammalian DGAT enzymes have been identified, DGAT1 and 2. Mice lacking DGAT2 have a drastic reduction in whole body TAG and die shortly after birth. Mice lacking DGAT1 are viable, have improved insulin sensitivity, modest reductions in tissue TAG and are resistant to diet-induced obesity.
In addition to acyl-coenzyme (Co)A-dependent reactions, acyl-CoA-independent pathways of TAG synthesis from DAG also exist, and the enzymes catalyzing these reactions need to be identified and studied.
Hydrolysis of triacylglycerol
Until recently, hormone sensitive lipase (HSL) was believed to be the rate-limiting enzyme in adipocyte lipolysis. Studies in HSL-null mice, however, indicated the presence of significant residual TAG-lipase activity, suggesting the presence of additional distinct, but yet unidentified, neutral lipase(s).
The hydrolytic action of HSL, and possibly other non-HSL TAG lipases, is regulated by perilipin, a lipid-droplet-associated protein. Phosphorylation of perilipin increases rates of lipolysis. While recent evidence suggests that perilipin phosphorylation, per se, may not be necessary for HSL translocation, it is required for increased activity by HSL under stimulated conditions.
Recently, a novel TAG lipase, desnutrin/adipose triglyceride lipase (ATGL), was identified. Studies in desnutrin/ATGL-null mice show TAG accumulation in multiple tissues, indicating an important function for this enzyme in lipolysis. In mice, desnutrin is induced by fasting and suppressed by refeeding. Glucocorticoids increase desnutrin/ATGL expression, while insulin downregulates desnutrin/ATGL expression.
Contributor Information
Maryam Ahmadian, University of California, Department of Nutritional Sciences & Toxicology, Berkeley, CA 94720, USA, Tel.: +1 510 642 3978; Fax: +1 510 642 0535; mahmadia@berkeley.edu.
Robin E Duncan, University of California, Department of Nutritional Sciences & Toxicology, Berkeley, CA 94720, USA, Tel.: +1 510 642 3978; Fax: +1 510 642 0535; robin_duncan@berkeley.edu.
Kathy Jaworski, University of California, Department of Nutritional Sciences & Toxicology, Berkeley, CA 94720, USA, Tel.: +1 510 642 3978; Fax: +1 510 642 0535; kathyjaworski@hotmail.com.
Eszter Sarkadi-Nagy, University of California, Department of Nutritional Sciences & Toxicology, Berkeley, CA 94720, USA, Tel.: +1 510 642 3978; Fax: +1 510 642 0535; sarkadi@berkeley.edu.
Hei Sook Sul, University of California, Department of Nutritional Sciences & Toxicology, Berkeley, CA 94720, USA, Tel.: +1 510 642 3978; Fax: +1 510 642 0535; hsul@nature.berkeley.edu.
Bibliography
- 1.Gregoire FM, Smas CM, Sul HS. Understanding adipocyte differentiation. Physiol. Rev. 1998;78(3):783–809. doi: 10.1152/physrev.1998.78.3.783. [DOI] [PubMed] [Google Scholar]
- 2.Walley AJ, Blakemore AI, Froguel P. Genetics of obesity and the prediction of risk for health. Hum. Mol. Genet. 2006;15(Suppl 2):R124–R130. doi: 10.1093/hmg/ddl215. [DOI] [PubMed] [Google Scholar]
- 3.Agarwal AK, Garg A. Congenital generalized lipodystrophy: significance of triglyceride biosynthetic pathways. Trends Endocrinol. Metab. 2003;14(5):214–221. doi: 10.1016/s1043-2760(03)00078-x. [DOI] [PubMed] [Google Scholar]
- 4.Bhayana S, Hegele RA. The molecular basis of genetic lipodystrophies. Clin. Biochem. 2002;35(3):171–177. doi: 10.1016/s0009-9120(02)00297-7. [DOI] [PubMed] [Google Scholar]
- 5.Reitman ML. Metabolic lessons from genetically lean mice. Annu. Rev. Nutr. 2002;22:459–482. doi: 10.1146/annurev.nutr.22.010402.102849. [DOI] [PubMed] [Google Scholar]
- 6.Shimomura I, Hammer RE, Richardson JA, et al. Insulin resistance and diabetes mellitus in transgenic mice expressing nuclear SREBP-1c in adipose tissue: model for congenital generalized lipodystrophy. Genes Dev. 1998;12(20):3182–3194. doi: 10.1101/gad.12.20.3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heilbronn L, Smith SR, Ravussin E. Failure of fat cell proliferation, mitochondrial function and fat oxidation results in ectopic fat storage, insulin resistance and Type II diabetes mellitus. Int. J. Obes. Relat. Metab. Disord. 2004;28(Suppl 4):S12–S21. doi: 10.1038/sj.ijo.0802853. [DOI] [PubMed] [Google Scholar]
- 8.Perseghin G. Muscle lipid metabolism in the metabolic syndrome. Curr. Opin. Lipidol. 2005;16(4):416–420. doi: 10.1097/01.mol.0000174401.07056.56. [DOI] [PubMed] [Google Scholar]
- 9.Raz I, Eldor R, Cernea S, et al. Diabetes: insulin resistance and derangements in lipid metabolism. Cure through intervention in fat transport and storage. Diabetes Metab. Res. Rev. 2005;21(1):3–14. doi: 10.1002/dmrr.493. [DOI] [PubMed] [Google Scholar]
- 10.Weiss SB, Kennedy EP, Kiyasu JY. The enzymatic synthesis of triglycerides. J. Biol. Chem. 1960;235:40–44. [PubMed] [Google Scholar]
- 11.Lehner R, Kuksis A. Biosynthesis of triacylglycerols. Prog. Lipid Res. 1996;35(2):169–201. doi: 10.1016/0163-7827(96)00005-7. [DOI] [PubMed] [Google Scholar]
- 12.Dircks L, Sul HS. Acyltransferases of de novo glycerophospholipid biosynthesis. Prog. Lipid Res. 1999;38(56):461–479. doi: 10.1016/s0163-7827(99)00012-0. [DOI] [PubMed] [Google Scholar]
- 13.Coleman RA, Lee DP. Enzymes of triacylglycerol synthesis and their regulation. Prog. Lipid Res. 2004;43(2):134–176. doi: 10.1016/s0163-7827(03)00051-1. [DOI] [PubMed] [Google Scholar]
- 14.Soma MR, Mims MP, Chari MV, et al. Triglyceride metabolism in 3t3-L1 cells. An in vivo 13c Nmr study. J. Biol. Chem. 1992;267(16):11168–11175. [PubMed] [Google Scholar]
- 15.Dircks LK, Ke J, Sul HS. A conserved seven amino acid stretch important for murine mitochondrial glycerol-3-phosphate acyltransferase activity. Significance of arginine 318 in catalysis. J. Biol. Chem. 1999;274(49):34728–34734. doi: 10.1074/jbc.274.49.34728. [DOI] [PubMed] [Google Scholar]
- 16.Yet SF, Lee S, Hahm YT, et al. Expression and identification of P90 as the murine mitochondrial glycerol-3-phosphate acyltransferase. Biochemistry. 1993;32(36):9486–9491. doi: 10.1021/bi00087a029. [DOI] [PubMed] [Google Scholar]
- 17.Yet SF, Moon YK, Sul HS. Purification and reconstitution of murine mitochondrial glycerol-3-phosphate acyltransferase. Functional expression in baculovirus-infected insect cells. Biochemistry. 1995;34(22):7303–7310. doi: 10.1021/bi00022a003. [DOI] [PubMed] [Google Scholar]
- 18.Lewin TM, Schwerbrock NM, Lee DP, et al. Identification of a new glycerol-3-phosphate acyltransferase isoenzyme, mtGPAT2, in mitochondria. J. Biol. Chem. 2004;279(14):13488–13495. doi: 10.1074/jbc.M314032200. [DOI] [PubMed] [Google Scholar]
- 19.Shin DH, Paulauskis JD, Moustaid N, et al. Transcriptional regulation of P90 with sequence homology to Escherichia Coli glycerol-3-phosphate acyltransferase. J. Biol. Chem. 1991;266(35):23834–23839. [PubMed] [Google Scholar]
- 20.Hammond LE, Gallagher PA, Wang S, et al. Mitochondrial glycerol-3-phosphate acyltransferase-deficient mice have reduced weight and liver triacylglycerol content and altered glycerolipid fatty acid composition. Mol. Cell Biol. 2002;22(23):8204–8214. doi: 10.1128/MCB.22.23.8204-8214.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harada N, Hara S, Yoshida M, et al. Molecular cloning of a murine glycerol-3-phosphate acyltransferase-like protein 1 (Xgpat1) Mol. Cell Biochem. 2007 doi: 10.1007/s11010-006-9321-5. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 22.Cases S, Smith SJ, Zheng YW, et al. Identification of a gene encoding an acyl coa:diacylglycerol acyltransferase, a key enzyme in triacylglycerol synthesis. Proc. Natl Acad. Sci. USA. 1998;95(22):13018–13023. doi: 10.1073/pnas.95.22.13018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buhman KK, Smith SJ, Stone SJ, et al. DGAT1 is not essential for intestinal triacylglycerol absorption or chylomicron synthesis. J. Biol. Chem. 2002;277(28):25474–25479. doi: 10.1074/jbc.M202013200. [DOI] [PubMed] [Google Scholar]
- 24.Buhman KK, Chen HC, Farese RV., Jr The enzymes of neutral lipid synthesis. J. Biol. Chem. 2001;276(44):40369–40372. doi: 10.1074/jbc.R100050200. [DOI] [PubMed] [Google Scholar]
- 25.Cases S, Stone SJ, Zhou P, et al. Cloning of DGAT2, a second mammalian diacylglycerol acyltransferase, and related family members. J. Biol. Chem. 2001;276(42):38870–38876. doi: 10.1074/jbc.M106219200. [DOI] [PubMed] [Google Scholar]
- 26.Lu B, Jiang YJ, Zhou Y, et al. Cloning and characterization of murine 1-acyl-sn-glycerol 3-phosphate acyltransferases and their regulation by PPARα in murine heart. Biochem. J. 2005;385(Pt 2):469–477. doi: 10.1042/BJ20041348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.West J, Tompkins CK, Balantac N, et al. Cloning and expression of two human lysophosphatidic acid acyltransferase cDNAs that enhance cytokine-induced signaling responses in cells. DNA Cell Biol. 1997;16(6):691–701. doi: 10.1089/dna.1997.16.691. [DOI] [PubMed] [Google Scholar]
- 28.Agarwal AK, Barnes RI, Garg A. Functional characterization of human 1-acylglycerol-3-phosphate acyltransferase isoform 8: cloning, tissue distribution, gene structure, and enzymatic activity. Arch. Biochem. Biophys. 2006;449(12):64–76. doi: 10.1016/j.abb.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 29.Vergnes L, Beigneux AP, Davis R, et al. AGPAT6 deficiency causes subdermal lipodystrophy and resistance to obesity. J. Lipid Res. 2006;47(4):745–754. doi: 10.1194/jlr.M500553-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carman GM, Han GS. Roles of phosphatidate phosphatase enzymes in lipid metabolism. Trends Biochem. Sci. 2006;31(12):694–699. doi: 10.1016/j.tibs.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Han GS, Wu WI, Carman GM. The Saccharomyces cerevisiae lipin homolog is a Mg2+-dependent phosphatidate phosphatase enzyme. J. Biol. Chem. 2006;281(14):9210–9218. doi: 10.1074/jbc.M600425200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Phan J, Reue K. Lipin, a lipodystrophy and obesity gene. Cell Metab. 2005;1(1):73–83. doi: 10.1016/j.cmet.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 33.Harris TE, Huffman TA, Chi A, et al. Insulin controls subcellular localization and multisite phosphorylation of the phosphatidiic acid phosphatase, lipin 1. J. Biol. Chem. 2006;282(1):277–286. doi: 10.1074/jbc.M609537200. [DOI] [PubMed] [Google Scholar]
- 34.Stone SJ, Levin M, Farese RV., Jr Murine acyl coa:diacylglycerol acyltransferase-2 (DGAT2): membrane topology and identification of key functional amino acid residues. J. Biol. Chem. 2007 doi: 10.1074/jbc.M607986200. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 35.Yen CL, Brown CH, 4th, Monetti M, et al. A human skin multifunctional O-acyltransferase that catalyzes the synthesis of acylglycerols, waxes, and retinyl esters. J. Lipid Res. 2005;46(11):2388–2397. doi: 10.1194/jlr.M500168-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen HC. Enhancing energy and glucose metabolism by disrupting triglyceride synthesis: lessons from mice lacking DGAT1. Nutr. Metab. (Lond.) 2006;3(1):10. doi: 10.1186/1743-7075-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen HC, Farese RV., Jr Inhibition of triglyceride synthesis as a treatment strategy for obesity: lessons from DGAT1-deficient mice. Arterioscler. Thromb. Vasc. Biol. 2005;25(3):482–486. doi: 10.1161/01.ATV.0000151874.81059.ad. [DOI] [PubMed] [Google Scholar]
- 38.Smith SJ, Cases S, Jensen DR, et al. Obesity resistance and multiple mechanisms of triglyceride synthesis in mice lacking DGAT. Nat. Genet. 2000;25(1):87–90. doi: 10.1038/75651. [DOI] [PubMed] [Google Scholar]
- 39.Chen HC, Smith SJ, Ladha Z, et al. increased insulin and leptin sensitivity in mice lacking acyl Coa:diacylglycerol acyltransferase 1. J. Clin. Invest. 2002;109(8):1049–1055. doi: 10.1172/JCI14672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stahl U, Carlsson AS, Lenman M, et al. Cloning and functional characterization of a phospholipid:diacylglycerol acyltransferase from arabidopsis. Plant Physiol. 2004;135(3):1324–1335. doi: 10.1104/pp.104.044354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dahlqvist A, Stahl U, Lenman M, et al. Phospholipid:diacylglycerol acyltransferase: an enzyme that catalyzes the acyl-Coa-independent formation of triacylglycerol in yeast and plants. Proc. Natl Acad. Sci. USA. 2000;97(12):6487–6492. doi: 10.1073/pnas.120067297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lehner R, Kuksis A. Triacylglycerol synthesis by an sn-1,2(2,3)-diacylglycerol transacylase from rat intestinalmicrosomes. J. Biol. Chem. 1993;268(12):8781–8786. [PubMed] [Google Scholar]
- 43.Jenkins CM, Mancuso DJ, Yan W, et al. Identification, cloning, expression, and purification of three novel human calcium-independent phospholipase A2 family members possessing triacylglycerol lipase and acylglycerol transacylase activities. J. Biol. Chem. 2004;279(47):48968–48975. doi: 10.1074/jbc.M407841200. [DOI] [PubMed] [Google Scholar]
- 44.Haemmerle G, Lass A, Zimmermann R, et al. Defective lipolysis and altered energy metabolism in mice lacking adipose triglyceride lipase. Science. 2006;312(5774):734–737. doi: 10.1126/science.1123965. [DOI] [PubMed] [Google Scholar]
- 45.Villena JA, Roy S, Sarkadi-Nagy E, et al. Desnutrin, an adipocyte gene encoding a novel patatin domain-containing protein, is induced by fasting and glucocorticoids: ectopic expression of desnutrin increases triglyceride hydrolysis. J. Biol. Chem. 2004;279(45):47066–47075. doi: 10.1074/jbc.M403855200. [DOI] [PubMed] [Google Scholar]
- 46.Gibbons GF, Islam K, Pease RJ. Mobilisation of triacylglycerol stores. Biochim. Biophys. Acta. 2000;1483(1):37–57. doi: 10.1016/s1388-1981(99)00182-1. [DOI] [PubMed] [Google Scholar]
- 47.Gilham D, Lehner R. The physiological role of triacylglycerol hydrolase in lipid metabolism. Rev. Endocr. Metab. Disord. 2004;5(4):303–309. doi: 10.1023/B:REMD.0000045101.42431.c7. [DOI] [PubMed] [Google Scholar]
- 48.Fredrikson G, Tornqvist H, Belfrage P. Hormone-sensitive lipase and monoacylglycerol lipase are both required for complete degradation of adipocyte triacylglycerol. Biochim. Biophys. Acta. 1986;876(2):288–293. doi: 10.1016/0005-2760(86)90286-9. [DOI] [PubMed] [Google Scholar]
- 49.Holm C. Molecular mechanisms regulating hormone-sensitive lipase and lipolysis. Biochem. Soc. Trans. 2003;31(Pt 6):1120–1124. doi: 10.1042/bst0311120. [DOI] [PubMed] [Google Scholar]
- 50.Karlsson M, Contreras JA, Hellman U, et al. Cdna cloning, tissue distribution, and identification of the catalytic triad of monoglyceride lipase. Evolutionary relationship to esterases, lysophospholipases, and haloperoxidases. J. Biol. Chem. 1997;272(43):27218–27223. doi: 10.1074/jbc.272.43.27218. [DOI] [PubMed] [Google Scholar]
- 51.Fredrikson G, Strålfors P, Nilsson NÖ, et al. Hormone-sensitive lipase of rat apidose tissue. J. Biol. Chem. 1981;256(12):6631–6320. [PubMed] [Google Scholar]
- 52.Greenberg AS, Shen WJ, Muliro K, et al. Stimulation of lipolysis and hormone-sensitive lipase via the extracellular signal-regulated kinase pathway. J. Biol. Chem. 2001;276(48):45456–45461. doi: 10.1074/jbc.M104436200. [DOI] [PubMed] [Google Scholar]
- 53.Rossmeisl M, Flachs P, Brauner P, et al. Role of energy charge and AMP-activated protein kinase in adipocytes in the control of body fat stores. Int. J. Obes. Relat. Metab. Disord. 2004;28(Suppl 4):S38–S44. doi: 10.1038/sj.ijo.0802855. [DOI] [PubMed] [Google Scholar]
- 54.Greenberg AS, Egan JJ, Wek SA, et al. Perilipin, a major hormonally regulated adipocyte-specific phosphoprotein associated with the periphery of lipid storage droplets. J. Biol. Chem. 1991;266(17):11341–11346. [PubMed] [Google Scholar]
- 55.Brasaemle DL, Rubin B, Harten IA, et al. Perilipin a increases triacylglycerol storage by decreasing the rate of triacylglycerol hydrolysis. J. Biol. Chem. 2000;275(49):38486–38493. doi: 10.1074/jbc.M007322200. [DOI] [PubMed] [Google Scholar]
- 56.Miyoshi H, Souza SC, Zhang HH, et al. Perilipin promotes hormone-sensitive lipase-mediated adipocyte lipolysis via phosphorylation-dependent and -independent mechanisms. J. Biol. Chem. 2006;281(23):15837–15844. doi: 10.1074/jbc.M601097200. [DOI] [PubMed] [Google Scholar]
- 57.Souza SC, Muliro KV, Liscum L, et al. Modulation of hormone-sensitive lipase and protein kinase a-mediated lipolysis by perilipin a in an adenoviral reconstituted system. J. Biol. Chem. 2002;277(10):8267–8272. doi: 10.1074/jbc.M108329200. [DOI] [PubMed] [Google Scholar]
- 58.Tansey JT, Huml AM, Vogt R, et al. Functional studies on native and mutated forms of perilipins. A role in protein kinase α-mediated lipolysis of triacylglycerols. J. Biol. Chem. 2003;278(10):8401–8406. doi: 10.1074/jbc.M211005200. [DOI] [PubMed] [Google Scholar]
- 59.Sztalryd C, Xu G, Dorward H, et al. Perilipin a is essential for the translocation of hormone-sensitive lipase during lipolytic activation. J. Cell Biol. 2003;161(6):1093–1103. doi: 10.1083/jcb.200210169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tansey JT, Sztalryd C, Hlavin EM, et al. The central role of perilipin a in lipid metabolism and adipocyte lipolysis. IUBMB Life. 2004;56(7):379–385. doi: 10.1080/15216540400009968. [DOI] [PubMed] [Google Scholar]
- 61.Miyoshi H, Perfield JW, 2nd, Souza SC, et al. Control of ATGL action by Serine 517 of perilipin a globally regulates PKA-stimulated lipolysis in adipocytes. J. Biol. Chem. 2006;282(2):99–1002. doi: 10.1074/jbc.M605770200. [DOI] [PubMed] [Google Scholar]
- 62.Brasaemle DL, Dolios G, Shapiro L, et al. Proteomic analysis of proteins associated with lipid droplets of basal and lipolytically stimulated 3t3-L1 adipocytes. J. Biol. Chem. 2004;279(45):46835–46842. doi: 10.1074/jbc.M409340200. [DOI] [PubMed] [Google Scholar]
- 63.Subramanian V, Rothenberg A, Gomez C, et al. Perilipin a mediates the reversible binding of CGI-58 to lipid droplets in 3t3-L1 adipocytes. J. Biol. Chem. 2004;279(40):42062–42071. doi: 10.1074/jbc.M407462200. [DOI] [PubMed] [Google Scholar]
- 64.Yamaguchi T, Omatsu N, Matsushita S, et al. CGI-58 interacts with perilipin and is localized to lipid droplets. Possible involvement of CGI-58 mislocalization in Chanarin-Dorfman syndrome. J. Biol. Chem. 2004;279(29):30490–30497. doi: 10.1074/jbc.M403920200. [DOI] [PubMed] [Google Scholar]
- 65.Lass A, Zimmermann R, Haemmerle G, et al. Adipose triglyceride lipase-mediated lipolysis of cellular fat stores is activated by CGI-58 and defective in Chanarin-Dorfman syndrome. Cell Metab. 2006;3(5):309–319. doi: 10.1016/j.cmet.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 66.Kraemer FB, Shen WJ. Hormone-sensitive lipase: control of intracellular tri-(di)acylglycerol and cholesteryl ester hydrolysis. J. Lipid Res. 2002;43(10):1585–1594. doi: 10.1194/jlr.r200009-jlr200. [DOI] [PubMed] [Google Scholar]
- 67.Ben Ali Y, Chahinian H, Petry S, et al. Might the kinetic behavior of hormone-sensitive lipase reflect the absence of the lid domain? Biochemistry. 2004;43(29):9298–9306. doi: 10.1021/bi049479o. [DOI] [PubMed] [Google Scholar]
- 68.Harada K, Shen WJ, Patel S, et al. Resistance to high-fat diet-induced obesity and altered expression of adipose-specific genes in HSL-deficient mice. Am. J. Physiol. Endocrinol. Metab. 2003;285(6):E1182–E1195. doi: 10.1152/ajpendo.00259.2003. [DOI] [PubMed] [Google Scholar]
- 69.Haemmerle G, Zimmermann R, Hayn M, et al. Hormone-sensitive lipase deficiency in mice causes diglyceride accumulation in adipose tissue, muscle, and testis. J. Biol. Chem. 2002;277(7):4806–4815. doi: 10.1074/jbc.M110355200. [DOI] [PubMed] [Google Scholar]
- 70.Haemmerle G, Zimmermann R, Zechner R. Letting lipids go: hormone-sensitive lipase. Curr. Opin. Lipidol. 2003;14(3):289–297. doi: 10.1097/00041433-200306000-00009. [DOI] [PubMed] [Google Scholar]
- 71.Osuga J, Ishibashi S, Oka T, et al. Targeted disruption of hormone-sensitive lipase results in male sterility and adipocyte hypertrophy, but not in obesity. Proc. Natl Acad. Sci. USA. 2000;97(2):787–792. doi: 10.1073/pnas.97.2.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Raclot T, Leray C, Bach AC, et al. The selective mobilization of fatty acids is not based on their positional distribution in white-fat-cell triacylglycerols. Biochem. J. 1995;311(Pt 3):911–916. doi: 10.1042/bj3110911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zimmermann R, Strauss JG, Haemmerle G, et al. Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase. Science. 2004;306(5700):1383–1386. doi: 10.1126/science.1100747. [DOI] [PubMed] [Google Scholar]
- 74.Smirnova E, Goldberg EB, Makarova KS, et al. ATGL has a key role in lipid droplet/adiposome degradation in mammalian cells. EMBO Rep. 2006;7(1):106–113. doi: 10.1038/sj.embor.7400559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kershaw EE, Hamm JK, Verhagen LA, et al. Adipose triglyceride lipase: function, regulation by insulin, and comparison with adiponutrin. Diabetes. 2006;55(1):148–157. [PMC free article] [PubMed] [Google Scholar]
- 76.Lake AC, Sun Y, Li JL, et al. Expression, regulation, and triglyceride hydrolase activity of adiponutrin family members. J. Lipid Res. 2005;46(11):2477–2487. doi: 10.1194/jlr.M500290-JLR200. [DOI] [PubMed] [Google Scholar]
- 77.Gronck S, Mildner A, Fellert S, et al. Brummer lipase is an evolutionary conserved fat storage regulator in Drosophila. Cell Metabolism. 2005;1:323–329. doi: 10.1016/j.cmet.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 78.Kurat CF, Natter K, Petschnigg J, et al. Obese yeast: triglyceride lipolysis is functionally conserved from mammals to yeast. J. Biol. Chem. 2006;281(1):491–500. doi: 10.1074/jbc.M508414200. [DOI] [PubMed] [Google Scholar]
- 79.Schweiger M, Schreiber R, Haemmerle G, et al. Adipose triglyceride lipase and hormone-sensitive lipase are the major enzymes in adipose tissue triacylglycerol catabolism. J. Biol. Chem. 2006;281(52):40236–40241. doi: 10.1074/jbc.M608048200. [DOI] [PubMed] [Google Scholar]
- 80.Soni KG, Lehner R, Metalnikov P, et al. Carboxylesterase 3 (Ec 3.1.1.1) is a major adipocyte lipase. J. Biol. Chem. 2004;279(39):40683–40689. doi: 10.1074/jbc.M400541200. [DOI] [PubMed] [Google Scholar]
- 81.Okazaki H, Igarashi M, Nishi M, et al. Identification of a novel member of the carboxylesterase family that hydrolyzes triacylglycerol: a potential role in adipocyte lipolysis. Diabetes. 2006;55(7):2091–2097. doi: 10.2337/db05-0585. [DOI] [PubMed] [Google Scholar]